Abstract
Exposure to traumatic events can increase the risk for major depressive disorder (MDD) as well as posttraumatic stress disorder (PTSD), and pharmacological treatments for these disorders often involve the modulation of serotonergic (5-HT) systems. Several behavioral paradigms in rodents produce changes in behavior that resemble symptoms of MDD and these behavioral changes are sensitive to antidepressant treatments. Here we review two animal models in which MDD-like behavioral changes are elicited by exposure to an acute traumatic event during adulthood, learned helplessness (LH) and conditioned defeat. In LH, exposure of rats to inescapable, but not escapable, tailshock produces a constellation of behavioral changes that include deficits in fight/flight responding and enhanced anxiety-like behavior. In conditioned defeat, exposure of Syrian hamsters to a social defeat by a more aggressive animal leads to a loss of territorial aggression and an increase in submissive and defensive behaviors in subsequent encounters with non-aggressive conspecifics. Investigations into the neural substrates that control LH and conditioned defeat revealed that increased 5-HT activity in the dorsal raphe nucleus (DRN) is critical for both models. Other key brain regions that regulate the acquisition and/or expression of behavior in these two paradigms include the basolateral amygdala (BLA), central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST). In this review, we compare and contrast the role of each of these neural structures in mediating LH and conditioned defeat, and discuss the relevance of these data in developing a better understanding of the mechanisms underlying trauma-related depression.
Keywords: Learned Helplessness, Conditioned Defeat, Serotonin, Corticotropin-Releasing Hormone, Dorsal Raphe Nucleus, Bed Nucleus of the Stria Terminalis, Amygdala, Social Defeat, Fear, Anxiety, Stress
Posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) are highly comorbid psychopathologies, such that, depending on the study, more than 50% of subjects meeting criteria for PTSD also meet criteria for MDD at the time of diagnoses, and 95% of those with PTSD will be diagnosed with MDD in their lifetime (Bleich et al., 1997) (see (Shalev et al., 1998) for review). Multiple factors have been proposed to explain the high levels of comorbidity between these two diagnoses, including the similarity in the symptoms required for diagnosis as well as similar etiologies for both disorders. Exposure to stressors has been recognized as a contributing factor in the onset of both PTSD and MDD. In fact, diagnostic criteria for PTSD require exposure to a traumatic event or events that are typically characterized by actual or threatened death, serious injury, or a threat to the physical integrity of the individual or someone close to the individual (American et al., 2000). Notably, PTSD can be diagnosed following exposure to acute or chronic trauma, such as an assault or a life-threatening accident or following exposure to child abuse or domestic violence (see (Vieweg et al., 2006)). While stressor exposure has also been recognized as an important factor in the etiology of MDD, the stressor characteristics that influence risk for MDD are less clear. Exposure to traumatic events, such as those that can produce PTSD, also increase the risk for MDD (Goodyer et al., 2000; Heim et al., 2000; Kohn et al., 2001; Mayou et al., 2001; Shalev et al., 1998); however, risk for MDD might also been linked to a variety of negative life events or adversities that do not meet the criteria for trauma as described above (Kohn et al., 2001). It has also been proposed that these negative life events may alter the response of an individual to a subsequent traumatic experience increasing the likelihood that latter trauma will lead to PTSD or MDD (Yehuda et al., 2004).
Pharmacological treatments for PTSD and MDD also suggest that these disorders share similar causal mechanisms. Selective serotonin reuptake inhibitors (SSRIs) are typically the first class of pharmacotherapies used to treat PTSD or MDD, and are effective in the treatment of both disorders (see (Vaswani et al., 2003)). Also,the disruption of central serotonin (5-HT) systems has been argued to be a critical component of the etiologies for both disorders (Drevets et al., 2007; Neumeister et al., 2004).
While many similarities exist between PTSD and MDD regarding their symptomology, treatment, and etiology, there are notable differences between these disorders. For example, subjects meeting criteria for PTSD have been shown to have disruptions in the function of their hypothalamic-pituitary-adrenal axis (HPA axis), exhibiting reduced basal cortisol levels (Boscarino, 1996; Goenjian et al., 1996) and enhanced cortisol suppression in response to dexamethasone (DEX, (Goenjian et al., 1996; Yehuda et al., 1993), but see (Meewisse et al., 2007) for a critical review). In contrast, MDD is associated with elevated basal cortisol and reduced DEX suppression (Nemeroff et al., 1984). The differences in HPA function between these two disorders may reflect different central mechanisms; however, these differences may also reflect different environmental factors, such as the severity of the traumatic experience, a history of repeated trauma, or whether trauma was experienced in childhood or adulthood (Yehuda et al., 2004).
Animal Models
Multiple animal paradigms have been argued to model both PTSD and MDD, which may reflect that these disorders share similar symptoms and/or have a similar biological basis. Animal models of depression have often been evaluated on the basis of several criteria: whether they produce behavioral changes that are similar to those observed in depression, whether these changes can be objectively measured, and whether these changes can be reversed by treatments used to treat depression (McKinney and Bunney, 1969). Based on these criteria, several strategies have been utilized to study the neural changes associated with depression-like behavior in animals, including olfactory bulbectomy (Leonard, 1984), genetic alteration and/or breeding for desired behaviors (King et al., 2001; Overstreet et al., 2005), chronic social defeat or subordination in social groups (Blanchard et al., 2002; Blanchard et al., 1995; Fuchs, 2005; Fuchs and Flugge, 2002; Heinrichs et al., 1992), acute exposure to stressful stimuli early in development ((Arborelius et al., 1999; Heim et al., 2004), but see (Schmidt et al., 2011)), and acute exposure to stressful stimuli in adulthood (Maier, 1984). Of these, models that use acute stressors have also been argued to represent PTSD ((Foa et al., 1992), but see (Yehuda and Antelman, 1993)). Hence, in most cases, animal models of MDD (and PTSD) require exposure to a stressor either early in development, in adulthood, or both. The effects of early life stressors on depression- and anxiety-like behavior in adulthood have been reviewed previously (Heim et al., 2004), as have studies of chronic social stress in group-living animals (Blanchard et al., 2002; Fuchs and Flugge, 2002). The scope of the current review focuses on changes within neural circuits that mediate anxiety-like and depression-like behavior that occur as a consequence of exposure to a traumatic stressful experience during adulthood.
The exposure of adult rodents to stressors can produce lasting behavioral changes that resemble symptoms of MDD such as increased behavioral immobility (Anisman et al., 1978; Rittenhouse et al., 2002), disrupted circadian rhythms (Meerlo et al., 2002), and changes in feeding and body weight (Bartolomucci et al., 2004; Maier, 1984). Furthermore, similar neurochemical mediators seem to be involved in mediating depression-like behavior in rodents and in clinical populations with MDD. Similar to the treatment of MDD within clinical populations, MDD-like behavioral changes within animal models can also be prevented by antidepressant treatment (Berton and Nestler, 2006; Sherman et al., 1982). Exposure to inescapable shock leads to dysfunction of the HPA-axis resembling MDD such as elevated basal levels of glucocorticoids and reduced DEX suppression (Maier et al., 1986; O'Connor et al., 2003). Similarly, a single social defeat produces robust activation of the HPA axis (Huhman et al., 1991; Koolhaas et al., 1997). The effects of repeated social defeat are more complex, as chronic social defeat has been shown to elevate basal glucocorticoid levels (Berton et al., 1998; Miczek et al., 1990), whereas chronic subordination in a group of rats leads to a blunted glucocorticoid response to a subsequent stressor in a subset of subordinates (Blanchard et al., 1995). Because of these behavioral and neuroendocrine changes, the behavioral paradigms described below have been argued to model MDD in humans. In addition, the use of acute traumatic stressful events in these paradigms reflects the similarities between MDD and other types of stress-related mental illness such as PTSD.
Learned Helplessness
In humans, trauma requires the experience or witnessing an actual or threatened death, serious injury or threat to the physical integrity of self or others, and the response to this event must involve intense fear, helplessness or horror (APA, 2000). While it is not possible to determine how animals interpret laboratory stressors, behavioral correlates of fear can be assessed, and the controllability of laboratory stressors can be manipulated to produce situations in which animals are made “helpless.” Exposure to inescapable, but not equivalent escapable, shock produces a constellation of behavioral changes that have been called learned helplessness (LH, (Maier and Seligman, 1976)) or behavioral depression (Weiss and Simson, 1985). Importantly, the behavioral changes associated with LH are mediated by the inescapable/uncontrollable nature of the shock, and not the shock itself, and include increases in fear- and anxiety-like behavior, reductions in fight/flight responding, disrupted sleep patterns, disrupted food and water intake, and the subsequent failure to learn to escape aversive stimuli in subsequent tasks where escape is possible (for review, see (Maier, 1984)). Because these changes mimic symptoms of mood disorders in humans, LH has been reviewed extensively as a powerful animal model of several human disorders, including MDD and PTSD ((Foa et al., 1992; Maier, 1984), but see (Yehuda and Antelman, 1993)). Moreover, many treatments effective in treating these disorders in humans such as electroconvulsive shock therapy (Sherman et al., 1982), benzodiazepines (Short and Maier, 1993), and chronic antidepressant administration (Sherman et al., 1982), are also effective in blocking LH in animals.
One criticism of LH is the short duration of LH-associated behavioral changes. The behavioral consequences of LH have been observed 24–48 hr after the inescapable shock session, but typically dissipate within 72-hr, and this timecourse does not resemble the sustained changes in mood characteristic of PTSD or MDD. However, the behavioral consequences associated with LH have been shown to be prolonged simply by re-exposing organisms to the original stressor environment; that is, the subsequent presentation of contextual cues associated with the original experience of inescapability can prolong the behavioral consequences of LH, even if shock is not delivered during these “reminders” (Maier, 2001).
Recently, Maier and colleagues have argued that LH models the pathological changes induced when environmental stressors are severe and uncontrollable, which may be associated with multiple mood disorders in humans (Maier and Watkins, 2005); hence, Maier and colleagues posit that (1) the perception that stress is uncontrollable underlies the etiology of multiple mood disorders, and (2) the mechanisms that mediate LH are activated in mood disorders associated with uncontrollable stress. Notably, exposure to inescapable shock activates the HPA-axis and elevates basal levels of circulating glucocorticoids, which is consistent with the elevated basal glucocorticoid levels observed in MDD; however, these glucocorticoid elevations are also observed after escapable shock (unlike the behavioral consequences of LH), and are not true LH effects, since they do not depend on the escapability of the stressor.
The Dorsal Raphe Nucleus (DRN)
While glucocorticoid release is necessary, but not sufficient, to produce LH, the behavioral changes associated with LH are critically dependent on the activation of serotonin (5-HT) cells in the caudal aspect of the dorsal raphe nucleus (DRN). The DRN is the primary 5-HT source to the forebrain and has been argued to be topographically organized such that the activation of different DRN subregions mediate different functions (Lowry et al., 2005), with the caudal aspect mediating anxiety-associated behavioral states. Inescapable, relative to escapable, shock activates 5-HT neurons in the caudal region of the DRN (Grahn et al., 1999), and this activation is associated with a significant increase in 5-HT release both in the DRN and its projection regions (Amat et al., 1998a, b). The attenuation of DRN 5-HT activity via intra-DRN infusion of benzodiazepines (Maier et al., 1994), or the 5-HT1A autoreceptor agonist 8-OH-DPAT, blocks both the development and expression of LH (Maier et al., 1995b). Also, pharmacologically activating 5-HT neurons in the caudal, but not rostral, DRN produces LH-like behavioral changes 24-hr later (Hammack et al., 2002). Based on these data, Maier and colleagues (2005) have suggested that elevated 5-HT sensitizes the caudal DRN for a period of time after treatment, and that caudal DRN 5-HT sensitization mediates the behavioral effects of LH (Maier and Watkins, 2005). While substantial evidence suggests caudal DRN 5-HT activation mediates LH, the input(s) that activate the DRN during inescapable shock are not well understood.
The data described above suggesting that increased 5-HT release mediates the behavioral consequences of LH appear inconsistent with the therapeutic effects of SSRI treatment, which are also associated with increased levels of central 5-HT. Notably, effective SSRI treatment typically requires several weeks of administration, and the acute effects of these drugs are often associated with increased anxiety and depressive behavior in humans and animals (Bagdy et al., 2001; Burghardt et al., 2004), consistent with the behavioral effects of 5-HT activation observed in LH. We and others have argued that the efficacy of long-term SSRI treatment depends on changes in the function of pre- and/or post-synaptic receptors in brain regions that mediate emotional behavior; hence, the response to 5-HT release after long-term SSRI treatment may be dramatically altered, and these same mechanisms may mediate the efficacy of these drugs in preventing LH ((Berendsen, 1995; Hammack et al., 2009b; Stahl, 1994; Strome et al., 2005; Yatham et al., 1999), see below).
Corticotropin-releasing hormone (CRH) has been heavily studied for its role in initiating peripheral stress responses and emotional behavior (see (Koob and Heinrichs, 1999; Owens and Nemeroff, 1993) for review). While CRH-positive cell bodies can be found throughout the brain, they are dense in regions associated with both stressor responding and emotional behavior, including the paraventricular nucleus of the hypothalamus (PVN), central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST). Generally, the activation of CRH neurons in these regions has been associated with behavioral phenotypes resembling stress, fear and/or anxiety.
CRH receptors are found in the DRN, with the CRH type 2 receptor more highly expressed than the CRH type 1 receptor (Chalmers et al., 1995; Day et al., 2004). CRH type 1 receptors are expressed at low levels throughout the rostral-caudal axis of the DRN, whereas the CRH type 2 is expressed predominantly by 5-HT neurons at mid-levels and on both 5-HT neurons and gamma amino butyric acid (GABA)-expressing neurons at caudal levels (Day et al., 2004). Several reports have suggested that CRH inhibits DRN 5-HT activity in rostral DRN regions (Kirby et al., 2000; Price et al., 1998), likely via activation of CRH type 1 receptors. In contrast, CRH may selectively excite DRN 5-HT neuronal activity only in the middle-to-caudal regions of the DRN, the very same neuronal population activated by inescapable, but not escapable, shock (Lowry et al., 2000). Moreover, while systemic administration of a CRH type 1 receptor antagonist systemically (Deak et al., 1999), or directly into the DRN (Hammack et al., 2003b) does not alter LH, DRN CRH type 2 receptor blockade during inescapable shock blocks the development of LH (Hammack et al., 2003b). Also, CRH 2 receptor activation directly into the caudal, but not rostral, DRN activates middle-to-caudal DRN 5-HT neurons (Abrams et al., 2004; Amat et al., 2004; Forster et al., 2008; Kirby et al., 2008; Staub et al., 2005), and produces LH-like behavioral changes 24-hours later in the absence of shock (Hammack et al., 2003b). Hence, CRH type 2 receptor activation in the DRN is both necessary and sufficient to produce LH (see (Maier and Watkins, 2005) for review). Notably, DRN CRH release is critical for the development of LH, but does not mediate the expression of LH-associated behaviors after the inescapable shock session (Hammack et al., 2002).
In many of the reports that found CRH inhibitory effects on DRN 5-HT activity, the inhibitory effects were localized to the rostral DRN and attenuated at high CRH doses. These data suggest that low doses of CRH inhibit DRN 5-HT activity whereas high doses of CRH excite DRN 5-HT activity. If the behavioral consequences of LH depend on caudal DRN 5-HT activation, then low CRH doses might be expected to block LH, whereas high CRH doses should produce LH-like behavioral changes, and indeed this prediction has been confirmed (Hammack et al., 2003a).
As described above, DRN CRH receptor subtypes are organized topographically, so that CRH type 2 receptors are highly expressed only in the middle-to-caudal DRN. Hence, the release of CRH can selectively activate only this DRN subregion, which is the same subregion selectively activated by inescapable shock. While many DRN inputs tightly regulate DRN 5-HT activity, to date only the CRH system has been shown to selectively activate the anxiety-associated middle-to-caudal region.
The sources of DRN CRH during inescapable shock are unknown. A small percentage of DRN neurons coexpress CRH (Commons et al., 2003), and we have found that DRN CRH mRNA is upregulated following an acute stressor (unpublished data). Moreover, CRH analogues, such as the urocortin family of peptides, may preferentially activate DRN CRH type 2 receptors to produce LH, and are capable of producing LH-like behavioral changes at low doses (Hammack et al., 2003b). However, for reasons discussed below, DRN-projecting CRH neurons in the BNST likely contribute to the development of LH following inescapable shock.
The Bed Nucleus of the Stria Terminalis (BNST)
The extended amygdala has been implicated in emotional responses, with separate extended amygdala subregions mediating fear- and anxiety-like behaviors. A consideration of the paradigms sensitive to manipulations of the CeA and BNST led Walker et al. to argue that the CeA mediates responding when both the fear-eliciting stimulus and the behavioral response are short in duration, whereas the BNST mediates responding when both the stimulus and response have a long duration (Walker and Davis, 2008; Walker et al., 2009; Walker et al., 2003). They have further argued that CeA-mediated responding resembles “fear”, and is anatomically and phenomenologically dissociable from BNST-mediated responding, which they argue more resembles “anxiety.” Consistent with this argument, Waddell et al. (2006), showed that the BNST mediates responding to a 10-min tone that was previously paired with shock, but not a 1-min tone, and argued that anxiety was conditioned to the 10-min tone that was dissociable from the fear state conditioned to the shorter tone (Waddell et al., 2006). In sum, the BNST has been argued to mediate anxiety-like responding to stimuli that are of long duration.
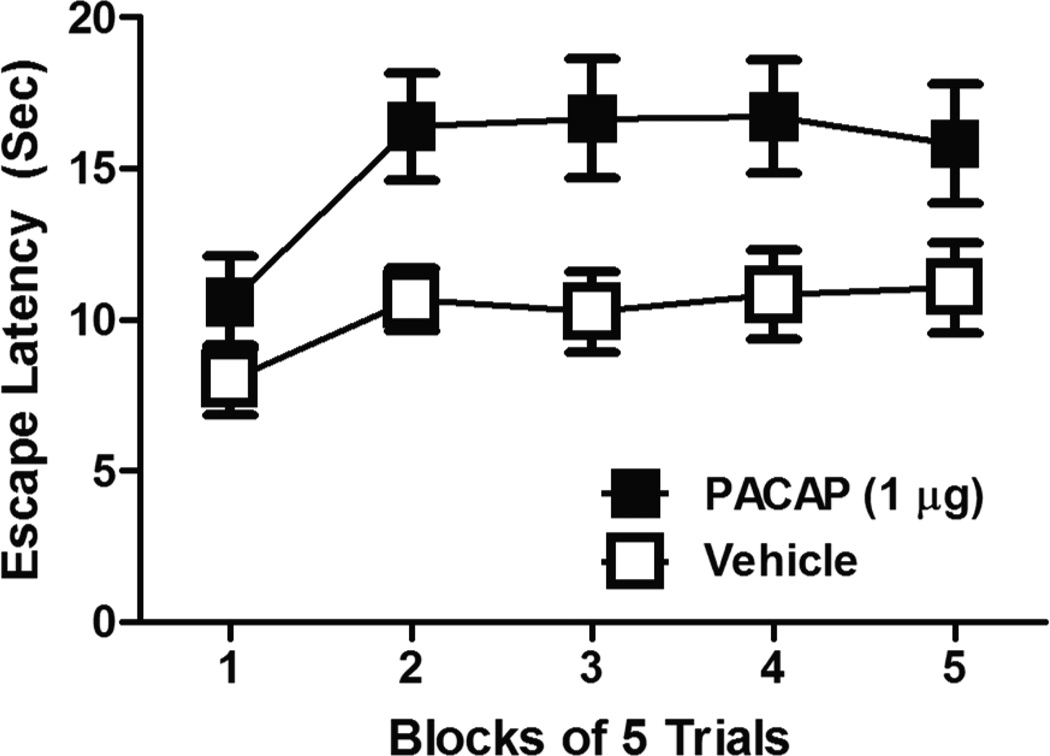
Importantly, the BNST has also been implicated in behavioral changes associated with depression. BNST CRH activity has been correlated with anhedonic behavior after chronic mild stress (Stout et al., 2000), inescapable shock activates and sensitizes anterior BNST neurons (Greenwood et al., 2005) and BNST lesions block the development of LH (Hammack et al., 2004). While BNST lesions blocked the exaggerated fear conditioning observed 24 hours after inescapable shock such that fear conditioning in inescapably shocked rats did not differ from fear conditioning in control rats, BNST lesioned rats still displayed normal fear conditioning. Notably, BNST lesions blocked more than just the increased fear conditioning observed after inescapable shock, also blocking the escape deficits normally associated with LH. Hence, BNST activity appears necessary for the development of several behavioral consequences of LH, and not just LH effects that are associated with fear and anxiety. In support, we have found that local infusion of pituitary-adenylate cyclase activating polypeptide (PACAP) into the BNST can mimic LH-like increases in anxiety-like behavior as well as escape deficits, likely by activating BNST CRH neurons (Figure 1), and suggesting that BNST activity is both necessary and sufficient to produce LH. Moreover, the over-expression of CRH in the BNST using a lentiviral based system for site-specific CRH-expression increased depressive behaviors on the forced swim and tail-suspension tests (Regev et al.). Hence, these data implicate the BNST as critical for depressive behaviors in addition to anxiety. In contrast to these studies, which suggest that BNST activity promotes depressive behavior, BNST lesions have also been shown to aggravate behavioral despair on the forced-swim test in male and female rats (Pezuk et al., 2008; Pezuk et al., 2006). Notably, in these studies BNST lesions extended through the entire rostral-caudal extent of the BNST. Choi et al (Choi et al., 2007) have shown that posterior regions of the BNST may inhibit, while anterior BNST regions excite, stressor responding. Hence, the anterior aspects of the BNST, which contain BNST subregions that highly express CRH, may promote anxiety- and depression-like behavior while posterior regions of the BNST may reduce it, although this has yet to be tested.
Figure 1.
Rats were infused into the BNST with 1 µg PACAP or equivolume (0.5 µg) vehicle. 24-hr later, rats were tested in a shuttlebox escape task for the latency to escape footshock (FR-2 schedule). Prior BNST PACAP infusion produced a learned-helplessness like behavioral phenotype; 2-way analysis of variance revealed a main effect of drug F(4,132) = 8.588, p < 0.05, and time F(4,132) = 8.046, p < 0.05.
CRH afferents from the BNST may modulate DRN 5-HT neuronal activity during LH. As described above, the brain regions exhibiting the highest expression of CRH include the PVN, CeA, and BNST. Notably, PVN CRH expression and glucocorticoid release are not sensitive to shock escapability (Helmreich et al., 1999; Maier et al., 1986), and large electrolytic lesions of the CeA do not block the development/expression of LH (Maier et al., 1993), suggesting that neither the PVN nor the CeA mediate LH. We have found DRN afferents originating in CRH-rich areas of the BNST (unpublished data), and as noted above, excitotoxic BNST lesions blocked both the increases in anxiety-like behavior and escape deficits associated with LH (Hammack et al., 2004). Hence, exposure to inescapable shock activates BNST neurons (Greenwood et al., 2005), likely leading to the release of CRH in the caudal DRN. Substantial caudal DRN CRH release activates 5-HT neurons and produces the behavioral consequences of LH (Figure 2A).
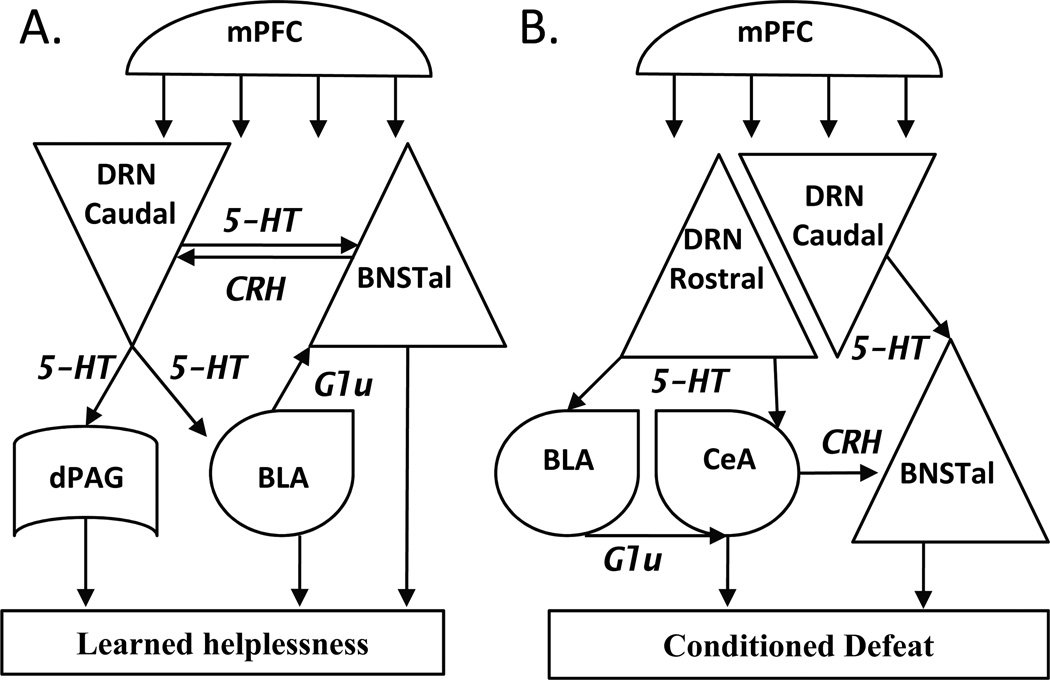
Figure 2.
Hypothesized circuits for A. Learned helplessness and B. Conditioned defeat. Abbreviations: 5-HT, serotonin; BLA, basolateral amygdala; BNSTal, anterolateral bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; CRH, corticotropin-releasing hormone; dPAG, dorsal periaqueductal gray; DRN, dorsal raphe nucleus; Glu, glutamate; mPFC, medial prefrontal cortex.
Notably, the BNST also receives 5-HT projections from the caudal DRN (Commons et al., 2003; Phelix et al., 1992), contains multiple inhibitory and excitatory 5-HT receptor subtypes, and we have shown that 5-HT release in the BNST can both inhibit and excite BNST neuronal activity (see (Hammack et al., 2009b) for review). Because multiple inhibitory and excitatory 5-HT receptor subtypes can be expressed by single BNST neurons, we have argued that treatments that shift the balance of these receptor subtypes towards excitation may produce an anxiogenic and/or depression-like phenotype, whereas treatments that shift the balance of these receptor subtypes towards inhibition may be protective and/or therapeutic (Hammack et al., 2009b).
The central nucleus of the amygdala (CeA)
Because of their many similarities, neurons in the anterolateral BNST and CeA have been argued to form the rostral and caudal extensions of the central extended amygdala (Alheid, 2003). Substantial data has implicated the CeA in mediating the expression of learned fear (see (Davis et al., 1993; LeDoux, 1993) for review), and the CeA highly expresses CRH (Cummings et al., 1983) and projects to and receives input from the DRN (Rizvi et al., 1991). However, while large electrolytic lesions of the amygdala completely blocked the expression of learned fear in a LH paradigm, they did not attenuate the inescapable shock-induced escape deficits (Maier et al., 1993). These data are in contrast to BNST lesions which only attenuated the exaggerated fear conditioning normally observed after inescapable shock to levels observed in controls (Hammack et al., 2004). Hence, while the CeA is critical for fear expression in many paradigms, CeA activity does not appear to be necessary for LH.
The basolateral nucleus of the amygdala (BLA)
Many studies have implicated BLA plasticity as critical for the acquisition of learned fear (see (Davis et al., 1993; LeDoux, 1993)), and the BLA projects to and modulates activity in both the BNST and CeA. However, while BLA activity seems to be critical for mediating the expression of anxiety-like behavior following inescapable shock, BLA activity does not appear necessary for the development of LH. The BLA receives 5-HT input from the DRN, and substantially more 5-HT is released within the BLA following inescapable shock than equivalent escapable shock (Amat et al., 1998a). Moreover, 5-HT release was potentiated in the BLA in rats exposed to a stressor 24 hr after inescapable shock, suggesting that serotonin release is sensitized during IS (Amat et al., 1998a). Lastly, BLA 5-HT2C antagonism during testing blocked the anxiogenic effects of prior inescapable shock and BLA 5-HT2C agonism mimicks the anxiogenic effects of prior inescapable shock (Christianson et al.). While these data implicate BLA 5-HT2C receptors as mediating the expression of the anxiogenic consequences of LH, it is unknown whether BLA 5-HT2C receptors mediate other behavioral consequences of LH not related to fear or anxiety. Moreover, BLA 5-HT2C antagonism prior to inescapable shock had no effect on the development of LH (Christianson et al.). Together, these results suggest that the BLA is efferent to the DRN and critical for mediating the expression of exaggerated fear and anxiety observed following the development of LH. These data stand in contrast to the BNST, whose activity is both necessary and sufficient for LH development.
Conditioned Defeat
Social defeat is a robust stressor that, like LH, produces an array of behavioral changes including anxiety- and depression-like behavior (Berton et al., 1998; Heinrichs et al., 1992; Keeney et al., 2006; Krishnan et al., 2007; Rodgers and Cole, 1993). In Syrian hamsters, social defeat leads to a loss of species-typical territorial aggression and increased submissive and defensive behavior in subsequent social encounters with smaller non-aggressive intruders. This phenomenon has been called conditioned defeat (Huhman et al., 2003). Like LH, conditioned defeat can be produced by a single treatment session such that a single 15 min social defeat leads to changes in agonistic behavior the next day. On the surface the changes in agonistic behavior that characterize conditioned defeat are reminiscent of LH because defeated hamsters submit to non-threatening conspecifics as if they were helpless. Interestingly, inescapable shock can produce social avoidance as well. Rats that receive inescapable, but not equivalent escapable, shock show reduced social investigation with juvenile conspecifics (Christianson et al., 2008). Although social defeat activates the HPA axis in hamsters (Huhman et al., 1991), blocking glucocorticoid synthesis prior to social defeat does not alter the acquisition of conditioned defeat (Cooper and Huhman, 2010). Thus, consistent with LH glucocorticoids do not appear to be critical for the developmental of conditioned defeat.
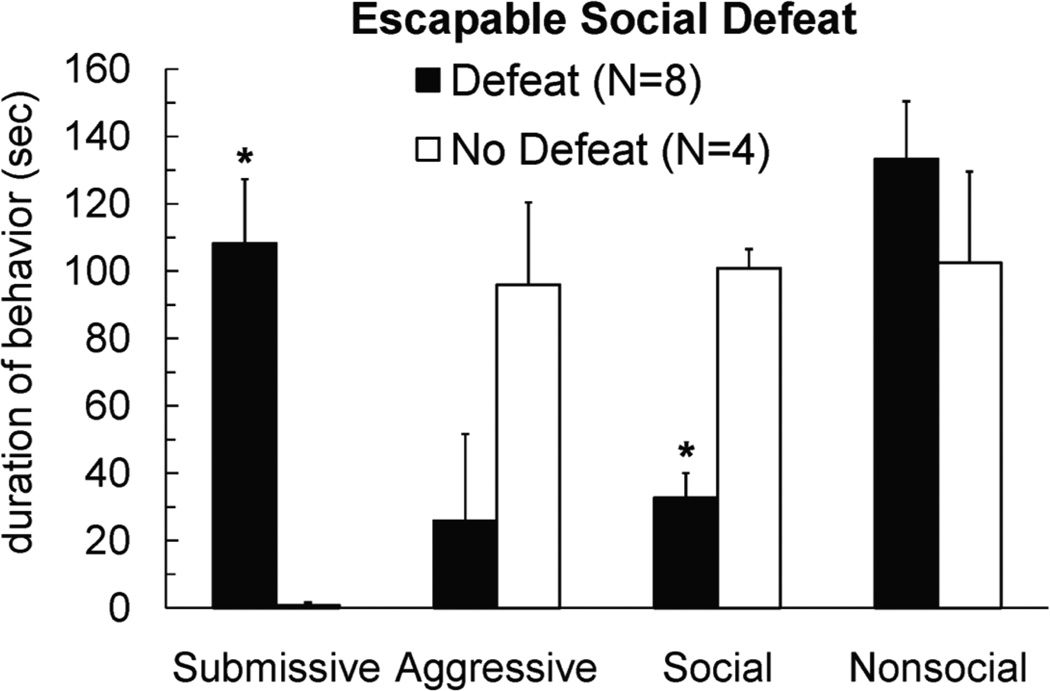
While conditioned defeat and LH paradigms have important similarities, there are also some noteworthy differences. Stressor controllability is critical for LH, but may not be critical for conditioned defeat. We have found that hamsters still show conditioned defeat behavior when they are allowed to escape from the aggressor during social defeat training (Figure 3). Also, unlike LH, the changes in agonistic behavior that characterize conditioned defeat can persist for at least a month (Huhman et al., 2003). The consequences of social defeat also may depend on how animals are tested, as defeated hamsters do not avoid novel conspecifics when tested in a Y-maze (Lai et al., 2005). In sum, the LH and conditioned defeat paradigms have some important similarities and in this review we suggest that the neural structures mediating the respective behavioral changes are remarkably similar as well.
Figure 3.
Male Syrian hamsters received 3 social defeats in a neutral arena at 3-min intervals. Subjects were able to jump out of the arena during social defeats, and individuals were removed from the arena by the experimenter after 3 failed jumps. Subjects escaped from the arena more quickly during the 2nd and 3rd defeats compared to the 1st (ANOVA, p < .05). No defeat controls received corresponding exposure to an empty neutral arena. Subjects were tested for conditioned defeat 24-hours later in a 5-min social encounter with a non-aggressive intruder. * indicates that defeated subjects showed increased submissive and defensive behavior and reduced social behavior during conditioned defeat testing compared to no defeat controls (t-tests, p < .05).
As discussed below, many of the brain regions that mediate LH are also important for conditioned defeat; however, a careful examination of the subregions implicated in conditioned defeat in some of these areas (i.e. the DRN) reveal important differences between these two models. The reasons for these differences are provocative, but currently unclear, and will be discussed in the following sections.
The Dorsal Raphe Nucleus (DRN)
Social defeat has been shown to increase neural activation in the DRN as indexed by c-Fos expression (Kollack-Walker et al., 1997; Martinez et al., 1998). Also, social defeat has been shown to decrease 5-HT1A receptor mRNA expression in the DRN (Cooper et al., 2009). Thus, social defeat appears to activate DRN 5-HT neurons and may sensitize them to future stressors as has been proposed for LH. Researchers have also investigated c-Fos expression in separate DRN subregions and found that social defeat activates 5-HT neurons in middle and caudal portions of the DRN (Gardner et al., 2005). These data are consistent with the effects of inescapable shock on caudal DRN activation; however, in hamsters, social defeat selectively increases c-Fos expression in rostral portions of the ventral DRN (Cooper et al., 2009). Similarly, social defeat interacts with maternal separation in rats to increase tryptophan hydroxylase mRNA (Gardner et al., 2009b) and serotonin transporter mRNA (Gardner et al., 2009a) in rostral portions of the ventral DRN. Although the mid and caudal DRN are well-known for reciprocal connections with limbic structures, the rostral DRN may also have connections with the amygdala and BNST (Commons et al., 2003; Imai et al., 1986; Rizvi et al., 1991). Urocortin 1 administration into the rat BLA was shown to increase c-Fos expression in the rostral DRN (Spiga et al., 2006). However, the BLA does not have major direct projections to the DRN, and a BLA-DRN pathway likely involves other brain regions such as the BNST, central amygdala, or medial prefrontal cortex (Peyron et al., 1998). Glutamatergic projections from the lateral ventral BNST to the rostral-mid DRN have been proposed to contribute to the ability of wheel running to attenuate stress-induced c-Fos expression in DRN 5-HT neurons and prevent LH (Greenwood et al., 2005). The rostral DRN also sends serotonergic projections to brain regions critical for aggressive behavior including the medial preoptic area and anterior hypothalamus (Ferris et al., 1999; Rizvi et al., 1991). Thus, the rostral DRN has the necessary neuroanatomical connections to regulate agonistic behavior in potentially threatening situations.
The role of the DRN in modulating the acquisition and expression of conditioned defeat has been examined using similar strategies to those described for LH. Inhibition of DRN 5-HT neurons via local infusion of a 5-HT1A autoreceptor agonist blocks both the development and expression of conditioned defeat (Cooper et al., 2008). Moreover, the activation of DRN 5-HT neurons via 5-HT1A autoreceptor antagonism exacerbates conditioned defeat following a suboptimal social defeat experience (Cooper et al., 2008). Notably, the infusion sites in these studies extended throughout the rostral-caudal axis of the DRN, targeting all DRN subregions. Together, these data suggest that, like LH, DRN 5-HT activation is necessary and sufficient to modulate the behavioral consequences of social defeat in Syrian hamsters. The DRN inputs that drive 5-HT activity during the acquisition and expression of conditioned defeat are unknown, although CRH is likely a key factor.
The combined blockade of CRH type 1 and type 2 receptors in the DRN reduces the acquisition and expression of conditioned defeat (Cooper and Huhman, 2007). In contrast, infusion of a selective CRH type 2 receptor antagonist into the DRN reduces the expression, but not acquisition, of conditioned defeat (Cooper and Huhman, 2007). The immunohistochemical and pharmacological data together suggest that, unlike LH, the development of conditioned defeat is likely modulated by neurochemical signaling at CRH type 1 receptors in the rostral DRN. In contrast, CRH type 2, and not type 1, receptors in the forebrain appear to modulate the development of conditioned defeat. Injection of a CRH type 2 receptor antagonist into the lateral ventricle reduces the acquisition of conditioned defeat, whereas injection of a CRH type 1 receptor antagonist does not (Cooper and Huhman, 2010). As discussed below, one potential forebrain region that could mediate the effect of CRH type 2 receptor blockade is the medial amygdala.
The Bed Nucleus of the Stria Terminalis (BNST)
BNST activation is necessary for the expression, but not acquisition, of conditioned defeat. Inhibiting the BNST with local infusion of the GABA(A) receptor agonist muscimol prior to testing blocks the production of conditioned defeat behavior, while infusion prior to social defeat does not disrupt the development of conditioned defeat (Markham et al., 2009). Consistent with this role for the BNST, injection of a CRH type 2 receptor antagonist into the BNST reduces the expression of conditioned defeat, whereas injection of a CRH type 1 receptor antagonist into the lateral ventricle does not (Cooper and Huhman, 2005). These data suggest that CRH type 2 receptors are activated in the BNST at the time of conditioned defeat testing. The CRH or urocortin input to the BNST likely originates in the CeA, because unilateral blockade of CRH receptors in the BNST together with a contralateral CeA lesion also impairs the expression of conditioned defeat (Jasnow et al., 2004).
The basolateral and central nuclei of the amygdala
Several studies indicate that the BLA is a critical brain region for the neural plasticity underlying the formation of conditioned defeat. Selective blockade of the NR2B subunit of N-methyl-D-aspartate (NMDA) receptors in the BLA by infusion of ifenprodil impairs the acquisition, but not expression, of conditioned defeat (Day et al., in press). Also, inhibition of protein synthesis in the BLA by infusion of anisomycin impairs the acquisition of conditioned defeat (Markham and Huhman, 2008). Furthermore, using a viral vector to over-express cyclic AMP response element binding protein (CREB) in the BLA was shown to enhance the acquisition of conditioned defeat (Jasnow et al., 2005). These findings suggest the neural structures and neurochemical signals that control the formation of conditioned defeat overlap with those that underlie the formation of conditioned fear.
The BLA has significant projections to the CeA and, as mentioned above, CeA activation is critical for the expression of some forms of conditioned fear. Inhibition of protein synthesis in the CeA also has been shown to impair the consolidation of conditioned fear, which suggests that the formation of fear memories may depend on neural plasticity in the CeA as well as the BLA (Wilensky et al., 2006). Inactivation of the CeA with infusion of muscimol reduces both the acquisition and expression of conditioned defeat (Jasnow and Huhman, 2001). Several injections in this study occurred at the border between the CeA and BLA, and therefore it is difficult to know whether muscimol blocked the acquisition of conditioned defeat by diffusing into the BLA. Thus, the precise role the CeA plays in the neural plasticity underlying conditioned defeat remains uncertain.
The BLA receives input from several brain regions important for the development of conditioned defeat including the medial amygdala (MeA) and hippocampus (Coolen and Wood, 1998; Petrovich et al., 2001). The MeA is known to modulate fearful and defensive behavior (Blanchard et al., 2005; Li et al., 2004), and social defeat activates MeA neurons that express CRH type 2 receptor mRNA (Fekete et al., 2009). Pharmacological inactivation of the MeA (Markham and Huhman, 2008) and ventral hippocampus (Markham et al., 2010) prior to social defeat has been shown to disrupt the acquisition of conditioned defeat. However, injection of a protein synthesis inhibitor into the MeA (Markham and Huhman, 2008) and ventral hippocampus (Markham et al., 2010) does not impair the acquisition of conditioned defeat. All together, data on the acquisition of conditioned defeat suggest that several brain regions provide crucial input to the BLA during social defeat training, including the MeA, ventral hippocampus, and DRN. This input likely modulates the critical neural plasticity in the BLA that underlies the formation of conditioned defeat. In conclusion, the neural structures implicated in the development of conditioned defeat are a combination of those known to be critical for the development of LH and conditioned fear (Figure 2B).
Developing a circuit (BNST-DRN interactions)
A direct comparison of the anatomical and pharmacological similarities and differences of LH and conditioned defeat is described in Table 1. While the neural mechanisms that mediate LH and conditioned defeat are not identical, the similarities should be noted. First, activity in the DRN, BNST, and BLA is critical for the acquisition and/or expression of the behavioral changes associated with both models. Second, the activation of 5-HT neurons in select subregions of the DRN is necessary for both the acquisition and expression of LH and conditioned defeat, and the activation of DRN 5-HT is sufficient to produce LH behavioral changes in the absence of shock (Hammack et al., 2003a; Hammack et al., 2002; Maier et al., 1995a) or exacerbate the effects of defeat (Cooper et al., 2008). Third, the activation of CRH type 2 receptors is critical for the acquisition of learned helplessness and modulates both the acquisition and expression of conditioned defeat.
Table 1.
| Learned Helplessness (LH) | Conditioned Defeat | Manuscript | |
|---|---|---|---|
|
Basolateral Amygdala (BLA) |
-↑ 5-HT within the BLA following IS1 -BLA 5-HT release is sensitized 24-hr after inescapable shock (IS) 1 -BLA 5-HT2C activation mimics the anxiogenic effects of IS2 -BLA 5-HT2C blockade during IS blocks the subsequent anxiogenic effects of IS2 |
-BLA NR2B subunit blockade impairs acquisition but not expression of conditioned defeat3 -Intra-BLA anisomycin impairs acquisition of conditioned defeat4 -Intra-BLA CREB over-expression enhances acquisition of defeat5 |
1Amat et al., 1998a 2Christianson et al., in press 3Day et al., in press 4Markham & Huhman, 2008 5Jasnow et al., 2005 |
|
Other Amygdala Subregions |
Large electrolytic lesions of the amygdala centered around the central nucleus of the amygdala (CeA) block fear conditioning, but do not block the escape deficits produced by IS6 |
-CeA Muscimol blocks acquisition and expression of conditioned defeat7 -MeA inactivation impairs acquisition of conditioned defeat4 |
6Maier et al., 1993 7Jasnow & Huhman, 2001 |
|
Bed Nucleus of the Stria Terminalis (BNST) |
-Electrolytic lesions block LH8 -Inescapable shock sensitizes BNST neuronal activity9 -PACAP infusion produces LH-like behaviors 24-hr later[unpublished] |
-Muscimol blocks the expression but not aquistion of conditioned defeat10 -CRHR2 antagonism blocks the expression of conditioned defeat11 |
8Hammack et al., 2004 9Greenwood et al., 2005 10Markham et al., 2009 11Cooper & Huhman, 2005 |
|
Dorsal Raphe Nucleus (DRN) |
-↑ 5-HT release within the DRN and its projection regions following IS1,12 -Increased 5-HT activity in the middle- caudal DRN regions after IS13 -5-HT1A activation blocks development and expression of LH14 -Caudal DRN 5-HT activation produces LH-like behaviors 24-hr later15 -Caudal DRN CRH-R2 blockade blocks development16 -Caudal DRN CRH-2R activation produces LH-like behaviors 24 hrs later 16 |
-5-HT1A receptor activation blocks acquisition and expression of conditioned defeat17 -Decreased 5-HT1A receptor mRNA following defeat18 -Increased activity in rostral ventral DRN following social defeat18 -Blockade of CRHR1 & R2 reduces acquisition and expression of conditioned defeat19 |
12Amat et al., 1998b 13Grahn et al., 1999 14Maier et al., 1995 15Hammack et al., 2002 16Hammack et al., 2003 17Cooper et al., 2008 18Cooper et al., 2009 19Cooper & Huhman, 2007 |
| Other Areas | Inactivation of ventral medial prefrontal cortex (mPFC) in escapable rats ↑ LH behavior20 |
Inactivation of ventral hippocampus reduces acquisition of conditioned defeat21 |
20Christianson et al., 2009 21Markham et al. 2010 |
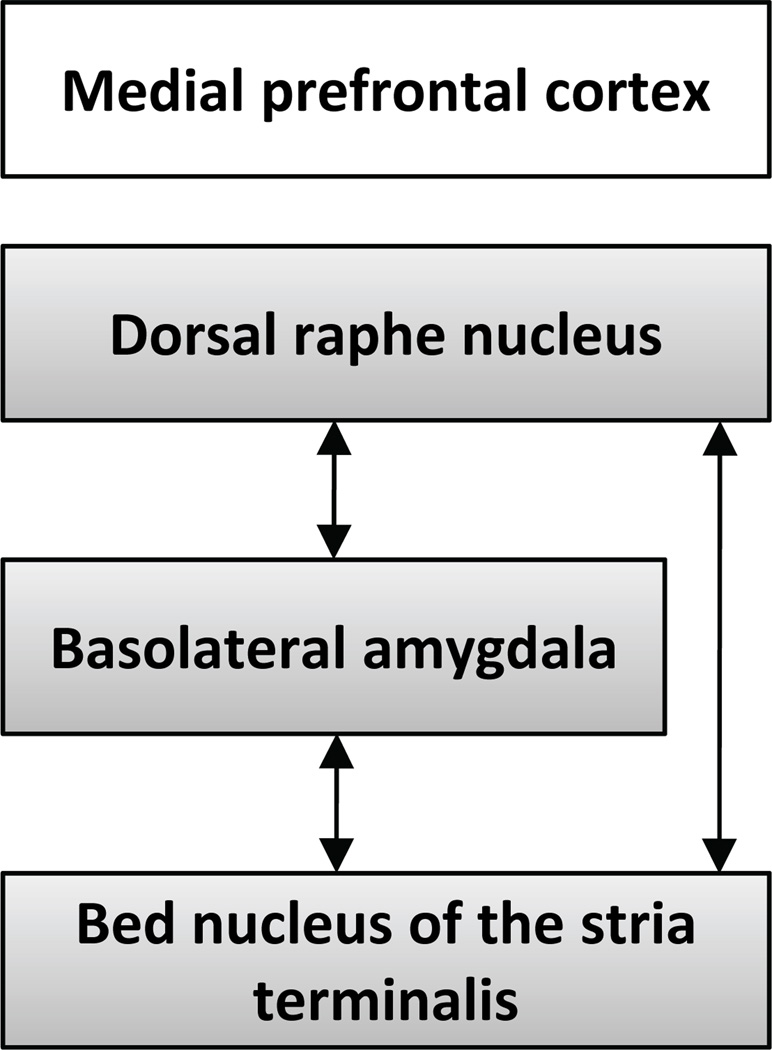
Repeated stress models extend findings from LH and conditioned defeat, and together suggest that interactions between the serotonergic DRN, BLA, and BNST play a prominent role in mediating depressive symptoms associated with stressor exposure (Figure 4). The nature of the interaction between these systems may depend in part on characteristics of the stress exposure. For example, when an organism interprets a stressor as uncontrollable, the BNST may be activated prior to BLA activation, whereas following a social defeat, the BLA may be activated prior to the BNST. Likely, frontal brain regions such as the medial prefrontal cortex may coordinate activity in the ventral forebrain and brainstem differently depending on the behavioral paradigm. Notably, when activity in the ventral aspects of the medial prefrontal cortex (mPFC) is suppressed, escapably shocked rats exhibit marked DRN 5-HT activity and develop LH (Amat et al., 2005). These results suggest that the mPFC actively inhibits the neurocircuitry that mediates LH when an organism learns it has control over stress. In addition to its modulation of DRN activity, the mPFC may project to and modulate activity in the BLA, BNST and CeA (McDonald et al., 1999); hence, the expression of depressive behaviors may result from a failure of the mPFC to inhibit activity in these emotional circuits during stressor exposure.
Figure 4.
A review of the literature investigating the brain regions that mediate both learned helplessness and conditioned defeat suggest that interactions between the dorsal raphe nucleus, basolateral amygdala and bed nucleus of the stria terminalis are critical for both behavioral paradigms. Activity within this hypothesized circuit is tightly regulated by projections from the medial prefrontal cortex.
New Directions
As discussed above, it is likely that the brain regions implicated in LH and conditioned defeat are important targets for current pharmacological approaches in the treatment of MDD and PTSD, and we have previously suggested that changes in the 5-HT response of brain regions like the BNST and DRN mediate the efficacy of some of these treatments ((Hammack et al., 2009b), also see (Berendsen, 1995; Stahl, 1994; Strome et al., 2005; Yatham et al., 1999)). However, the data described above suggest that other mechanisms for modulating these neural circuits may represent novel treatments for trauma-related disorders; some of these are discussed below.
CRH type 2 receptor knockout mice exhibit an anxiogenic profile, suggesting that the expression of the CRH type 2 receptor might be anxiolytic (Bakshi et al., 2002; Takahashi, 2001). Because substantial evidence suggests the opposite role for the CRH type 1 receptors, and CRH type 1 receptors have been shown to mediate the effects of CRH activity on the HPA-axis (see (Takahashi, 2001) for review), considerable interest has focused on the development of CRH type 1 receptor antagonists for the treatment of anxiety disorders and MDD. While results from these studies have been mixed, there likely remains a valuable role for the use of CRH type 1 antagonists in the treatment of these disorders. Interestingly, there has been considerably less interest in the development of CRH type 2 receptor antagonists for the treatment of anxiety disorders or MDD, despite the fact that many pharmacological studies also show a role for CRH type 2 receptors in mediating anxiogenic states. The data reviewed here showing that CRH regulates the development of LH and conditioned defeat indicates a critical role for CRH type 2 receptors. One important consideration for future research on antidepressant treatments is that activation of these receptor subtypes likely depends on the brain region targeted.
Moreover, the action of other therapeutic treatments may be mediated by changes in the neurocircuitry depicted in Figure 4. For example, voluntary exercise has been shown to have many therapeutic benefits on mood in both humans and animals (see (Greenwood and Fleshner, 2008)), and 6 weeks of voluntary wheel running in rodents has been shown to block behavioral consequences of LH (Greenwood et al., 2003). Notably, voluntary exercise upregulated 5-HT1A receptors in the DRN, reducing both DRN and BNST activity (Greenwood et al., 2005; Greenwood et al., 2003). We have shown that prior exercise blocks the anxiogenic effects of the 5-HT2C agonist m-chloro-phenylpiperazine (mCPP, (Fox et al., 2008)), and we have localized this protective effect of exercise on mCPP-induced anxiety to the BNST (unpublished data). Hence a shift in the BNST response to 5-HT towards inhibition (via a decrease in function of excitatory 5-HT2C receptors) may mediate a behavioral phenotype of resistance to stress.
The recent finding that PACAP may mediate behavioral changes associated with LH as well as those associated with repeated stress (Hammack et al., 2009a) suggests that this peptidergic system may represent a promising new target for the development of therapeutic drugs in the treatment of disorders associated with stressor exposure. To date most of the literature investigating the physiology of PACAP-related peptides has focused on the neuroprotective and neurotrophic actions of these molecules (see (Shioda et al., 2006)); however, accumulating evidence is suggesting a role for PACAP in mediating the physiological response to stress exposure (see (Hammack et al.)), and elevated PACAP levels and alterations in PAC1 receptor expression and methylation were recently shown to predict PTSD symptoms and diagnosis in women (Ressler et al., 2011). More studies are necessary to determine the potential benefits of targeting this system in the treatment of anxiety disorders and MDD.
Conclusions
The LH and conditioned defeat paradigms produce behavioral changes that may be associated with the symptomology of trauma-related depression. A consideration of the neural circuitry underlying each of these behavioral paradigms suggests that the DRN, BNST, BLA, and CeA play a critical role in the acquisition and expression of these behavioral changes, although the precise role of these structures may differ depending on the behavioral paradigm studied. Based on these studies, it seems clear that interactions between the serotonergic DRN and emotion-related brain regions such as subregions of the amygdala and BNST play a key role in the development and/or expression of LH and conditioned defeat. Hence the efficacy of treatments for mental disorders associated with traumatic stress will likely depend on a modulation of activity in this neural circuit.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Annals of the New York Academy of Sciences. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus.[see comment] Nature Neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998a;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Research. 1998b;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- American, Psychological, Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Anisman H, de Catanzaro D, Remington G. Escape performance deficits following exposre to inescapable shock: Deficits in motor response maintenance. J. Exper. Psychol. Anim. Behav. Proc. 1978;4:197–218. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Washington, DC: 2000. [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. International Journal of Neuropsychopharmacology. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. Journal of Neuroscience. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Berendsen HH. Interactions between 5-hydroxytryptamine receptor subtypes: is a disturbed receptor balance contributing to the symptomatology of depression in humans? Pharmacology & Therapeutics. 1995;66:17–37. doi: 10.1016/0163-7258(94)00075-e. [DOI] [PubMed] [Google Scholar]
- Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82:147–159. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat.Rev.Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, McKittrick CR, Hardy MP, Blanchard RJ. Effects of social stress on hormones, brain, and behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior, vol. 1. San Diego: Academic Press; 2002. pp. 735–772. [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Bleich A, Koslowsky M, Dolev A, Lerer B. Post-traumatic stress disorder and depression. An analysis of comorbidity. Br J Psychiatry. 1997;170:479–482. doi: 10.1192/bjp.170.5.479. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: findings and clinical implications. J Consult Clin Psychol. 1996;64:191–201. doi: 10.1037//0022-006x.64.1.191. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. Journal of Neuroscience. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. Journal of Neuroscience. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: Simultaneous anterograde and retrograde tract tracing. Journal of Comparative Neurology. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2005;119:1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology (Berl) 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Blocking corticotropin-releasing factor-2 receptors, but not corticotropin-releasing factor-1 receptors or glucocorticoid feedback, disrupts the development of conditioned defeat. Physiol Behav. 2010;101:527–532. doi: 10.1016/j.physbeh.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. Journal of Neuroscience. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behavioural Brain Research. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Day DE, Cooper MA, Markham CM, Huhman KL. NR2B subunit of the NMDA receptor in the basolateral amygdala is necessary for the acquisition of conditioned defeat in Syrian hamsters. Behav Brain Res. doi: 10.1016/j.bbr.2010.09.034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRFR1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong ML, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamster (Mesocricetus auratus) Behav Neurosci. 1999;113:804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychological Bulletin. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. European Journal of Neuroscience. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behavioral Neuroscience. 2008;122:943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectr. 2005;10:182–190. doi: 10.1017/s1092852900010038. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol Biochem Behav. 2002;73:247–258. doi: 10.1016/s0091-3057(02)00795-5. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 2009a;1305:47–63. doi: 10.1016/j.brainres.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA. Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009b;163:991–1001. doi: 10.1016/j.neuroscience.2009.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry. 1996;153:929–934. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PM. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br J Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stressresistant brain. NeuroMolecular Medicine. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009a;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009b;33:1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behavioural Brain Research. 2003a;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. Journal of Neuroscience. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci. 2010;42:327–340. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. Journal of Neuroscience. 2003b;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Research. 1992:581, 190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology. 1999;11:121–128. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Moore TO, Ferris CF, Mougey EH, Meyerhoff JL. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreases in plasma testosterone. Horm Behav. 1991;25:206–216. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. Journal of Comparative Neurology. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005:119, 1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- King JA, Abend S, Edwards E. Genetic predisposition and the development of posttraumatic stress disorder in an animal model. Biol Psychiatry. 2001;50:231–237. doi: 10.1016/s0006-3223(01)01071-x. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. Journal of Neuroscience. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus.[erratum appears in Neuropsychopharmacology 2000 Apr;22(4):449] Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kohn Y, Zislin J, Agid O, Hanin B, Troudart T, Shapira B, Bloch M, Gur E, Ritsner M, Lerer B. Increased prevalence of negative life events in subtypes of major depressive disorder. Compr Psychiatry. 2001;42:57–63. doi: 10.1053/comp.2001.19753. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. Journal of Neuroscience. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Research. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. Journal of Neuroscience. 2005;25:11239–11247. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The olfactory bulbectomized rat as a model of depression. Pol J Pharmacol Pharm. 1984;36:561–569. [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. Journal of Neuroscience. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF. Learned helplessness and animal models of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biological Psychiatry. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behavioral Neuroscience. 1995a;109:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995b;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Kalman BA, Grahn RE. Chlordiazepoxide microinjected into the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescapable shock whether administered before inescapable shock or escape testing. Behavioral Neuroscience. 1994;108:121–130. doi: 10.1037//0735-7044.108.1.121. [DOI] [PubMed] [Google Scholar]
- Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behavioral Neuroscience. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman MEP. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience & Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learning and Memory. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. European Journal of Neuroscience. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Mayou R, Bryant B, Ehlers A. Prediction of psychological outcomes one year after a motor vehicle accident. Am J Psychiatry. 2001;158:1231–1238. doi: 10.1176/appi.ajp.158.8.1231. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- McKinney WT, Jr, Bunney WE., Jr Animal model of depressionIReview of evidence: implications for research. Arch Gen Psychiatry. 1969;21:240–248. doi: 10.1001/archpsyc.1969.01740200112015. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Turek FW. The effects of social defeat and other stressors on the expression of circadian rhythms. Stress. 2002;5:15–22. doi: 10.1080/102538902900012323. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Tornatzky W. Short and long term physiological and neurochemical adaptations to social conflict. In: Puglisi-Allegra S, Oliveirio A, editors. Psychobiology of Stress. Kluwer Academic Publishers; 1990. pp. 15–30. [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropinreleasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. Journal of Neuroscience. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KA, Johnson JD, Hammack SE, Brooks LM, Spencer RL, Watkins LR, Maier SF. Inescapable shock induces resistance to the effects of dexamethasone. Psychoneuroendocrinology. 2003;28:481–500. doi: 10.1016/s0306-4530(02)00035-5. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Foundation Symposium. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion 308-216. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pezuk P, Aydin E, Aksoy A, Canbeyli R. Effects of BNST lesions in female rats on forced swimming and navigational learning. Brain Res. 2008;1228:199–207. doi: 10.1016/j.brainres.2008.06.071. [DOI] [PubMed] [Google Scholar]
- Pezuk P, Goz D, Aksoy A, Canbeyli R. BNST lesions aggravate behavioral despair but do not impair navigational learning in rats. Brain Res Bull. 2006;69:416–421. doi: 10.1016/j.brainresbull.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Serotonin-CRF interaction in the bed nucleus of the stria terminalis: a light microscopic double-label immunocytochemical analysis. Brain Research Bulletin. 1992;28:943–948. doi: 10.1016/0361-9230(92)90217-l. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.64. in press. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with the PAC1 receptor and dysregulation of PACAP. Nature. 2011 doi: 10.1038/nature09856. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. Anxiety enhancement in the murine elevated plus maze by immediate prior exposure to social stressors. Physiol Behav. 1993;53:383–388. doi: 10.1016/0031-9384(93)90222-2. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155:630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- Sherman AD, Sacquitne JL, Petty F. Specificity of the learned helplessness model of depression. Pharmacol Biochem Behav. 1982;16:449–454. doi: 10.1016/0091-3057(82)90451-8. [DOI] [PubMed] [Google Scholar]
- Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, Arata S, Kitamura S, Okuda H, Takenoya F, Kitamura Y. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Annals of the New York Academy of Sciences. 2006;1070:550–560. doi: 10.1196/annals.1317.080. [DOI] [PubMed] [Google Scholar]
- Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacology, Biochemistry & Behavior. 1993;45:827–835. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- Spiga F, Lightman SL, Shekhar A, Lowry CA. Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-Fos expression in brainstem serotonergic neurons. Neuroscience. 2006;138:1265–1276. doi: 10.1016/j.neuroscience.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Stahl S. 5HT1A receptors and pharmacotherapy. Is serotonin receptor downregulation linked to the mechanism of action of antidepressant drugs? Psychopharmacology Bulletin. 1994;30:39–43. [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Research. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. European Journal of Pharmacology. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Strome EM, Clark CM, Zis AP, Doudet DJ. Electroconvulsive shock decreases binding to 5-HT2 receptors in nonhuman primates: an in vivo positron emission tomography study with [18F]setoperone. Biological Psychiatry. 2005;57:1004–1010. doi: 10.1016/j.biopsych.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neuroscience & Biobehavioral Reviews. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Vieweg WV, Julius DA, Fernandez A, Beatty-Brooks M, Hettema JM, Pandurangi AK. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am J Med. 2006;119:383–390. doi: 10.1016/j.amjmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Structure & Function. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PG. Neurochemical mechanisms underlying stressinduced depression. In: Field TM, McCabe PM, Schneiderman N, editors. Stress and Coping. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1985. pp. 93–116. [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. Journal of Neuroscience. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Dennie J, Shiah IS, Adam MJ, Lane CJ, Lam RW, Ruth TJ. Decrease in brain serotonin 2 receptor binding in patients with major depression following desipramine treatment: a positron emission tomography study with fluorine-18-labeled setoperone. Archives of General Psychiatry. 1999;56:705–711. doi: 10.1001/archpsyc.56.8.705. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biological Psychiatry. 1993;33:479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]