Abstract
Dysregulated hypothalamic-pituitary-adrenocortical (HPA) axis stress response has been reported among individuals with prenatal substance exposure and those with early adversity. However, few researchers have examined the combined effects of these risk factors. Patterns of HPA reactivity among maltreated foster children with and without prenatal substance exposure (N = 53; ages 9–12 years) were examined using the Trier Social Stress Test for Children. Area under the curve with respect to increase (AUCI) analyses revealed that prenatal substance exposure or physical abuse significantly increased the likelihood of a negative AUCI (i.e., little or no HPA reactivity). Among children with prenatal substance exposure and physical abuse, 85% exhibited a negative AUCI. The results underscore the importance of addressing this combined risk.
Keywords: maltreatment, cortisol, prenatal substance exposure
Introduction
The hypothalamic-pituitary-adrenocortical (HPA) axis is a neuroendocrine system that plays a central role in maintaining the body’s homeostatic balance in response to stress (Stansbury & Gunnar, 1994). Activation of the HPA axis in response to stress involves a hormonal cascade that begins with the stimulation of the paraventricular (PVN) region of the hypothalamus from a number of inputs from other brain regions. Such stimulation might arise from either physical or psychological threat, a characteristic that has led the HPA axis to be described as a “final common pathway” for stress (Levine, 1993). Stimulation of the PVN leads to the secretion of corticotrophin-releasing hormone (CRH), which is carried to the anterior region of the pituitary (located directly below the PVN) via capillaries of the hypophysial artery. CRH in the anterior pituitary stimulates the secretion of adrenocorticotrophin-releasing hormone (ACTH), which enters the systemic blood flow via the hypophyseal vein. ACTH in the circulatory system reaches the adrenal gland, leading to the secretion of glucocorticoid hormones such as cortisol in the adrenal cortex.
Cortisol acts in a number of ways to maintain homeostasis following stress (Sapolsky, Romero, & Munck, 2000). Metabolically, cortisol increases concentrations of glucose in the blood through several interrelated mechanisms, including the stimulation of gluconeogenesis in the liver, the mobilization of amino acids that form the substrates for hepatic gluconeogenesis in tissues outside the liver, the stimulation of fat breakdown in fatty tissue, and the inhibition of glucose uptake in muscle and fatty tissue. Cortisol also acts on the immune system, producing an anti-inflammatory response and (at high levels) immunosuppression. Finally, cortisol completes a negative feedback loop within the HPA axis, effectively shutting down secretion of CRH and terminating the stress response.
The HPA axis also exhibits a diurnal rhythm (Fries, Dettenborn, & Kirschbaum, 2009), with cortisol levels typically peaking approximately 30 min after awakening and dropping quickly over the morning, reaching their nadir in the evening around bedtime. The mechanisms controlling this diurnal rhythm originate in areas of the brain not associated with the stress response (e.g., hippocampus and suprachiasmatic nucleus).
Although the HPA axis is exceptionally well sculpted to help regulate short-term stress, it is also highly sensitive to the effects of chronic adversity over the lifespan. In a vast literature of animal and human studies, researchers have documented alterations in HPA axis activity associated with a multitude of stressors, including prenatal adversity, early maternal separation and deprivation, malnutrition, chronic occupational stress, and disease (McCrory, De Brito, & Viding, 2010). HPA axis dysregulation has also been observed in a number of populations with psychiatric disorders, including children and adults with anxiety and affective disorders and adolescents with externalizing disorders (Lopez-Duran, Kovacs, & George, 2009; Simeon et al., 2007). As such, alterations in HPA axis activity arising from exposure to chronic adversity can be considered a risk factor for psychopathology.
However, it is interesting to note that there is no single characteristic signature of stress upon HPA axis functioning. For example, patterns of hypercortisolism (elevated cortisol) and hypocortisolism (suppressed cortisol) have been reported (Heim, Ehlert, & Hellhammer, 2000; Ronsaville et al., 2006). Moreover, these respective patterns have been observed in the diurnal activity and stress reactivity of the HPA axis. The lack of consistency in the literature with respect to stress-induced alterations in HPA axis function has led researchers to postulate that variations in specific dimensions of adversity —for example, the type, duration, severity, and developmental timing of adversity —account for different patterns of HPA axis dysregulation (Fisher & Gunnar, 2010). Thus, it has become important to identify specific experiential profiles associated with patterns of HPA axis dysregulation. If suppressed (vs. elevated) HPA axis activity can be shown to be associated with risk for different psychopathologies, such findings could inform future prevention and intervention programs.
In the current study, we examined alterations in HPA axis stress responsivity in the context of prenatal substance exposure and early adversity. Alterations in HPA axis functioning have been observed in infants prenatally exposed to alcohol and drugs. Several mechanisms have been proposed for these alterations, including teratogenic effects of illicit substances on the development key brain regions (Lester et al., 2010) and the rather intriguing possibility that these substances act by increasing maternal stress levels and producing elevated glucocorticoids in the prenatal environment (Lester & Padbury 2009). Notably, however, the results of studies on prenatal substance exposure effects on HPA axis function are somewhat inconsistent. For example, Ramsay, Bendersky, and Lewis (1996) compared infants prenatally exposed to alcohol and cigarettes to nonexposed infants and found an increased cortisol response to an inoculation among the exposed infants at age 2 months but not at age 6 months. Jacobson, Bihun, and Chiodo (1999) examined cortisol levels in a sample of 83 African American infants prenatally exposed to alcohol, cocaine, and other drugs. Prenatal alcohol exposure was associated with elevated basal cortisol levels and elevated cortisol levels in response to a heel-stick blood draw. Prenatal cocaine exposure was associated with lower basal cortisol levels but not the blood draw. In contrast, Magnano, Gardner, and Karmel (1992) found no differences in basal cortisol levels but lower cortisol levels in response to stress in infants prenatally exposed to cocaine compared to healthy, full-term infants. Relatively little research has been conducted on HPA axis function among older children and adolescents with prenatal substance exposure, although such investigations are beginning to appear in the literature. Chaplin et al. (in press) found that adolescents prenatally exposed to cocaine had elevated basal cortisol levels and elevated cortisol levels in response to a laboratory stressor compared to nonexposed adolescents.
In addition to the studies on prenatal substance exposure, alterations in HPA axis function have been observed in a number of studies of children who have experienced early adversity, including children reared in institutional settings, abused and neglected children, and foster children (Bruce, Fisher, Pears, & Levine, 2009; Carlson & Earls, 1997; Cicchetti & Rogosch, 2001; Dozier et al., 2006; Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; MacMillan et al., 2009). Most of these studies —especially those involving young children — have been focused on diurnal HPA axis activity. The findings from some, but not all, of these studies have shown a characteristic pattern of low morning cortisol levels that remain low throughout the day. This diurnal pattern has been explained as a potential downregulation of the HPA axis in the context of nonresponsive early life care (Fisher & Gunnar, 2010). Consistent with this speculation, severity of physical neglect is associated with blunted morning cortisol levels among preschool-aged foster children (Bruce et al., 2009).
Researchers studying older children, adolescents, and adults exposed to early adversity have examined alterations in HPA axis stress responsivity. For example, MacMillan et al. (2009) compared a sample of 12- to 16-year-old females with histories of childhood maltreatment to a sample of aged-matched, nonmaltreated females. The maltreated females showed an attenuated cortisol response to a psychosocial stressor (notably, the differences in cortisol responsivity were not associated with psychiatric symptomatology). Similarly, Carpenter et al. (2007) found that healthy adults with histories of significant childhood maltreatment showed lower elevations in cortisol and ACTH following a psychosocial stressor.
The results from the above studies demonstrate the impact of prenatal substance exposure and early adversity on the development and functioning of the HPA axis. Alterations in the HPA axis might help to explain why these populations are at risk for later psychopathology. However, a clear gap exists in the present literature: although these two risk factors have been investigated separately, the effect of their co-occurrence is not well understood. This is particularly problematic because prenatal substance exposure and early adversity are likely to occur at high rates in certain contexts. For example, in the U.S. foster care system, over 80% of children appear to come from families with substance abuse problems (Besinger, Garland, Litrownik, & Landsverk, 1999; Young, Boles, & Otero, 2007). Although the proportion of these families involving prenatal substance exposure is unknown, it is likely that many foster children are prenatally exposed to alcohol and drugs. Moreover, early adversity (particularly neglect but also physical and sexual abuse) are nearly ubiquitous among foster children. Similarly, children reared in institutional care in some countries are likely to have experienced prenatal substance exposure and early adversity. Although rates of maternal substance abuse vary greatly from country to country, some countries, especially in Eastern Europe, have exceptionally high rates of substance abuse. As in the United States, women with severe alcohol and drug problems in these countries might continue to abuse substances during pregnancy and become unable to care for their children. In addition, the institutions in these countries often provide highly neglectful care due to limited resources and a high child:caregiver ratio. Thus, many foster children and institutionally reared children might be doubly at risk for prenatal substance exposure and early adversity.
To our knowledge, only one prior study has been conducted to examine the combined effects of prenatal substance exposure and early adversity on HPA axis functioning. Lester et al. (2010) examined HPA axis responsivity to a laboratory psychosocial stressor in a sample of 743 11-year-olds with and without prenatal cocaine exposure. Overall, the children who were prenatally exposed to cocaine were less likely to show an elevation in cortisol in response to the stressor than the nonexposed children. Most relevant to the current study, among the prenatally exposed children, the children who had also experienced domestic violence were the most likely to show blunted HPA axis responsivity. Lester et al. conclude that the combined effects of prenatal substance exposure and early adversity appear worse than either stressor alone. These results might best be understood within an allostatic load framework. This concept, introduced by McEwen and Stellar (1993), is based on the idea that neuroendocrine systems such as the HPA axis play a central role in facilitating a bodily balance (i.e., allostasis) even in the face of stress. However, chronic adversity exerts an additive load on these systems, ultimately impacting the body’s ability to maintain an allostatic balance. Within this framework, a compromised prenatal environment and continued stress in the postnatal environment might overextend this balance, leading to a less responsive (or downregulated) HPA axis, in turn placing these individuals at increased risk for psychopathology.
We aimed to follow-up Lester et al.’s findings, focusing on whether foster children in a similar age range to the children in the prior study (with varying profiles of prenatal substance exposure and maltreatment) would show a similar pattern of blunted cortisol responsivity to a laboratory psychosocial stressor. Given Chaplin et al.’s (in press) findings of increased HPA axis reactivity during a stressor in children prenatally exposed to cocaine, it was important to clarify the nature of HPA axis dysregulation in this population. Equally important, we aimed to investigate whether this pattern of HPA axis hyporesponsiveness is most prevalent among the prenatally exposed children who also had experienced significant early adversity. Replicating this potentially important finding in an independent sample of children would underscore the need to allocate resources to children exposed to both risk factors.
Method
Participants
Our participants included 57 children placed in foster care during the preschool period. In the U.S., children are placed in foster care due to concerns about their physical or emotional safety and well-being. Time in foster care varies greatly, from a few days to many years, although a federal law was enacted in 1997 to decrease the proportion of children spending more than 12 months in out-of-home care during a single foster care episode. Of the children in our study, 1 child was excluded due to the child’s difficulty with the saliva sampling, 1 child was excluded due to the family’s inability to complete the afternoon laboratory assessment, and 2 children were excluded because they did not have a valid prestressor cortisol values due to technical issues (the first value being required to compute the area under the curve with respect to increase measure). Thus, the resulting analytical sample was 53 children, (male n = 27 [51%]). The mean child age at assessment was 10.36 years (SD = 0.82, range = 9.02 12.88). The ethnicity of the sample was representative of the community in which the study was conducted: 87% (n = 46) European American, 8% (n = 4) Latino, and 6% (n = 3) Native American.
Procedure
The study was reviewed and approved by the Institutional Review Boards at the Center for Research to Practice and the Oregon Department of Human Services, Public Health Division/Multnomah County Health Department. The children and their caregivers were asked to attend a 2-hr laboratory assessment. The assessments occurred in the afternoon (M = 4:01 p.m., SD = 65 min, range = 2:25 6:50 p.m.) to control for the diurnal rhythm in cortisol and to assess cortisol responsivity during a responsive phase of this rhythm. After informed consent was obtained, the caregivers were taken to a nearby room to complete a number of questionnaires and interviews. The children were photographed and were asked to complete social cognition tasks similar to those completed during previous assessments. This period of time permitted the children to adapt to the laboratory prior to obtaining a baseline (i.e., prestressor) cortisol level.
After the adaptation period, the children completed the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., 1997). This standardized protocol is designed to induce moderate psychosocial stress in laboratory settings and has been shown to produce elevations in cortisol in adults and children (Dickerson & Kemeny, 2004; Gunnar, Talge, & Herrera, 2009). At the beginning of the TSST-C, the children are asked to give a speech and perform mental arithmetic aloud in front of two unfamiliar adult judges. For the speech portion of the task, the children are asked to imagine that they have been accused of stealing money from a friend and need to prepare a speech explaining that they could not have done it. They are also told to pretend that the two judges are their teacher and principal. After a 5-min preparation period that included scripted reminders about the remaining time, the children are asked to give their speech in front of the judges. The judges use scripted prompts to ensure a 5-min speech period. After their speech, the children are asked to perform serial subtractions aloud for 5 min with the judges correcting any errors. The judges maintain a somber demeanor throughout the speech and math portions of the task. After the math portion, the judges adopt a friendly demeanor, remind the children that the scenario is only pretend, and congratulate the children on their performance. After completing the TSST-C, the children are asked to complete a cognitive task and an interview.
Measures
Salivary cortisol response
Five salivary cortisol samples were collected at 15-min intervals beginning immediately prior to the TSST-C. The saliva samples were collected by having each child chew a piece of Trident Original sugarless gum, which stimulates salivation and has been shown to have little effect on cortisol levels (Schwartz, Granger, Susman, Gunnar, & Laird, 1998). Each child then used a straw to expel the saliva into a prelabeled vial. The samples were stored at 20° C until shipped for assaying. The samples were assayed for cortisol determination at the Department of Pathology and Laboratory Medicine at the Emory University School of Medicine using the High Sensitivity Salivary Cortisol Enzyme Immunoassay (Salimetrics, LLC, State College, PA). All of the samples from a child were included in the same assay batch. The samples were assayed in duplicate and averaged. Duplicates varying by more than 10% were reassayed. The intraassay and interassay coefficients of variance were 7.69% and 9.78%, respectively.
To estimate each child’s cortisol response, a trapezoidal formula was used to compute area under the curve with respect to increase (AUCI; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). AUCI is calculated with reference to the first value and thus is a good indicator of change over time, especially elevations over prestressor cortisol levels. The actual time between the collections were included in the formula rather than assuming what the time distances were. As was suggested by Pruessner et al., negative areas were maintained to avoid potential loss of information and were considered an index of decline.
Early adversity
Early adversity (i.e., abuse and neglect experienced prior to placement in foster care during the preschool period) was coded from child welfare system records using the Maltreatment Classification System (MCS; Barnett, Manly, & Cicchetti, 1993), which allows for the classification of each maltreatment incident according to type (i.e., physical abuse, sexual abuse, physical neglect, supervisory neglect, and emotional maltreatment) and severity: 1 (less serious incidents) to 5 (potentially life-threatening incidents).
Training in the use of this coding system was provided by one of the MCS authors (Manly). During the data collection, 20% of the records were coded by two coders to compute interrater agreement. Agreement on the identification of incidents of maltreatment was high (80%). Similarly, interrater agreement for the severity of each type of maltreatment was high (average κ = .72; range for individual categories = .82 .65). In the current study, all of the children experienced multiple types of maltreatment. The specific types of maltreatment included the following: 42% (n = 22) physical abuse, 28% (n = 15) sexual abuse, 83% (n = 44) physical neglect, 87% (n = 46) supervisory neglect, and 91% (n = 48) emotional maltreatment. The mean severity and frequency across the different types of maltreatment were 2.89 (SD = .64) and 12.72 (SD = 8.13), respectively.
Prenatal substance exposure
An indicator of the children’s prenatal substance exposure was developed using child welfare system records and the FAS Facial Photographic Analysis Software (Astley & Clarren, 2001), which was developed to measure the expression of the three key diagnostic facial features of FAS. Prior research has shown high sensitivity (100%) and specificity (99.8%) of this tool (Astley, Stachowiak, Clarren, & Clausen, 2002). The software has been successfully used as a screening tool by the Washington State FAS Diagnostic and Prevention Network (Clarren & Astley, 1997) and to study prevalence rates of FAS among foster children (Astley et al., 2002). The software requires three standardized digital photographs of a child’s face to compute a 4-digit facial rank: 1 (complete absence of the FAS facial features) to 4 (strong presence of the FAS facial features). For this study, the facial rank was recoded to 0 (complete absence of the FAS facial features) and 1 (some degree of presence of the FAS facial features). The child welfare system records were reviewed and rated as follows: 0 (prenatal alcohol and drug use were not mentioned in these records) and 1 (prenatal alcohol or drug was mentioned in these records). The information from these two sources was then combined to create an indicator of the children’s prenatal substance exposure; 32% of the children (n = 16) were coded as negative for prenatal substance exposure, and 68% of the children (n = 34) were coded as positive for prenatal substance exposure.
Pubertal status
The caregivers completed the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988), a five-item measure of growth spurt in height, pubic hair growth, and skin change (males and females); facial hair growth and voice change (males); and breast development and menarche (females). The average score across items was examined as a potential covariate.
Data Analysis
Preliminary analyses were conducted to examine the associations between potential covariates (e.g., age, sex, and pubertal status) and the AUCI measure. The children’s AUCI scores were then dichotomized to compare children with increases and decreases in cortisol levels over time. Pearson’s chi-square tests were conducted to examine the relations between early adversity, prenatal substance exposure, and salivary cortisol response over time.
Results
Preliminary Analyses
The relations between the salivary cortisol response over time and potential covariates were examined. The results of independent-sample t-tests revealed that the AUCI scores did not differ by sex, t(51) = 0.68, ns, and were not significantly correlated with age or pubertal status, r(51) = .089, ns, and r(51) = .005, ns, respectively. Therefore, these variables were not considered in the subsequent analyses, and the results reported in the study were not adjusted for sex, age, or pubertal status.
Associations Between Salivary Cortisol Response, Early Adversity, and Prenatal Substance Exposure
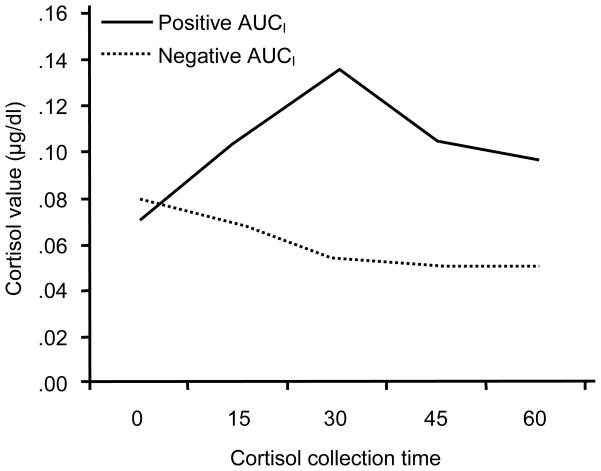
To examine the effects of prenatal substance exposure and early adversity, the AUCI measure was dichotomized: positive AUCI score (a stronger increase than decrease in cortisol levels over time) and negative AUCI score (a stronger decrease than increase in cortisol levels over time). In the current study, 40% of the children (n = 21) had a positive AUCI score, and 60% of the children (n = 32) had a negative AUCI score. The observed cortisol levels at each collection time for these two groups are shown in Figure 1. The groups had similar prestressor cortisol levels, t(51) = 0.79, ns, but their subsequent cortisol levels significantly differed at 15 min, t(50) = 2.28, p = .03; at 30 min, t(51) = 4.51, p = .00; at 45 min, t(51) = 5.33, p = .00; and at 60 min, t(50) = 4.97, p = .00.
Figure 1.
Cortisol levels over time for children with positive AUCI and negative AUCI. Note. AUCI = area under the curve with respect to increase.
As shown in Table 1, there was a significant main effect of prenatal substance exposure on salivary cortisol response, Pearson χ2(1, N = 50) = 4.06, p = .04. The percentage of children who showed a decrease in cortisol levels over time was greater among children who were prenatally exposed to substances. Among these children, 68% (n = 23) displayed a decreased cortisol response after the stressor. Additionally, as shown in Table 2, there was a significant main effect of early adversity, physical abuse in particular, Pearson χ2(1, N = 53) = 4.49, p = .03. Among the children who experienced physical abuse, 77% (n = 17) showed a decreased cortisol response over time. None of the other types of maltreatment were significantly associated with salivary cortisol response.
Table 1.
Number of Children with Positive and Negative AUCI based on Prenatal Substance Exposure Status
| Prenatal substance exposure (N = 50)a
|
||
|---|---|---|
| No | Yes | |
| Positive AUCI | 10 | 11 |
| Negative AUCI | 6 | 23 |
The facial photographs were missing for 3 children.
Note. AUCI = area under the curve with respect to increase.
Table 2.
Number of Children with Positive and Negative AUCI based on Physical Abuse Status
| Physical abuse (N = 53)
|
||
|---|---|---|
| No | Yes | |
| Positive AUCI | 16 | 5 |
| Negative AUCI | 15 | 17 |
Note. AUCI = area under the curve with respect to increase.
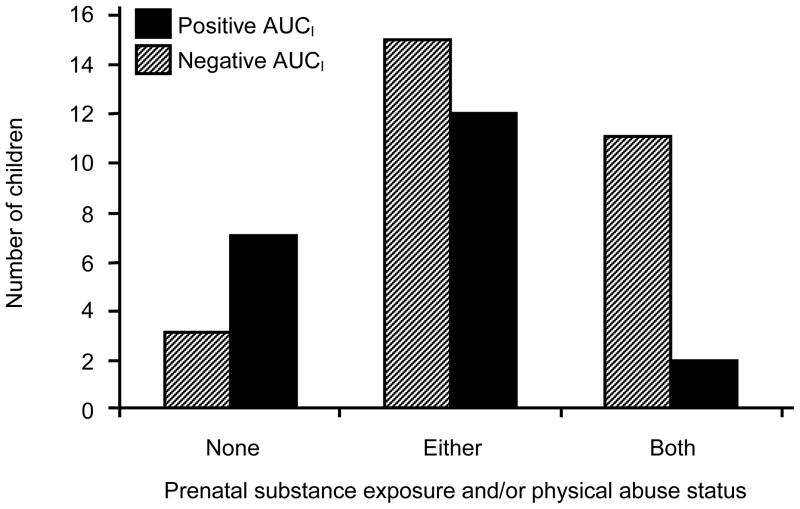
Finally, there was a significant interaction effect between prenatal substance exposure and physical abuse on salivary cortisol response, Pearson χ2(2, N = 50) = 7.07, p = .03. The nature of this interaction is illustrated in Figure 2. The children who experienced both risk factors (vs. only one risk factor) were significantly more likely to show a decreased cortisol response over time: 85% of the children who experienced both risk factors displayed a decreased cortisol response after the stressor (vs. 30% of the children who experienced only one risk factor).
Figure 2.
Number of children with positive and negative AUCI based on combined prenatal substance exposure and physical abuse status.
Discussion
In the current study, a significantly higher proportion of children with histories of child welfare system involvement who had experienced prenatal substance exposure exhibited a stronger decrease in cortisol over time in response to a psychosocial stressor. More than two thirds of the children who were prenatally exposed to substances showed this pattern of cortisol levels compared with just over half of the nonexposed children. These proportions suggest that prenatal substance exposure negatively impacts the development of the HPA system. Notably, these results are consistent with Lester et al.’s (2010) finding that children prenatally exposed to cocaine show a propensity to exhibit an attenuated HPA axis response to a laboratory psychosocial stressor compared with nonexposed children.
It is important to note that the specific mechanisms by which prenatal substance exposure alters HPA axis activity are not well understood; however, Lester and Padbury (2009) recently proposed a model in which maternal substance use during pregnancy increases physical and psychological stress, which leads to elevated glucocorticoid levels in the mother and consequently in the fetal environment. Other researchers have found a link between high maternal stress during pregnancy and altered HPA function in their offspring (Austin, Leader, & Reilly, 2005; Egliston, McMahon, & Austin, 2007). Overall, our results support the speculation that prenatal substance exposure is a significant source of allostatic load that has the potential to produce long-term alterations in a key stress response and regulatory system. This information highlights the need for early identification of and preventive intervention for children who have been prenatally exposed to substances.
A similar conclusion can be drawn from the analyses examining the effects of early adversity on cortisol responsivity. Specifically, although we examined the effects of different forms of maltreatment, only physical abuse was associated with an attenuated HPA axis response to stress. In previous research with foster children, severity of physical neglect as opposed to physical abuse was associated with blunted cortisol levels (Bruce et al., 2009). However, Bruce et al. focused on diurnal cortisol levels rather than cortisol responsivity; and as noted above, the neural mechanisms may differ for these two HPA axis functions. It is also important to note that very few children in the current study did not experience physical neglect, supervisory neglect, and emotional maltreatment. Thus, additional research examining the differential effects of specific types of maltreatment on HPA axis responsivity is warranted.
In addition to the independent effects of prenatal substance exposure and physical abuse, the most noteworthy aspect of the results of the current study was the frequency of an attenuated HPA axis response to the stressor among children with both prenatal substance exposure and physical abuse. Indeed, within this group of children, a typical cortisol response was extremely uncommon (i.e., 15%). These results replicate those reported by Lester et al. (2010) and provide further support for the idea that prenatal substance exposure and early adversity create a cumulative allostatic load, as indexed by the likelihood of disruption of HPA axis regulation. Despite the growing evidence base that prenatal substance exposure and early adversity commonly co-occur and that the combined effects of these stressors are quite deleterious on a system specifically designed to help the body maintain homeostatic balance under stress, there is still very little awareness or policy directed to this area. Put simply, children who experience prenatal substance exposure and early adversity appear to be at increased risk for psychopathology, but their need for services is largely unrecognized. Additional attention to this area in terms of basic descriptive and applied prevention/intervention research is necessary.
It is important to note that 60% of the children in the current sample showed little or no elevation in cortisol levels in response to a psychosocial stressor that has been shown to elevate cortisol levels in children and adults (Dickerson, & Kemeny, 2004; Gunnar, Talge, et al., 2009). One possible explanation for this high nonresponse rate is that children in this age range are less likely to demonstrate an elevation in cortisol in response to the TSST-C than adolescents (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Stroud et al., 2009). However, in the current study, the preliminary analyses did not reveal any differences for older or more developmentally mature children. An alternative explanation is that the base rate of altered HPA axis function in the foster care population, in which prenatal substance exposure and early adversity are very common, is quite high. Thus, the percentage of nonresponders in the current study might be another indicator of risk in this population.
The limitations of the current study include our relatively small sample size and the age range of the sample. The small sample size might have also reduced the variability in the prenatal substance exposure and early adversity measures. These issues might have been expected to reduce effect sizes, so the fact that the observed effects were statistically significant and consistent with prior findings is indicative of the robustness of these results. Further, although the MCS has been widely used in the maltreatment literature, the use of child welfare records to code prenatal substance exposure and early adversity should be noted as a limitation: the information in these records can be incomplete. Future research involving larger samples and examining HPA axis responsivity to stress through adolescence, a period during which serious psychopathology emerges for many at-risk individuals, will further inform the field.
Acknowledgments
The authors thank the participating families and project staff, Kristen Greenley for project management, and Matthew Rabel for editorial assistance.
Funding: Support for this project was provided by the following grants: MH059780 and MH078105, NIMH, U.S. PHS; HD045894, NICHD, U.S. PHS; and DA021424 and DA023920, NIDA, U.S. PHS.
Footnotes
Declaration of Conflicting Interests
The authors declare that they have no conflicts of interest.
Contributor Information
Philip A. Fisher, Oregon Social Learning Center and University of Oregon
Hyoun K. Kim, Oregon Social Learning Center
Jacqueline Bruce, Oregon Social Learning Center.
Katherine C. Pears, Oregon Social Learning Center
References
- Astley SJ, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: Correlations with brain dysfunction. Alcohol and Alcoholism. 2001;36:147–159. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Stachowiak J, Clarren SK, Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. Journal of Pediatrics. 2002;141:712–717. doi: 10.1067/mpd.2002.129030. [DOI] [PubMed] [Google Scholar]
- Austin MP, Leader LR, Reilly N. Prenatal stress, the hypothalamic-pituitary-adrenal axis, and fetal and infant neurobehaviour. Early Human Development. 2005;81:917–926. doi: 10.1016/j.earlhumdev.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Vol. 8. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Besinger B, Garland A, Litrownik A, Landsverk J. Caretaker substance abuse among maltreated children placed in foster care. Child Welfare. 1999;78:221–239. [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer DH. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, …Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Freiburger MB, Mayes LC, Sihna R. Prenatal cocaine exposure, gender, and adolescent stress response: A prospective longitudinal study. Neurotoxicology and Teratology. doi: 10.1016/j.ntt.2010.08.007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Astley SJ. Development of the FAS Diagnostic and Prevention Network in Washington State. In: Streissguth A, Kanter J, editors. The challenge of fetal alcohol syndrome: Overcoming secondary disabilities. Seattle: University of Washington Press; 1997. pp. 40–51. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, …Levine S. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Egliston KA, McMahon C, Austin MP. Stress in pregnancy and infant HPA axis function: Conceptual and methodological issues relating to the use of salivary cortisol as an outcome measure. Psychoneuroendocrinology. 2007;32:1–13. doi: 10.1016/j.psyneuen.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR. Early life stress as a risk factor for disease in adulthood. In: Vermetten E, Lanius R, Pain C, editors. The impact of early life trauma on health and disease: The hidden epidemic. Cambridge, UK: Cambridge University Press; 2010. pp. 133–141. [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Talge N, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Development and Psychopathology. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Lester BM, LaGasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, …Higgins R. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. Journal of Pediatrics. 2010;157:288–295. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Levine S. The psychoendocrinology of stress. Annals of the New York Academy of Sciences. 1993;697:61–69. doi: 10.1111/j.1749-6632.1993.tb49923.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, …Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the Youth Mood Project. Biological Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnano CL, Gardner JM, Karmel BZ. Differences in salivary cortisol levels in cocaine-exposed and noncocaine-exposed NICU infants. Developmental Psychobiology. 1992;25:93–103. doi: 10.1002/dev.420250203. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants’ adrenocortical reactivity to stress. Journal of Pediatric Psychology. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsaville DS, Municchi G, Laney C, Cizza G, Meyer SE, Haim A, …Martinez PE. Maternal and environmental factors influence the hypothalamic-pituitary-adrenal axis response to corticotropin-releasing hormone infusion in offspring of mothers with or without mood disorders. Development and Psychopathology. 2006;18:173–194. doi: 10.1017/S095457940606010X. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biological Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:108–134. [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NK, Boles SM, Otero C. Parental substance use disorders and child maltreatment: Overlap, gaps, and opportunities. Child Maltreatment. 2007;12:137–149. doi: 10.1177/1077559507300322. [DOI] [PubMed] [Google Scholar]