Abstract
Diabetes causes a number of metabolic and physiologic abnormalities in the retina, but which of these abnormalities contribute to recognized features of diabetic retinopathy (DR) is less clear. Many of the molecular and physiologic abnormalities that have been found to develop in the retina in diabetes are consistent with inflammation. Moreover, a number of anti-inflammatory therapies have been found to significantly inhibit development of different aspects of DR in animal models. Herein, we review the inflammatory mediators and their relationship to early and late DR, and discuss the potential of anti-inflammatory approaches to inhibit development of different stages of the retinopathy. We focus primarily on information derived from in vivo studies, supplementing with information from in vitro studies were important.
1. Introduction
About 8 percent of the U.S. population has diabetes, and the number of people diagnosed with this disease is increasing rapidly in the US and the world. DR is a major cause of visual impairment, and is the leading cause of blindness in the United States for individuals 20-75 years of age (Kempen et al., 2004). The prevalence of DR in adult diabetic patients is greater than 40%, with approximately 5%-10% developing vision-threatening complications, including proliferative diabetic retinopathy (PDR), severe non-proliferative diabetic retinopathy, or macular edema (Kempen et al., 2004).
Many mechanisms have been postulated to explain the pathogenesis of the retinopathy, but many of these postulated mechanisms are focused on particular molecular abnormalities. In this review, we review evidence that supports a hypothesis that inflammatory-like processes play a critical role in the development of the early and late stages of the retinopathy, and that the inflammation hypothesis can encompass many of the previously postulated mechanisms under a broad “umbrella” hypothesis of the pathogenesis of diabetic retinopathy. We will first review the lesions of the retinopathy, then discuss studies that support the postulated role of inflammatory processes in the pathogenesis of diabetic retinopathy, as well as weaknesses of the present inflammatory hypothesis, and future directions.
2. Diabetic Retinopathy
The clinically visible lesions of diabetic retinopathy are mainly vascular in nature. Consequently, diabetic retinopathy has been regarded as a vascular disorder for many years. The natural history of the retinopathy has been divided into two stages based on the proliferative status of the retinal vasculature: an early, nonproliferative stage (NPDR; Fig1A), and an advanced, proliferative or neovascular stage (PDR; Fig1B). Neural abnormalities have also been recognized, and are now being explored to determine their clinical significance.
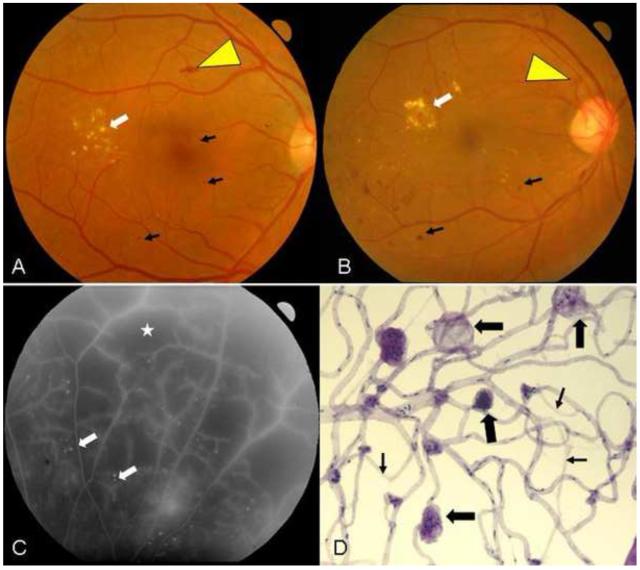
Fig 1.
A. Fundus image of a patient with moderate nonproliferative diabetic retinopathy (NPDR). Microaneurysms can be seen (black arrows) along with an area containing a flame-hemorrhage (yellow arrowhead) and exudates (white arrow) temporal to the fovea. B. Fundus image of a patient with proliferative diabetic retinopathy (PDR). Multiple areas containing microaneurysms can be seen here (black arrows) along with an area of neovascularization of the optic disc (yellow arrowhead) and exudates (white arrow) superotemporal to the fovea. C. Fluorescein angiogram of patient seen in B. Areas of profound retinal nonperfusion are identified by a star. Numerous white dots (white arrows) indicate the presence of microaneurysms, which are more easily visualized on fluorescein angiograms than color photos. D. Isolated retinal microvessels from a diabetic patient demonstrating numerous capillary microaneurysms (thick arrows) and degenerate capillaries (thin arrows).
2A. Early stages of diabetic retinopathy
Changes during the nonproliferative stage of the retinopathy rarely have clinical significance themselves, but increases in their presence and severity tend to predict progression towards the more advanced and clinically significant stages of the disease. Patients with early diabetic retinopathy commonly have retinal microaneurysms, which appear as red dots on dilated funduscopic examination. These microaneurysms are localized dilatations of the microvasculature which have been postulated to have developed as a result of localized weaknesses in the vessel wall, pressure disturbances, or glial retraction/death (Kern, 2007). An increase in the rate of appearance and disappearance of microaneurysms has been found to mark progression of the retinopathy, and to predict future reductions in visual function (Nunes et al., 2009). Microaneurysms have been detected also in diabetic dogs, cats, and primates, but have not been found to develop reproducibly in diabetic rodents (Kern, 2008; Zheng and Kern, 2010).
Capillary nonperfusion and degeneration also are important lesions of the early retinopathy (de Venecia et al., 1976; Kohner and Henkind, 1970), because they have been regarded as causal in the eventual progression to neovascularization (Shimizu et al., 1981) as summarized in this simple flowchart: 
Hypoxia stimulates the release of hypoxia-regulated vasoproliferative factors, such as Vascular Endothelial Growth Factor (VEGF), but VEGF has been found to be increased in retinas of diabetic animals also before capillary degeneration, indicating that also other factors regulate its induction in diabetes. Capillary nonperfusion is not detectable clinically without infusion of a fluorescent dye (fluorescein) into the blood (Fig 1C), but degenerate capillaries are very apparent in isolated preparations of the retinal microvasculature (Fig 1D). Diabetes-induced degeneration of retinal capillaries has been observed to develop in all animal species tested to date (Kern, 2008; Zheng and Kern, 2010), but the extent of capillary nonperfusion and degeneration that has developed in diabetic animal models studied for only a few years or less is modest compared to that in some diabetic patients (likely explaining the failure of animal models to progress to preretinal neovascularization).
Retinal edema or thickening of the retina occurs in some diabetic patients, and is believed to be due to breakdown of the blood-retinal barrier, resulting in localized increases in vascular permeability that exceed the pumping capacity of the retinal pigment epithelium. This increase in permeability occurs at the level of the vascular endothelium, and is both correlated with and secondary to increases in expression of VEGF (Ehrlich et al., 2010). In patients with early NPDR, the leakage seems to arise primarily from microaneurysms, and result in focal areas of edema.
Neural function and structure also are altered in the retina in diabetes. Diabetes results in a reduction in contrast sensitivity and electroretinogram (ERG) in diabetic patients and animals. Several studies of histologic material have demonstrated also that some retinal neuroglia are lost in diabetic patients and rodents (Barber et al., 1998). In vivo use of scanning laser polarimetry, optical coherence tomography and other techniques found a thinning of the thickness of the nerve fiber layer or retina in diabetic patients, further consistent with loss of retinal ganglion cells and their axons in diabetes (Kern and Barber, 2008).
2B. Advanced stages of diabetic retinopathy
The more advanced stages of diabetic retinopathy commonly are defined by retinal neovascular events and impairment of vision. The mechanisms of DR-related vision loss include vitreous hemorrhage, tractional retinal detachment from proliferative diabetic retinopathy, development of a fibrovascular membrane in the vitreous, and macular edema. Study of diabetic neovascularization and macular edema in laboratory animals has been problematic, as most laboratory species lack a macula, and have not shown the retinal neovascularization and thickening characteristic of advanced diabetic retinopathy in patients.
2C. Current therapies for diabetic retinopathy
Several therapeutic approaches are in use clinically to inhibit the development or progression of the retinopathy. The earlier stages of the retinopathy can be reduced by aggressive intervention to control hyperglycemia (Diabetes Control and Complications Trial Research Group, 1993; UK Prospective Diabetes Study Group, 1998), blood pressure, and lipids (Chaturvedi et al., 1998; Mauer et al., 2009; UK Prospective Diabetes Study Group, 1998). Unfortunately, maintaining normal metabolic control has been very difficult to accomplish in many diabetic patients. Data from studies showing a beneficial effect of lipid or blood pressure control recently have been challenged (Mancia, 2010; Mitka, 2010).
Treatments to inhibit advanced stages of the retinopathy include laser and vitrectomy, anti-VEGF therapies, and steroids. When used appropriately and in a timely manner, laser and vitrectomy help reduce the risk of catastrophic vision loss from DR (The Diabetic Retinopathy Study Research Group, 1981), although laser therapy is inherently destructive. A number of studies have implicated VEGF as a major causative factor in diabetic macular edema, retinal neovascularization and related complications (including vitreous hemorrhage and tractional retinal detachments) (Zhang et al., 2009b). Macular edema in diabetic patients can be significantly reduced by intravitreal administration of VEGF antagonists (Elman et al., 2010; Kashani et al., 2010), or steroids (Gillies et al., 2006; Yilmaz et al., 2009). Unfortunately, the beneficial effects of intravitreal steroids have been found to be temporary compared to effects of standard laser photocoagulation (Grover et al., 2008), and complications (cataract formation and steroid-induced glaucoma) have developed after intravitreal steroids (Jones and Rhee, 2006).
Given the limitations and side effects of current treatments of diabetic retinopathy, there has been a continuing effort to understand the molecular mechanisms that contribute to the early changes seen in the retinas of diabetics. One hypothesis that is gaining considerable experimental support as a cause of diabetic retinopathy is inflammation.
3. Inflammation and diabetic retinopathy
3A. What is inflammation?
Inflammation is a nonspecific response to injury that includes a variety of functional and molecular mediators, including recruitment and/or activation of leukocytes. Inflammation typically has beneficial effects on an acute basis, but can have undesirable effects if persisting chronically. The classic cellular inflammation model has been recognized for decades, but current discussions of inflammation include also molecular changes and mechanisms (Fig 2). Inflammation is one of the means by which the innate immune system of a host rapidly protects itself after exposure to an antigen or microorganism. Recognition of pathogens by the innate immune system is mediated by specific binding of the pathogen to pattern recognition receptors, such as Toll-like receptors (TLR) and Receptor for Advanced Glycation Endproducts (RAGE). The ligands for these receptors are categorized as classes of molecules, termed “pathogen-associated molecular patterns” (PAMPs). Activation of TLRs results in the production of cytokines such as Tumor Necrosis Factor-alpha (TNFα) and interleukin-1-beta (IL-1β), which act to induce the expression of pro-inflammatory proteins. Inflammation normally resolves promptly through a coordinated program that includes resolvins, lipoxins, and protectins (Serhan, 2007).
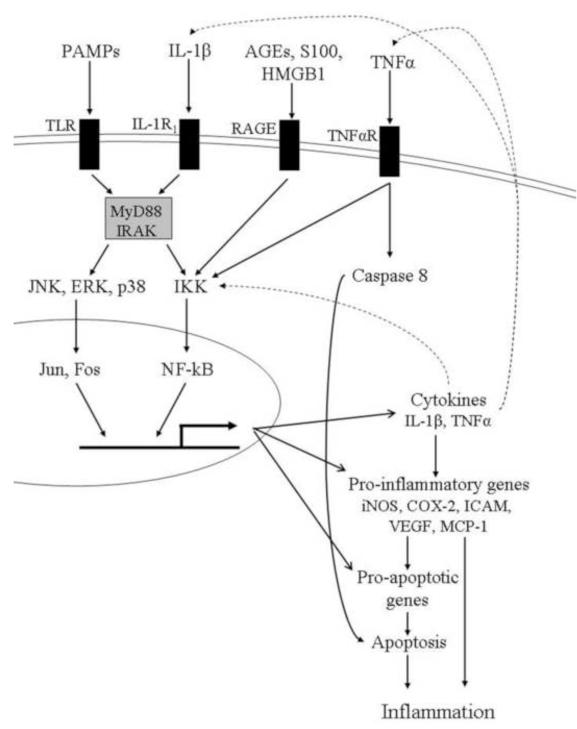
Fig 2.
Summary of the relation of the innate immune system to inflammation. As indicated in the text, many of the components of this system are found to be abnormal in retinas of diabetic animals. 1l-1R1, interleukin-1 receptor; AGEs, advanced glycation endproducts; HMGB1, high mobility box group 1; TNFαR, receptor for TNFα; MyD88, Myeloid differentiation primary response gene (88); IRAK, Interleukin-1 receptor-associated kinases; IKK, IκB kinase; p38; p38 MAP kinase
The increased expression of many inflammatory proteins is regulated at the level of gene transcription through the activation of proinflammatory transcription factors, including Nuclear Factor-kappa-B (NF-κB). NF-κB activation eventually leads to the synthesis of many cytokines, chemokines, acute phase proteins, and pro-inflammatory molecules. In autoimmune disease and inflammatory conditions, proinflammatory proteins such as cyclooxygenase-2 (COX-2), IL-1β, the inducible isoform by nitric oxide synthetase (iNOS), and TNFα are induced.
3B. Inflammation and early stages of diabetic retinopathy
A possible contribution of inflammation to the development of diabetic retinopathy developed out of initial reports that diabetic patients taking salicylates to treat rheumatoid arthritis had a lower-than-expected incidence of DR (Powell and Field, 1964). Since then, a variety of physiologic and molecular abnormalities that are consistent with inflammation have been found to be increased in the retinas or vitreous humor of diabetic animals and patients. Microarray analyses likewise have shown an inflammatory response in retinas from diabetic rodents (Brucklacher et al., 2008).
These pro-inflammatory changes are consistent with the innate immune pathway and have been reviewed also elsewhere (Adamis and Berman, 2008; Kaul et al., 2010; Kern, 2007). Many of these inflammatory changes seem important in the development of diabetic retinopathy because inhibiting them blocks the development of lesions characteristic of the retinopathy in animals. Inflammatory molecules that have been shown to contribute to structural or functional alterations that are characteristic of the retinopathy are summarized in Table 1, and more detailed information about each of these abnormalities follows in Sections 3B1 and 3B2. Subsequently, this chapter includes a discussion of how these abnormalities apparently interact (Section 4), and a discussion of which of these inflammatory abnormalities might be good therapeutic targets at which to inhibit the retinopathy (Section 5). Our present understanding of the role of inflammatory processes in the pathogenesis of diabetic retinopathy is at an early stage, and needs to be expanded.
Table 1.
Inflammatory molecules that have been shown to be involved in the development of clinically recognized lesions of diabetic retinopathy
3B1. Molecular changes in diabetic retinopathy
iNOS and nitric oxide (NO)
Upregulation of iNOS has been found in retinas of experimental diabetic rodents and patients in most studies (Zheng and Kern, 2009). A possible role of this enzyme in the pathogenesis of diabetic retinopathy was suggested initially by the studies using aminoguanidine. Aminoguanidine is an inhibitor of iNOS, and has been found to inhibit the diabetes-induced increase in NO production and iNOS expression in retina (Du et al., 2002), as well as the development of the microvascular lesions of diabetic retinopathy in diabetic rats, dogs, and mice (Zheng and Kern, 2009). Nevertheless, aminoguanidine also has other effects, so this therapy does not prove a role of iNOS in the pathogenesis of the retinopathy. The role of iNOS in the development of the early stages of diabetic retinopathy recently has been demonstrated directly using mice genetically deficient in iNOS (Leal et al., 2007; Zheng et al., 2007a) (Fig 3). In those studies, diabetic mice in which iNOS had been deleted or inhibited did not develop diabetes-induced structural (including capillary degeneration) or functional (permeability) abnormalities in the retina. This contribution of iNOS to development of the retinopathy seems not to be necessarily true of other nitric oxide synthases, because deletion of endothelial nitric oxide synthase exacerbates the retinopathy (Li et al., 2010b).
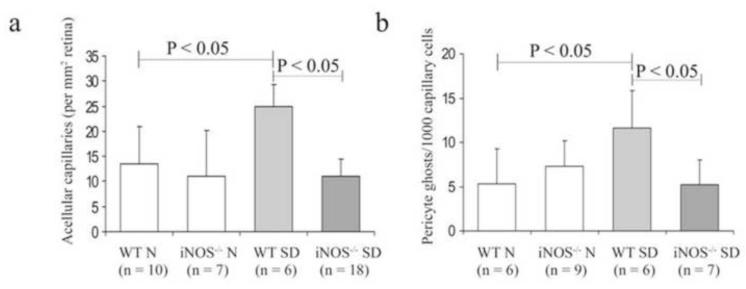
Fig 3.
Genetic deletion of the proinflammatory protein, iNOS, inhibits diabetes-induced (a) capillary degeneration and (b) pericyte loss in retinal vessels from mice diabetic for 9 months. N, nondiabetic; SD, streptozotocin diabetic; WT, wildtype; iNOS-/-, iNOS deficient. (Used with kind permission from Springer Science+Business Media: Diabetologia. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. 2007 50(9):1987-96; Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA, Kern TS; Fig 4).
Production of nitric oxide results in both nitration and nitrosylation of retinal proteins (Ali et al., 2008; El-Remessy et al., 2003a; El-Remessy et al., 2005; El-Remessy et al., 2003b; Zhan et al., 2007), resulting in potentially toxic effects (Ali et al., 2008).
Eicosanoids and lipids
Diabetes alters the lipid profile of the retina (Tikhonenko et al., 2010). Eicosanoids are metabolites of arachidonic acid, and are known mediators of inflammation. Two major families of eicosanoids are the prostaglandins (synthesized by cyclooxygenases) and leukotrienes (synthesized via lipoxygenases).
In retinas of diabetic animals, induction of COX-2 as well as increased production of prostaglandins has been reported. In advanced stages of diabetic retinopathy, COX-2 was identified in vascular endothelial cells in fibrovascular epiretinal membranes removed from diabetic patients (El-Asrar et al., 2008). PGE2 production by retinas from diabetic rats was significantly inhibited by celecoxib (a selective COX-2 inhibitor), but not by a COX-1 inhibitor (Ayalasomayajula and Kompella, 2004), suggesting that COX-2 is responsible for the diabetes-induced increase in retinal prostaglandin production. Inhibition of COX-2 also inhibited the diabetes-induced upregulation of retinal VEGF (Ayalasomayajula and Kompella, 2003), increase in retinal vessel permeability and leukostasis (Joussen et al., 2002), and death of retinal endothelial cells cultured in diabetic-like concentrations of glucose (Du et al., 2004). The COX-2 inhibitor, Meloxicam, also reduced eNOS levels, inhibited NF-κB activation in the diabetic retina, and partially reduced TNFα levels in the retina (Joussen et al., 2002). The effect of selective COX inhibitors on histologic lesions of diabetic retinopathy has not been studied, but less selective COX inhibitors (such as salicylates) have inhibited the development of the retinal vascular histopathology in diabetic dogs and rodents (Kern and Engerman, 2001; Zheng et al., 2007b). The COX inhibitor, Nepafenac, inhibited the diabetes-induced increases in retinal prostaglandin production and leukocyte adhesion in retinal vessels, as well as apoptosis of retinal capillary cells, and degeneration of retinal pericytes and capillaries (Kern et al., 2007).
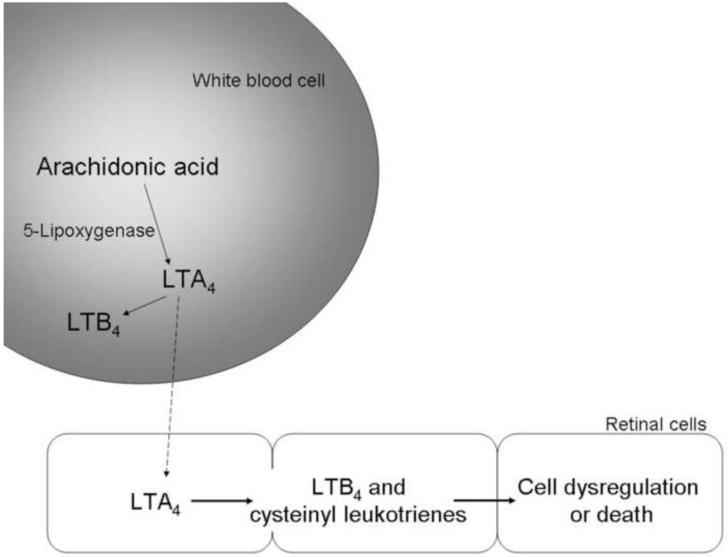
Products of 5-lipoxygenase, including leukotriene B4 (LTB4) and leukotrienes C4/D4/E4, are important in leukocyte recruitment and vascular permeability, respectively. Lipoxygenase-derived 5-hydroxyeicosatetraenoic acid (5-HETE) and other arachidonate and docosahexanoate-derived lipid autacoids are increased in the vitreous of diabetic patients (Schwartzman et al., 2010). Deficiency of 5-lipoxygenase inhibited the diabetes-induced degeneration of retinal capillaries, as well as leukostasis and superoxide generation in mice (Gubitosi-Klug et al., 2008) (Fig 4). In contrast, deficiency of 12-lipoxygenase inhibited leukostasis in the retinal vasculature of diabetic mice, but did not inhibit the degeneration of retinal capillaries. Antagonism of the BLT1 receptor inhibited LTB4-induced death of retinal endothelial cells in culture (Talahalli et al., 2010).
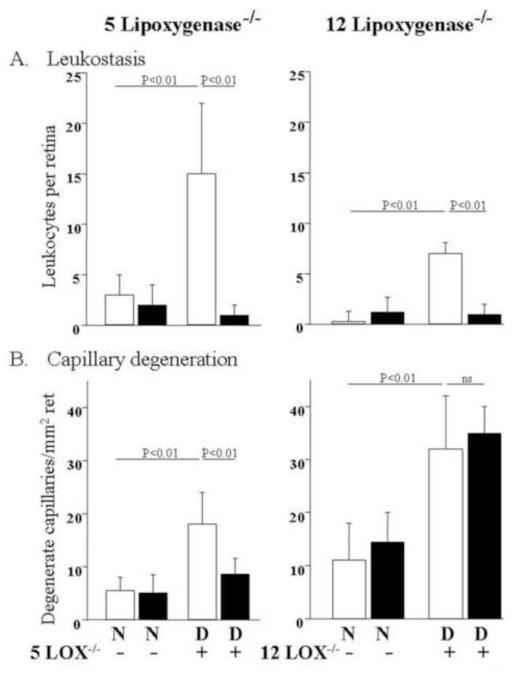
Fig 4.
A. Deletion of either 5- or 12 lipoxygenase significantly inhibited diabetes-induced leukostasis compared to nondiabetic controls. Wildtype mice and mice genetically deficient in 5-lipoxygenase or 12-lipoxygensase were made diabetic for 9 months or kept as nondiabetic controls. B. Inhibition of diabetes-induced capillary degeneration by deficiency of 5-lipoxygenase, but not 12-lipoxygenase. N, nondiabetic; D, diabetic. (Copyright 2008 American Diabetes Association From Diabetes, Vol. 57, 2009; 1387-1393 Reprinted with permission from the American Diabetes Association).
Interestingly, retinas from nondiabetic or diabetic mice produce neither leukotrienes nor 5-lipoxygenase mRNA, but addition of exogenous leukotriene A(4) to retina or retinal glial cells results in robust production of leukotriene B(4) or cysteinyl leukotrienes. Thus, retinal cells can produce pro-inflammatory/ toxic prostanoids, but only if they are provided the initial substrate (LTA4) from another cell type (Fig 5). In contrast to retinal cells, bone marrow cells (which mature into white blood cells) from diabetic mice produced greater than normal amounts of LTB4 (Talahalli et al., 2010). This data suggests that marrow-derived cells can generate LTA4, and that transcellular delivery of prostanoid precursors from blood-borne cells to the retina can contribute to the death of endothelial cells, and likely also, the chronic inflammation in diabetic retinopathy (Talahalli et al., 2010).
Fig 5.
Schematic summarizing transcellular transfer between the retina and marrow-derived cells that is postulated to contribute to inflammatory changes and cell death in diabetic retinopathy.
In contrast to pro-inflammatory effects of some lipids, docosohexanoic acid, resolvins and other autocoids have been shown to have anti-inflammatory actions in retinal cells (Chen et al., 2005; Opreanu et al., 2010). Busik and collaborators have reported also that administration of docosahexanoic acid inhibits diabetes-induced degeneration of retinal capillaries in animals (unpublished), but whether or not this is related to anti-inflammatory effects remains to be learned.
Adhesion molecules and integrins
White blood cells bind to ICAM-1 on the surface of endothelial cells in a multi-step process leading to adherence of the blood cells to the endothelial wall, a characteristic of inflammation. ICAM-1 is upregulated by several stimuli, including VEGF, PARP activation, oxidative stress, and dyslipidemia, at least in part via NF-κB. VCAM expression also is increased in the retinal vasculature in diabetes. Diabetic mice genetically deficient in ICAM-1 or its ligand (CD18) were protected from the expected development of lesions of early diabetic retinopathy (including capillary degeneration, pericyte loss and increased permeability) as well as leukostasis (Joussen et al., 2004). Topical administration of a small molecule antagonist of leukocyte function associated antigen-1 (LFA-1) to diabetic rats has been shown to significantly reduce retinal leukostasis and blood-retinal-barrier breakdown (Rao et al., 2010).
Integrin alpha 4/CD49d has been identified as another mediator of leukocyte adhesion and alterations of retinal vascular physiology in early diabetic retinopathy. Blockade of this integrin attenuated the diabetes-induced inflammatory changes in retina, including activation of NF-κB, upregulation of VEGF and TNFα, leukostasis and vascular leakage (Iliaki et al., 2009).
VEGF
VEGF is known to be a pro-inflammatory molecule whose vitreal levels are highly correlated with retinal neovascularization and edema. Intraocular delivery of anti-VEGF therapies are now used widely to treat advanced diabetic retinopathy (for a review see (Wirostko et al., 2008). The actions of VEGF to enhance permeability and endothelial cell migration/proliferation during angiogenesis are well documented, and might occur via vascular inflammation. VEGF has been shown to promote endothelial cell expression of ICAM-1), leading to leukocyte activation and cytokine release, thereby causing further increases in VEGF expression and amplification of the inflammatory response. Specific blockade of endogenous VEGF(164) resulted in a significant suppression of retinal leukostasis and BRB breakdown in both early and established diabetes (Ishida et al., 2003a). VEGF is produced to a large degree in Müeller (glial) cells of the retina, and inhibition of Müeller cell-derived VEGF significantly decreased expression of TNFα, ICAM-1 and NF-κB in diabetic mice (Wang et al., 2010). Inhibition of VEGF in the retina using a sulfonated oligosaccharide was associated with inhibition of leukostasis and ERG changes in diabetic rats (Ma et al., 2009).
Cytokines and chemokines
Levels of IL-1β and TNFα are increased in retinas from diabetic animals. Caspase-1 is the enzyme that generates active IL-1β from its precursor, and the biological activity of IL-1β is mediated by binding to the cell surface receptor, IL-1R1. Activity of caspase-1 is increased in retinas of diabetic mice, galactose-fed mice, diabetic humans, and in retinal Müller cells incubated in elevated glucose concentration (Mohr et al., 2002). Dietary antioxidants (Kowluru and Odenbach, 2004; Yulek et al., 2007) or inhibition of caspase-1 using minocycline (Vincent and Mohr, 2007) inhibited the diabetes-induced increase in IL-1β in retina, and inhibited degeneration of retinal capillaries in those animals (Vincent and Mohr, 2007). As a further confirmation of the role of IL-1β in degeneration of retinal capillaries, mice lacking the IL-1β receptor were protected from degeneration of retinal capillaries in diabetes (Vincent and Mohr, 2007). One known action of IL-1β is to activate NF-κB.
Eternacept, a soluble TNFα receptor that acts as competitive inhibitor to block effects of TNFα binding to cells, reduced leukocyte adherence in retinal blood vessels (Joussen et al., 2002) and blood-retinal barrier breakdown and NF-κB activation in the diabetic retina (Joussen et al., 2009). Intravitreal injection of another TNFα-specific inhibitor, pegsunercept, led to a significant reduction in pericyte loss and capillary degeneration in diabetic rats (Behl et al., 2008; Behl et al., 2009), and mice genetically deficient in TNFα were reported to have less diabetes-induced increase in vascular permeability and leukostasis in diabetes (Huang et al., 2011), and pericyte and endothelial cell loss in experimental galactosemia (Joussen et al., 2009). Consistent with a role of TNFα in the diabetes-induced degeneration of retinal capillaries, DNA binding of transcription factor Forkhead box O1 (FOXO1), which is regulated by TNFα, is elevated in retinas of animals having type 1 and type 2 diabetes, and the diabetes-induced degeneration of retinal capillaries and pericyte loss were inhibited by intravitreal injection of FOXO1 siRNA (Behl et al., 2009).
Vitreal concentrations of proinflammatory cytokines (TNFα, IL-8,and IL-6), chemokines (monocyte chemotactic protein-1 (MCP-1) and other proteins (endothelin-1, sE-selectin, VEGF, ICAM-1, CXCL10/IP-10) have been found to be higher in patients with PDR or diabetic macular edema than in controls. Vitreous samples and epiretinal membranes obtained by vitrectomy in advanced DR also have significantly increased levels of IL-6, IL-8, and MCP-1 in diabetic macular edema (Kocak et al., 2010).
Complement activation
Deposition of C5b-9, the terminal product of complement activation, has been observed within retinal blood vessels of diabetic humans (Dagher et al., 2004; Zhang et al., 2002), and complement C3 and complement factor I, as well as prothrombin, alpha-1-antitrypsin, antithrombin III and Factor XIII were increased in vitreous of patients having PDR (Gao et al., 2008). Immunohistological study of pre-retinal membranes from diabetic patients showed deposition of complement components within the connective stroma and along new vessels (Baudouin et al., 1993), as well as the presence of C3d, C5b-9, and vitronectin in the choriocapillaris of eyes with DR (Gerl et al., 2002).
Fas
Fas levels are increased in retinas of rats diabetic for 2 weeks, and blocking FasL in vivo inhibited endothelial cell damage, vascular leakage, and platelet accumulation in diabetes (Joussen et al., 2003).
NF-κB and other transcription factors
NF-κB is a widely expressed inducible transcription factor that is an important regulator of many genes involved in inflammatory and immune responses, cellular proliferation and apoptosis. Activation of NF-κB results most commonly in the translocation of p50-p65 heterodimers into the nucleus, where transcription of a variety of pro-inflammatory proteins (including iNOS, ICAM, and cytokines) subsequently are induced. Diabetes has been shown to activate NF-κB in rodent retinas ( Zheng et al., 2004; Kowluru et al., 2006), and to cause migration of the p65 subunit into nuclei of retinal endothelial cells, pericytes, ganglion cells, or cells of the inner nuclear layer (Romeo et al., 2002; Zheng et al., 2007b). DNA-binding experiments also have demonstrated increased DNA-binding activity of NF-κB in retinal endothelial cells or pericytes exposed to elevated glucose concentration. NF-κB expression (mRNA and immunohistochemical analysis) was higher than normal in epiretinal membranes of patients with PDR (Harada et al., 2004; Mitamura et al., 2003).
There is increasing evidence in support of an important role of NF-κB in the pathogenesis of early stages of DR. Seemingly selective inhibition of NF-κB activation using dehydroxymethylepoxyquinomicin inhibited diabetes-induced increases in retinal leukostasis and expression of ICAM-1 and VEGF in vivo (Nagai et al., 2007), but studies on long-term histopathology were not conducted. Diabetes-induced degeneration of retinal capillaries and expression of inflammatory proteins nevertheless were inhibited by less selective therapies that inhibited activation of retinal NF-κB in diabetes (salicylates such as aspirin, sodium salicylate, and sulfasalazine (Zheng et al., 2007b) or antioxidants (Kowluru et al., 2003)). Deletion of p105, a precursor to the p50 subunit of NF-κB, resulted in accelerated degeneration of retinal capillaries in diabetes (Veenstra and Kern, in preparation). We postulate that deletion of p105 in our diabetic mice removes an important potential regulator of NF-κB-dependent transcription, thus resulting in supranormal retinal inflammation and subsequent histopathology.In addition to its well-recognized role in target gene transactivation by forming heterodimers with RelA, RelB, or c-Rel , the p50 subunit also can form p50-50 homodimers that block transactivation by the classical NF-κB (Ziegler-Heitbrock, 2001).
A variety of other transcription factors are altered in the retina in diabetes (Kern, unpublished), but these have not yet been implicated in the events that lead to diabetic retinopathy. Additional research is expected to provide additional information about which transcription factors contribute to the development of the retinopathy.
CCl2 (CC motif, ligand 2, also known as monocyte chemotactic protein1)
Levels of CCL2 have been detected in the vitreous of patients with proliferative DR (Hernandez et al., 2005), increased levels of CCL2 mRNA or protein have been found to be increased in the retina of diabetic rodents (Brucklacher et al., 2008; Zhang et al., 2009a). In vitro studies indicate that NADPH oxidase, Akt and NF-κB are required for the CCL2 production. Whether or not this change is causally related to development of lesions of the retinopathy is not known at present.
Pigment epithelium-derived factor (PEDF)
Pigment epithelium-derived factor (PEDF) is a member of the superfamily of serine protease inhibitors with complex neurotrophic, neuroprotective, anti-angiogenic, anti-oxidative, and anti-inflammatory properties (Yamagishi et al., 2008; Yoshida et al., 2009). High glucose decreased the expressions of PEDF in retinal Müller cells, and vitreous levels of PEDF were significantly lower in patients with DME or PDR than in nondiabetic patients or diabetic patients without retinopathy. This deficiency likely has proinflammatory effects, since intravitreal injection of PEDF significantly reduced vascular hyper-permeability in rat models of diabetes and oxygen-induced retinopathy, correlating with the decreased levels of retinal inflammatory factors (including VEGF, VEGF receptor-2, MCP-1, TNFα, and ICAM-1) (Zhang et al., 2006). Moreover, down-regulation of PEDF expression by siRNA resulted in significantly increasing the expression of IL-1β in retinal Müller cells (Shen et al., 2010). Long-term administration of an angiotensin-converting enzyme inhibitor to diabetic rats inhibited the diabetes-induced increase in VEGF-to-PEDF ratio, and inhibited capillary degeneration (Zheng et al., 2009).
Angiotensin II
Angiotensin II (Ang II), a major effector of the renin-angiotensin system, is now recognized as a pro-inflammatory mediator. This Ang II signaling causes transcription of pro-inflammatory genes via NF-κB.(Ghattas et al., 2011; Jeganathan, 2011; Zhou and Yang, 2010)
Rho/Rho kinase (ROCK) pathway
The Rho/Rho kinase pathway has been implicated in diabetic microvascular disease via inflammatory mechanisms. Intravitreal injection of a selective ROCK inhibitor significantly inhibited ICAM-1 expression, leukocyte adhesion, and the number of damaged endothelium in retinas of diabetic rats (Arita et al., 2009).
RAGE
RAGE is significantly elevated in the diabetic retina (especially in Muller glia) (Barile et al., 2005; Zong et al., 2010), and its inhibition reduced diabetes-induced capillary degeneration (Barile et al., 2005; Li et al., 2011). RAGE signaling induces inflammatory changes, as shown by the ability of RAGE inhibitors to block cytokine responses induced by high glucose in vitro (Zong et al., 2010) and diabetes-induced upregulation of retinal ICAM in vivo (Li et al., 2011).
3B2. Functional changes in diabetic retinopathy
Permeability
Breakdown of the blood-retinal barrier in diabetes has been attributed to increases in leukostasis, cytokines and growth factors (Antonetti et al., 1999; Harhaj et al., 2006; Joussen et al., 2001). Molecular alterations, such as in proteins of the tight junction complex, also have been demonstrated to play a significant role in the diabetes-induced increase in capillary permeability (Erickson et al., 2007). VEGF is known to be a key molecule leading to retinal permeability in diabetes and other retinal diseases, and there has been considerable clinical effort to inhibit DME using VEGF antagonists or traps. TNFα likewise has been shown to increase retinal endothelial permeability increase via protein kinase C zeta (Aveleira et al., 2010). Permeability has been reported to increase also in diabetic animals, and a variety of therapies having anti-inflammatory effects have been reported to inhibit the diabetes-induced increase in retinal vascular permeability. Whether increased permeability causes retinal inflammation in diabetes, or if inflammatory changes cause the diabetes-induced increase in permeability, or both, has not been adequately addressed at present.
Leukostasis
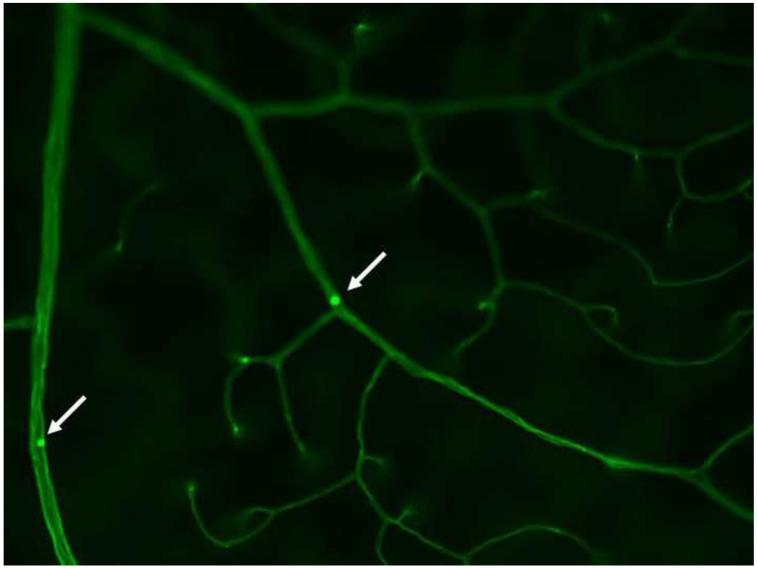
Leukocytes might contribute to microvascular damage by releasing cytokines and superoxide via the respiratory burst, or by physically occluding the capillaries (Fig 6), thereby causing a local ischemia downstream of the blockage. White blood cells interact with, and bind to, ICAM-1 and VCAM on the surface of endothelial cells in a multi-step process leading to adherence of the blood cells to the endothelial wall (leukostasis). This leukostasis is known to be increased in retinal blood vessels of diabetic rats, mice and monkeys, and is influenced by a variety of diabetes-induced abnormalities, including oxidative stress, inflammatory molecules, and the renin-angiotensin system. Elevated numbers of intravascular polymorphonuclear leukocytes have been detected adjacent to areas of capillary nonperfusion in retinas of diabetic monkeys (Kim et al., 2005), and leukocytes accumulated in choroidal vessels of diabetic humans (Lutty et al., 1997). Leukostasis commonly is associated with diabetic retinopathy in animal models, and deletion of proteins important in adherence of white blood cells to endothelium (ICAM-1 and CD-18) significantly inhibited diabetes-induced capillary degeneration (Joussen et al., 2004). Additionally, leukocytes from diabetic, but not control, rats induced endothelial cell apoptosis in vitro (Joussen et al., 2003).
Fig 6.
Adherence of a leukocyte to the wall of a retinal capillary (leukostasis). The vasculature has been perfused to remove all free blood cells and plasma, and then the vasculature perfused with Concanavalin A-FITC, which stains the endothelium light green, and adherent leukocytes bright green (arrow).
Nevertheless, other studies (Gubitosi-Klug et al., 2008; Kern et al., 2010) (Fig 5) suggest that leukostasis as measured by the ex vivo technique (Joussen et al., 2002) probably is not the cause of retinal capillary degeneration, because diabetes-induced degeneration of retinal capillaries was not inhibited in some studies even though leukostasis was inhibited. Some leukocytes do occlude retinal capillaries in diabetes (as demonstrated in vivo (Azuma et al., 1998), but the ex vivo method to measure leukostasis has an additional potentially confounding variable that comes from perfusion itself; it seems possible that perfusion to wash free blood and leukocytes out of the vessels might artifacticiously lodge relatively stiff white blood cells in the capillary bed.
Vision
Reductions in vision are a major cause of morbidity in diabetes, and diabetes impairs visual acuity and contrast sensitivity also in mice (Barber et al., 2010; Li et al., 2010a). Pharmacologic inhibition of p38 MAPK or RAGE from the onset of diabetes had no effect on the defect in contrast sensitivity (Li et al., 2010a), raising questions about the contribution of these inflammatory changes in the pathogenesis of these diabetes-induced defects in visual function in diabetic mice. Additional work is needed to better determine whether or not other diabetes-induced inflammatory processes in the retina play a role in the development of diabetes-induced alterations in visual function.
3B3. Inflammatory changes in specific cell types
Endothelial cells
ICAM is known to be upregulated on retinal endothelial cells in diabetes (McLeod et al., 1995; Miyamoto et al., 1999). In BREC, elevated glucose increased NO and PGE(2) significantly, whereas expression of iNOS and COX-2 were unchanged (Du et al., 2004). Interaction of AGEs with RAGE on endothelial cells enhances vascular activation, vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and E-selectin, and stimulated leukocyte adherence to the endothelium (Massaro et al., 2002; Schmidt et al., 1995). Deposition of C5b-9, the terminal product of complement activation, has been detected on endothelial cells of the retina and choriocapillaris in diabetic patients or animals (Gerl et al., 2002; Zhang et al., 2002). In contrast to a number of studies using animals cells, human retinal endothelial cells (unlike retinal pericytes or Muller cells) did not stimulate endogenous ROS production, activation of NF-κB, or other pro-inflammatory changes when exposed to elevated glucose, although they did show these pro-inflammatory changes after exposure to proinflammatory cytokines (Busik et al., 2008). Whether or not the apparent difference among species with respect to response to hyperglycemia is due to true species differences or differences in the degree of contamination of the preparations remains to be learned.
Pericytes
Continuous high glucose exposure for 2-12 days significantly elevated gene expressions and protein concentrations of IL-1β , NF-κB, VEGF, TNFα, TGF-beta and ICAM-1 in retinal pericytes (Kowluru et al., 2010; Romeo et al., 2002), and these inflammatory changes persisted even after restoration of normal glucose concentrations (Kowluru et al., 2010).
Müller (glial) cells
VEGF is produced in Müller cells of the retina, and inhibition of Müller cell-derived VEGF significantly decreased retinal expression of TNFα, ICAM-1 and NF-κB in diabetic mice (Wang et al., 2010). Other inflammatory proteins, including iNOS and nitric oxide, ICAM, cytokines, and PGE2 are produced by Müller cells exposed to elevated levels of glucose (Du et al., 2004). Diabetes significantly elevated RAGE expression in Muller glia (Barile et al., 2005; Zong et al., 2010), and pro-inflammatory responses by retinal Müller glia in elevated glucose are regulated by RAGE (Zong et al., 2010).
Microglia
Microglia are considered one of the principal cells sensing abnormal stimuli to neural tissue, and they release proinflammatory and neurotoxic substances when activated. Microglial activation was observed In recent animal studies of early diabetic retinopathy (Krady et al., 2005; Rungger-Brandle et al., 2000; Zeng et al., 2008), and therapies that inhibited microglial activation (although not selectively) attenuated retinal inflammation in diabetes (Ibrahim et al., 2010; Krady et al., 2005). A recent in vitro study suggests that glycated compounds that react with microglial contribute to activation of the cells, and secretion of TNFα (Ibrahim et al., 2011).
Bone marrow-derived cells
Diabetes-induced inflammatory changes, superoxide production, and degeneration of retinal capillaries were inhibited in diabetic mice in which inflammatory proteins (PARP-1 or iNOS) were deleted only from bone marrow cells (Li, Veenstra, Talahalli, Wang, Gubitosi-Klug, Sheibani, Kern; under review). This provides strong evidence that marrow-derived cells such as leukocytes play a critical role in development of the retinopathy in animals.
4. Inflammatory molecules and the vascular lesions of diabetic retinopathy; multiple mechanisms or a common pathway?
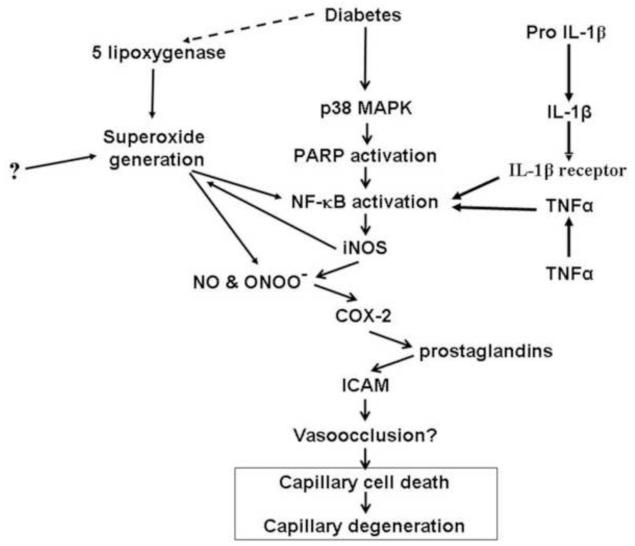
Inflammatory proteins described in this chapter have been associated with the diabetes-induced microvascular disease in animal models, and inhibition of these proteins inhibits development of the retinal microvascular disease. It seems unlikely that these different inflammatory proteins cause capillary degeneration by different mechanisms, so we postulate that these pro-inflammatory steps are part of a sequential pathway like that summarized in Fig 7. This sequence of molecular steps was deduced by inhibiting or deleting a particular enzyme, and then determining which additional molecular abnormalities also are inhibited (those would be downstream of the targeted reaction). For example, inhibition of p38 MAPK inhibited the diabetes-induced alterations in expression of retinal iNOS and ICAM, as well as leukostasis and superoxide generation (Du et al., 2010). Likewise, inhibition of iNOS inhibited the hyperglycemia-induced generation of prostaglandin (Du et al., 2004), whereas the converse was not true (inhibition of cyclooxygenase did not inhibit nitric oxide production). Thus, iNOS and ICAM, leukostasis and superoxide generation likely are downstream of (and regulated by) p38 MAPK, and iNOS regulates prostaglandin generation, but cyclooxygenase apparently does not regulate nitric oxide production. Recent evidence indicates also that cyclooxygenase-2 and nitric oxide interact with the VEGF system with respect to vascular permeability and angiogenesis.
Fig 7.
Postulated scheme by which inflammation contributes to retinal capillary degeneration in diabetes. The scheme shows a series of steps which were elucidated by inhibiting a specific protein (such as iNOS), and then determining which other steps (or proteins) also were inhibited (and thus were regulated by that protein). RAGE also fits into this scheme, but its position relative to many of these other abnormalities is not yet clear.
Many cytokines and other signaling molecules are known to activate NF-κB and other proinflammatory mediators, thus indicating that the inflammatory system and its relation to diabetic retinopathy are considerably more complex than what is noted in the figure. For example, NF-κB is able to directly induce expression of ICAM-1 and COX2. This working model clearly will have to be updated in the future. Many of the steps identified in Fig 7 were represented also in Fig 2, suggesting that the molecular abnormalities that contribute to the vascular abnormalities of diabetic retinopathy are consistent with a likely role of the innate immune system in the development of some aspects of the retinopathy.
5. What are good inflammation targets at which to inhibit the retinopathy?
Good glycemic control remains the best accepted means to inhibit diabetic complications, but inhibition of inflammation might help inhibit the retinopathy even in the presence of hyperglycemia. Based on animal studies to date, we have yet to see a strong advantage or disadvantage for any particular anti-inflammatory therapy, at least to inhibit the diabetes-induced degeneration of retinal capillaries. One exception to this is that inhibition of 5-lipoxygenase was more beneficial at inhibiting capillary degeneration in diabetic retinopathy than was inhibition of 12-lipoxygenase. There also are differences with regard to side-effects that make some therapeutic approaches less desirable than others. Steroids, COX2 inhibitors and high doses of aspirin have been reported to have undesirable side-effects that should be avoided. Drugs that seem worthy of further examination for their ability to inhibit at least the vascular abnormalities of early diabetic retinopathy include derivatives of salicylates (such as salsalate) or minocycline, RAGE inhibitors, and inhibitors (or antagonists) of p38 MAPK, 5-lipoxygenase, or TNFα. Lipid mediators, including eicosanoids, can play important roles in the regulation of inflammation in other tissues (Wall et al., 2010), but evidence is now accumulating that supplementation with lipids like lutein or docosahexanoic also show a beneficial effect in diabetic retinopathy (Arnal et al., 2009; Kowluru et al., 2008a).
Inflammatory changes might contribute also to degeneration of retinal neurons in diabetes. The potential role of inflammation in diabetes-induced neurodegeneration in the retina is only beginning to be explored, but it is interesting that drugs with known anti-inflammatory actions (minocycline and salicylates) inhibit death of cells in the retinal ganglion cell layer in diabetic animals (Krady et al., 2005; Zheng et al., 2007b).
Immunohistochemical studies have demonstrated migration of NF-κB subunits into nuclei of retinal neurons in diabetes (Zheng et al., 2007b), suggesting that this proinflammatory transcription factor was activated in neurons in diabetes. This nuclear translocation (and presumably activation) of NF-κB in retinal neurons was inhibited by salicylates (Zheng et al., 2007b).
6. Therapies used clinically which also have anti-inflammatory actions in the retina in diabetes
Diabetes-induced inflammatory changes in retina have been found to be inhibited also by therapies whose major effect was believed to be on other targets. Retinal leukostasis and expression of ICAM-1, VEGF, angiotensin II, and angiotensin II type 1 receptor were significantly suppressed by blockade of the angiotensin II type 1 receptor (telmisartan), but leukostasis was not inhibited by a angiotensin II type 2 receptor (valsartan) (Kim et al., 2009; Nagai et al., 2007). A (pro)renin receptor blocker inhibited the diabetes-induced increases in VEGF and ICAM expression, and leukostasis (Satofuka et al., 2009). In diabetic Ren-2 rats, candesartan reduced retinal acellular capillaries, inflammation and iNOS and NO (Miller et al., 2010). Administration of lovastatin and simvastatin to diabetic animals normalized the expression of the diabetes-induced increase in ICAM-1, VEGF and TNFα, and inhibited the decrease of tight junction (occludin) and adherens junction (VE-cadherin) proteins (Al-Shabrawey et al., 2008; Li et al., 2009a). The mechanism by which statins mediate this effect might involve mitochondrial-derived ROS (Zheng et al., 2010). Newer coumarin derivatives have also been shown to attenuate diabetes-induced alterations in retinal permeability, adhesion molecules, and cytokines (Bucolo et al., 2009).
If inflammation does indeed contribute to development of the retinopathy, it seems that these therapies should inhibit the morphologic lesions of DR. It is well known that anti-VEGF therapies and steroids have potent effects on retinal edema and/or neovascularization, and intravitreal steroids downregulate VEGF and ICAM-1 expression and inhibit the activation of NF-κB (Wang et al., 2008). Similarly, blood pressure medications (such as captopril (Zhang et al., 2007) and perindopril (Zheng et al., 2009) and lipid lowering drugs (Zheng et al., 2010) inhibit capillary degeneration in diabetic retinopathy. These studies do not prove that beneficial effects of these therapies on retinopathy are mediated via anti-inflammatory actions, but it is worth further testing.
Salicylates are an anti-inflammatory group of drugs worth discussing, since their effect on DR already has been studied in clinical trials and animal studies. Administration of aspirin (dogs, rats) or other salicylates (rats) from the onset of diabetes significantly inhibited the diabetes-induced degeneration of retinal capillaries (Kern and Engerman, 2001; Zheng et al., 2007b). Prospective clinical trials in humans, however, yielded contradictory conclusions, with one study showing a significantly lower mean yearly increase in the number of definite microaneurysms in the aspirin-treated group (DAMAD Study Group, 1989), and the other showing no benefit (or harm) of aspirin on the retinopathy (Early Treatment Diabetic Retinopathy Research Group, 1991). The failure to inhibit retinopathy by the Early Treatment Diabetic Retinopathy Research study might indicate that inflammation is not primary in the development of the retinopathy, but this conclusion seems premature since the dose of aspirin used was not high enough to have had anti-inflammatory effects, and the severity of retinopathy likely was too advanced at the onset of the study to have been promptly inhibited. The postulate that salicylates can inhibit the retinopathy if delivered at anti-inflammatory doses is supported by a recent prospective, randomized study where treatment with the NSAID, sulindac, inhibited development and progression of DR (Hattori et al., 2007).
7. Inflammation in PDR and diabetic-like retinal neovascularization
Retinas or vitreous from patients with PDR have been found to contain elevated levels of a variety of inflammatory mediators, including ET-1, TNFα, IL-6, and VEGF (Adamiec-Mroczek and Oficjalska-Mlynczak, 2008; Adamiec-Mroczek et al., 2010; Aiello et al., 1994). Experimentally diabetic laboratory animals have not been found to develop preretinal neovascularization, so investigations of neovascularization instead have used models like the oxygen-induced retinopathy model (Madan and Penn, 2003). In angiogenic models like this, extensive leukocyte adhesion was observed at the leading edge of pathological, but not physiological, neovascularization (Ishida et al., 2003b). Depletion of phagocytic cells (including monocytes) by intravitreal injection of clodronate led to a reduction in pathological neovascularization (Ishida et al., 2003b). In a model of choroidal neovascularization, inhibiting monocyte recruitment by deleting the receptor for monocyte chemoattractant protein-1 (Tsutsumi et al., 2003) or ICAM-1 or CD18 (Sakurai et al., 2003) also led to significant inhibition of neovascularization. Prostanoids generated by COX-2 can induce the expression of VEGF and other pro-angiogenic factors (Cheng et al., 1998), and inhibition of COX reduced the production of VEGF and retinal neovascularization (Ayalasomayajula and Kompella, 2003; Sennlaub et al., 2003; Wilkinson-Berka et al., 2003). Thus, the inflammatory system can contribute to aspects of the neovascular response, especially in the presence of hypoxia.
8. How does diabetes cause retinal inflammation?
Cell death is known to occur in the retina in diabetes, and this might induce an inflammatory response. Retinal cell death in diabetes, however, seems to occur largely by apoptosis, thus raising a possibility that the signal(s) required to induce the inflammatory state likely are largely metabolic in origin.
Hyperglycemia
Hyperglycemia itself has been regarded as a proinflammatory environment. Incubation of retinal cells in high glucose causes upregulation of proinflammatory iNOS, COX-2 and leukotrienes (Du et al., 2004; El-Remessy et al., 2005; Kowluru and Kowluru, 2007; Madsen-Bouterse et al., 2010; Talahalli et al., 2010; Tawfik et al., 2009; Zheng et al., 2004). Moreover, long-term experimental hyperglycemia (via a sugar (galactose)-rich diet) in the absence of diabetes resulted in diabetes-like retinopathy, as well as increases in retinal leukostasis and vascular permeability (Joussen et al., 2004).
In apparent contrast to the concept that endothelial cells respond to hyperglycemia, Busik and coworkers have presented evidence that retinal endothelial cells do not respond to hyperglycemia per se, but instead to cytokines produced by adjacent cells (Busik et al., 2008) (see below).
Lipids
Diabetes-induced changes in retinal fatty acid metabolism lead to a significant decrease in retinal n-3 polyunsaturated fatty acids (PUFAs), especially docosohexanoic acid (DHA) (Tikhonenko et al., 2010), and these changes in fatty acid compositions may be related to the chronic inflammation that occurs in the diabetic retina (Byeon et al., 2010). Hammes and collaborators found that long-term administration of omega-3 fatty acids to diabetic rats caused a significant increase in degeneration of retinal capillaries (Hammes et al., 1996). Vitreous lipids, including proinflammatory lipoxygenase- and cytochrome P450 epoxygenase-derived prostenoids have been detected also in the vitreous of diabetic patients (Schwartzman et al., 2010).
In contrast to pro-inflammatory effects of some lipids, docosohexanoic acid, resolvins and a small number of other autocoids have been shown to have anti-inflammatory actions. Busik and collaborators have reported that administration of docosohexanoic acid inhibits diabetes-induced degeneration of retinal capillaries in animals (unpublished). Dietary carotenoids inhibited diabetes-induced increases in retinal ICAM-1 (Kowluru et al., 2008b), and administration of a HMG-CoA inhibitor (statin) inhibited diabetes-induced increases in retinal inflammatory status and blood-retinal barrier (Li et al., 2009a).
Oxidative stress
Diabetes-induced oxidative stress clearly plays a role in development of the inflammatory processes in the retina. Two months of diabetes in rats significantly increased retinal levels of IL-1β and NF-κB, and antioxidants inhibited those increases (Kowluru and Odenbach, 2004). The diabetes-induced increase in retinal NF-κB activation also could be inhibited by inhibiting activity of the pro-oxidant NADPH oxidase (Tawfik et al., 2009). Others have demonstrated administration of N-acetylcysteine, baicalein and lutein, inhibited activation of macrophage/microglia and VEGF increases in the retinas of diabetic animals (Sasaki et al., 2010; Tsai et al., 2009; Yang et al., 2009). Oxidative stress has been postulated to be a cause for the diabetes-induced increase in retinal inflammation and vascular permeability via Wnt pathway activation (Chen et al., 2009).
AGE/RAGE
Interaction of advanced glycation endproducts with RAGE is known is known to have pro-inflammatory consequences, and inhibitors of RAGE have been shown to have anti-inflammatory effects in retina (Li et al., 2011) and other tissues. In a human retinal Müller cell line, RAGE signaling via MAPK pathway caused cytokine production in high glucose, and blockade of RAGE prevented cytokine responses induced by high glucose and S100B in Müller glia (Zong et al., 2010). Pharmacologic inhibition of RAGE signaling (Barile et al., 2005; Li et al., 2011) significantly inhibited degeneration of retinal capillaries and other lesions of early diabetic retinopathy in animals.
Cytokines
Busik and coworkers have presented evidence that retinal endothelial cells do not respond to hyperglycemia per se, but instead to cytokines produced by adjacent cells (Busik et al., 2008). In contrast to effects of incubating human retinal endothelial cells in high glucose, exposure of the cells to the proinflammatory cytokines IL-1β or TNFα increased glucose consumption, mitochondrial superoxide production, ERK and JNK phosphorylation, tyrosine phosphorylation, NF-κB, and caspase activation (Busik et al., 2008).
Blood pressure
Hypertension exacerbates diabetes-induced retinal inflammation, as assessed by expression of VEGF and ICAM-1 (Silva et al., 2007). Consistent with this, administration of candesartan to Ren-2 diabetic rats attenuated retinal inflammation and vascular pathology, apparently by restoring glyoxalase-I function. Whether or not the beneficial effects of antihypertensive medications act solely through effects on blood pressure is not yet clear.
Endoplasmic reticulum (ER) stress
The diabetes-induced increase in retinal inflammation is associated with ER stress, and inflammatory molecules in the retina were increased using a chemical stimulator of the stress (tunicamycin) and decreased by a chemical chaperone (4-phenyl butyric acid) (Li et al., 2009b).
9. Weaknesses of the inflammation hypothesis
In spite of a growing amount of data that is consistent with a contribution of inflammatory processes to the development of diabetic retinopathy, there are short-comings in the current hypothesis. If inflammatory processes do contribute to the development of diabetic retinopathy, why don’t other inflammatory conditions in nondiabetic patients or animals cause a diabetic-like retinopathy? For example, an increase in retinal leukostasis (which is consistent with inflammation) has been observed in insulin-resistant animals who are not diabetic (Abiko et al., 2003), yet insulin resistance generally does not cause a diabetic-like retinopathy. Aging or hypertension in the absence of diabetes increase expression of pro-inflammatory molecules in the retina (Silva et al., 2007; Xu et al., 2009), yet these conditions do not cause a diabetic-like retinopathy. Moreover, induction of systemic inflammation (produced by footpad injection of lipopolysaccharide) caused a reduction in the severity of diabetes-induced retinal inflammation (as assessed by leukostasis and mRNA expression of adhesion molecules) (Tamura et al., 2005).
Thus, it seems likely that systemic inflammation does not cause the retinal inflammation that develops in diabetes (and which we postulate contributes to the development of early diabetic retinopathy). The pro-inflammatory environment which we postulate initiates the retinopathy must develop locally in the retina. An example of this is that diabetes-induced increases in retinal vascular permeability and leukostasis were inhibited by blocking NF-κB activation solely in glial cells (such as retinal Muller cells) (Bethea and Kern, unpublished). Since both of these measured parameters involve the retinal vasculature, this indicates that retinal glial cells contribute to local development of inflammatory changes that adversely influence the retinal vasculature in diabetic animals.
Several other issues are worth considering in relation to the postulated role of inflammation in the development or progression of diabetic retinopathy. An obvious weakness of the inflammatory hypothesis is that the inflammatory changes develop quickly in the retina in diabetes, but the histopathology does not develop until considerably later (and pre-retinal neovascularization has not developed reproducibly in animal models). This difference remains to be explained. Another unanswered question pertains to why the retinal inflammation doesn’t resolve in diabetes. Inflammation normally resolves with time, but the abnormal environment of diabetes seems to create a non-resolving inflammation which needs to be explained. Diabetes-induced increases in expression of inflammatory proteins have been found to persist at elevated levels even after reestablishment of near-normal blood sugars (Chan et al., 2010). This persistence is important because it parallels the tendency of diabetic retinopathy to progress even after hyperglycemia is corrected (called “metabolic memory”), and might provide new insight into the pathogenesis of the retinopathy. The mechanism(s) by which diabetic retinopathy resists arrest by improved glycemia, and whether or not inflammation contributes to metabolic memory, is not yet clear.
10. Future directions
Research topics that need to be addressed in order to more fully understand the significance of inflammation in the pathogenesis of diabetic retinopathy are numerous, and some of these are summarized below.
Laboratory research
Which metabolic abnormalities initiate diabetes-induced inflammation in the retina? Are there advantages in inhibiting certain of these inflammatory processes as opposed others?
Which retinal cell types exhibit or cause inflammation in diabetic retinopathy? Accumulating evidence that nonretinal cells play a role in the pathogenesis of diabetic retinopathy seems particularly noteworthy. This suggests that investigations will need to expand beyond the traditional view of the retinopathy, to include also leukocytes, stem cells, and possibly also other cell types. What is the role of other aspects of the innate immune system (such as toll-like receptors and PAMPs) in the etiology of diabetic retinopathy?
Do inflammatory processes play a role in diabetes-induced dysfunction of retinal nerves?
What are the mechanisms by which pro-inflammatory changes in diabetes result in dysfunction or death of retinal nerve and/or vessel cells?
Does inflammation contribute to metabolic memory, and by what mechanisms?
Why doesn’t retinal inflammation resolve in diabetes, and does correction of that abnormality have beneficial effects on the retina?
Clinical research
The most serious weakness of the postulated role of inflammation in the pathogenesis of diabetic retinopathy in patients is the paucity of clinical data in patients supporting the concept. A critical need is to assess the validity of the concept that inflammatory molecules play a critical role in the early stages of the retinopathy. Thus, clinical questions to be addressed include:
Can markers of inflammation be demonstrated (preferably noninvasively) in retinas of diabetic patients?
What are the best anti-inflammatory therapies to test the postulated role of inflammation in development of diabetic retinopathy?
Can the development and progression of diabetic retinopathy be inhibited with anti-inflammatory doses of drugs?
What is the best route of administration of this therapy?
11. Conclusion and perspectives
We and others have postulated that inflammatory processes play an important role in the development of early (and possibly also later) stages of diabetic retinopathy. Unlike in uveitis, this diabetes-induced inflammation is not grossly apparent in the retina, and the concept of DR having an inflammatory pathogenesis is based on the molecular characteristics of inflammation (as opposed to the classical cellular definition of inflammation). These molecular changes seem causally related to the development of at least the diabetes-induced leakage and degeneration of retinal capillaries, since inhibition of the inflammatory cascade at any of multiple points has inhibited these abnormalities that are characteristic of the retinopathy in animals. It is possible that “inflammation” does not perfectly describe the changes that ultimately cause the retinopathy, but this term seems to describe the pathogenesis of the retinopathy better than previous concepts. It is likely that this concept will become better focused with future research.
Two questions seem to have particular importance for understanding the role of inflammation in the development of diabetic retinopathy. First, what is the mechanism(s) by which inflammatory processes cause retinal cell death or visual dysfunction. and second, why doesn’t retinal inflammation resolve in diabetes? Understanding these questions is expected to provide new therapeutic targets at which to inhibit or prevent the retinopathy.
Administration of anti-inflammatory steroids or VEGF inhibitors reduce diabetic macular edema in patients, but whether or not these or other anti-inflammatory therapies will inhibit also earlier stages of diabetic retinopathy has not been adequately tested in patients. Rigorous testing of this postulate will require clinical studies, but this seems worth the investment since the available evidence indicates that inflammation can contribute both to the early and late stages of diabetic retinopathy.
Acknowledgements
This work was funded by research grants from the Veteran Affairs Career Development and Foundation Awards (JT), and a Veterans Affairs Merit Award and PHS grant EY00300 (TK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abiko T, Abiko A, Clermont AC, Shoelson B, Horio N, Takahashi J, Adamis AP, King GL, Bursell SE. Characterization of retinal leukostasis and hemodynamics in insulin resistance and diabetes: role of oxidants and protein kinase-C activation. Diabetes. 2003;52:829–837. doi: 10.2337/diabetes.52.3.829. [DOI] [PubMed] [Google Scholar]
- Adamiec-Mroczek J, Oficjalska-Mlynczak J. Assessment of selected adhesion molecule and proinflammatory cytokine levels in the vitreous body of patients with type 2 diabetes--role of the inflammatory-immune process in the pathogenesis of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1665–1670. doi: 10.1007/s00417-008-0868-6. [DOI] [PubMed] [Google Scholar]
- Adamiec-Mroczek J, Oficjalska-Mlynczak J, Misiuk-Hojlo M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: Analysis of vitreous samples. Cytokine. 2010;49:269–274. doi: 10.1016/j.cyto.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30:65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali TK, Matragoon S, Pillai BA, Liou GI, El-Remessy AB. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57:889–898. doi: 10.2337/db07-1669. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, Zandi S, Almulki L, Tayyari F, Shimokawa H, Hafezi-Moghadam A, Ishibashi T. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal E, Miranda M, Johnsen-Soriano S, Alvarez-Nolting R, Diaz-Llopis M, Araiz J, Cervera E, Bosch-Morell F, Romero FJ. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr Eye Res. 2009;34:928–938. doi: 10.3109/02713680903205238. [DOI] [PubMed] [Google Scholar]
- Aveleira CA, Lin CM, Abcouwer SF, Ambrosio AF, Antonetti DA. TNF-alpha signals through PKCzeta/NF-kappaB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59:2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003;458:283–289. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Kompella UB. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm Res. 2004;21:1797–1804. doi: 10.1023/b:pham.0000045231.51924.e8. [DOI] [PubMed] [Google Scholar]
- Azuma N, Yoshimasa Y, Nishimura H, Yamamoto Y, Masuzaki H, Suga J, Shigemoto M, Matsuoka N, Tanaka T, Satoh N, Igaki T, Miyamoto Y, Itoh H, Yoshimasa T, Hosoda K, Nishi S, Nakao K. The significance of the Trp 64 Arg mutation of the beta3-adrenergic receptor gene in impaired glucose tolerance, non-insulin-dependent diabetes mellitus, and insulin resistance in Japanese subjects. Metabolism. 1998;47:456–460. doi: 10.1016/s0026-0495(98)90059-2. [DOI] [PubMed] [Google Scholar]
- Barber A, Schuller K, Bridi M, Bronson S. Visual Acuity and Contrast Sensitivity Are Reduced in the Ins2Akita. Diabetic Mouse ARVO. 2010 abstract A237. [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile GR, Pachydaki SI, Tari SR, Lee SE, Donmoyer CM, Ma W, Rong LL, Buciarelli LG, Wendt T, Horig H, Hudson BI, Qu W, Weinberg AD, Yan SF, Schmidt AM. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Fredj-Reygrobellet D, Brignole F, Lapalus P, Gastaud P. MHC class II antigen expression by ocular cells in proliferative diabetic retinopathy. Fundam Clin Pharmacol. 1993;7:523–530. doi: 10.1111/j.1472-8206.1993.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Behl Y, Krothapalli P, Desta T, DiPiazza A, Roy S, Graves DT. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–1418. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl Y, Krothapalli P, Desta T, Roy S, Graves DT. FOXO1 plays an important role in enhanced microvascular cell apoptosis and microvascular cell loss in type 1 and type 2 diabetic rats. Diabetes. 2009;58:917–925. doi: 10.2337/db08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucklacher RM, Patel KM, VanGuilder HD, Bixler GV, Barber AJ, Antonetti DA, Lin CM, LaNoue KF, Gardner TW, Bronson SK, Freeman WM. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC Med Genomics. 2008;1:26. doi: 10.1186/1755-8794-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo C, Ward KW, Mazzon E, Cuzzocrea S, Drago F. Protective effects of a coumarin derivative in diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:3846–3852. doi: 10.1167/iovs.08-3328. [DOI] [PubMed] [Google Scholar]
- Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeon SH, Chung HY, Kwon OW. Polyunsaturated Fatty Acid Composition of Surgically Removed Hard Exudates in Diabetic Macular Edema. Ophthalmic Surg Lasers Imaging. 2010:1–3. doi: 10.3928/15428877-20100215-29. [DOI] [PubMed] [Google Scholar]
- Cao R, Xue Y, Hedlund EM, Zhong Z, Tritsaris K, Tondelli B, Lucchini F, Zhu Z, Dissing S, Cao Y. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci U S A. 2010;107:856–861. doi: 10.1073/pnas.0911661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PS, Kanwar M, Kowluru RA. Resistance of retinal inflammatory mediators to suppress after reinstitution of good glycemic control: novel mechanism for metabolic memory. J Diabetes Complications. 2010;24:55–63. doi: 10.1016/j.jdiacomp.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, Rogulja-Pepeonik Z, Fuller JH. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- Chen W, Esselman WJ, Jump DB, Busik JV. Anti-inflammatory effect of docosahexaenoic acid on cytokine-induced adhesion molecule expression in human retinal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:4342–4347. doi: 10.1167/iovs.05-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, Boulton M, Lyons TJ, Gao G, Ma JX. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol. 2009;175:2676–2685. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39:581–591. [PubMed] [Google Scholar]
- Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53:2404–2411. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- DAMAD Study Group Effect of aspirin alone and aspirin plus dipyridamole in early diabetic retinopathy: a multicenter rendomized controlled clinical trial. Diabetes. 1989;38:491–498. [PubMed] [Google Scholar]
- de Venecia G, Davis MD, Engerman RL. Clinicopathologic correlations in diabetic retinopathy. 1. Histology and fluorescein angiography of microaneurysms. Arch Ophthalmol. 1976;94:1766–1773. doi: 10.1001/archopht.1976.03910040540013. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Du Y, Sarthy V, Kern T. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol. 2004;287:R735–741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Tang J, Li G, Berti-Mattera L, Lee CA, Bartkowski D, Gale D, Monahan J, Niesman MR, Alton G, Kern TS. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci. 2010;51:2158–2164. doi: 10.1167/iovs.09-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Research Group Effects of aspirin treatment on diabetic retinopathy. Ophthalmol. 1991;98:757–765. [PubMed] [Google Scholar]
- Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular oedema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmol. 2010;88:279–291. doi: 10.1111/j.1755-3768.2008.01501.x. [DOI] [PubMed] [Google Scholar]
- El-Asrar AM, Missotten L, Geboes K. Expression of cyclo-oxygenase-2 and downstream enzymes in diabetic fibrovascular epiretinal membranes. Br J Ophthalmol. 2008;92:1534–1539. doi: 10.1136/bjo.2008.142182. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003a;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci. 2005;118:243–252. doi: 10.1242/jcs.01612. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003b;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2002;43:1104–1108. [PubMed] [Google Scholar]
- Ghattas A, Lip PL, Lip GY. Renin-angiotensin blockade in diabetic retinopathy. Int J Clin Pract. 2011;65:113–116. doi: 10.1111/j.1742-1241.2010.02592.x. [DOI] [PubMed] [Google Scholar]
- Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–1538. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- Grover D, Li TJ, Chong CC. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005656.pub2. CD005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-Lipoxygenase, Contributes to Degeneration of Retinal Capillaries in a Mouse Model of Diabetic Retinopathy. Diabetes. 2008;57:1387–1393. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Weiss A, Fuhrer D, Kramer HJ, Papavassilis C, Grimminger F. Acceleration of experimental diabetic retinopathy in the rat by omega-3 fatty acids. Diabetologia. 1996;39:251–255. doi: 10.1007/BF00418338. [DOI] [PubMed] [Google Scholar]
- Harada C, Harada T, Mitamura Y, Quah HM, Ohtsuka K, Kotake S, Ohno S, Wada K, Takeuchi S, Tanaka K. Diverse NF-kappaB expression in epiretinal membranes after human diabetic retinopathy and proliferative vitreoretinopathy. Mol Vis. 2004;10:31–36. [PubMed] [Google Scholar]
- Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Hashizume K, Nakajima K, Nishimura Y, Naka M, Miyanaga K. The effect of long-term treatment with sulindac on the progression of diabetic retinopathy. Curr Med Res Opin. 2007;23:1913–1917. doi: 10.1185/030079907X218770. [DOI] [PubMed] [Google Scholar]
- Hernandez C, Carrasco E, Casamitjana R, Deulofeu R, Garcia-Arumi J, Simo R. Somatostatin molecular variants in the vitreous fluid: a comparative study between diabetic patients with proliferative diabetic retinopathy and nondiabetic control subjects. Diabetes Care. 2005;28:1941–1947. doi: 10.2337/diacare.28.8.1941. [DOI] [PubMed] [Google Scholar]
- Huang H, Gandhi JK, Zhong X, Wei Y, Gong J, Duh EJ, Vinores SA. TNF{alpha} is required for late BRB breakdown in diabetic retinopathy and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Invest Ophthalmol Vis Sci. 2011;52:1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono P, Battaglia Parodi M, Bandello F. Antivascular endothelial growth factor in diabetic retinopathy. Dev Ophthalmol. 2010;46:39–53. doi: 10.1159/000320008. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar MM, El-Shishtawy MM, Liou GI. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes. 2011;60:1122–1133. doi: 10.2337/db10-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, El-Shishtawy MM, Pena A, Jr., Liou GI. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol Vis. 2010;16:2033–2042. [PMC free article] [PubMed] [Google Scholar]
- Iliaki E, Poulaki V, Mitsiades N, Mitsiades CS, Miller JW, Gragoudas ES. Role of alpha 4 integrin (CD49d) in the pathogenesis of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:4898–4904. doi: 10.1167/iovs.08-2013. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003a;44:2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D’Amore PA, Shima DT, Adamis AP. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003b;198:483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan VS. The therapeutic implications of renin-angiotensin system blockade in diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:392–395. doi: 10.2174/138920111794480615. [DOI] [PubMed] [Google Scholar]
- Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163–167. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, Poulaki V, Semkova I, Kociok N. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009;15:1418–1428. [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Cai WY, Suzuma I, Pak J, Ju ST, Rook SL, Esser P, Mitsiades CS, Kirchhof B, Adamis AP, Aiello LP. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. Faseb J. 2003;17:76–78. doi: 10.1096/fj.02-0157fje. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Zimmer-Galler IE, Shah SM, Dustin L, Do DV, Eliott D, Haller JA, Nguyen QD. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol. 2010;149:496–502. e491. doi: 10.1016/j.ajo.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul K, Hodgkinson A, Tarr J, Kohner EM, Chibber R. Is Inflammation a Common Retinal-Renal-Nerve Pathogenic Link in Diabetes? Curr Diabetes Rev. 2010;6:294–303. doi: 10.2174/157339910793360851. [DOI] [PubMed] [Google Scholar]
- Kempen JH, O’Colmain BJ, Leske MC, Haffner SM, Klein R, Moss SE, Taylor HR, Hamman RF. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS. In vivo models of diabetic retinopathy. In: Duh EJ, editor. Diabetic Retinopathy. Humana Press; Totowa, New Jersey: 2008. pp. 137–156. [Google Scholar]
- Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;15:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Du Y, Miller CM, Hatala DA, Levin LA. Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol. 2010;176:2550–2558. doi: 10.2353/ajpath.2010.091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Pharmacologic inhibition of diabetic retinopathy: Aminoguanidine and aspirin. Diabetes. 2001;50:1636–1642. doi: 10.2337/diabetes.50.7.1636. [DOI] [PubMed] [Google Scholar]
- Kern TS, Miller CM, Du Y, Zheng L, Mohr S, Ball SL, Kim M, Jamison JA, Bingaman DP. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007;56:373–379. doi: 10.2337/db05-1621. [DOI] [PubMed] [Google Scholar]
- Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin II attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009;29:621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, Lutty GA. Neutrophils are associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes. 2005;54:1534–1542. doi: 10.2337/diabetes.54.5.1534. [DOI] [PubMed] [Google Scholar]
- Kocak N, Alacacioglu I, Kaynak S, Ozcan MA, Celik O, Yuksel F, Piskin O, Oner H, Saatci AO, Ergin M. Comparison of vitreous and plasma levels of vascular endothelial growth factor, interleukin-6 and hepatocyte growth factor in diabetic and non-diabetic retinal detachment cases. Ann Ophthalmol (Skokie) 2010;42(Spec No, 10-14) [PubMed] [Google Scholar]
- Kohner EM, Henkind P. Correlation of fluorescein angiogram and retinal digest in diabetic retinopathy. Am J Ophthalmol. 1970;69:403–414. doi: 10.1016/0002-9394(70)92273-7. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kanwar M, Chan PS, Zhang JP. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch Ophthalmol. 2008a;126:1266–1272. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Menon B, Gierhart DL. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Invest Ophthalmol Vis Sci. 2008b;49:1645–1651. doi: 10.1167/iovs.07-0764. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45:4161–4166. doi: 10.1167/iovs.04-0633. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Zhong Q, Kanwar M. Metabolic memory and diabetic retinopathy: role of inflammatory mediators in retinal pericytes. Exp Eye Res. 2010;90:617–623. doi: 10.1016/j.exer.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru V, Kowluru RA. Increased oxidative stress in diabetes regulates activation of a small molecular weight G-protein, H-Ras, in the retina. Mol Vis. 2007;13:602–610. [PMC free article] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- Le LM, Poulaki V, Koizumi K, Fauser S, Kirchhof B, Joussen AM. Reduced Histopathological Alterations in Long-Term Diabetic TNF-R Deficient Mice (ARVO abstract) Invest. Ophthalmol. Vis. Sci. 2003;44:3894. [Google Scholar]
- Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrosio AF, Forrester JV. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- Li G, Lee CA, Patel MD, Petrash JM, Benetz B, Tang J, Kern TS. Diabetes-induced impairment in contrast sensitivity and visual acuity in mice: contributions of p38 MAPK, RAGE, and aldose reductase. Diabetes. 2010a doi: 10.1167/iovs.13-11659. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Tang J, Du Y, Lee CA, Kern TS. Beneficial effects of RAGE-Ig fusion protein on early diabetic retinopathy and tactile allodynia. Molecular Vision. 2011 (in revision) [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009a;89:71–78. doi: 10.1016/j.exer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009b;583:1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma A, Han PY, Nakagawa T, Johnson RJ, Grant MB, Campbell-Thompson M, Jarajapu YP, Lei B, Hauswirth WW. Diabetic eNOS-knockout mice develop accelerated retinopathy. Invest Ophthalmol Vis Sci. 2010b;51:5240–5246. doi: 10.1167/iovs.09-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear leukocytes to capillary dropout in the human diabetic choroid. Am J Pathol. 1997;151:707–714. [PMC free article] [PubMed] [Google Scholar]
- Ma P, Luo Y, Zhu X, Ma H, Hu J, Tang S. Phosphomannopentaose sulfate (PI-88) inhibits retinal leukostasis in diabetic rat. Biochem Biophys Res Commun. 2009;380:402–406. doi: 10.1016/j.bbrc.2009.01.092. [DOI] [PubMed] [Google Scholar]
- Madan A, Penn JS. Animal models of oxygen-induced retinopathy. Front Biosci. 2003;8:d1030–1043. doi: 10.2741/1056. [DOI] [PubMed] [Google Scholar]
- Madsen-Bouterse SA, Zhong Q, Mohammad G, Ho YS, Kowluru RA. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic Res. 2010;44:313–321. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:847–849. doi: 10.1161/CIRCULATIONAHA.110.960120. [DOI] [PubMed] [Google Scholar]
- Massaro M, Basta G, Lazzerini G, Carluccio MA, Bosetti F, Solaini G, Visioli F, Paolicchi A, De Caterina R. Quenching of intracellular ROS generation as a mechanism for oleate-induced reduction of endothelial activation and early atherogenesis. Thromb Haemost. 2002;88:335–344. [PubMed] [Google Scholar]
- Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intercellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am. J. Pathol. 1995;147:642–653. [PMC free article] [PubMed] [Google Scholar]
- Miller AG, Tan G, Binger KJ, Pickering RJ, Thomas MC, Nagaraj RH, Cooper ME, Wilkinson-Berka JL. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function. Diabetes. 2010;59:3208–3215. doi: 10.2337/db10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura Y, Harada T, Harada C, Ohtsuka K, Kotake S, Ohno S, Tanaka K, Takeuchi S, Wada K. NF-kappaB in epiretinal membranes after human diabetic retinopathy. Diabetologia. 2003;46:699–703. doi: 10.1007/s00125-003-1084-x. [DOI] [PubMed] [Google Scholar]
- Mitka M. Aggressive lipid, hypertension targeting yields no benefit for some with diabetes. JAMA. 2010;303:1681–1683. doi: 10.1001/jama.2010.492. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S, Tang J, Kern TS. Caspase activation in retinas of diabetic and galactosemic mice and diabetic patients. Diabetes. 2002;51:1172–1179. doi: 10.2337/diabetes.51.4.1172. [DOI] [PubMed] [Google Scholar]
- Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, Yamashiro K, Inoue M, Tsubota K, Umezawa K, Ishida S. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- Nunes S, Pires I, Rosa A, Duarte L, Bernardes R, Cunha-Vaz J. Microaneurysm turnover is a biomarker for diabetic retinopathy progression to clinically significant macular edema: findings for type 2 diabetics with nonproliferative retinopathy. Ophthalmologica. 2009;223:292–297. doi: 10.1159/000213639. [DOI] [PubMed] [Google Scholar]
- Opreanu M, Lydic TA, Reid GE, McSorley KM, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2010;51:3253–3263. doi: 10.1167/iovs.09-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ED, Field RA. Diabetic retinopathy and rheumatoid arthritis. Lancet. 1964;2:17–18. doi: 10.1016/s0140-6736(64)90008-x. [DOI] [PubMed] [Google Scholar]
- Rao VR, Prescott E, Shelke NB, Trivedi R, Thomas P, Struble C, Gadek T, O’Neill CA, Kompella U. Delivery of SAR 1118 to Retina Via Ophthalmic Drops and its Effectiveness in Reduction of Retinal Leukostasis and Vascular Leakiness in Rat Streptozotocin (STZ) Model of Diabetic Retinopathy (DR) Invest Ophthalmol Vis Sci. 2010;51:5198–5204. doi: 10.1167/iovs.09-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of Nuclear Factor-kappaB Induced by Diabetes and High Glucose Regulates a Proapoptotic Program in Retinal Pericytes. Diabetes. 2002;51:2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980. [PubMed] [Google Scholar]
- Sakurai E, Taguchi H, Anand A, Ambati BK, Gragoudas ES, Miller JW, Adamis AP, Ambati J. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:2743–2749. doi: 10.1167/iovs.02-1246. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971–979. doi: 10.1007/s00125-009-1655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S. Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–1633. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman ML, Iserovich P, Gotlinger K, Bellner L, Dunn MW, Sartore M, Grazia Pertile M, Leonardi A, Sathe S, Beaton A, Trieu L, Sack R. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010;59:1780–1788. doi: 10.2337/db10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennlaub F, Valamanesh F, Vazquez-Tello A, El-Asrar AM, Checchin D, Brault S, Gobeil F, Beauchamp MH, Mwaikambo B, Courtois Y, Geboes K, Varma DR, Lachapelle P, Ong H, Behar-Cohen F, Chemtob S. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 2003;108:198–204. doi: 10.1161/01.CIR.0000080735.93327.00. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Shen X, Zhong Y, Xie B, Cheng Y, Jiao Q. Pigment epithelium derived factor as an anti-inflammatory factor against decrease of glutamine synthetase expression in retinal Muller cells under high glucose conditions. Graefes Arch Clin Exp Ophthalmol. 2010;248:1127–1136. doi: 10.1007/s00417-010-1362-5. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601–612. doi: 10.1016/s0161-6420(81)34983-5. [DOI] [PubMed] [Google Scholar]
- Silva KC, Pinto CC, Biswas SK, de Faria JB, de Faria JM. Hypertension increases retinal inflammation in experimental diabetes: a possible mechanism for aggravation of diabetic retinopathy by hypertension. Curr Eye Res. 2007;32:533–541. doi: 10.1080/02713680701435391. [DOI] [PubMed] [Google Scholar]
- Talahalli R, Zarini S, Sheibani N, Murphy RC, Gubitosi-Klug RA. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:1699–1708. doi: 10.1167/iovs.09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Kiryu J, Miyamoto K, Nishijima K, Katsuta H, Miyahara S, Hirose F, Honda Y, Yoshimura N. In vivo evaluation of ocular inflammatory responses in experimental diabetes. Br J Ophthalmol. 2005;89:1052–1057. doi: 10.1136/bjo.2004.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik A, Sanders T, Kahook K, Akeel S, Elmarakby A, Al-Shabrawey M. Suppression of retinal peroxisome proliferator-activated receptor gamma in experimental diabetes and oxygen-induced retinopathy: role of NADPH oxidase. Invest Ophthalmol Vis Sci. 2009;50:878–884. doi: 10.1167/iovs.08-2005. [DOI] [PubMed] [Google Scholar]
- The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorley KM, Renis RL, Kern T, Jump DB, Reid GE, Busik JV. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59:219–227. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai GY, Cui JZ, Syed H, Xia Z, Ozerdem U, McNeill JH, Matsubara JA. Effect of N-acetylcysteine on the early expression of inflammatory markers in the retina and plasma of diabetic rats. Clin Experiment Ophthalmol. 2009;37:223–231. doi: 10.1111/j.1442-9071.2009.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi C, Sonoda KH, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo IF, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- Vincent JA, Mohr S. Inhibition of Caspase-1/Interleukin-1β Signaling Prevents Degeneration of Retinal Capillaries in Diabetes and Galactosemia. Diabetes. 2007;56:224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang Y, Gao L, Li X, Li M, Guo J. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull. 2008;31:1541–1546. doi: 10.1248/bpb.31.1541. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003;44:974–979. doi: 10.1167/iovs.02-0392. [DOI] [PubMed] [Google Scholar]
- Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008;27:608–621. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Matsui T, Nakamura K, Ueda S, Noda Y, Imaizumi T. Pigment epithelium-derived factor (PEDF): its potential therapeutic implication in diabetic vascular complications. Curr Drug Targets. 2008;9:1025–1029. doi: 10.2174/138945008786786154. [DOI] [PubMed] [Google Scholar]
- Yang LP, Sun HL, Wu LM, Guo XJ, Dou HL, Tso MO, Zhao L, Li SM. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, Lavaque AJ, Larson RJ. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116:902–911. doi: 10.1016/j.ophtha.2009.02.002. quiz 912-903. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yamagishi S, Matsui T, Jinnouchi Y, Fukami K, Imaizumi T, Yamakawa R. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab Res Rev. 2009;25:678–686. doi: 10.1002/dmrr.1007. [DOI] [PubMed] [Google Scholar]
- Yulek F, Or M, Ozogul C, Isik AC, Ari N, Stefek M, Bauer V, Karasu C. Effects of stobadine and vitamin E in diabetes-induced retinal abnormalities: involvement of oxidative stress. Arch Med Res. 2007;38:503–511. doi: 10.1016/j.arcmed.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- Zhan X, Du Y, Crabb JS, Gu X, Kern TS, Crabb JW. Targets of tyrosine nitration in diabetic rat retina. Mol Cell Proteomics. 2007;7:864–874. doi: 10.1074/mcp.M700417-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang J, Gerhardinger C, Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes. 2002;51:3499–3504. doi: 10.2337/diabetes.51.12.3499. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Xi X, Gao L, Kern TS. Captopril inhibits capillary degeneration in the early stages of diabetic retinopathy. Curr Eye Res. 2007;32:883–889. doi: 10.1080/02713680701584123. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. Faseb J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- Zhang W, Rojas M, Lilly B, Tsai NT, Lemtalsi T, Liou GI, Caldwell RW, Caldwell RB. NAD(P)H oxidase-dependent regulation of CCL2 production during retinal inflammation. Invest Ophthalmol Vis Sci. 2009a;50:3033–3040. doi: 10.1167/iovs.08-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao S, Hambly BD, Gillies MC. Vascular endothelial growth factor-A: a multifunctional molecular player in diabetic retinopathy. Int J Biochem Cell Biol. 2009b;41:2368–2371. doi: 10.1016/j.biocel.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA, Kern TS. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007a;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- Zheng L, Howell SJ, Hatala DA, Huang K, Kern TS. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes. 2007b;56:337–345. doi: 10.2337/db06-0789. [DOI] [PubMed] [Google Scholar]
- Zheng L, Kern T. Role of nitric oxide, superoxide, peroxynitrite and poly(ADP-ribose) polymerase in diabetic retinopathy. Front Biosci. 2009;14:3974–3987. doi: 10.2741/3505. [DOI] [PubMed] [Google Scholar]
- Zheng L, Kern T. In vivo animal models of diabetic retinopathy. In: HP H, M P, editors. Experimental Approaches to Diabetic Retinopathy. Karger; Basel: 2010. pp. 42–60. [Google Scholar]
- Zheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes. 2004;53:2960–2967. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Chen H, Ke G, Fan Y, Zou H, Sun X, Gu Q, Xu X, Ho PC. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria-reactive oxygen species pathway. Diabetes. 2009;58:954–964. doi: 10.2337/db07-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Chen H, Wang H, Ke B, Zheng B, Li Q, Li P, Su L, Gu Q, Xu X. Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor gamma coactivator 1alpha. Diabetes. 2010;59:2315–2325. doi: 10.2337/db10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JB, Yang JK. Angiotensin-converting enzyme gene polymorphism is associated with proliferative diabetic retinopathy: a meta-analysis. Acta Diabetol. 2010;47:187–193. doi: 10.1007/s00592-009-0160-1. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L. The p50-homodimer mechanism in tolerance to LPS. J Endotoxin Res. 2001;7:219–222. [PubMed] [Google Scholar]
- Zong H, Ward M, Madden A, Yong PH, Limb GA, Curtis TM, Stitt AW. Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE) Diabetologia. 2010;53:2656–2666. doi: 10.1007/s00125-010-1900-z. [DOI] [PubMed] [Google Scholar]