Abstract
Background
Lower respiratory viral infections predispose to Bronchiolitis Obliterans Syndrome (BOS). In addition, there is emerging evidence to support the role of autoimmunity in the pathogenesis of BOS. Since CD4+CD25+Foxp3+ T-cell (Treg) control autoimmunity, we tested the hypothesis that respiratory virus-induced Treg dysfunction leads to BOS.
Methodology
Treg frequency was monitored using flow-cytometry. Apoptosis, cytokines, and antibodies were analyzed using annexin V assay, LUMINEX, and ELISA, respectively. Murine studies were performed using orthotopic tracheal transplant model.
Results A)
Human studies: Treg troughs (decrease >50% of baseline) were found in 13 (43.3%) of 30 lung transplant recipients. Treg isolated during troughs revealed increased apoptosis (37.8%). Patients with Treg troughs had increased prevalence of antibodies to self antigens collagen type I (23.1% Vs 5.8% pre-trough), collagen V (7.7% Vs 0%), and k-alpha tubulin (30.7% Vs 11.7%, p<0.01) at 6-months. Increased number of Treg troughs correlated with more rapid onset of BOS. B) Murine studies: Infection of tracheal transplant recipients with murine parainfleunza sendai virus led to increased Treg apoptosis (50.5%) in the draining lymph nodes. Vaccination against sendai virus prior to transplant abrogated apoptosis of Treg. In vitro, sendai virus infected, but not naive, tracheal epithelial cells demonstrated upregulation of FasL (>3.5 fold) and induction of co-cultured Treg apoptosis (5.6 fold increase).
Conclusions
RVI cause Treg apoptosis which leads to the development of de novo autoimmunity that may play a role in the pathogenesis of BOS.
Keywords: Viruses, Regulatory T-cells, Autoimmunity, BOS
Introduction
Bronchiolitis obliterans syndrome (BOS), chronic lung allograft rejection, accounts for about one-third of the mortality after one year post-transplant and develops in over 50 –70% of lung transplant recipients after 5 years (1). Several risk factors have been identified for the development of BOS (2). These include acute rejection, respiratory viral infections (RVI) , lymphocytic bronchitis, cytomegalovirus pneumonitis, anti-HLA antibodies, primary lung allograft dysfunction, gastroesophageal reflux, and autoimmunity (2–4).
RVI represent one of the strongest but preventable risk factors for the development of BOS (5). In a cohort of 259 patients, Khalifah et al demonstrated that respiratory viral infections were distinct risk factors for BOS (5). Furthermore, in our previous report we demonstrated that murine parainfluenza sendai viral (SdV) infection, a correlate of human RVI, increased the severity of obliterative airway disease (OAD), the correlate of human BOS (6).
LTR can also develop autoimmunity against collagen type V (4, 7) and k-alpha tubulin (8) that predisposes to BOS. Previous reports have also demonstrated that there is loss of regulatory CD4+CD25+foxp3+ regulatory T-cells (Treg) in patients with BOS (4). Treg play a major role in peripheral tolerance against self-antigens and loss of Treg leads to autoimmunity (9). Since many viruses have also been implicated in autoimmune disorders (10), we tested the hypothesis that RVI predisposed to BOS by inducing Treg dysfunction and promoting an immune response to self-antigens. We demonstrate that respiratory viruses induce Treg apoptosis both in humans and murine models of chronic rejection. Treg apoptosis leads to development of autoimmunity that contributes to the immunopathogenesis of BOS following lung transplantation.
Methods
Human subjects
Blood specimens were collected from patients undergoing lung transplantation and normal volunteers at the Barnes-Jewish Hospital after obtaining informed consent in accordance with a protocol approved by the Institutional Review Board. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation, and stored at −135°C. Plasma was stored at −70°C. BOS was defined according to the standard ISHLT guidelines (11). RVI was defined as a positive microbial culture for respiratory syncitial virus (RSV), parainfluenza, influenza, and adenovirus from bronchoalveolar lavage, bronchial wash, tracheal aspirate, sputum culture, or nasopharyngeal swab specimens (5).
Reagents
Mouse anti-human CD4 (clone RPA-T4), CD25 (clone M-A251), mouse anti-human foxp3 (clone 259D/C7), rat anti-mouse CD4 (clone GK1.5), rat anti-mouse CD25 (clone 3C7), rat anti-mouse foxp3 (clone MF23), annexin-V staining kit, anti-mouse FasL (clone MFL3), and isotype control antibodies were purchased from BD Biosciences (Pharmingen, San Diego, CA). Ham's media comprised of Ham's F-12 (Sigma Aldrich, St Louis, MO) with 100U/mL penicillin and 100 μg/mL streptomycin. Tracheal epithelial cell culture (TEC) medium was formulated in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS). LUMINEX, ELISA, [3H]-thymidine proliferation, and annexin V (BD Pharmingen, San Jose, CA) assays were performed according to methods described in our earlier publications (12).
Orthotopic Tracheal Transplantation
Orthotopic tracheal transplants (OTT) were performed according the technique described before (6) using 6–8 week old age and weight matched BALB/c (H2d) or C57BL/6 (H2b) animals (Jackson Laboratories, Bar Harbor, ME). MMP7-KO mice were a gift William C. Park, Washington University, St. Louis, MO. All animal studies were performed in accordance with the Animal Studies Committee, Washington University, guidelines. Percent fibrosis was calculated with computer assistance (Image Pro Express v 4.0, Media Cybernetics; Silver Springs, MD) by dividing the lamina propria, the area below the basement membrane and above the cartilage, by the total area above the cartilage. Sendai virus infection was done by intranasal injection of a sublethal (5K) dose of the virus as discussed in our prior manuscript (6).
Murine tracheal epithelial cell culture
Tracheal epithelial cells were isolated and cultured as previously described (13). Briefly, the murine tracheal segments were harvested from the larynx down to the carina. The tracheas were split and placed into ice cold Ham's media containing pronase (Roche Applied Science, Indianapolis, IN) overnight at 4°C. The harvested tracheas were gently inverted over a shaker after mixing with 10% FBS for 2 hrs. The cells were washed and resuspended in Ham's media containing DNase I and incubated for 10 minutes on ice. The cells were then resuspended in TEC medium in non-coated culture plates for 2 hours to allow fibroblasts to adhere. Subsequently, non-adherent cells were collected by centrifugation and resuspended in TEC media. Cells were seeded in culture flasks coated with human collagen type I at 37°C in 5% CO2. Murine tracheal epithelial cells were harvested between third and fifth passage and cultured into 24-well plates.
Statistical Analysis
For all parametric data, one-way analysis of variance with post hoc comparisons was performed using the Fisher's least significant difference test. Student t test was used to compare the means of continuous variables. Contingency tables 2×2 were analyzed using Fisher's test and 2×n by chi square test. Statistical significance in all cases was defined as p< 0.05.
Results
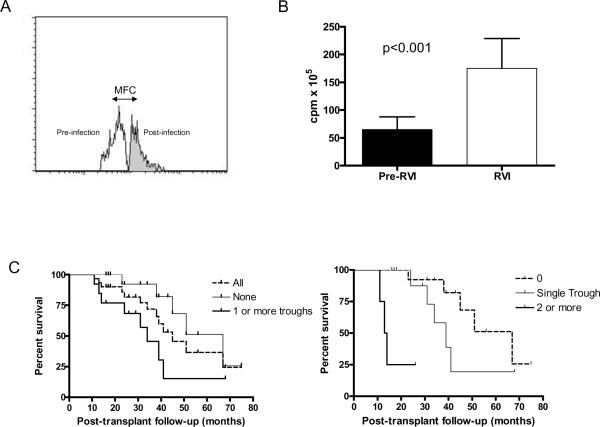
Loss of Treg during respiratory viral infections in human lung transplant recipients
The clinical and demographic profile of study subjects (n=30) is shown in Table 1. We determined the frequency of Treg in lung transplant recipients at 2–4 month intervals using PBMC. The median onset of BOS was 45 months. As shown in Table 2, 13 of the thirty patients (43.3%) revealed a trough as defined by a drop in the frequency of Treg greater than 50% of the baseline, prior to the development of BOS. Of the patients that revealed Treg trough (n=13), 9 (69.2%) had a single trough, 3 (23.1%) had two troughs, and 1 (7.7%) had three troughs. Therefore, overall 18 troughs were observed in the 13 patients. Further, 11 (61.1%) of these 18 troughs were associated with RVI within 2 weeks of the onset of trough (Table 2). Treg during these troughs demonstrated increased level of apoptosis [Mean Fluorescent Channel (MFC) increase 37.8±12.5%, p=0.013, Figure 1A). The PBMC during this period also showed increased proliferative response to mitogenic stimulation (Figure 1B). Six months following detection of Treg troughs, patients showed increased incidence of autoantibodies to collagen I (23.1% Vs 5.8% pre-trough), collagen V (7.7% Vs 0% pre-trough), and k-alpha tubulin (30.7% Vs 11.7% pre-trough). However, there was no significant difference in the titer of antibodies in patients that were positive prior to the development of troughs. Only one patient in the group without troughs developed RVI. This patient remained negative for all three antibodies before and 6-months after the infection. There was higher prevalence of BOS among patients with Treg trough [9 out of 13 (69.2%) Vs 6 of 17 (35.3%), chi p=0.06]. The number of Treg troughs correlated with more rapid onset of BOS (No Trough: Median 67 months, Single: 9 months, Two or more: 13.9 months; p<0.001, Figure 1C).
Table 1.
Clinical and demographic profile of LT patients
| All n=30 | No Troughs (n=17) | Treg troughs (n=13) | p | |
|---|---|---|---|---|
| Age | 50.1±13.7 | 49.8±12.2 | 51.2±13.5 | 0.8 |
| Sex | ||||
| Female | 13 | 6 | 7 | 0.1 |
| Male | 17 | 11 | 6 | |
| Race | ||||
| Caucasian | 27 | 14 | 13 | 0.5 |
| African-American | 2 | 2 | 0 | |
| Others | 1 | 1 | 0 | |
| Pathology * | ||||
| COPD | 16 | 10 | 6 | 0.8 |
| A1A | 5 | 2 | 3 | |
| CF | 3 | 1 | 2 | |
| IPF | 5 | 3 | 2 | |
| Others | 1 | 1 | 0 | |
| HLA mismatch | ||||
| Class-I | 2.82±1.5 | 2.91±1.8 | 2.78±1.2 | 0.2 |
| Class-II | 0.77±0.6 | 0.81±0.8 | 0.66±0.8 | 0.1 |
| # Acute rejections | 0.77±0.9 | 0.71±0.4 | 0.82±0.5 | 0.1 |
| Ischemia time (min) | ||||
| Right | 279.0±13.3 | 273.1±17.5 | 291.0±16.8 | 0.08 |
| Left | 311.1±18.4 | 299.1±19.8 | 323.8±21.5 | 0.11 |
| Type of Transplant | ||||
| Bilateral | 28 | 16 | 12 | 0.8 |
| Single | 2 | 1 | 1 |
COPD= Chronic Obstructive Pulmonary Disease
A1A= Alpha-1 Antitrypsin deficiency
CF= Cystic Fibrosis
IPF= Idiopathic Pulmonary Fibrosis
Table 2.
Treg troughs and respiratory viral infections
| Groups | N | RVI | BOS | Median onset of BOS | ||
|---|---|---|---|---|---|---|
| No Treg Trough | 17 | 1 | 6 | 67 months | ||
| Single Trough | 9 | 4 | 6 | 39 months | ||
| Two Trough | 3 | 2 | 12 months | |||
| Patient 1 | ||||||
| Trough 1 | Pos | |||||
| Trough 2 | Pos | |||||
| Patient 2 | ||||||
| Trough 1 | Pos | |||||
| Trough 2 | Pos | |||||
| Patient 3 | ||||||
| Trough 1 | Neg | |||||
| Trough 2 | Pos | |||||
| Three Troughs | Patient 1 | 1 | 1 | 14 months | ||
| Trough 1 | Neg | |||||
| Trough 2 | Pos | |||||
| Trough 3 | Pos | |||||
RVI = Respiratory Viral Infection
Figure 1.
RVI and Treg. A) RVI induce Treg apoptosis. PBMC were analyzed for Treg apoptosis using annexin V assay. Cells were gated using CD4, CD25, Foxp3. B) Increase in mitogenic reponse of PBMC during Treg troughs. PBMCs (3×105) during and prior to Treg trough development were stimulated using CD3/CD28 beads and proliferation counts measured. C) Increase BOS development in patients with Treg troughs. Survival curves were statistically different (p<0.01)
Regeneration of recipient epithelium into murine orthotopic tracheal allografts induces Treg
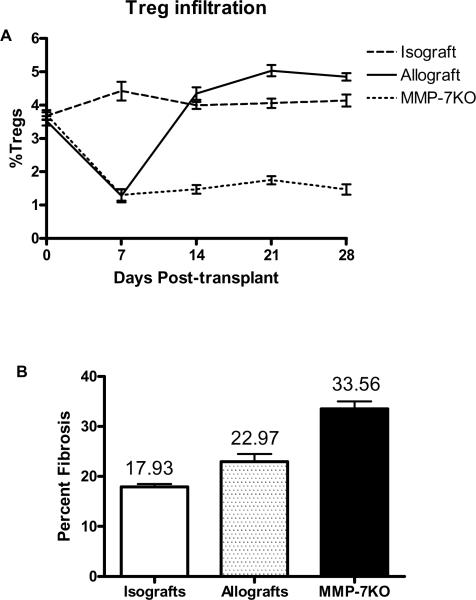
Following allogeneic murine OTT, there is in-growth of the recipient epithelium into the donor tracheal segment (14). The recipient epithelium re-populates the donor trachea by day 28. This has been postulated to limit further allograft fibrosis and occlusion, seen in the heterotopic tracheal transplantation (14, 15). We characterized the kinetics of Treg in the draining lymph node following OTT. Syngeneic C57Bl/6 (B6) or allogeneic Balb/c (B/c) to B6 transplants were performed. The draining paratracheal lymph nodes (DLN) were harvested on days 0, 7, 14, 21, 28, and 35. The frequency of CD4+CD25+foxp3+ Treg was analyzed. Following transplant, isograft recipients revealed a stable frequency of Treg (Figure 2A) between 3.4–4.5%. In contrast, tracheal allograft recipients revealed a marked loss of Treg from 3.8% to 1.1% (p=0.001) within the first week that correlated with epithelial denudation of the donor tracheal segments. By day 7 there was a rise in the frequency of Treg and by day 14, Treg reached baseline frequency (4.1%). Similar pattern was found in B/c recipients of B6 tracheas (data not shown). MMP-7KO mice (B6 background) that have defective epithelial regeneration, when transplanted with allogeneic B/c tracheas, developed initial decrease in Treg as seen in the wild-type B6 recipients. However, Treg in MMP-7KO mice failed to reach baseline even at day 28 (Figure 2A). MMP-7KO mice also revealed increased long-term tracheal allograft fibrosis (Percent Fibrosis 33.56±5.45%) compared to wild type B6 recipients (22.97±3.56%, p=0.002) and isograft (17.93 ±3.56%, p=0.001, Figure 2B).
Figure 2. Airway epithelium and Treg regeneration.
A). OTT recipients (Balb/c into C57Bl/6 and Balb/c into C57Bl/6 MMP-7KO, n=50 each) were sacrificed and the frequency of CD4+CD25+Foxp3+ Treg analyzed in the draining lymph nodes. B) Mice in each of the above groups were then sacrificed on day 60 and intramural fibrosis of the donor tracheas analyzed.
Infection of SdV induces Treg apoptosis and increases allograft fibrosis
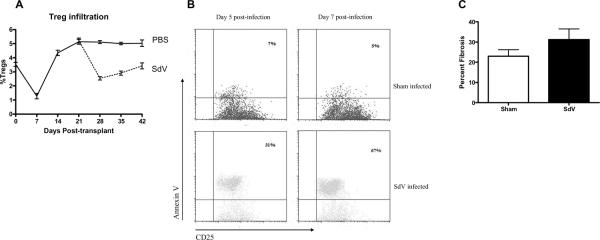
B/c donor tracheas were transplanted into B6 recipients. Following return of Treg to baseline by day 21, recipients were infected by 5K (sublethal) dose of SdV. As control, mice received intranasal injection of phosphated-buffered saline solution. Mice were then sacrificed and Treg in the DLN analyzed. There was a significant decrease in Treg following SdV-infection from 5.13±0.17% to 2.54±0.12% at day 28 (Figure 3A). This correlated with clinical symptoms of infection including weight loss and lethargy as previously described (6). As the recipients recovered from the infection, there was an increase in the frequency of Treg to 2.99±0.15% by day 35 and 3.42±0.21% by day 42. In contrast, mice that received a sham infection did not develop any decrease in Treg.
Figure 3. SdV-infection leads to Treg apoptosis in murine recipients.
A) Following epithelial regeneration, SdV or sham infection was induced (n=40 each group). Mice were then serially sacrificed and the frequency of Treg in the draining lymph nodes determined using flow-cytometry. B). At day 5 and day 7 (n=5 at each time point), Treg were stained with annexin V to determine apoptosis. C) At day 60, recipients were sacrificed and analyzed for intramural fibrosis of tracheal allografts.
Following SdV-infection, SdV-reactive T-cells were detected in the from the infected, but not sham mice (data not shown). Treg isolated from the infected recipients at day 26 (day 5 post-infection) as well as day 28 (day 7 post-infection) revealed increased apoptosis compared to the sham infected recipients (day 26: 38.2±5.8% Vs 7.4±2.5%; day 28: 69.2±8.4% Vs 6.8±1.9%, p<0.01 for both, Figure 3B). This suggested that the decrease in Treg following SdV-infection is not merely a result of proliferation of viral specific T-cells but due to cell death in the Treg population. Recipients infected with SdV also demonstrated significantly increased allograft fibrosis compared to sham-infected recipients (31.22±5.33% & Vs 22.97±3.22%, p=0.02, Figure 3C).
Pre-transplant vaccination prevents Treg apoptosis
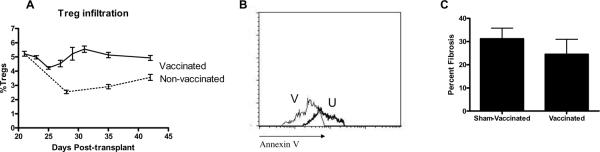
In order to further confirm that SdV was the cause of Treg apoptosis and allograft fibrosis, we induced SdV-specific immunity in recipients one month prior to transplantation. Mice were allowed to completely recover following the infection. Subsequently, these mice were transplanted with donor B/c tracheas. On day 21 post-transplant, recipients were infected for the second time with SdV. As expected, the convalescent phase was significantly shorter for the second compared to the first infection (5.23±2.11 days Vs 10.11±2.98 days, p<0.001). Vaccinated recipients did develop a transient decrease in the frequency of Treg (Figure 4A). However, these Treg did not undergo apoptosis, suggesting that the drop in Treg frequency is likely a “dilutional effect” from proliferation of effector T-cells (Figure 4B). Tracheal allografts from the vaccinated mice had decreased allograft fibrosis compared to non-vaccinated recipients (24.55±6.44% Vs 31.22±5.33%, p=0.02, p=0.01, Figure 4C).
Figure 4. Pre-transplant vaccination prevents Treg apoptosis and allograft fibrosis.
A) Vaccinated and sham-vaccinated OTT recipients received SdV-infection (n=45 in each group). Treg were serially measured. B) Treg apoptosis in vaccinated (V) and sham-vaccinated (U) recipients. C) At day 60 intramural allograft fibrosis analyzed.
Induction of Treg apoptosis by airway epithelial cells infected with respiratory viruses
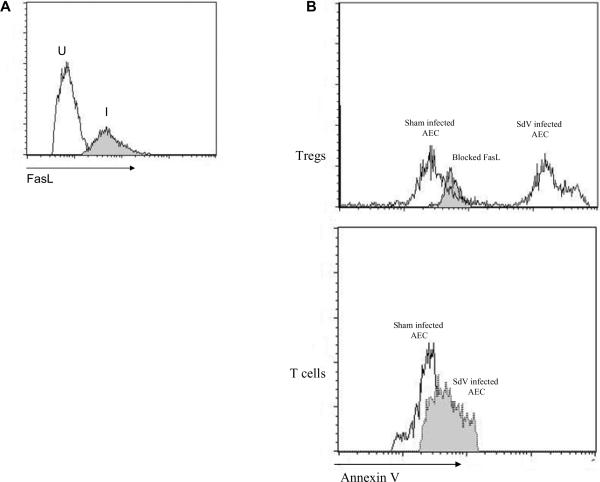
We next tested the association between SdV and Treg in vitro. Primary murine tracheal epithelial cell lines were established as previously described. Subsequently, the cells were infected with SdV. Freshly isolated CD4+CD25+ Treg or conventional CD4+25− T-cells were co-cultured with the airway epithelial cells. The infected airway epithelium, but not sham, upregulated expression of FasL (Mean 3.5 ± 1.3 fold increase, Figure 5A). Treg co-cultured with the SdV infected cells revealed increased apoptosis when compared to the sham infected AEC (5.6 fold increase). In contrast, CD4+CD25− T-cells did not undergo apoptosis when cultured with infected AEC. In order to test whether the Treg apoptosis was mediated through FasL-Fas, we blocked FasL on the infected AEC with soluble Fas prior to culturing with Treg. Blocking FasL on infected AEC significantly reduced Treg apoptosis (Figure 5B).
Figure 5. SdV infected tracheal epithelial cell lines induce Treg apoptosis through Fas-FasL mediated pathways.
A) Murine tracheal epithelial cells lines were infected with SdV and expression of FasL was analyzed at 36 hours following infection. I=Infected lines, U=Sham (inactivated SdV) infected lines. B) Treg or CD4+CD25− T-cells were co-cultured with infected lines. In parallel, SdV infected airway epithelial cells were blocked with Fas prior to co-culture with Treg. After 24 hrs, non-adherent cells were isolated from co-culture and then stained using annexin V to determine apoptosis.
Discussion
BOS remains the major cause of poor long-term lung allograft survival (11, 16). The development of BOS is highly complex and involves multiple pathways. It is generally believed that both alloimmune dependent and independent pathways contribute to BOS (1). There is increasing evidence that alterations in pathways responsible for maintenance of peripheral tolerance may play a crucial role in chronic rejection. In support of this, studies have shown that Treg play an important role in lung allograft tolerance (4, 17). Autoimmunity can be demonstrated in patients with BOS (4, 7, 8) implying loss of peripheral tolerance most likely maintained by Treg function. Respiratory viral infections have been shown to strongly predispose to BOS. Khalifah et al from our center demonstrated that the risk of BOS was increased 3-fold with lower respiratory tract viral infection (5). Viruses are also known to be associated with autoimmune diseases in immunocompetent individuals (10). Results presented in this manuscript provide a mechanistic link between respiratory viral infections and Treg dysfunction resulting in an immune response to various self-antigens and BOS both in human lung transplant recipients as well as in an animal model of OAD.
Longitudinal analysis of the frequency of Treg in human lung transplant recipients demonstrated that Treg troughs were associated with both increased incidence of BOS as well as rapid onset of BOS (Table 2 and Figure 1C). While the median onset of BOS in patients without troughs was 67 months, in those with troughs it was reduced to only 34 months, making the onset of BOS 1.9 times faster in the latter group. Further, patients with Treg trough revealed higher incidence of autoantibodies to collagen type I, type V, and k-alpha tubulin after the onset of Treg trough. Autoimmunity against collagen V and k-alpha tubulin have been previously associated with BOS (7, 8). Multiple clinical variables were evaluated but only microbiologically proven RVI was found to correlate with Treg trough. In the present report we evaluated only respiratory infections of viral etiology but a recent study also showed that bacterial as well as fungal pneumonias can also increase the risk of BOS (18). We found that 11 of the 18 (61.1%) Treg troughs were associated with RVI. Therefore, it is possible that the other troughs may be associated with pneumonias of bacterial etiology (10). In the present cohort, we had no evidence of fungal pneumonia.
Murine tracheal transplant model has been used to study the pathogenesis of obliterative airway disease, a correlate of BOS following human lung transplantation (15). Limitations of this model include lack of a vascularized pedicle and the fact that BOS is primarily a distal airway disease while here we use tracheal. Nevertheless, this remains the only model where the histopathological lesions of BOS can be reliably reproduced. Interestingly, the orthotopically transplanted tracheas, unlike heterotopic tracheal transplants, do not develop complete luminal obliteration. We have previously shown that this due to the result of re-epithelialization of the donor tracheal allograft by the recipient airway epithelium (14). In the present study, we characterized the proportion of Treg in the DLN. There was initial loss of Treg which is likely a result of epithelial loss on the donor segment and alloantigen exposure (Figure 2). As the epithelium regenerated in the donor segment, the number of Treg expanded and returned to baseline. Epithelial regeneration was crucial for the Treg expansion since MMP-7KO mice that have defective epithelial regeneration had persistently low Treg in the draining lymph nodes. Further, MMP-7KO animals had increased severity of rejection characterized by intramural allograft fibrosis (Figure 2). The mechanisms by which regenerating epithelial cell induce Treg are unclear and remain the subject for future investigation.
SdV-infection induced a rapid loss of Treg in the OTT recipients (Figure 3A). It can be argued that the percentage loss of Treg was due to a relative expansion of SdV-specific T-cells. Indeed, there was expansion of SdV reactive T-cells in the draining lymph nodes (data not shown). However, Treg isolated from the infected animals but not from sham-infected animals were undergoing apoptosis (Figure 3B). There was gradual re-expansion of Treg following recovery of the animals. Despite that, SdV associated injury caused a significant increase in the allograft fibrosis (Figure 3C). This correlates with the observation in human subjects where respiratory viral infections increased the risk of BOS that is characterized by fibrosis allograft (5). More significant is our data demonstrating that pre-transplant immunity prevented SdV-associated Treg apoptosis (Figure 4B). We noted an initial decrease in the percentage of Treg in the draining lymph nodes in recipients that were vaccinated. Our contention is that this was due to the proliferation of SdV reactive T-cells since there was no significant increase in the Treg apoptosis (Figure 4A). Furthermore, pre-transplant immunity decreased the intramural allograft fibrosis associated with the infection (Figure 4C).
Respiratory viruses have not been previously shown induce Treg apoptosis. Therefore, we determined the mechanisms that induced Treg apoptosis following SdV-infection. The primary target of respiratory viruses is airway epithelial cells. Therefore, primary murine tracheal epithelial cells lines were developed and then infected with SdV. There was a 2–4 fold increase in FasL in infected epithelial cells (Figure 5A). Upon co-culture of infected epithelial cells with purified Treg, we noted a significant apoptosis of the Treg. In contrast, freshly isolated T-cells which are predominantly effector population did not undergo apoptosis upon co-culture (Figure 5B). These results demonstrate that Fas-FasL pathway mediated apoptosis of Treg can occur following RVI of airway epithelial cells. Interestingly, Treg stimulated with cognate antigen were more resistant to the Fas-FasL mediated apoptosis from infected airway epithelial cells (data not shown). Ongoing work in our lab has also revealed that SdV-activated dendritic cells can covert Treg into Th17 cells which then lose their immuno-modulatory properties and this conversion is dependent both on IL-16 and cognate Treg antigens (Bharat et al, manuscript under preparation). Interestingly, Anz et al recently showed that immunostimulatory viral RNA can also mediate Treg suppression through IL-6 production (19). Hence, this may represent another pathway by which viruses induce Treg dysfunction, namely, conversion into “effector” Th-17 cells.
The above study demonstrates a novel pathway by which respiratory viruses predispose to chronic lung allograft rejection via induction of Treg apoptosis. We propose that this loss of Treg can increase both the allo- as well as auto- immune responses that are known to constitute distinct injury mechanisms for the pathogenesis of BOS. Therefore, development of therapeutic strategies to prevent respiratory infections, including vaccinations against known prevailing agents as well as abrogating the development of allo- and auto-immune responses by early detection of viral infections may facilitate the prevention of BOS and promote long-term lung allograft function.
Acknowledgements
This work was supported by ARRA Award HL056643 from the National Institutes of Health/National Heart Lung Blood Institute (TM), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Ms Billie Glascock for assistance in the preparation of this manuscript.
REFERENCES
- 1.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17(12):1255–1263. [PubMed] [Google Scholar]
- 2.Girgis RE, Tu I, Berry GJ, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15(12):1200–1208. [PubMed] [Google Scholar]
- 3.Snyder LD, Palmer SM. Immune mechanisms of lung allograft rejection. Semin Respir Crit Care Med. 2006;27(5):534–543. doi: 10.1055/s-2006-954610. [DOI] [PubMed] [Google Scholar]
- 4.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+CD25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human transplantation. American Journal of Transplantation. 2006;6(8):1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 5.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 6.Kuo E, Bharat A, Goers T, et al. Respiratory viral infection in obliterative airway disease after orthotopic tracheal transplantation. Ann Thorac Surg. 2006;82(3):1043–1050. doi: 10.1016/j.athoracsur.2006.03.120. [DOI] [PubMed] [Google Scholar]
- 7.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 10.Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155(1):1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 12.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 13.Davidson DJ, Kilanowski FM, Randell SH, Sheppard DN, Dorin JR. A primary culture model of differentiated murine tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 2000;279(4):L766–778. doi: 10.1152/ajplung.2000.279.4.L766. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez FG, Jaramillo A, Chen C, et al. Airway epithelium is the primary target of allograft rejection in murine obliterative airway disease. Am J Transplant. 2004;4(3):319–325. doi: 10.1111/j.1600-6143.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuo E, Bharat A, Dharmarajan S, Fernandez F, Patterson GA, Mohanakumar T. Animal models for bronchiolitis obliterans syndrome following human lung transplantation. Immunol Res. 2005;33(1):69–81. doi: 10.1385/IR:33:1:069. [DOI] [PubMed] [Google Scholar]
- 16.Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80(10):1406–1413. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 17.Mizobuchi T, Yasufuku K, Zheng Y, et al. Differential expression of Smad7 transcripts identifies the CD4+CD45RChigh regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol. 2003;171(3):1140–1147. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
- 18.Valentine VG, Gupta MR, Walker JE, Jr, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2009;28(2):163–169. doi: 10.1016/j.healun.2008.11.907. [DOI] [PubMed] [Google Scholar]
- 19.Anz D, Koelzer VH, Moder S, et al. Immunostimulatory RNA blocks suppression by regulatory T cells. J Immunol. 184(2):939–946. doi: 10.4049/jimmunol.0901245. [DOI] [PubMed] [Google Scholar]