Summary
Effective humoral immunity depends on the support of B cell responses by T-follicular helper (Tfh) cells. Whilst it has been proposed that Tfh cell differentiation requires T-B interactions, the relative contribution of specific populations of Ag presenting cells remains unknown. We employed three independent strategies that compromised interactions between CD4+ T cells and activated B cells in vivo. Whereas the expansion of CD4+ T cells was relatively unaffected, Tfh cell differentiation was completely blocked in all scenarios. Surprisingly, augmenting antigen presentation by non-B cells rescued Tfh cell differentiation, as determined by surface phenotype, gene expression and germinal center localization. We conclude that although Ag presentation by responding B cells is typically required for the generation of Tfh cells, this does not result from the provision of a unique B cell-derived signal, but rather because responding B cells rapidly become the primary source of antigen.
Introduction
B cell responses, such as germinal center (GC) formation and the generation of high affinity long-lived plasma cells and memory cells, are dependent on help provided by CD4+ T cells. T follicular helper (Tfh) cells are a specialized subset of T cells that provide help to B cells (Breitfeld et al., 2000; Schaerli et al., 2000). Tfh cells are characterized by increased expression of numerous molecules including the surface markers CXCR5, PD1, ICOS and CD40 ligand (CD40L), the cytokine IL-21 and the transcription factor Bcl-6 (King et al., 2008). These serve not only as markers of Tfh cells but also play important roles in their generation and function. The coordinated upregulation of CXCR5 and downregulation of CCR7 is important for positioning of Tfh cells in the B cell follicle (Ansel et al., 1999; Hardtke et al., 2005; Haynes et al., 2007). Similarly, CD40L and IL-21 are potent modulators of B cell differentiation (Armitage et al., 1992; Bryant et al., 2007; Ettinger et al., 2005; Noelle et al., 1992; Ozaki et al., 2002), while ICOS-ICOS-ligand (ICOS-L) interactions are required for eliciting T-dependent (TD) B cell responses (Mak et al., 2003; McAdam et al., 2001; Tafuri et al., 2001). Several recent studies have also demonstrated that Bcl-6 controls the commitment of CD4+ T cells to a Tfh fate in the same way that Th1, Th2, Th17 and Treg cells are controlled by T-bet, GATA3, RORγt and FoxP3, respectively (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009b).
Uncertainty exists in the steps involved in Tfh cell differentiation, although roles for several different molecules in their generation have been elucidated. For example, Tfh cells are reduced in mice deficient in ICOS (Akiba et al., 2005; Bossaller et al., 2006) and patients with immune deficiencies caused by mutations in ICOS and CD40LG (Bossaller et al., 2006) suggesting that these molecules play key roles in their generation and/or maintenance. It has also been proposed that Tfh cell generation is a multi-step process involving initial activation on dendritic cells (DC) within the T cell zone followed by interactions with B cells at the T-B border or within the follicle (King et al., 2008; Yu et al., 2009a).
X-linked lymphoproliferative disease (XLP) is a rare immunodeficiency caused by mutations in SH2D1A, which encodes SLAM-associated protein (SAP). SAP is an intracellular adaptor molecule that binds members of the SLAM family of surface receptors and mediates downstream signaling. The SLAM family of receptors is widely expressed on hematopoietic cells, including T and B cells. Both XLP patients and SAP-deficient mice display impaired TD Ab responses due to an inability of SAP-deficient CD4+ T cells to provide help to B cells (Crotty et al., 2003; Czar et al., 2001; Hron et al., 2004; Ma et al., 2005; Ma et al., 2007; Yin et al., 2003). Although several groups have recently examined Tfh cells in SAP-deficient mice, their conclusions have been contradictory, with some groups noting normal Tfh cell development but compromised function (Kamperschroer et al., 2008; Qi et al., 2008) yet others observing impaired development (Cannons et al., 2010; Linterman et al., 2009). Insight into the mechanism of defective help by SAP-deficient CD4+ T cells was provided by the finding that SAP-deficient CD4+ T cells had a reduced ability to form stable conjugates with B cells resulting in dramatically reduced interaction times between CD4+ T cells and B cells (Qi et al., 2008).
We sought to clarify the role of SAP in the different stages of CD4+ T cell activation and Tfh cell development, particularly in light of the observed defect in T-B interactions. We found that SAP-deficient CD4+ T cells were incapable of generating normal numbers of antigen (Ag) specific Tfh cells, thus revealing an important role for SAP in Tfh cell development. Similarly, when Ag was limiting, Ag-presentation by activated B cells was required for Tfh cell generation. Boosting with Ag, however, could substantially restore this defect inasmuch that CD4+ T cells no longer required Ag-presentation by B cells to become Tfh cells. Rather, this “rescue” of Tfh cell development reflected ongoing Ag-presentation by DC. Hence, the differences observed in Tfh cell development in SAP-deficient mice (Cannons et al., 2010; Kamperschroer et al., 2008; Linterman et al., 2009; Qi et al., 2008) could be explained by differences in Ag-presentation between DC and activated B cells.
Thus, this study not only provides an explanation for the disparate results observed for Tfh cell generation in the absence of SAP but also demonstrates that the requirement for interactions with B cells during Tfh cell formation reflects Ag availability rather than a unique B-cell derived activation signal. Together, our findings reveal an alternative pathway for Tfh cell development that is B-cell independent.
Results
SAP-deficient mice show a severe defect in the generation of Tfh cells
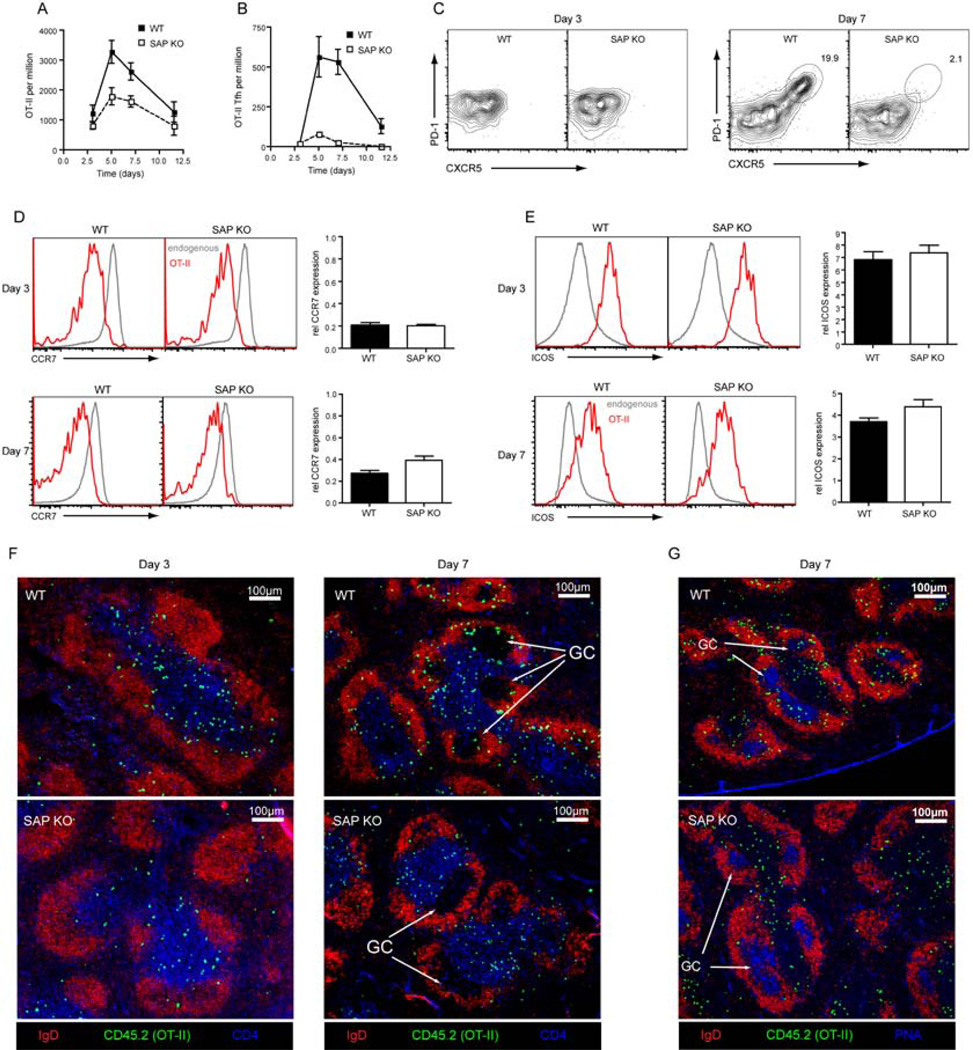
To study the role of SAP in Tfh cell development SAP-deficient mice were crossed with mice expressing the OT-II T cell receptor (TCR), which recognizes ovalbumin (OVA). Wild-type (WT) or SAP-deficient OT-II CD4+ T cells were then transferred into congenic WT hosts, which were immunized with OVA plus Alum. WT OT-II cells reached their peak of expansion at day 5 after which their numbers began to contract (Figure 1A). SAP-deficient OT-II cells also showed maximal expansion on day 5, however, the degree of expansion was only half that observed for WT cells (Figure 1A). To determine the kinetics of Tfh cell formation, expression of CXCR5 and PD1 was examined. Three days after immunization no CXCR5hiPD1hi cells were generated from either WT or SAP-deficient OT-II cells. However, by day 5 a population of CXCR5hiPD1hi (Tfh) cells could be distinguished in the WT OT-II cells (Figure 1B, C). Strikingly, this population was not seen for SAP-deficient OT-II cells (Figure 1B, C). Thus, whereas WT CD4+ T cells generated a robust Tfh cell response that peaked around day 5–7 and could still be observed at day 10, SAP-deficient CD4+ T cells were largely unable to form Tfh cells.
Figure 1. SAP deficiency severely compromises the development of Tfh cells.
WT or Sh2d1a−/− (SAP KO) OT-II cells were transferred to congenic recipients that were then immunized with OVA plus Alum i.p. (A) the proportion of OT-II cells in the spleen was determined at various times (Mean ± SEM, n=5–14). (B–C) Cells were stained for CXCR5 and PD1 and the number of CXCR5hiPD1hi cells was determined (Mean ± SEM, n=5–14). (C) Contour plots show CXCR5 and PD1 expression on OT-II cells on days 3 (left panel) and 7 (right panel) post-immunization. Cells were stained for CCR7 (D) or ICOS (E). Histograms show CCR7 or ICOS expression on endogenous CD4+ T cells (grey) and OT-II cells (red) after 3 (upper panel) or 7 (lower panel) days. Graphs show relative change in expression of CCR7 and ICOS on OT-II cells compared to endogenous CD4+T cells (Mean ± SEM, n=7–8). (F) Immunofluorescence staining of spleen sections was carried out to determine the positioning of OT-II T cells (CD45.2+) within the follicle (IgD) and T cells zone (CD4) after 3 (left panel) or 7 days (right panel). (G) Further staining confirmed positioning within the GC (PNA) at day 7.
Next, we studied changes in expression of ICOS and CCR7, which are also associated with CD4+ T cell activation. Early in the response, before CXCR5hiPD1hi cells appear, WT and SAP-deficient OT-II cells downregulated CCR7 (Figure 1D) and upregulated ICOS (Figure 1E) to a similar degree. Although SAP-deficient CD4+ T cells failed to generate Tfh cells by day 7, their expression of ICOS was similar to WT OT-II cells (Figure 1E) and they maintained substantial CCR7 downregulation (Figure 1D). Hence, early activation events are intact in SAP-deficient CD4+ T cells.
Tfh cells are characterized not only by expression of CXCR5 and PD1 but also by altered expression of other markers (King et al., 2008). Thus, to better understand the development of Tfh cells, their phenotype was determined (Figure S1). As previously described (Chtanova et al., 2004; Kim et al., 2004; Ma et al., 2009; Rasheed et al., 2006) they expressed high amounts of CXCR4, ICOS, CD200 and CD272 (BTLA), although these latter three were upregulated on all WT OT-II cells, albeit to a slightly lower degree than on Tfh cells. Tfh cells also showed decreased expression of CCR7, CD62L and CD127 (IL-7Rα). Importantly, the SLAM family members SLAM, CD84 and Ly108 were present on activated OT-II cells (both non Tfh and Tfh) at higher amounts than on endogenous (mostly naive) CD4+ T cells.
We next determined whether the detection of CXCR5hiPD1hi cells corresponded with the appearance of OT-II cells in the B-cell follicle. At day 3 the majority of WT and SAP-deficient OT-II cells localized to the T cell zone while only a few were observed in the follicle (Figure 1F). By day 7, GC were established and WT OT-II cells could be found within these structures, in addition to the T cell zone and B-cell follicle. In contrast, SAP-deficient OT-II cells could not be found in the GC (Figure 1F,G) with the majority of them remaining in the T cell zone. Thus, both phenotypic and histological analyses demonstrated that SAP-deficient T cells failed to form Tfh cells, particularly those localizing to GC.
Restoring GC does not induce Tfh cell development from SAP-deficient CD4+ T cells
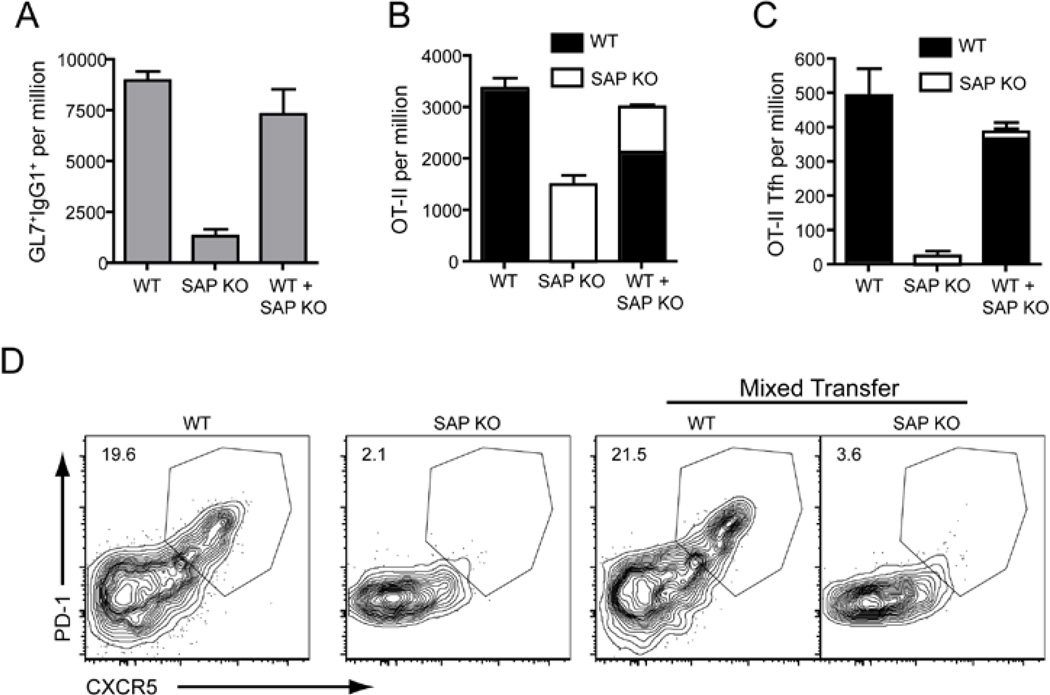
Although we could find GC in mice receiving SAP-deficient OT-II cells, GC formation was reduced compared to mice that received WT OT-II cells (Figure 2A, 3D). These residual GC observed in mice receiving SAP-deficient OT-II cells are largely supported by the endogenous SAP-sufficient CD4+ T cells that also respond to OVA, as demonstrated by a considerable decrease in GC cells when OT-II cells were transferred into SAP-deficient recipients (Figure S2). Thus, it was possible that the lack of GC-related Tfh cell development was secondary to poor GC development, and a reduction in GC B cells, to support these Tfh cells. This scenario would be consistent with the proposed role of B cells in providing appropriate signals for Tfh cell development (King et al., 2008; Yu et al., 2009a). To test this possibility Thy1.1− WT or Thy1.1+ SAP-deficient OT-II cells were transferred either separately or together into CD45.1-congenic recipients followed by challenge with OVA plus Alum. The co-transfer of WT OT-II cells was able to rescue the poor GC response induced by SAP-deficient OT-II cells as measured by the presence of IgG1+GL7+ cells (Figure 2A). Nevertheless, SAP-deficient CD4+ T cells still showed decreased expansion (Figure 2B) and the increased GC response did not induce formation of CXCR5hiPD1hi Tfh cells from SAP-deficient CD4+ T cells (Figure 2C, D). Therefore, defective formation of Tfh cells in the absence of SAP is not merely a consequence of the lack of a strong GC response.
Figure 2. Rescuing germinal centre formation does not facilitate Tfh cell development from SAP-deficient CD4+ T cells.
CD45.1-congenic mice were transferred with CD45.2+ OT-II cells. Mice received either Thy1.1− WT OT-II or Thy1.1+ SAP KO OT-II alone or a combination of equal numbers of Thy1.1− WT OT-II or Thy1.1+ SAP KO OT-II and were then immunized i.p with OVA plus Alum. The responses were examined 7 days later. (A) The proportion of IgG1+GL7+ (GC) B cells in recipient spleens. (B) The proportion of OT-II cells from each donor was determined – WT OT-II: filled bars, SAP KO OT-II: open bars. (C) The proportion of CXCR5hiPD1hi Tfh cells from each donor was determined. All graphs show Mean ± SEM, n=4 (D) Contour plots show expression of CXCR5 and PD1 on OT-II cells of each genotype.
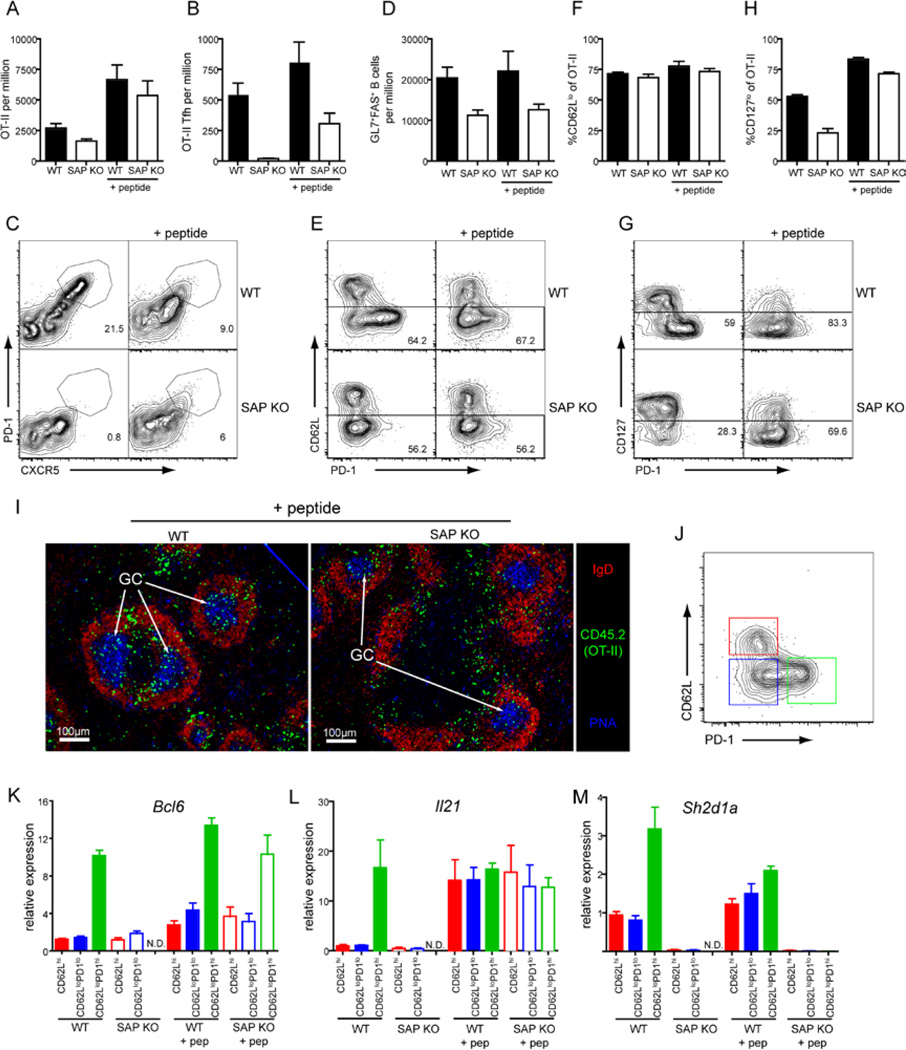
Figure 3. Treatment with peptide can rescue Tfh cell development from SAP-deficient T cells.
WT or SAP KO OT-II cells were transferred to congenic recipients that were then given OVA plus Alum i.p. at day 0. On day 3 some mice received additional OVA peptide i.v. and the mice then sacrificed on day 7. (A) The proportion of total OT-II cells in the spleen. (B) The proportion of CXCR5hiPD1hi Tfh OT-II cells was determined by (C) staining for CXCR5 and PD1 on OT-II cells. (D) Proportion of GC B cells as determined by staining for expression of GL7 and Fas. (E) Expression of CD62L and PD1 on OT-II cells. (F) Percentage of OT-II cells that are CD62Llo. (G) Expression of CD127 and PD1 on OT-II cells. (H) Percentage of cells OT-II cells that are CD127lo. All graphs show Mean±SEM, n=7–9, except (F) n=4–6. (I) Immunofluorescence staining of spleen sections was performed to determine the positioning of OT-II T cells (CD45.2+) within the follicle (IgD) and GC (PNA). (J-M) CD62Lhi, CD62LloPD1lo and CD62LloPD1hi populations of OT-II CD4+ T cells (CD4+B220−CD45.2+) were isolated by sorting. Expression of Bcl6 (K), Il21 (L) and Sh2d1a (M) in each of these populations was then determined by quantitative PCR. N.D. - Not done. The values represent the (Mean±SEM of 4 experiments for peptide boost, 3 experiments for non-boost).
SAP-deficient T cells can differentiate into Tfh cells following boosting with specific Ag
Several phenotypic markers of Tfh cells, such as high PD1 and CXCR5 expression and low CD127 expression, have been associated with ongoing T cell activation (Franchimont et al., 2002; Hammerbeck and Mescher, 2008) suggesting that Tfh cells may require sustained stimulation for their development or maintenance. Therefore, we determined whether the provision of a second dose of Ag might restore Tfh cell development from SAP-deficient CD4+ T cells. Accordingly, some mice were given OVA peptide three days after the primary OVA plus Alum challenge. Mice receiving the peptide boost showed increased numbers of OT-II cells regardless of whether or not the transferred cells expressed SAP (Figure 3A). More strikingly this peptide boost dramatically restored Tfh cell development from SAP-deficient precursors such that the number of CXCR5hiPD1hi CD4+ T cells approached that seen for WT cells in recipients immunized with OVA plus Alum alone (Figure 3B, C). This rescue of CXCR5hiPD1hi Tfh cells was not associated with an increase in GC B cells (Figure 3D), nor a substantial change in downregulation of CD62L (Figure 3E, F). This second dose of Ag did, however, result in an increase in the proportion of cells that downregulated CD127 (Figure 3G, H). The increase in CD127lo cells was particularly prominent in the SAP-deficient OT-II population, which had less than half the WT numbers of CD127lo cells if they received only OVA plus alum, but reached approximately 90% of WT numbers following the boost. Because loss of CD127 is associated with sustained TCR signaling (Franchimont et al., 2002; Hammerbeck and Mescher, 2008) SAP deficient T cells may lack the persistent Ag-stimulation necessary for Tfh cell formation.
SAP-deficient Tfh cell express Bcl-6 and localize to germinal centers
Although boosting with peptide increased the number of CXCR5hiPD1hi cells it was necessary to determine whether these cells possessed other characteristics of Tfh cells such as localization within the follicle and GC, and expression of Tfh cell-associated genes. This was particularly important as the maximum degree of CXCR5 and PD1 expressed by WT and SAP-deficient OT-II cells following the peptide boost was decreased compared to WT cells that had not received the boost (Figure 3C). In contrast to the initial OVA plus alum challenge, in which no SAP-deficient cells were found in the GC (Figure 1 F, G), following peptide boost both WT and SAP-deficient OT-II cells were detected in the follicle and the GC (Figure 3I). Thus, in terms of positioning within secondary lymphoid tissues, these cells generated from SAP-deficient CD4+ T cells following peptide boost appear to be bona fide Tfh cells.
The Tfh cell phenotype has also been associated with expression of the transcription factor Bcl-6 (Chtanova et al., 2004; Kim et al., 2004; Rasheed et al., 2006). Therefore, we isolated the three different subsets of OT-II cells (Figure 3J) generated in response to OVA plus Alum with or without the peptide boost – CD62Lhi, CD62LloPD1lo and CD62LloPD1hi (i.e. Tfh cells) - and determined their expression of Bcl6 (Figure 3K). Irrespective of the immunization strategy and genotype of the transferred OT-II cells, high expression of Bcl6 was only detected in the CD62LloPD1hi population (Figure 3K). This confirmed that the inability of SAP-deficient OT-II cells to form Tfh cells in the absence of the peptide Ag boost was not simply a consequence of a lack of surface CXCR5 and PD1 expression but also a failure to upregulate the Tfh cell “master regulator” Bcl-6. Interestingly, even though the peptide-boosted OT-II cells displayed reduced amounts of PD1 and CXCR5 compared to those responding to OVA plus Alum alone, Bcl-6 expression by Tfh (i.e. CD62LloPD1hi) cells generated by these different immunization strategies was similar. We also examined expression of IL-21 and SAP in the sorted populations. We found high Il21 expression in all of the CD62LloPD1hi populations consistent with a Tfh phenotype. However, we also observed elevated Il21 in the CD62Lhi and CD62LloPD1lo populations from mice that received a peptide boost (Figure 3L). Sh2d1a (encoding SAP) expression was also upregulated in the WT CD62LloPD1hi populations (Figure 3M) consistent with previous reports of increased expression of SAP mRNA or protien in Tfh cells (Chtanova et al., 2004; Ma et al., 2009; Rasheed et al., 2006).
Thus, although in the absence of SAP there is a paucity of Tfh cells, this deficiency can be partially rescued by the provision of peptide Ag. This rescue was not limited to peptide Ag alone as it was also achieved by boosting with whole OVA protein (Figure S3A, B) or peptide-pulsed in vitro activated B cells (data not shown). Interestingly, in both of these alternate boosting protocols, the effect on WT cells was superior than with peptide, thereby resulting in a much greater difference in the generation of Tfh cells between WT and SAP-deficient cells (Figure S3A, B). Consistent with the ability of SAP-deficient T cells to develop into Tfh cells if sufficient Ag is available, we also observed less severe defects in the numbers of SAP-deficient Tfh cells in response to alternative immunization strategies (Figure S3C, D). Similarly, Tfh-like CD4+CXCR5+ cells could be detected in the blood of XLP patients (Figure S3E).
Peptide boost overcomes the requirement for B cell activation via CD40 for Tfh cell development
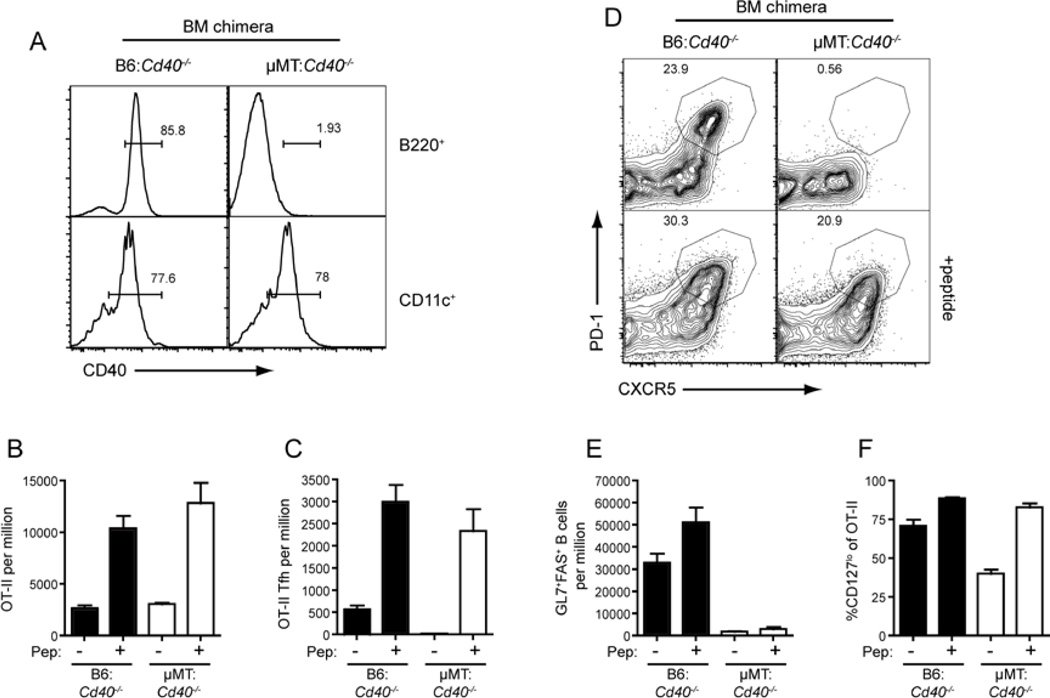
What does this ability of Ag boost to rescue SAP-deficient CD4+ T cells reveal about the generation of Tfh cells in general? It was recently shown that SAP-deficient CD4+ T cells are able to interact normally with DC, but not B cells (Qi et al., 2008). This is consistent with our observation that initial activation of SAP-deficient T cells, such as expansion, ICOS upregulation and CCR7 downregulation, was relatively normal. Thus, one explanation for the reduced expansion, CD127 downregulation and Tfh cell development observed at later time-points for SAP-deficient CD4+ T cells is that there is a lack of ongoing Ag-stimulation that would normally be provided by activated Ag-presenting B cells. To more closely examine the role of B cells in Tfh cell development we generated mixed BM chimeras in which the B cells lacked CD40 (µMT: Cd40−/−), and therefore could not be activated by CD40L-expressing CD4+ T cells, but CD40+ DC were present (Figure 4A, S4A). Chimeras received WT OT-II cells and were challenged with OVA plus Alum with or without the peptide boost. In mice that did not receive peptide boost we observed similar expansion of OT-II cells regardless of whether the B cells expressed CD40 (Figure 4B). However, in the absence of CD40+ B cells there was a complete absence of Tfh cells (Figure 4C, D). This was associated with a lack of GC B cells (Figure 4E) consistent with the need for CD40L-CD40 interactions for TD B-cell responses (Renshaw et al., 1994; Xu et al., 1994). When mice received a peptide boost there was an increase in the number of OT-II cells recovered. Strikingly, this was associated with the appearance of CXCR5hiPD1hi Tfh cells in the µMT: Cd40−/− chimeras, although GC still failed to be generated. In contrast, the peptide boost was unable to efficiently rescue Tfh cell development from CD40L-deficient OT-II cells (Figure S4B–G), indicating that CD40L:CD40 interactions, with either B cells or other APC, are required for Tfh cell development. OT-II cells in the µMT: Cd40−/− chimeric mice also showed decreased CD127 downregulation in response to OVA plus Alum alone, however, this could be increased by peptide injection (Figure 4F) mirroring the observations for SAP-deficient OT-II cells (Figure 3H). Thus, Tfh cells could be generated independently of TD B-cell activation and GC formation when mice received an Ag boost.
Figure 4. B cell activation via CD40 is not required for Tfh development.
Chimeras were generated as described in Experimental Procedures (see also Figure S4A) using 80:20 mix of µMT:Cd40−/− BM (to generate mice with B cells lacking CD40) or B6: Cd40−/− BM (controls). Thy1.1+ WT OT-II cells were transferred into the chimeras, which were then given OVA plus Alum i.p. at day 0. On day 3 some mice received additional OVA peptide i.v. and the mice were sacrificed on day 7 for analysis. (A) Reconstitution was determined by staining B cells (B220+) and DC (CD11c+) for CD40 expression. (B) The proportion of total OT-II cells in the spleen. (C) The proportion of CXCR5hiPD1hi Tfh OT-II cells was determined by (D) staining for CXCR5 and PD1 on OT-II cells. (E) Proportion of GC B cells as assessed by staining for expression of GL7 and Fas. (F) Percentage of cells OT-II cells that are CD127lo. All plots show Mean ± SEM, n=7–14.
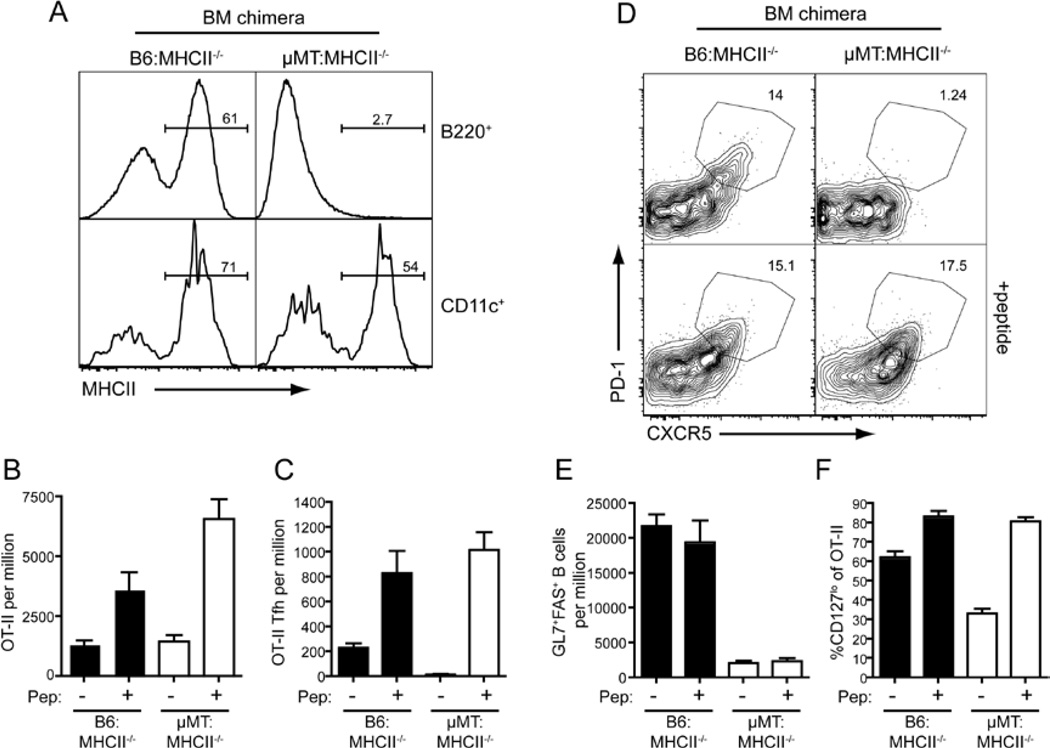
B cell mediated Ag-presentation is not required for Tfh cell development in the presence of excess Ag
These results with the µMT:Cd40−/−chimeras suggested that B cells may not be as critical to Tfh cell development as previously suggested. Hence, we generated another set of mixed BM chimeras in which the B cells - but not DC - lacked MHC class II (MHC II) and were therefore incapable of presenting Ag to CD4+ T cells (Figure 5A). As expected, OT-II cells transferred into B6: MHCII−/− control chimeras expanded and formed Tfh cells in response to OVA plus Alum (Figure 5B–D) and these numbers were further increased following peptide boost. Moreover, these mice developed strong GC responses (Figure 5E). In contrast, when B cells were unable to present Ag (i.e. µMT: MHCII−/−chimeras) the generation of Tfh cells in response to OVA plus Alum alone was abolished (Figure 5B–C). This lack of Tfh cell development was associated with a reduced proportion of CD127lo cells (Figure 5E). The peptide boost at day 3, however, induced greater downregulation of CD127 and was able to restore formation of Tfh cells in these mice as judged by surface phenotype (Figure 5D) and Bcl6 expression (Figure S5C). Boosting with whole OVA was able to rescue Tfh cell development in a similar manner (Figure S5A, B). Thus, responses of WT OT-II CD4+ T cells in the absence of B-cell mediated Ag presentation replicated responses of SAP-deficient OT-II cells, which is consistent with the reduced ability of SAP-deficient CD4+ T cells to interact with B cells (Qi et al., 2008). More strikingly, this result reveals that Tfh cell formation does not strictly require Ag presentation by B cells, but rather can be driven by other APC when there is abundant Ag. This is further supported by the finding that Tfh-like CD4+CXCR5+ cells can be detected in the blood of B-cell deficient patients with X-linked agammaglobulinemia due to mutations in BTK (Figure S5D).
Figure 5. B cell Ag-presentation is not required for Tfh development.
Chimeras were generated as described in Experimental Procedures using 80:20 mix of µMT:MHCII−/− BM (to generate mice with B cells lacking MHC class II) or B6:MHCII−/− BM (controls). Thy1.1+ WT OT-II cells were transferred into the chimeras, which were then given OVA plus Alum i.p. at day 0. On day 3 some mice received additional OVA peptide i.v. and the mice were sacrificed on day 7 for analysis. (A) Reconstitution was determined by staining B cells (B220+) and DC (CD11c+) for MHCII expression. (B) The proportion of total OT-II cells in the spleen. (C) The proportion of CXCR5hiPD1hi Tfh OT-II cells was determined by (D) staining for CXCR5 and PD1 on OT-II cells. (E) Proportion of GC B cells as assessed by staining for expression of GL7 and Fas. (F) Percentage of cells OT-II cells that are CD127lo. All plots show Mean ± SEM, n=6–8.
Limited Ag-presentation by DC is extended by peptide boost
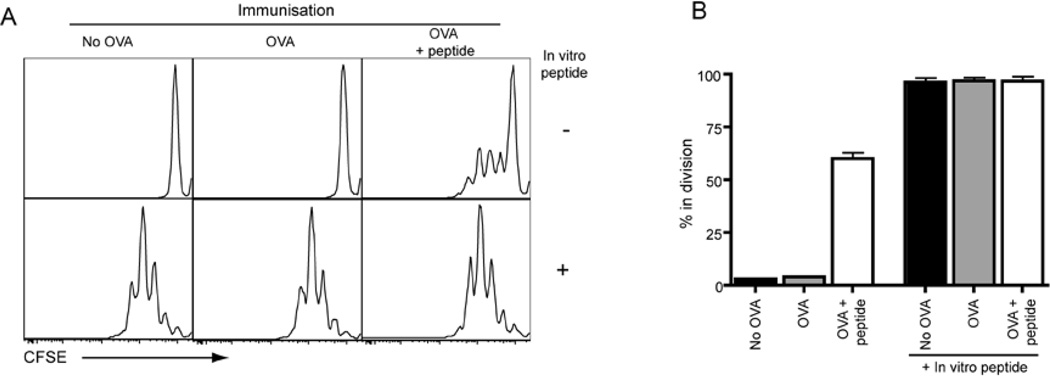
Our data using mixed BM chimeric mice extends that of other groups that have established a key role for B cells in Tfh cell generation under conditions of limiting Ag (Haynes et al., 2007; Johnston et al., 2009; Zaretsky et al., 2009). Because Tfh cells could be formed in the presence of excess Ag when Ag presentation by B cells was abolished, we hypothesized that DC were responsible for prolonged Ag presentation (and thus Tfh cell development) following the peptide boost. To test this we immunized mice and then boosted some with peptide. Two days later we determined whether there was ongoing Ag presentation by sorting DC and culturing them with naïve CFSE-labeled OT-II cells. When peptide was added to the in vitro cultures DC from all three groups of mice could promote proliferation, demonstrating that all the DC had equal APC capabilities (Figure 6A, B). In contrast, only DC isolated from mice that received the peptide boost induced OT-II cell proliferation in the absence of exogenous peptide. Peptide boost did not result in prolonged Ag-presentation by all MHC class II-expressing cells as non-activated B cells from boosted mice did not induce proliferation of OT-II cells in the absence of exogenous peptide (Figure S6). Thus, injection of Alum plus OVA i.p. results in only brief Ag presentation by DC that can no longer be detected by day 6. However, expression of peptide: MHCII complexes can be prolonged by i.v. administration of peptide thereby facilitating ongoing stimulation of CD4+ T cells on DC.
Figure 6. Peptide boost prolongs Ag-presentation by DC.
Mice were given Alum or OVA plus Alum i.p. at day 0. At day 4 mice some mice received additional OVA peptide i.v. On day 6 the mice were sacrificed and spleens removed. CD11c+ cells were isolated by cell sorting and were placed in culture with CFSE-labeled OT-II cells to detect presentation of OVA peptide. Cultures were harvested 3 days later and OT-II cells identified by staining for CD4. (A) Representative CFSE profiles of OT-II cells. (B) Plots show percentage of cells that are in division (Mean ± range of two experiments).
Boosting the Tfh response has limited effect on the B cell response
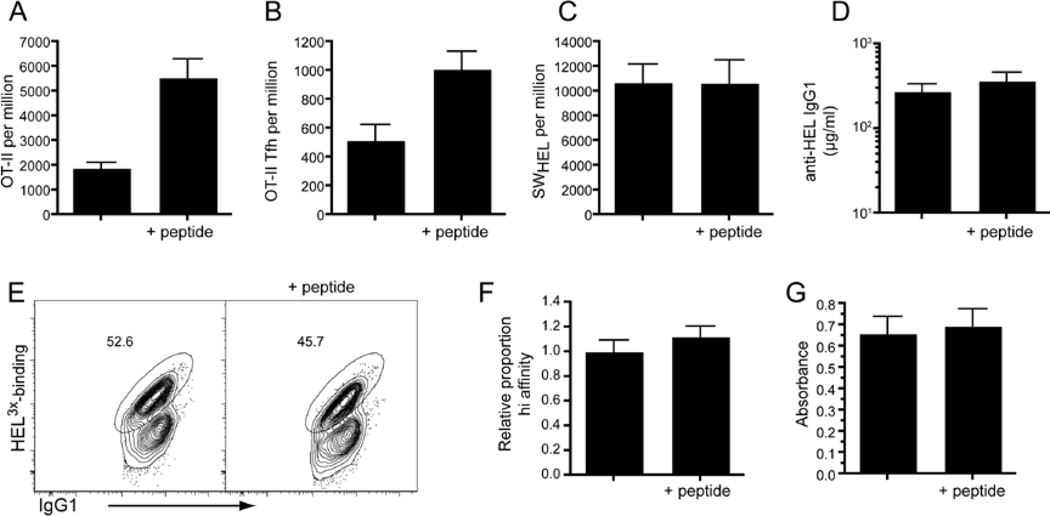
TD B cell responses require the generation and function of Tfh cells. It is unclear, however, whether increasing the number of Tfh cells is sufficient to increase the subsequent Ab response. Indeed, a recent study found that dramatically increasing the generation of Tfh cells by ectopically expressing Bcl6 in naïve CD4+ T cells only augmented the Ag-specific B-cell response by ~2-fold (Johnston et al., 2009). Thus, we examined the effect that boosting with peptide (and thus increasing the number of Ag-specific Tfh cells) would have on the B cell response. OT-II CD4+ T cells and hen egg lysozyme (HEL)-specific B cells (SWHEL) were adoptively transferred into recipient mice that were then challenged with HEL coupled to OVA peptide (HEL-OVApep). In order to also study any effect on affinity maturation we used a variant of HEL (HEL2X) that has approximately 200-times lower affinity for the transgenic B cell receptor than WT HEL (Paus et al., 2006). Boosting the mice with OVA peptide at day 3 and 6 resulted in a significant increase in both total OT-II cells (p=0.0003) and OT-II Tfh (p=0.0074) cells at day 10 (Figure 7A,B). However, even though Tfh cell numbers were augmented, no increase in the total number of HEL-binding B cells (Figure 7C), nor in the titers of serum anti-HEL IgG1 (Figure 7D) were observed. Because boosting Tfh cell numbers may alter the quality of the response, we stained the responding B cells with a different variant of HEL (ie HEL3X) that identifies cells that had undergone affinity maturation (Paus et al., 2006) (Figure 7E). This staining revealed that affinity maturation of the major isotype switched population (IgG1) was unaltered following peptide boost (Figure 7F). Similarly, titers of affinity matured IgG1 in the serum were not altered (Figure 7G). Thus, boosting Tfh cells during the course of an ongoing Ab response has little effect on the outcome of Ab production suggesting that the initial immunization in this case had already produced optimal numbers of these cells
Figure 7. Effect of peptide boost on B cell responses.
WT congenic mice received WT or SAP KO OT-II cells in combination with WT SWHEL cells. Mice were immunized i.p. with OVA plus Alum and i.v. with HEL2X-OVApep. Some mice received additional peptide at Day3 and 6. Mice were sacrificed at day 10 and spleens removed. (A) The proportions of total OT-II cells and (B) CXCR5hiPD1hi Tfh OT-II cells were determined. (C) Splenocytes were stained for CD45.1 and CD45.2 to detect donor cells and the proportion of total HEL-binders was determined by staining with HELWT. (D) The titers of anti-HEL IgG1 in the serum were measured by ELISA against HELWT (E) The proportion of IgG1-switched cells of high affinity was determined by staining for with HEL3X. Plots show donor SWHEL cells (gated using CD45.1 and CD45.2) that are IgG1+. Numbers show proportion of cells that bind HEL3X with high affinity (i.e. cells that have undergone affinity maturation). (F) Graphs show proportion of IgG1+ SWHEL cells that had undergone affinity maturation (numbers are expressed relative to the average of non-boosted controls in each experiment). (G) The degree of affinity matured anti-HEL in the serum was determined by coating plates with HEL4X. All plots show mean ± SEM of at least 12 animals combined from multiple experiments.
Discussion
The generation of Tfh cells is crucial to the development of long-lived effector B cells. Dysregulated Tfh cell development and/or function has been associated with both immune deficiencies and autoimmune diseases (King et al., 2008). Consequently, it is important to understand the mechanisms that regulate the differentiation of naïve CD4+ T cells to a Tfh cell fate. Recent studies have identified requirements for CD40-CD40L, ICOS-ICOS-L (Akiba et al., 2005; Bossaller et al., 2006; Nurieva et al., 2008), IL-21 (Nurieva et al., 2008; Vogelzang et al., 2008), IL-6 (Eddahri et al., 2009; Nurieva et al., 2008) and B cells (Haynes et al., 2007; Johnston et al., 2009; Zaretsky et al., 2009) in Tfh cell formation. However, many uncertainties about the differentiation pathway of Tfh cells remain. For instance, the relative roles of DC and B cells in inducing Tfh cell differentiation are unclear, whereas the role of IL-21 is controversial (Bessa et al., 2010; Linterman et al., 2010; Nurieva et al., 2008; Vogelzang et al., 2008; Zotos et al., 2010). Initial activation of T cells occurs on DC within the T cell zone. This interaction is thought to induce the concomitant upregulation of CXCR5 and downregulation of CCR7, which mediates the migration of T cells to the T-B border or follicle (Ansel et al., 1999; Hardtke et al., 2005; Haynes et al., 2007). Indeed it has been shown that entry of CD4+ T cells into the follicle can occur independently of interactions with B cells (Fillatreau and Gray, 2003).
Following this initial activation of CD4+ T cells by DC, however, subsequent interactions with B cells are suggested to provide the requisite signals for Tfh cell development (King et al., 2008). A key role for B cells in inducing Tfh cells is supported by several studies in which Tfh cell numbers were severely diminished in mice where B cells were absent or unable to present Ag to the responding CD4+ T cells (Haynes et al., 2007; Johnston et al., 2009; Zaretsky et al., 2009). Such a two-stage model of Tfh cell development raises the question of what the unique signal is that B cells provide to induce Tfh cell differentiation. For example, it was recently shown that in the absence of ICOS-L expression by B cells Tfh cell numbers were reduced (Nurieva et al., 2008). However, because many other cells - including DC - also express ICOS-L (Aicher et al., 2000), this does not fulfill the role of a unique B-cell specific signal. We also observed a severe defect in Tfh cell development following immunization of mice whose B cells were unable to present Ag. However, Tfh cell development could be rescued when these same mice were boosted with OVA peptide. This demonstrates that B cells do not express a unique set of co-stimulatory molecules or soluble signals that are required for Tfh cell generation. Rather, these data suggest that other APC, namely DC, can drive Tfh cell development autonomously.
We were able to demonstrate that boosting mice with peptide prolonged Ag presentation by DC. Thus, our findings imply that non-B cell APC possess all of the signals required for inducing Tfh cells, but the limiting factor is Ag availability. Tfh cells possess many hallmarks of cells receiving strong TCR stimulation, such as expression of CXCR5, PD1, IL-21 and downregulation of CD127 (Franchimont et al., 2002; Hammerbeck and Mescher, 2008). Recent work has also suggested a role for Ag affinity in Tfh cell generation (Fazilleau et al., 2009). These findings are consistent with the idea that Tfh cell development and/or maintenance requires ongoing Ag stimulation. Hence, the ability of any APC to direct Tfh cell differentiation will be determined by its ongoing capacity to present Ag. In conditions of limiting Ag availability it would be predicted that B cells would have an advantage over other APC in the acquisition of Ag due to the ability of the high affinity BCR to efficiently take up Ag (Macaulay et al., 1997) and their access to Ag depots in the form of immune complexes on FDC (Szakal et al., 1989).
This model also provides an explanation for the range of results observed for Tfh cell development in SAP-deficient mice. Whereas some groups have reported relatively normal Tfh cell development (Kamperschroer et al., 2008; Qi et al., 2008), others observed a substantial numerical defect (Cannons et al., 2010; Linterman et al., 2009). We also observed varying severity in the defect in Tfh cell numbers depending on the immunization strategy used. SAP-deficient CD4+ T cells are unable to form stable conjugates with B cells (Qi et al., 2008). Strikingly, we were able to modulate whether or not Tfh cells developed from SAP-deficient CD4+ T cells by using different immunization strategies that altered the amount of Ag presented by DC. Thus, the spectrum of Tfh cell responses seen in SAP-deficient mice likely reflects the relative ratio of B cell to DC Ag presentation with models that show relatively normal Tfh cells representing situations where APC other than B cells continue to present Ag for extended periods of time. An example of this is the finding of normal frequencies of Tfh cells in SAP-deficient mice infected with influenza virus, a setting that would presumably result in persistent Ag presentation by multiple types of APC (Kamperschroer et al., 2008). It is important to note that regardless of the increased generation of Tfh cells with increased Ag, these SAP-deficient CD4+ cells continued to be unable to support robust B cells responses, as evidenced by a lack of GC and Ag-specific Ab responses (Crotty et al., 2003; Czar et al., 2001; Hron et al., 2004; Ma et al., 2007; Yin et al., 2003), because of their inability to interact with the B cells to provide appropriate helper signals (Qi et al., 2008). This may explain the normal frequencies of circulating CXCR5+ Tfh-like cells in XLP patients despite the inability of these patients to elicit efficient TD Ab responses in vivo and their CD4+ T cells to provide help to B cells in vitro (Ma et al., 2005; Ma et al., 2006).
IL-21 has also been shown to be expressed by Tfh cells (Chtanova et al., 2004; Nurieva et al., 2008) and may have a role in their development (Nurieva et al., 2008; Vogelzang et al., 2008). Interestingly, we showed that a peptide boost was able to not only induce formation of IL-21-producing Tfh cells from SAP-deficient cells but also increase IL-21 production from all responding OT-II cells. Thus, it is conceivable that part of the ability of the peptide boost to rescue Tfh cell development is due to the ability of the increased TCR signaling to increase the amount of IL-21 available to the differentiating cells (Fazilleau et al., 2009).
Regulation of Tfh cell development is critical for generating appropriate B cell responses. Our studies shed further light on the mechanisms involved in their generation. Importantly, they reveal the role of ongoing Ag stimulation, which has particular relevance to situations of chronic Ag exposure, such as autoimmunity, where it has been shown that Tfh cell numbers are increased (Hu et al., 2009; Victoratos and Kollias, 2009; Vinuesa et al., 2005). Further, they reveal the ability of DC to fully drive Tfh cell development from naïve CD4+ T cells even in the absence of T-B cell interactions. Surprisingly, however, we found that increasing Tfh cells was not sufficient to boost Ab production. This may be because the GC response in the model investigated is already “saturated” with Tfh cells and thus further increasing Tfh cell numbers has no discernable effect on the behavior of the B cells. Thus, in cases where it might be desirable to boost Ab responses, such as vaccination, boosting Tfh cell numbers may only be an effective strategy when this component of the response is suboptimal, for example in immunocompromised individuals or immunodeficient conditions where Tfh cell generation is affected.
Experimental Procedures
Mice
OT-II (Barnden et al., 1998), SWHEL (Phan et al., 2003), MHCII−/− (Madsen et al., 1999), Cd40−/− (Kawabe et al., 1994), Sh2d1a−/− (Czar et al., 2001), Cd40lg−/− (Renshaw et al., 1994), Icos−/− (Tafuri et al., 2001) and Igh-6−/− (i.e. µMT) (Kitamura et al., 1991) mice were on C57Bl/6 backgrounds and have been described previously. Mice were bred and housed in specific pathogen-free conditions in the Garvan Institute Biological Testing Facility or Australian BioResources. C57BL/6 and SJL-Ptprca (CD45.1 congenic) C57BL/6 mice were purchased from the Animal Resources Centre or Australian BioResources. Experiments were approved by the Garvan Institute-St. Vincent’s Animal Experimentation Ethics Committee.
OT-II Adoptive transfers
For OT-II experiments spleen cells containing 3 × 104 Va2+CD4+ OT-II T cells were injected i.v. into recipient mice. Recipient mice were also immunized i.p. with 100 µg of OVA (Sigma-Aldrich) in Alum (Pierce) on the day of transfer. For BM chimera experiments CD4+ T cells were negatively selected from OT-II splenocytes using a MACS CD4+ isolation kit (Miltenyi). Some mice also received 10µg of OVA323–339 peptide (Mimotopes) intravenously.
Immunofluorescence histology
Sections (6–7 µm) were cut using a Leica CM1900 cryostat, fixed in acetone, and blocked with 30% normal horse serum. T cells were stained with anti-CD4 FITC, follicular B cells with anti-IgD Alexa Fluor 647, GC with PNA-FITC (Vector Laboratories) and OT-II transgenic T cells were detected with anti-CD45.2 biotin, followed by SA-A555 (Invitrogen). Slides were analyzed with a Zeiss Axiovert 200M microscope and Adobe Photoshop software.
Quantitative PCR
Spleens were taken from mice and stained for sorting. OT-II cells were identified as CD4+ B220− CD45.2+. Three different populations of OT-II cells were sorted based on CD62L and PD1 expression using a FACSAria or FACSVantage (BD). RNA was extracted using Qiagen RNeasy kit and transcribed into cDNA with SuperScript™ III using first strand synthesis protocol by Invitrogen. Expression of Bcl6, Il21 and Sh2d1a was then determined by Real Time PCR using the Roche LightCycler® 480 Probe Master Mix and System. All Real Time PCR primers were from Integrated DNA Technologies and designed using Roche UPL Primer Design Program. All reactions were standardized to the expression of GAPDH.
Bone Marrow chimeras
SJL-Ptprca (CD45.1 congenic) recipients were lethally irradiated (2 doses of 425 rads 4 hours apart) using an x-ray irradiator (General Electric). Mice then received an 80/20 mix of B6/MHCII−/−, Igh-6−/−/MHCII−/−, B6/ Cd40−/− or Igh-6−/−/ Cd40−/− BM cells (6–10 × 106 cells) intravenously. Mice were allowed to reconstitute for at least 8 weeks before experiments were performed. Reconstitution was tested by staining for CD45.1, B220, CD11c, CD40 and MHCII.
Isolation of DC and in vitro Ag presentation assay
Spleens were removed and digested in Collagenase D (Roche) for 30 minutes at 37°C. RBC were removed by centrifugation over Ficoll and cells were stained for CD11c and B220. CD11c+B220+ cells were sorted using a FACSAria or FACSVantage (BD). CD4+ cells were isolated from the spleens of OT-II mice using a MACS CD4+ selection kit (Miltenyi) and were labeled with 5µM CFSE. 4×104 CD11c+ cells were cultured together with 5×104 OT-II cells in 96-round bottom plates. To some cultures 3µM OVA323–339 was added. Three days later cells were harvested, stained for CD4 and run on a flow cytometer.
SWHEL experiments
Production of recombinant HEL proteins and adoptive transfer procedures have been described previously (Paus et al., 2006). For collaborative responses between SWHEL B cells and OT-II T cells, HEL2X was chemically conjugated to OVA323–339 peptide (CGGISQAVHAAHAEINEAGR) using the cross-linking agent succinimidyl-6- [β-maleimidopropionamido] hexanoate (Pierce). A mixture of spleen cells from SWHEL and OT-II mice containing 3 × 104 HEL-binding B cells and 3 × 104 Vα2+ CD4+ OT-II T cells was injected i.v. into recipient mice together with 4–6µg of HEL2X-OVApep conjugate. Mice also received 10µg of OVA emulsified in Alum i.p. To detect HEL-binding cells, splenocytes were stained with saturating amounts of HEL (100 ng/ml), followed by Alexa Fluor 647-conjugated anti-HEL (HyHEL9). To detect affinity-matured SWHEL cells, splenocytes were stained with HEL3X (50ng/ml) followed by HyHEL9 Alexa Fluor 647 as previously described (Phan et al., 2006). Serum levels of affinity-matured antibody were detected by coating plates with HEL WT or HEL4X (which is bound by the HyHEL10 with the Y53D mutation but not unmutated HyHEL10) and detected with an anti-IgG1 (see Supplemental methods).
Supplementary Material
Highlights.
SAP-deficiency impairs Tfh cell development
Lack of Ag-presentation by activated B cells impairs Tfh development
Ag-presentation by DC is short-lived
Prolonging Ag-presentation by DC abolishes the need for B cells in Tfh development
Acknowledgments
We thank the Garvan Flow Facility for cell sorting, T. Chan for the HEL4X, D. Fulcher, B. Gaspar and S. Riminton for patient samples, L. Corcoran for the Bcl-6 antibody and T. Phan for critically reviewing this manuscript. This work was funded by grants and fellowships awarded by the Australian NHMRC to EKD, CSM, DG, RB and SGT.
References
- Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- Akiba H, Takeda K, Kojima Y, Usui Y, Harada N, Yamazaki T, Ma J, Tezuka K, Yagita H, Okumura K. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–2348. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, Anderson DM, Gimpel SD, Davis-Smith T, Maliszewski CR. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Bessa J, Kopf M, Bachmann MF. Cutting Edge: IL-21 and TLR Signaling Regulate Germinal Center Responses in a B Cell-Intrinsic Manner. J Immunol. 2010;184:4615–4619. doi: 10.4049/jimmunol.0903949. [DOI] [PubMed] [Google Scholar]
- Bossaller L, Burger J, Draeger R, Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier M, Welcher AA, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, de Waal Malefyt R, Tangye SG. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahri F, Denanglaire S, Bureau F, Spolski R, Leonard WJ, Leo O, Andris F. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities. Blood. 2009;113:2426–2433. doi: 10.1182/blood-2008-04-154682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med. 2003;197:195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Vacchio MS, Fan S, Visconti R, Frucht DM, Geenen V, Chrousos GP, Ashwell JD, O'Shea JJ. Positive effects of glucocorticoids on T cell function by up-regulation of IL-7 receptor alpha. J Immunol. 2002;168:2212–2218. doi: 10.4049/jimmunol.168.5.2212. [DOI] [PubMed] [Google Scholar]
- Hammerbeck CD, Mescher MF. Antigen controls IL-7R alpha expression levels on CD8 T cells during full activation or tolerance induction. J Immunol. 2008;180:2107–2116. doi: 10.4049/jimmunol.180.4.2107. [DOI] [PubMed] [Google Scholar]
- Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, Peng SL. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200:261–266. doi: 10.1084/jem.20040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y-L, Metz DP, Chung J, Siu G, Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol. 2009;182:1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamperschroer C, Roberts DM, Zhang Y, Weng N-P, Swain SL. SAP enables T cells to help B cells by a mechanism distinct from Th cell programming or CD40 ligand regulation. J Immunol. 2008;181:3994–4003. doi: 10.4049/jimmunol.181.6.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104:1952–1960. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Hare NJ, Nichols KE, Dupre L, Andolfi G, Roncarolo MG, Adelstein S, Hodgkin PD, Tangye SG. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, Nichols KE, Tangye SG. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest. 2006;116:322–333. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunol Cell Biol. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- Macaulay AE, DeKruyff RH, Goodnow CC, Umetsu DT. Antigen-specific B cells preferentially induce CD4+ T cells to produce IL-4. J Immunol. 1997;158:4171–4179. [PubMed] [Google Scholar]
- Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher L-M, Pintilie M, Duncan G, Gajewska BU, Gronski M, Eriksson U, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat Immunol. 2003;4:765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Feng CG, Qi C-F, Cheng J, Sher A, Morse HC, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Amesbury M, Gardam S, Crosbie J, Hasbold J, Hodgkin PD, Basten A, Brink R. B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J Exp Med. 2003;197:845–860. doi: 10.1084/jem.20022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- Renshaw BR, Fanslow WC, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakal AK, Kosco MH, Tew JG. Microanatomy of lymphoid tissue during humoral immune responses: structure function relationships. Annu Rev Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- Victoratos P, Kollias G. Induction of autoantibody-mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity. 2009;30:130–142. doi: 10.1016/j.immuni.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Yin L, Al-Alem U, Liang J, Tong WM, Li C, Badiali M, Medard JJ, Sumegi J, Wang ZQ, Romeo G. Mice deficient in the X-linked lymphoproliferative disease gene sap exhibit increased susceptibility to murine gammaherpesvirus-68 and hypo-gammaglobulinemia. J Med Virol. 2003;71:446–455. doi: 10.1002/jmv.10504. [DOI] [PubMed] [Google Scholar]
- Yu D, Batten M, Mackay C, King C. Lineage specification and heterogeneity of T follicular helper cells. Curr Opin Immunol. 2009a;21:619–625. doi: 10.1016/j.coi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity. 2009b;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.