Abstract
Background
Primary graft dysfunction (PGD) is a known risk factor for bronchiolitis obliterans syndrome (BOS) following lung transplantation. Here we report that preformed antibodies to self-antigens increase PGD risk and promote BOS.
Methods
Adult lung transplant recipients (n=142) were included in the study. PGD and BOS were diagnosed based on ISHLT guidelines. Antibodies to self-antigens K alpha-1 tubulin, collagen type V and collagen I, were quantitated using standardized ELISA while cytokines were analyzed using Luminex. HLA-antibodies were measured using Flow-PRA.
Results
Lung transplant recipients with pre-transplant antibodies to self-antigens had increased risk of PGD (Odds 3.09, 95% CI 1.2 – 8.1, p=0.02) compared to those without. Conversely, in patients with PGD, 34.7% were positive for pre-transplant antibodies while in the PGD negative group only 14.6% had antibodies (p=0.03). Antibody positive patients demonstrated high levels of pro-inflammatory cytokines IL-1 (2.1 fold increase), IL-2 (3.0), IL-12 (2.5), IL-15 (3.0) and chemokines IP-10 (3.9) and MCP-1 (3.1, p<0.01 for all). On 5-yr follow-up, patients without antibodies showed greater freedom from development of HLAantibodies compared to those with antibodies (Class I:67% versus 38%, p=0.001; Class II: 71% Vs 41%, p<0.001 ). Patients with pre-transplant antibodies were found to have an independent relative risk of 2.3 (95% CI 1.7 – 4.5, p=0.009) for developing BOS.
Conclusions
Presence of antibodies to self-antigens pre-transplant increases the risk of PGD immediately post-transplant period and BOS on long-term follow-up. PGD is associated with an inflammatory cascade that augments the alloimmune (anti-HLA) response that predisposes to BOS.
Keywords: Autoantibodies, Chronic Rejection, HLA-antibodies, PGD
Introduction
Chronic rejection, also known as Broncholitis Obliterans Syndrome (BOS), remains the predominant cause for poor long-term survival following human lung transplantation. BOS develops in about 50% of human lung allograft recipients within three years and over 90% at nine years post transplantation (1). Several risk factors including HLA-antibodies, alloimmunity, viral infection, acute rejection, gastroesophageal reflux have been shown to promote BOS (2). These occur post-transplant and typically represent late injury mechanisms. Pre-transplant risk factors that lead to allograft injury immediately may also exist and contribute to BOS. However, the role of such pre-transplant injury mechanisms in chronic allograft rejection remains unclear.
In a retrospective review of 334 adult lung transplant recipients Daud et al found that primary allograft dysfunction (PGD) that occurs within 24–48 hrs following lung transplantation is associated with increased risk of BOS (RR 1.73–2.53), independent of acute rejection, lymphocytic bronchitis, and respiratory viral infections (3). We previously hypothesized that PGD induced inflammation and upregulated MHC on the allograft leading to increased alloantigen presentation and production of anti-donor HLA-antibodies can contribute to the immunopathogenesis of BOS. In support of that hypothesis, we demonstrated that PGD was associated with elevated pro-inflammatory mediators (4). These patients showed increased development of HLA-antibodies that were independent predictors for BOS (5).
Nevertheless, the question that remains is why patients develop PGD that triggers these events. Several etiologies have been postulated for PGD. These include ischemia-reperfusion injury, donor-death related injuries, allograft injury related to cardiopulmonary bypass, or infections (3, 6, 7). Native lungs are in a state of inflammation and tissue remodeling due to the primary lung disease. Such an inflammatory milieu will be conducive for the development of autoimmunity. The surgical stress can lead to the expression of sequestered antigenic epitopes of various self-antigens present in the lung. Wilkes and Burlingham’s group has shown that collagen V expression is increased in rat lung allograft following transplantation (8). Therefore, if there are pre-formed antibodies to these self-antigens, they will react to the antigens or their determinants that are exposed following transplantation and lead to PGD. In this study, we hypothesized that preformed antibodies to self-antigens k-alpha tubulin, collagen I, and collagen V (autoantibodies) would bind to exposed antigenic epitopes and lead to PGD of lung allografts. PGD, in turn, will initiate the inflammatory cascade, promoting alloimmune responses and development of antibodies to mismatched donor HLA that are known to predispose to BOS.
Material and Methods
Study Subjects
Adult patients undergoing lung transplantation at Washington University Medical Center/ Barnes-Jewish Hospital were prospectively enrolled in the study between 1995 and 2005 after obtaining informed consent, in accordance with a protocol approved by the Institutional Review Board. The peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (Pharmacia, Sweden), and stored at −135°C. The plasma separated from peripheral blood was stored at −70°C. Immunotherapy consisted of cyclosporine, azathioprine, and prednisone. After BOS was diagnosed, the immunotherapy was modified to FK506 (Tacrolimus), mycophenolate mofetil, and prednisone.
Definitions
BOS was diagnosed according to the ISHLT criteria (9) based on the percentage decline in forced expiratory volume in 1 second (FEV1) compared to baseline and graded as follows: 1=80–66% of baseline value, 2=65–51% of baseline value, and 3=50% or less of baseline value. Other causes of decreased lung function such as infection and bronchial anastomotic stricture were ruled out. PGD was diagnosed following transplant on arrival of the patient to the intensive care unit according to ISHLT guidelines (10). The absence of other causes of lung allograft dysfunction such as hyperacute rejection, venous anastomotic complications, cardiogenic pulmonary edema, and pneumonia are implicit in this definition.
Assays and reagents
Flow-PRA for the detection of HLA-antibodies was done using flow-cytometry as per the manufacturer’s protocol (One Lambda Inc, CA). The percent PRA is determined by the percent of microparticles that are bound by the antibodies in the serum. PRA ≥ 2.9% for HLA class-I and 2.4% for HLA class-II was considered positive. Serum levels of IL-1β, IL-2, IL-12, IL-15, MCP-1, and IP-10 were analyzed using LUMINEX immunoassays (Biosource International Inc, CA) according to the manufacturer’s protocols. These techniques have been described in detail in our previous publication (11).
ELISA
ELISA plates (Thermo Fisher Scientific, Rochester, NY) were coated with collagen I (Cell Sciences, Canton, CA), Collagen V (BD Biosciences, San Jose, CA) or K-alpha 1 tubulin (recombinant purified) at a concentration of 1µg/ml in phosphated buffered saline overnight at 4°C. Patient and normal human sera were tested (1:250, 1:750 and 1:1250) for binding to collagen I, collagen V, and k-alpha 1 tubulin. Detection was done with anti-human IgG, IgM – Horseradish peroxidase (HRP), developed using tetramethylbenzidine (TMB) substrate for 10 minutes and read at 450nm. Concentration of Abs was calculated based on a standard curve using the binding of known concentration of anti-Collagen I, anti-k alpha 1 tubulin or anti-collagen V Abs (Santa Cruz Inc, Santa Cruz, CA). Positive cutoffs were determined at values two standard deviations above the mean values of the normal subjects. The cut off values were determined to be 32ng/ml for collagen type I, 125ng/ml for collagen type V, and 190ng/ml for k-alpha tubulin. ELISA for soluble C4d was performed according to manufacturer’s instructions (QUIDEL, San Diego, CA). Bronchoalveolar lavage (BAL) was processed by centrifugation at 1000g for 10 minutes to precipitate the cellular debris and supernatant was used for analysis. All samples were analyzed in triplicates and read at 405nm wavelength.
Statistical analysis
Continuous data was checked for normality using Shapiro-Wilk test. Non-normal data were transformed with a log transformation. Type 1 error was controlled when performing multiple t-tests using Dunn-Sidak correction. Tabular data were compared using the Fisher’s exact test for 2 × 2 tables and chi-square for 2 × n tables. Relative risk and confidence intervals were calculated using the contingency tables. For more than two group comparisons and analyzing multiple dependent variables, MANOVA was used. Serial development of HLA-antibodies on Flow-PRA was analyzed using the Kaplan-Meier analysis and the groups were compared using logrank test. Freedom from BOS was analyzed using Kaplan Meier method. Univariate and multivariate Cox proportional hazard models were constructed to identify risk factors for BOS. Software used for the above analyses were GraphPad Prism 4 (GraphPad Inc, La Jolla, CA), GraphPad Instat 3 (GraphPad Inc, La Jolla, CA), and SYSTAT V 11.0 (SYSTAT Inc, Richmond, CA).
Results
Pre-transplant antibodies and the risk of PGD
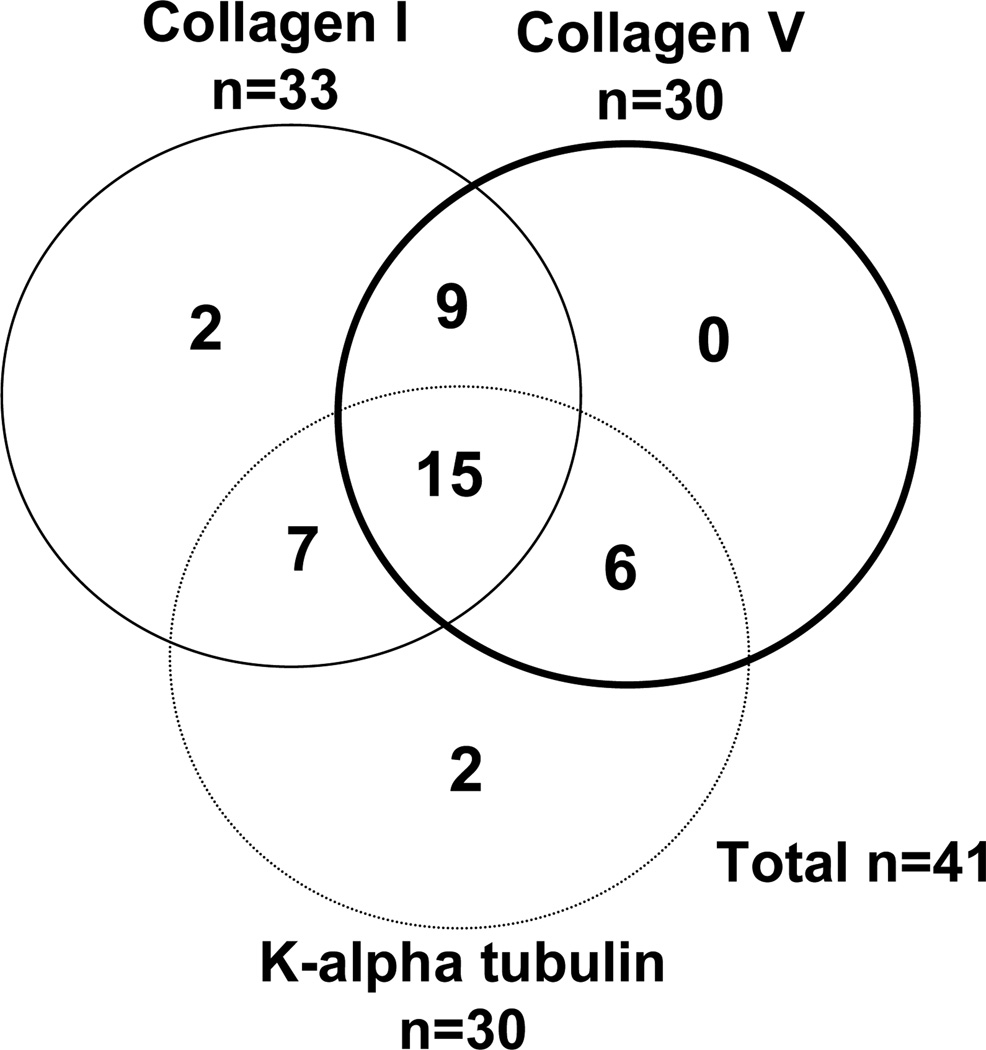
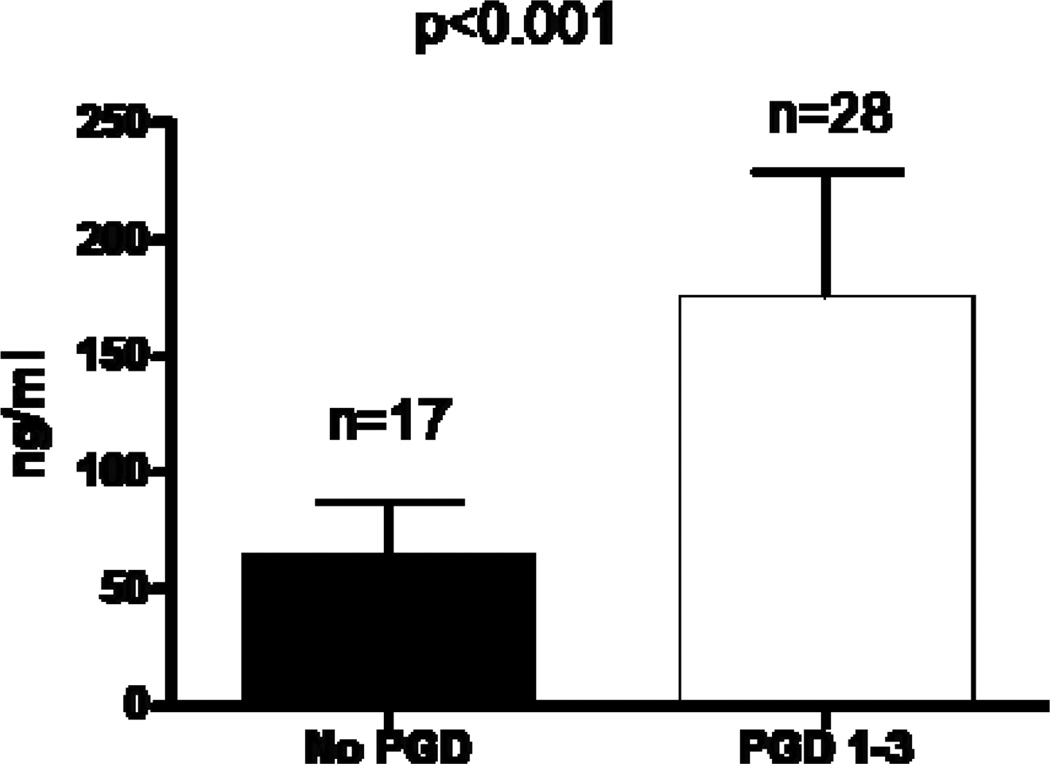
The study included 142 lung transplant patients. The clinical and demographic profile of the recipients and donors is shown in Table 1. Of the patients included in the study, 101 were negative for all antibodies while 41 had one or more of the three antibodies (Figure 1). There were no significant differences in the clinico-demographic variables between the study groups. We first screened BAL from HLA-antibody negative patients with (n=28) and without PGD (n=17) for soluble C4d. Patients with PGD revealed higher C4d in the BAL (175±54 ng/ml Vs 65±23 ng/ml, p<0.001, Figure 2). There was no difference in the clinical variables between groups (data not shown). Soluble C4d in the BAL strongly suggests an antigen-antibody complex mediated pathology. Since the patients were negative for HLA-antibodies, this suggested that PGD may be a consequence of antibodies to non-HLA antigens. Previous studies from our lab and others have shown that antibodies against self-antigens develop post-transplantation and correlate with the development of BOS. To test the hypothesis that preformed antibodies to self-antigens would increase PGD risk, we tested for antibodies against three self-antigens present in the lung, collagen I, collagen V, and k-alpha 1 tubulin.
Table 1.
Clinical Profile of study subjects
| All n=142 |
Antibody Negative N=101 |
Antibody Positive n=41 |
p | ||
|---|---|---|---|---|---|
|
RECIPIENT FACTORS |
|||||
| AGE | 55.33±13.77 | 56.18±12.11 | 54.51±14.48 | NS | |
|
GENDER M F |
75(52.8%) 67(47.2%) |
52(53.1%) 49(46.9%) |
23(34.5%) 18(65.5%) |
NS |
|
|
TRANSPLANT Single Bilateral |
14(9.8%) 128(90.2%) |
10(9.9%) 91(90.1%) |
4(9.7%) 37(90.3%) |
NS |
|
|
PATHOLOGY COPD A1A CF IPF Bronchiectasis Others |
88(61.9%) 16(11.3%) 21(14.7%) 10(7.0%) 3(2.1%) 4(2.8%) |
68(67.5%) 10(9.9%) 13(12.9%) 7(6.9%) 1(0.9%) 2(1.9%) |
20(48.8%) 6(14.6%) 8(19.5%) 3(7.3%) 2(4.8%) 2(5.0%) |
NS |
|
|
ISCHEMIA (mins) R L |
284.1±37.5 311.3±31.3 |
285.6±45.3 323.5±38.6 |
281.6±35.8 309.2±29.5 |
NS NS |
|
|
ACUTE REJECTION |
0.85±0.81 |
0.81±0.55 |
0.86±0.61 |
NS |
|
|
RACE Caucasian AA Others |
128(90.1%) 10(7.0%) 4(2.9%) |
93(92.1%) 6(5.9%) 2(2.0%) |
35(85.4%) 4(9.7%) 2(4.9%) |
NS |
|
|
HLA I mismatch |
2.43±1.9 | 2.51±1.7 | 2.41±1.6 | NS | |
|
HLA II mismatch |
0.86±0.9 | 0.84±0.7 | 0.87±0.6 | NS | |
|
DONOR FACTORS |
|||||
| AGE | 32.41±11.83 | 34.18±10.29 | 31.71±13.56 | NS | |
|
GENDER M F |
81(57%) 61(43%) |
55(54.5%) 46(45.5%) |
26(63.4%) 15(36.6%) |
||
|
RACE Caucasian AA Others |
77(54.2%) 55(38.7%) 10(7.1%) |
58(57.4%) 38(37.6%) 5(4.9%) |
19(46.4%) 17(41.5%) 5(12.1%) |
NS |
|
| SMOKING | 44(31%) | 30 (29.7%) | 14(34%) | NS | |
| TRAUMA | 86(60.6%) | 62(61.4%) | 24(58.6%) | NS | |
Figure 1.
Distribution of autoantibodies in the study subjects
Figure 2.
Elevated C4d levels in BAL specimens of HLA antibody negative PGD positive patients. BAL specimens from patients with PGD (n=28, white bars) and those without PGD (n=17, black bars) were tested for soluble C4d using ELISA. The levels are expressed as ng/ml. The difference was statistically significant with o<0.001.
Of the 41 patients in the autoantibody positive group, 35 (85.4%) had some grade of PGD (1–3) while 6 (14.6%) had no PGD. In contrast, in the autoantibody negative group, 66 (65.3%) had PGD while 35 (34.7%) did not. Hence, the risk of PGD was increased in patients with preformed antibodies to self-antigens compared to those with no antibodies (Table 2RR 3.1, 95% CI 1.2 to 8.1, p=0.02). The risk with all three antibodies was higher (RR 7.4, 95% CI 0.93 to 58.9, p=0.03). In patients with PGD, 34.7% were positive for pre-transplant antibodies while in the PGD negative group only 14.6% had these antibodies (p=0.03).
Table 2.
Autoantibodies and risk of PGD
| PGD −ve |
PGD +ve |
Odds Ratio |
CI | p | |
|---|---|---|---|---|---|
| All (n=142) | 41(28.9%) | 101(71.1%) | |||
| Antibody −ve | 35 (34.5%) | 66(65.5%) | |||
|
Antibody +ve All Two Positive Three Positive |
6(19.4%) 3(13.6%) 1(6.7%) |
35(80.6%) 19(86.4%) 14(93.3%) |
3.09 0.07 7.4 |
1.2–8.1 0.9–12.1 0.9–58.9 |
0.02 0.07 0.03 |
Preformed antibodies to self-antigens lead to inflammation and promote development of HLA-antibodies
In our previous report we demonstrated that PGD leads to increased pro-inflammatory IL-1β, IL-2, IL-12, IL-15, IP-10, and MCP-1 (4). If antibodies to self-antigens increased the risk of PGD, we hypothesized that higher levels of these pro-inflammatory mediators would be found in the autoantibody positive group. Sera was collected within one week of transplantation and analyzed for the presence of pro-inflammatory mediators. Samples of 14 patients in the antibody positive group and 23 patients in the antibody negative group were not available. As represented in Table 3, antibody positive group had elevated levels of IP-10, MCP-1, IL-1β, IL-2, IL-12, and IL-17 (p<0.04 for all).
Table 3.
Elevated levels of cytokines in Autoantibody positive group
| Autoantibody Positive (pg/ml) |
Autoantibody Negative (pg/ml) |
p value | |
|---|---|---|---|
| IP-10 | 298 ± 34 | 75 ± 23 | 0.001 |
| MCP-1 | 655 ± 102 | 212 ± 78 | 0.032 |
| IL-1β | 1121 ± 287 | 532 ± 134 | 0.003 |
| IL-2 | 677 ± 211 | 223 ± 123 | 0.090 |
| IL-12 | 443 ± 89 | 176 ± 56 | 0.010 |
| IL-17 | 967 ± 288 | 321 ± 101 | 0.040 |
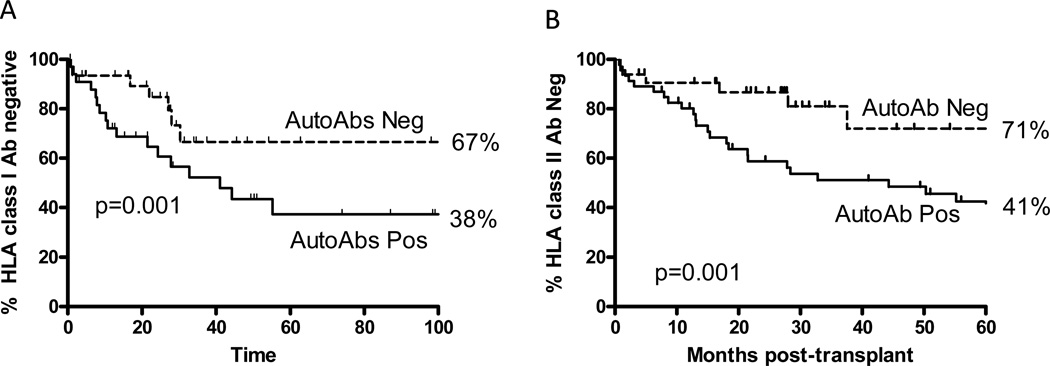
Sera collected at 1–2 monthly intervals were analyzed for the development of HLA class I as well as HLA class II antibodies using Flow-PRA. Patients were censored in case of death or development of BOS. All the patients in the study were negative for HLA-antibodies prior to transplantation. Using Kaplan-Meier analysis at 5-years, freedom from HLA class I antibody was 67% in autoantibody negative group and 38% in autoantibody positive group. For HLA class II antibody, at 5yrs freedom from alloantibody development was 71% in the autoantibody negative group and 41% in the autoantibody positive group (Figure 3p=0.001 for both).
Figure 3.
Development of HLA class I (A) and class II (B) antibodies in the patients with autoantibodies positive (solid lines) and autoantibodies negative (broken lines).
Preformed antibodies to self-antigens increase risk of BOS
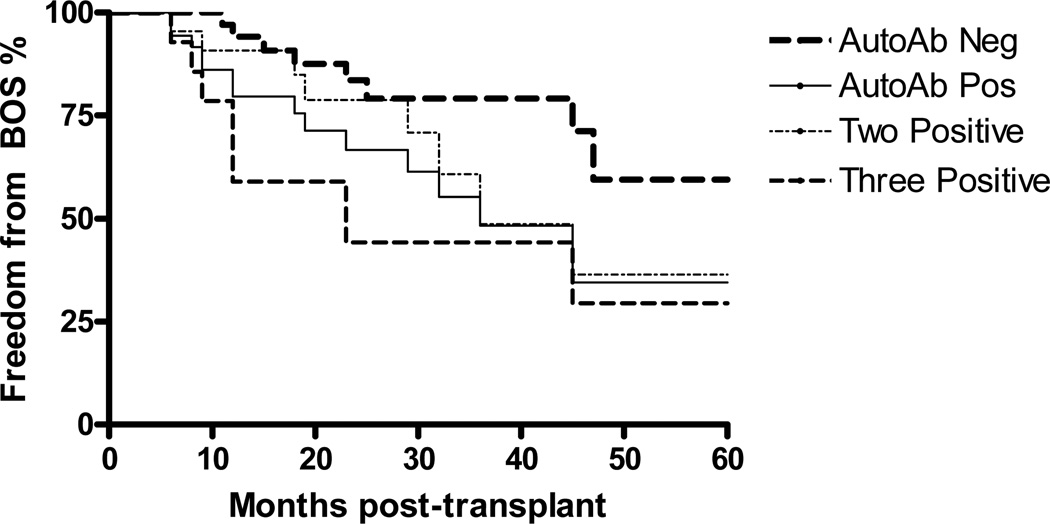
Patients were monitored for the development of BOS. Three patients in the antibody positive and six patients in the antibody negative group died within the first six months and were excluded. Forty-eight of the remaining 133 developed BOS with a median time to onset of 44 months. Prospective analysis of the remaining patients showed that antibody positive group had significantly increased incidence of BOS (Figure 4). Furthermore, patients with two and three antibodies had even higher incidence BOS compared to the antibody negative group. Univariate Cox regression analysis identified acute rejection, lymphocytic bronchitis, PGD, two or all three autoantibodies positivity, respiratory viral infections as significant risk factors for the development of BOS (Table 4). These risk factors were then fitted in a multivariate Cox proportional hazard model. Presence of two or more antibodies persisted to be independent risk factor for the development of BOS. In addition, acute rejection, respiratory viral infections, PGD remained independent risk factors (Table 5) while lymphocytic bronchitis lost statistical significance.
Figure 4.
Development of BOS in patients with autoantibodies. Differences between autoantibody negative and the autoantibody positive, two and three positive groups were statistically significant (p<0.05) for all.
Table 4.
Univariate Cox Proportional Analysis
| RR | 95% CI | p | |
|---|---|---|---|
| Age | 1.00 | 0.78–1.0 | 0.76 |
| Gender (Male Sex) | 1.11 | 0.65–2.6 | 0.59 |
|
Primary Lung disease COPD (reference) CF A1AT Def IPF Others |
1.00 1.3 0.79 1.5 0.78 |
0.8–1.9 0.2–3.4 0.75–2.3 0.4–2.9 |
0.76 0.88 0.32 0.43 |
|
HLA Mismatch 1 antigen 2 antigens 3 antigens |
0.9 1.1 1.4 |
0.77–2.3 0.5–3.1 0.8–4.1 |
0.77 0.11 0.08 |
| Single Lung Transplantation | 1.3 | 0.66–1.8 | 0.78 |
| Acute Rejection | 1.8 | 1.2–2.1 | 0.03 |
| Respiratory Viral infections | 1.9 | 1.2–2.6 | 0.02 |
| Lymphocytic Bronchitis | 1.4 | 1.2–2.1 | 0.03 |
| Primary Graft Dysfunction | 2.4 | 1.6–3.1 | 0.01 |
|
Antibodies One Positive Two Positive Three Positive |
1.5 1.9 2.8 |
0.8–3.9 1.3–4.3 1.5–6.5 |
0.09 0.02 0.01 |
Table 5.
Multivariate Cox Hazard Analysis
| RR | 95% CI | p | |
|---|---|---|---|
| Acute Rejection | 1.5 | 1.3–2.7 | 0.02 |
| Respiratory Viral Infection | 2.1 | 1.5–2.6 | 0.04 |
| Primary Graft Dysfunction | 2.5 | 1.3–3.8 | 0.01 |
|
Antibodies Two Positive Three Positive |
1.5 1.7 2.3 |
1.2–3.1 1.1–3.4 1.7–4.5 |
0.02 0.03 0.009 |
Comments
Land et al 12) initially introduced the concept of “response to injury” hypothesis (13). They proposed the role for early post-transplant inflammation in increasing allograft immunogenicity and risk for chronic rejection. In human lung transplantation, PGD is known to be a major risk factor for BOS (3). All grades of PGD (1–3) independently increase the risk. The reported incidence of PGD following lung transplantation approaches over 80% (3). Since PGD is a major risk for BOS, it is likely that prevention of PGD will have a major impact on lung allograft survival. Nevertheless, the immuno-pathogenesis of PGD largely remains unknown. In this report, we demonstrate that pre-existing antibodies to lung specific self-antigens can increase PGD risk. We also present evidence that autoantibodies can lead to inflammation, augment alloimmunity and thereby promote BOS.
Many factors have been shown to predispose to BOS including acute rejection, viral infections, alloimmunity, gastroesophageal reflux (2). Each of these constitutes an injury that leads to tissue remodeling and obliteration of small airways that is hallmark of BOS. However, these injuries occur in the post-transplant period and are typically late events. By the time these are diagnosed, the immunopathogenic cascade which can lead to BOS is already set. Therefore, identification of peri- and pre-transplant risk factors would enable timely intervention for prevention of BOS.
Recent evidence has shown that the inflammatory milieu in the allograft can lead to the development of de novo autoimmunity that is an independent risk factor for chronic rejection (14). Studies from our lab and others have demonstrated that both humoral and cellular immunity to self-antigens predispose to BOS (8, 14). Since the native lungs are also under a state of inflammation due to the ongoing insult by the primary lung disease, we hypothesized that these patients would develop autoimmunity prior to transplantation. Westall et al made an important observation that lung biopsy specimens in patients with primary graft dysfunction showed complement deposition (15). Furthermore, a small cohort of these patients developed early BOS and showed evidence of antibody mediated rejection. However, it was unclear whether these antibodies were alloantibodies to mismatched HLA or autoantibodies to self antigens. To answer this, we tested lung transplant recipients that were negative for HLA-antibodies for presence of soluble Cd4 (Figure 2). PGD patients had higher levels of complement in the BAL which is a marker for antibody mediated activation of complement pathways. Since the patients were negative for alloantibodies against the HLA antigens, it strongly suggests that PGD may be a consequence of autoantibody mediated activation of complement. Therefore, we analyzed for the presence of antibodies against collagen I, collagen V, and k-alpha 1 tubulin. Prior studies have shown the development of collagen V and K-alpha 1 tubulin antibodies in the post-transplant period strongly correlates with the development of BOS (14, 16, 17). Collagen type I is present in the lung tissue and is co-assembled with collagen type V into hetrotypic fibrils (18). Since there is evidence of collagen V autoantibodies in lung transplant patients, we hypothesized that collagen I would be also be a target.
Preformed antibodies to the self-antigens were found in 41 (28.9%) of the 142 patients in the pre-transplant sera. Not surprisingly, majority of the patients had more than one autoantibody (Figure 1). Patients with autoantibodies had elevated risk for PGD (Table 2). The risk was higher in patients with all three antibodies. Even in patients with two autoantibodies, the risk was elevated but this did not reach statistical significance. We attribute this to the smaller sample size. PGD is postulated to result from a variety of insults that start with donor brain death, ventilator-associated lung injury, cold ischemia, and ischemia-reperfusion during transplantation (3). In our study there was no difference in donor variables between the antibody positive and negative groups. Therefore, these donor factors cannot account for the difference in PGD rates between these two groups. We recently demonstrated that patients that develop autoantibodies post-transplant also reveal complement deposition on lung allografts (19). Contrastingly, despite the presence of autoantibodies, native lungs may not show antigen deposition. The surgical stress, and ischemia reperfusion augments the expression of sequestered self-antigens that can then lead to the antigen-antibody reactions and complement activation. Hence, we propose that the reason why patients only develop PGD post-transplant and not while on the waitlist may be because the surgical stress significantly increases the expression of self-antigens leading to a clinically identifiable syndrome. Studies from Wilkes and Burlingham group demonstrated that expression of collagen V is promoted due to the ischemia-reperfusion injury (8). Taken together, we postulate that pre-existing autoantibodies will bind to the self-antigens that are expressed following transplantation in lung allografts which can lead to complement activation and tissue injury that is manifested as PGD.
Patients that had pre-transplant autoantibodies were also found to have significantly higher levels of pro-inflammatory cytokines. These cytokines upregulate HLA on donor cells and promote antigen presentation thereby promoting alloimmunity. It is generally accepted that binding of alloantibodies to the allograft can produce deleterious effects by complement-mediated cytotoxicity, apoptosis, as well as by activation of epithelial and endothelial cells to increase stress proteins and growth factors that cause smooth muscle cell proliferation and fibrosis (20). Although there was increased alloantibody development in autoantibody group, we did not find any difference in acute rejection. This may be a result of effective immunosuppression or inadequate power of this study to detect such a difference.
To determine whether there was a relationship between pre-existing autoantibodies and chronic rejection, we developed Cox regression models. Our results identified acute rejection, respiratory viral infections, PGD and presence of pre-transplant autoantibodies as independent risk factors for BOS (Table 4). Interestingly, autoantibodies and PGD were identified as independent risk factors for BOS that may suggest that induction of PGD is not the only mechanism by which autoantibodies increase BOS risk. Even if not profound enough to manifest as PGD, it is likely that ligation of self-antigens by specific antibodies results in ongoing inflammation that can augment alloantigen presentation and promotion of donor-specific immunity.
Our study has certain limitations related to the lack of data availability and lack of randomization. Furthermore, our sample size was not large enough to determine the risk of individual autoantibodies in PGD and BOS. The study size also did not permit risk analysis of autoantibodies and different PGD and BOS grades. However, in spite of these shortcomings, the analysis we present here is extensive and includes close follow-up of patients and the HLA antibody status spanning over 5 years. All patients in the study were classified according to the latest definitions of PGD and BOS. The results presented clearly demonstrate an important clinically relevant and novel finding that pre-transplant autoantibodies increase the risk of PGD. PGD can then lead to inflammation, augment the development of alloimmunity and promote chronic rejection.
Acknowledgements
This work was supported by ARRA Award HL056643 from the National Institutes of Health/National Heart Lung Blood Institute (TM), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Ms Billie Glascock for assistance in the preparation of this manuscript.
Footnotes
Presented at the 46th annual Meeting of the Society of Thoracic Surgeons, Fort Lauderdale, FL, January 25–27th, 2010.
References
- 1.Trulock EP, Christie JD, Edwards LB, et al. Registry of the international society for heart and lung transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26(8):782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Hachem RR, Trulock EP. Bronchiolitis obliterans syndrome: pathogenesis and management. Semin Thorac Cardiovasc Surg. 2004;16(4):350–355. doi: 10.1053/j.semtcvs.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 4.Bharat A, Kuo E, Steward N, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86(1):189–195. doi: 10.1016/j.athoracsur.2008.03.073. discussion 196-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65(5):648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 6.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 7.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata T, Philipovskiy A, Fisher AJ, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181(8):5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 10.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 11.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 12.Land W, Schneeberger H, Schleibner S, et al. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57(2):211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 13.Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation. 2005;79(5):505–514. doi: 10.1097/01.tp.0000153160.82975.86. [DOI] [PubMed] [Google Scholar]
- 14.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westall GP, Snell GI, McLean C, Kotsimbos T, Williams T, Magro C. C3d and C4d deposition early after lung transplantation. J Heart Lung Transplant. 2008;27(7):722–728. doi: 10.1016/j.healun.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+CD25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human transplantation. American Journal of Transplantation. 2006;6(8):1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 18.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32(3):223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 19.Golocheikine A, Nath DS, Basha HI, et al. Increased erythrocyte C4D is associated with known alloantibody and autoantibody markers of antibody-mediated rejection in human lung transplant recipients. J Heart Lung Transplant. 2009 doi: 10.1016/j.healun.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64(5):521–529. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]