Abstract
Respiratory epithelial cells are exposed to complex mechanical forces which are often modulated during pathological conditions such as Otitis Media and acute lung injury. The transduction of these mechanical forces into altered inflammatory signaling may play an important role in the persistence of disease conditions and inflammation. In this study, we investigated how static and oscillatory pressures altered the activation of NF-κB inflammatory pathways and how changes in the actin cytoskeleton influenced the mechanotransduction of pressure into NF-κB activation. An in vitro system was used to apply static and oscillatory pressures to alveolar epithelial cells cultured at an air–liquid interface. Latrunculin A and Jasplakinolide were used to alter the cytoskeleton and tight-junction structure and ELISA was used to monitor activation of NF-κB. Results indicate that both static and oscillatory pressures can activate NF-κB and that this activation is magnitude-dependent at low oscillation frequencies only. Jasplakinolide treated cells did not exhibit significant changes in normalized NF-κB activation compared to unloaded controls while Latrunculin treated cells exhibited increases in normalized NF-κB activation only at low frequency or static pressures. These results indicate that altering the actin cytoskeleton may be a useful way to mitigate the mechanotransduction of pressure forces into inflammatory signaling.
Keywords: Mechanotransduction, Otitis Media, Acute lung injury, Ventilation induced lung injury, Inflammation, Compressive stress, Calcium signaling, IκBα
INTRODUCTION
Epithelial cells in the respiratory system are exposed to a wide-variety of complex mechanical forces and these forces are often modulated during disease conditions. For example, epithelial cells lining the middle ear (ME) cavity are normally exposed to static ambient pressures.4 However, during acute ME infections, i.e. Otitis Media, ME epithelial cells are exposed to large sub-ambient pressures as well as periodic and rapid changes in ME pressure.14 Bronchial epithelial cells in the lung are normally exposed to low levels of shear and pressure due to airflow. However, during asthma, buckling of the airway wall exposes the epithelial cells to complex compressive, tensile and shear stresses.36 Experimental studies indicate that the compressive stress experienced by epithelial cells during broncho-constriction causes the most damage.30,37 Finally, alveolar epithelial cells are normally exposed to low levels of cyclic strain and oscillatory pressure during tidal ventilation. However, during acute lung injury and artificial ventilation, these cells may be exposed to abnormally large cyclic strains40 and complex surface tension forces due to the cyclic closure and reopening of fluid-filled airways/alveoli.16 Experimental and computational studies indicate that the normal pressure gradient generated during airway reopening is responsible for cell necrosis and detachment in this system.3,21,44
In addition to physical injury, several investigators have demonstrated that mechanical forces can be transduced into altered biochemical signaling events and/or altered cell behaviors.35 For example, cyclic stretching of pulmonary epithelial cells results in altered cell migration rates,13 altered surfactant secretion profiles,2 changes in protein and gene expression,7 the up-regulation of inflammatory pathways29 and the secretion of inflammatory cytokines.20,41 In addition to stretching forces, exposing pulmonary or respiratory epithelial cells to fluid shear stress results in disassembly of keratin intermediate filaments,31 increased mucus secretion15 and reduced paracellular permeability. 32 Several previous studies have also investigated the response of epithelial cells to transmural pressure forces. Exposure of tracheal epithelial cells to static pressures results in a time and magnitude dependent increase in early growth response-1 (EGR1) and transforming growth factor-β1 (TGFβ1) gene expression.30 Tschumperlin and colleagues37,38 demonstrated that static transmembrane pressures can up-regulate pro-fibrotic pathways and activate the epidermal growth factor receptor (EGFR).39 However, there is limited information about how alveolar epithelial cells respond to static and oscillatory transmural pressure.
The development and maintenance of an inflammatory state plays a key role in several respiratory disorders. For example, several pro-inflammatory cytokines have been implicated as critical molecular regulators which are responsible for chronic inflammation in the ME and the development of Otitis Media.33 In addition, the inflammatory response of alveolar epithelial cells to pathologic stretching deformations during mechanical ventilation has been recognized as a key factor in the development of ventilation-induced lung injury.25 In particular, cyclic stretching of lung epithelial cells results in the production of pro-inflammatory cytokines IL-8 and IL-620,41 and the nuclear translocation of NF-κB,7 a “rapid-acting” primary transcription factor for a large number of inflammatory genes.26 The role of stretch-induced inflammation in the lung has also been demonstrated in in vivo.8 However, there is limited information about how exposure to static or oscillatory compressive forces (i.e. pressure), as opposed to strain (i.e. stretching), influences the activation of NF-κB inflammatory pathways in alveolar epithelial cells.
Although the use of low volume mechanical ventilation can be used to minimize the stretching deformations that cause cell necrosis and activate inflammatory pathways,43 it is difficult to eliminate all of the pathological mechanical forces generated during ventilation (i.e. pressure and shear stress). An alternative way to prevent injury is to modify the way epithelial cells respond to the damaging mechanical stimuli. For example, Yalcin et al.45 demonstrated that changes in cytoskeletal structure may be useful in preventing cell necrosis and detachment during airway reopening. Changes in cytoskeletal structure may also be useful in modifying the mechanotransduction mechanisms by which cells sense and transduce forces into inflammatory signals. Mechanical forces applied at the apical surface of cells may transmitted along the cytoskeleton to various signaling sites including focal adhesions,18 cell–cell junctions27 and stretch-sensitive ion channels.1 In addition, force-transmission along the cytoskeleton may be sensed and transduced into biochemical signals by proteins directly associated with cytoskeletal filaments.17 Finally, changes in cytoskeletal structure will result in concurrent changes in cell rheology23 which may in-turn influence the amount of intracellular strain/deformation for a given amount of mechanical force.11,45 Although the cytoskeleton has been implicated in mechanotransduction, there is limited information about how changes in actin cytoskeletal structure influences the transduction of static and oscillatory pressure into the activation of NF-κB inflammatory pathways in alveolar epithelial cells.
The goal of this study is to test two specific hypotheses: (1) Application of static and oscillatory pressure to alveolar epithelial cells activates NF-κB inflammatory pathways in a frequency and magnitude dependent fashion and (2) Alterations in the actin cytoskeleton can be used to modulate the mechanotransduction of pressure forces into NF-κB activation. To test these hypotheses, we used an in vitro cell culture system to expose epithelial cells to static and oscillatory pressures, cytoskeletal agents (Latrunculin A and Jasplakinolide) to alter the cytoskeleton and enzyme-linked immunosorbent assay (ELISA) kit to monitor the level of activated NF-κB in the nucleus.
MATERIALS AND METHODS
Cell Culture
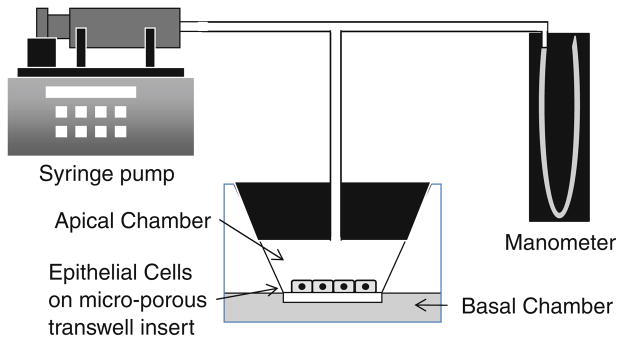
Human A549 alveolar epithelial cells (CCL-185, American Type Culture Collection, Manassas, VA) were maintained in Ham’s F12 K medium supplemented with 10% fetal bovine serum (FBS) and a 1% antibiotic and antifungal solution (100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B, Invitrogen, Carlsbad, CA). A549 cells were incubated at 37 °C in a humidified atmosphere of 5% CO2. To obtain monolayers of polarized cells with functional tight-junctions, A549 cells were seeded onto Transwell inserts (23 mm diameter, polyester membrane, 0.4 μm pore size, Costar, Corning, NY) in 6-well tissue culture plate at a density of 2 × 105 cells/ insert. As shown in Fig. 1, cells were grown with 1.5 mL cell culture medium in the upper (apical) chamber and 2.5 mL in the lower (basal) chamber. Medium in the upper compartment was removed prior to the application of pressure.
FIGURE 1.
Schematic diagram of in vitro system used to apply static and oscillatory pressure to A549 epithelial cells.
Immunofluorescence Microscopy
To visualize actin filaments, cells were fixed in formalin for 10 min and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Cells were then incubated with Alexa 488-labeled phalloidin (Invitrogen) for 20 min at room temperature. For Jasplakinolide treated cells, actin staining by phalloidin was weak due to the competition of phalloidin and Jasplakinolide for the same binding site on the actin filament.24 Therefore, cells treated with Jasplakinolide were fixed in −20°C methanol and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Cells were then incubated with a mouse anti-actin antibody (Sigma–Aldrich, St. Louis, MO) for 1 h at room temperature and incubated with a goat anti-mouse Alexa Fluor 567 antibody. Cell nuclei were counter stained with DAPI (Sigma–Aldrich, St. Louis, MO) (0.1 μg/mL) for 5 min.
To visualize tight-junction proteins ZO-1 and occludin, cells were fixed and permeabilized in 1:1 (vol) methanol:actone solution at −20 °C for 6 min and then double stained with primary antibodies (anti-ZO-1 and anti-occludin) followed by secondary antibodies (goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 567, Invitrogen). The inset membranes were then mounted and sealed on glass slides before being visualized on an IX-81 inverted epifluorescence microscope (Olympus).
Application of Static and Oscillatory Pressure
To expose cells to transcellular pressure, fitted stoppers were plugged tightly to the top of the Transwell inserts, forming a hermetically sealed pressure chamber in the apical compartment (see Fig. 1). Access ports in the plugs were connected to a manometer and a gas tight syringe attached to a PHD2000 programmable syringe pump (Harvard Apparatus, Holliston, MA). For static pressure experiments, the apical compartment was pressurized to positive or negative 14 cmH2O which was maintained for 1 h. Note that air was used in all experiments to pressurize the chamber. For oscillatory pressure experiments, the syringe pump was programmed to execute repeated infusion and withdrawal at a constant flow rate for a given amount of time per cycle. This produced a triangular pressure waveform that varied from positive to negative pressure relative to ambient conditions. In this study, we used waveforms with pressure magnitudes of ±10, ±12 and ±14 cmH2O and two separate oscillation frequencies, 0.125 and 0.18 Hz. These pressure magnitudes are similar to the mean airway pressures used during mechanical ventilation5 and the frequencies are based on normal breathing rates (~12 breaths/min) and hypo-ventilation conditions. All oscillatory pressures were applied for a total duration of ½ h and for these studies unloaded cells were used as controls.
Cytoskeletal Agents and Cell Viability Assay
To investigate how changes in the actin cytoskeleton influence the mechanotransduction of static and oscillatory pressure into NF-κB signaling, cells were pretreated with specific cytoskeletal agents, Latrunculin A and Jasplakinolide. Latrunculin A typically results in the de-polymerization of actin filaments while Jasplakinolide can either stabilize actin filaments at low concentrations or increase actin mass in the perinuclear region at higher concentrations. One hour prior to pressure exposure, cells were pretreated with 0.5 μM Latrunculin A (Biomol, Plymouth Meeting, PA) or 0.5 μM Jasplakinolide (Invitrogen). To investigate the effect of these drugs alone on NF-κB activation, non-treated samples were used as controls. In addition, for pressure loading experiments, unloaded, drug-treated samples were used as controls to evaluate the relative increase or decrease in NF-κB activation due to changes in mechanotransduction via the actin cytoskeleton.
After exposure to static or oscillatory pressure, cell viability was assessed with a LIVE/DEAD Viability Assay Kit (Invitrogen). Briefly, cells were incubated in 2 μM calcein-AM to label live cells and 4 μM EthD-1 to label dead cells for 30 min at room temperature after washing with PBS. Samples were visualized via fluorescence microscopy (Olympus, IX81) and live/dead cell numbers were counted in six 10× images. The percentage of cell death was determined as the number of dead cells divided by the total number of cells in each image.
Quantification of Actin Staining
To quantify that amount of actin stating, A549 cells were cultured on cell culture inserts until they were confluent. They were treated with 0.5 μM Latrunculin A or Jasplakinolide for 1 h as described above. For control cells and Latrunculin A treated cells, actin was labeled with phalloidin. However, due to the weak phalloidin signal in Jasplakinolide cells, control cells and Jasplakinolide treated cells were incubated with an actin antibody as mentioned above. After labeling actin, the cell culture inserts were cut into 6 mm circles and transferred onto a black plate. Fluorescence intensity was determined with a plate reader (BioTek Synergy HT) with excitation at 590 nm and emission at 645 nm.
Quantification of Paracellular Permeability
Trans-epithelial transport experiments were conducted to quantify paracellular permeability. A solution containing 1 mg/mL FITC-labeled dextran (molecular weight 4 kDa) was placed in the apical chamber of the transwell insert. 100 μL Samples were withdrawn from the lower compartment after 1 h and fluorescence intensity was determined with a spectrofluorimeter (BioTek Synergy HT) with excitation at 485 nm and emission at 538 nm. An apparent permeability coefficient (Papp) was calculated according to the following equation: Papp = ΔQ/(A × Δt × C0), where ΔQ is the change in concentration, A is the surface area of the monolayer, Δt is the time, and C0 is the initial concentration in the upper chamber. Note that ΔQ was determined using a calibration curve that relates fluorescent intensity with concentration.
ELISA for Activated NF-κB/p65
The commercially available Trans-AM kit (Active Motif, Carlsbad, CA) was used to detect and quantify NF-κB/p65 by ELISA. This system uses an oligonucleotide containing the NF-κB consensus binding site (5′-GGGACTTTCC-3′) that binds specifically to activated NF-κB in the nuclear extract. A NF-κB p65 antibody was used to detect the p65 subunit activation. The absorbance of the final solutions was read on a plate reader (Biotek Synergy HT) within 5 min at 450 nm with a reference wavelength of 655 nm. NF-κB activation was normalized by folds when compared with control cells. After treating A549 cells with cytoskeletal agents or applying static/oscillatory pressure, nuclear or cytoplasmic proteins were obtained by washing cells twice with PBS and incubating cells in 0.15 mL ice-cold lysis buffer [HEPES (10 mM, pH 7.9), KCl (10 mM), EDTA (0.1 mM), EGTA (0.1 mM), DTT (1 mM), PMSF (0.5 mM)] with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Sigma, USA) for 15 min. 10 μL of 10% of Igepal CA-630 was added to lyse the cells and cells were then scraped, vortexed at a high speed and centrifuged for 3 min (15,000×g) at 4 °C. The nuclear pellet was re-suspended in 25 μL of ice-cold nuclear extraction buffer [HEPES (20 mM, pH 7.9), NaCl (0.4 M), EDTA (1 mM), DTT (1 mM), PMSF (0.5 mM), 10% glycerol] with freshly added protease inhibitor cocktail. After vigorous votexing, lysates were placed on a shaker for 40 min with ice. The tubes were then centrifuged for 10 min (15,000×g) at 4 °C, and the supernatant was used as the nuclear fraction and stored at −80 °C. Protein concentration was determined by the BCA Protein assay kit obtained from Pierce.
Western Blot Analysis
Nuclear or cytoplasmic fractions (20 μg) were resolved by electrophoresis on 8% sodium dodecyl sulfate (SDS)-polyacrylamide gel and electrotransferred to a nitrocellulose membrane. After blocking with 5% milk in TBST (0.1% Tween-20) for 1 h, membranes were probed with IκBα and NF-κB (p65) antibodies (Cell signaling, USA) at 4 °C overnight. The membranes were then incubated with horseradish peroxidase conjugated goat anti-rabbit or anti-mouse antibodies (Biorad) for 1 h at room temperature and washed/incubated with the enhanced chemiluminescence system (ECL, Pierce).
Calcium Signaling Studies
To determine if calcium is an important mediator in pressure induced NFκB activation, 30 μM of the intracellular calcium chelator 1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) (EMD) or 1 mM of the extracellular calcium chelator EGTA (ethylene glycol tetraacetic acid) (Sigma) was added to the medium 30 min prior to static pressure exposure of +14 cmH2O.
Statistical Analysis
Univariate analysis of variance (ANOVA) followed by post hoc least significant difference (LSD) was used to document statistical differences in NF-κB activation levels. All data was plotted as the mean fold difference relative to unloaded or untreated controls ± the 95% confidence level. The significance level was set at p < 0.05 and all F-values (i.e. the ANOVA F-statistic) were reported at the p = 0.05 level.
RESULTS
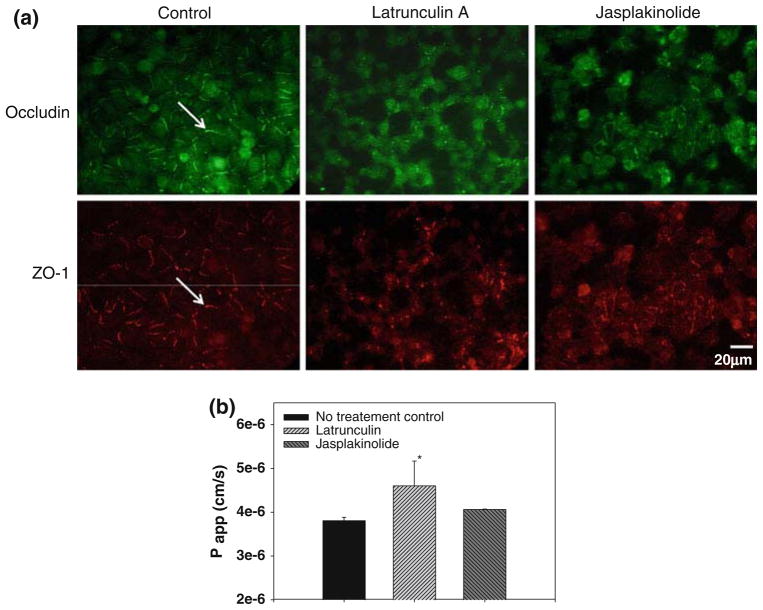
Latrunculin A and Jasplakinolide had distinct effects on the actin cytoskeleton as shown in Fig. 2a. Confluent monolayers of untreated control cells exhibited actin stress fibers as well as peripheral distribution of actin. Treatment with Latrunculin resulted in a loss of stress fibers and actin de-polymerization. This de-polymerization was confirmed by the reduction in fluorescent intensity shown in Fig. 2b (p = 0.06). Conversely, Jasplakinolide treated cells did not exhibit a significant change in actin fluorescent intensity. However, Jasplakinolide treated cells did exhibit a redistribution of actin to the perinuclear region. Although pressure-loading experiments in this study utilized confluent conditions, actin stating for sub-confluent conditions is also shown in Fig. 2a to clearly depict the effects of Latrunculin and Jasplakinolide on the actin cytoskeleton.
FIGURE 2.
(a) Influence of Latrunculin A and Jasplakinolide on the actin cytoskeleton in polarized epithelial cells grown on transwell inserts. Note: Phallodin staining techniques were used to label actin in Latrunculin cells while an anti-actin antibody technique was used to label actin in Jasplakinolide cells. Cell nuclei were counter-stained with DAPI. (b) Quantification of changes in actin fluorescent intensity for the confluent cells during Latrunculin or Jasplakinolide treatment. Data are mean ± 95% confidence interval (n = 6 per group).
Latrunculin A and Jasplakinolide also had significant effects on tight junction structure/organization as shown in Fig. 3a. Untreated cells grown on the transwell insert exhibit staining of the ZO-1 and occludin tight-junction proteins at the cell periphery. Untreated cells also exhibited paracellular resistance as assessed by the permeability of FITC labeled dextran, indicating the presence of functional tight-junctions (see Fig. 3b). Treatment with Latrunculin A resulted in a significant loss of peripheral ZO-1 and occludin staining and also resulted in a statistically significant increase in paracellular permeability (p < 0.05). Jasplakinolide treatment also resulted in altered tight junction organization but did not result in a significant change in paracellular permeability.
FIGURE 3.
(a) Cells grown on transwell inserts exhibit tight junction staining for occludin and ZO-1 (arrows) which was not observed in cells treated with Latrunculin A or Jasplakinolide. (b) Measurements of paracellular permeability indicate significant tight-junction disruption in Latrunculin treated cells. Statistically significant differences with no treatment control is indicated by * p < 0.05 and data are mean ± 95% confidence interval (n = 3 per group).
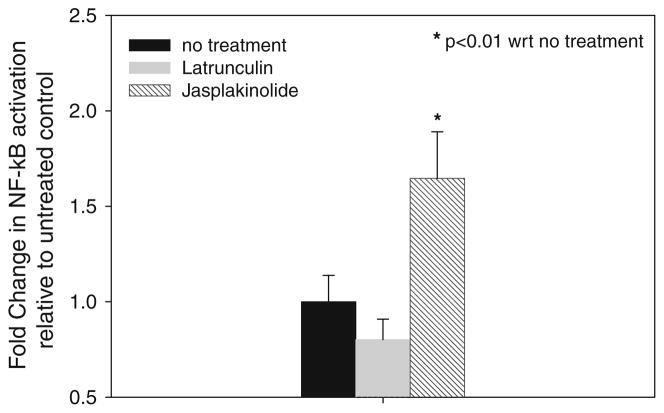
The influence of Latrunculin A or Jasplakinolide on the amount of NF-κB activation in the absence of an applied pressure load is shown in Fig. 4. ANOVA indicated that the mean levels of activation were significantly different (F = 27.1) and post hoc LSD indicated that the mean level of NF-κB activation in Jasplakinolide treated cells was significantly different than untreated controls (p < 0.01). Although Jasplakinolide caused a significant increase in NF-κB activation, Latrunculin treatment resulted in a small decrease in activation that was not statistically different from untreated controls (p = 0.203).
FIGURE 4.
Effect of Latrunculin and Jasplakinolide treatment alone on NF-κB activation. Data are mean ± 95% confidence interval (n = 5 per group). * Significant difference compared to untreated control (p < 0.01).
Prior to assessing NF-κB activation levels during static/oscillatory loading, cellular viability was assessed for both static and oscillatory pressure conditions. For static pressures the maximum amount of mean cellular death was 1.8% and was observed at −14 cmH2O. For oscillatory pressures, the maximum amount of mean cellular death was 3.6% and was observed at ±14 cmH2O and a frequency of 0.18 Hz.
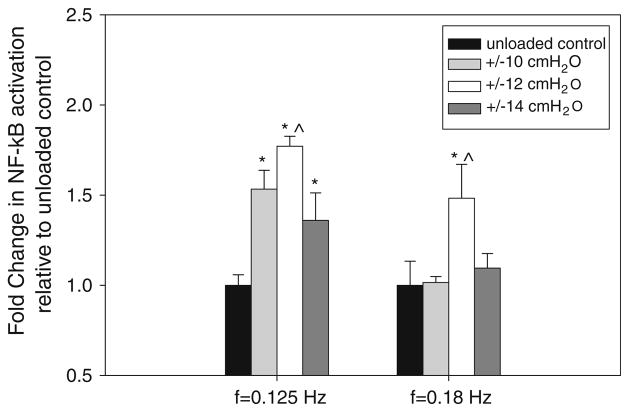
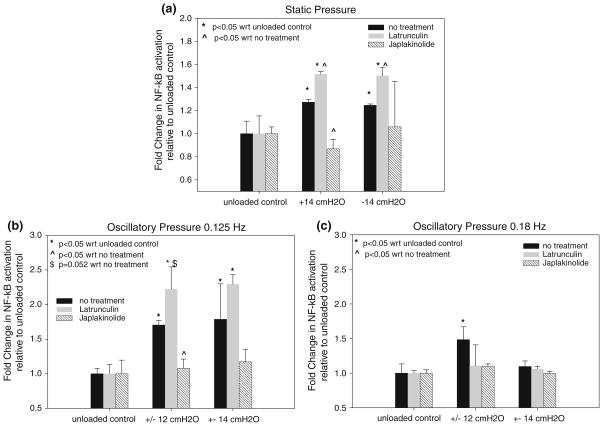
The application of oscillatory pressure to untreated epithelial cells at different frequencies and pressure magnitudes resulted in differential activation of NF-κB as shown in Fig. 5. Data are mean fold difference relative to unloaded controls. At a frequency of 0.125 Hz, ANOVA indicated that the amount of NF-κB activation depends on the pressure magnitude (F = 40) and post hoc LSD indicated that the mean level of activation at the ±10, ±12 and ±14 cmH2O pressure magnitudes were all significantly different than the amount of activation in unloaded controls (p < 0.01). In addition, there was more NF-κB activation at the ±12 cmH2O pressure level compared to the ±10 and ±14 cmH2O pressure levels (p < 0.05). At a frequency of 0.18 Hz, ANOVA indicated that differences between mean activation levels exist (F = 11.7) but post hoc LSD indicated that the only significant difference was a higher mean activation at ±12 cmH2O pressure level compared to activation in unloaded controls (p < 0.01) or activation at the ±10 cmH2O or ±14 cmH2O pressure levels (p < 0.05).
FIGURE 5.
Effect of oscillatory pressure on NF-κB activation at different frequencies and pressure magnitudes. Data are mean ± 95% confidence interval (n = 4 per group) and represent fold change in activation with respect to unloaded control in each group. Statistically significant differences with unloaded controls in each frequency group are indicated by * p <0.01. ^ Statistically significant differences (p <0.05) between activation at ±12 cmH2O and activation at ±10 cmH2O or ±14 cmH2O pressure levels in each frequency group.
The effect of +14 and −14 cmH2O static pressure on NF-κB activation in untreated, Latrunculin A treated and Jasplakinolide treated cells is shown in Fig. 6a. Note that all data are the mean fold difference relative to the unloaded control within each treatment group. For untreated cells, ANOVA indicated that the application of +14 cmH2O or −14 cmH2O static pressure results in a statistically significant increase in NF-κB activation compared to unloaded controls (F = 20.9, p < 0.05). For Latrunculin A treated cells, +14 and −14 cmH2O pressure also resulted in a statistically significant increase in NF-κB activation compared to unloaded controls (F = 33.9, p < 0.05). In contrast, there was no statistically significant difference in NF-κB activation in the Jasplakinolide-treated cells compared with unloaded controls (F = 0.664). At the +14 cmH2O pressure level, there was a statistically significant difference in mean NF-κB activation between treatment groups (F = 160). Post hoc LSD indicates that Latrunculin treated cells have significantly more activation at +14 cmH2O than untreated cells at the same pressure (p < 0.05) while Jasplakinolide treated cells exhibited less activation than untreated cells (p < 0.05). Finally, at the −14 cmH2O pressure level, the mean level of NF-κB activation in the Latrunculin treated cells was higher than the activation in untreated cells (p < 0.05).
FIGURE 6.
(a) Effect of static pressure and cytoskeletal agents on NF-κB activation. (b) Effect of cytoskeletal agents on NF-κB activation at an oscillation frequency of 0.125 Hz. (c) Effect of cytoskeletal agents on NF-κB activation at an oscillation frequency of 0.18 Hz. All data are mean ± 95% confidence interval (n = 2–4 per group) and represent fold change in activation with respect to unloaded controls in each treatment group. * Statistically significant differences with unloaded controls (p < 0.05). ^ Statistically significant differences with activation in the no treatment group (p < 0.05).
The effect of 0.125 Hz oscillatory pressure on NF-κB activation in untreated, Latrunculin A treated and Jasplakinolide treated cells is shown in Fig. 6b. For these studies oscillatory pressure magnitudes of ±12 and ±14 cmH2O were utilized. For Latrunculin A treated cells, ±12 and ±14 cmH2O oscillatory pressures resulted in a statistically significant increase in NF-κB activation compared to unloaded controls (F = 41.3, p < 0.01) and post hoc LSD indicated no significant difference in activation between the ±12 and ±14 cmH2O conditions (p = 0.79). In contrast, there was no statistically significant difference in NF-κB activation in the Jasplakinolide-treated cells compared with unloaded controls (F = 0.97). Additionally, ANOVA indicated that significant differences between treatment groups at the ±12 cmH2O pressure level exists (F = 31.5). Post hoc LSD indicated that the lower level of activation in Jasplakinolide treated cells compared to untreated cells was statistically significant (p < 0.05) while the higher level of activation in Latrunculin treated cells compared to untreated cells was nearly significant (p = 0.052).
The effect of 0.18 Hz oscillatory pressure on NF-κB activation in untreated, Latrunculin A treated and Jasplakinolide treated cells is shown in Fig. 6c. For these studies oscillatory pressure magnitudes of ±12 and ±14 cmH2O were utilized. Unlike the data at lower frequency (Fig. 6b), ANOVA indicates that at a higher frequency of 0.18 Hz there was no significant difference in NF-κB activation within the Latrunculin group (F = 0.77) or within the Jasplakinolide group (F = 4.8). In addition, ANOVA indicates that there were no significant differences between the treatment groups at the ±12 cmH2O or ±14 cmH2O pressure level (F = 4.6). The only statistically significant change in NF-κB activation at 0.18 Hz was in untreated cells between the unloaded and the ±12 cmH2O condition (p < 0.05).
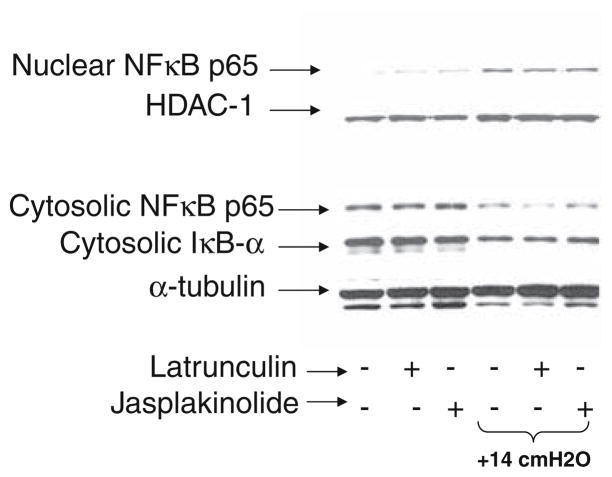
Western blots of both the nuclear and cytoplasmic fractions of NF-κB and the cytoplasmic amount of the inhibitory protein IκBα were obtained as shown in Fig. 7. Data was obtained for control cells and cells exposed +14 cmH2O static pressure and for untreated, Latrunculin treated and Jasplakinolide treated conditions. Application of +14 cmH2O for all treatment conditions resulted in a reduction in cytoplasmic NF-κB, a reduction in IκBα and an increase in nuclear NF-κB. This data indicates that pressure activates NFκB via the canonical pathway in which degradation of IκBα allows for the nuclear translocation of NF-κB.
FIGURE 7.
Western blots of key proteins associated with the canonical NF-κB pathway.
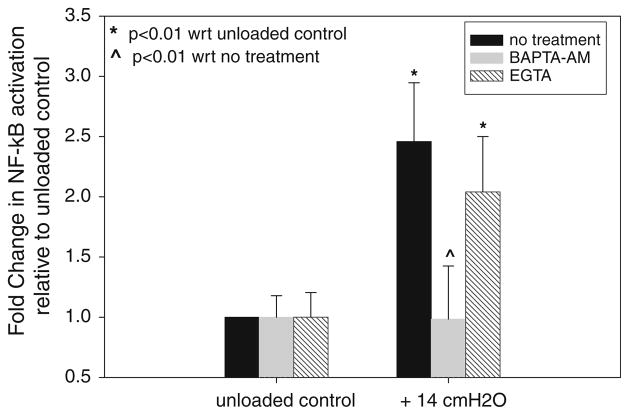
The effect of extracellular and intracellular calcium chelators (EGTA and BAPTA-AM, respectively) on pressure-induced activation of NF-κB is shown in Fig. 8. Data was obtained for unloaded cells and at the +14 cmH2O static pressure level and all data are reported relative to unloaded controls. ANOVA indicates that untreated cells and cells treated with EGTA exhibit statistically significant increases in NF-κB activation in response to +14 cmH2O pressure (p < 0.01). In contrast, cells treated with BAPTA-AM and exposed to +14 cmH2O pressure did not exhibit any statistically significant change in NF-κB activation relative to unloaded controls (p = 0.951). Finally, the amount of NF-κB activation for BAPTA-AM treated cells at +14 cmH2O pressure was statistically lower than the amount of activation in untreated and EGTA treated cells exposed to the same pressure (p < 0.01). These results indicate that intracellular Ca2+ release, not the influx of Ca2+ through the plasma membrane, is important for NF-κB activation.
FIGURE 8.
Influence of intracellular (BAPTA-AM) and extracellular (EGTA) calcium chealators on pressure induced NF-κB activation. All data are mean ± 95% confidence interval (n = 2 per group) and represent fold change in activation with respect to unloaded controls in each treatment group. * Statistically significant differences with unloaded controls (p < 0.01). ^ Statistically significant differences with activation in the no treatment group (p < 0.01).
DISCUSSION
The motivation for this study is that several respiratory disorders, i.e. Otitis Media and acute lung injury, are characterized by prolonged inflammation and subsequent tissue and organ damage.4,43 Although the initial inflammatory response is generally due to bacterial/viral toxins and/or other chemical irritants, the transduction of mechanical forces into additional inflammatory signaling may play a key role in exacerbating the existing injury. Although it is well established that exposing respiratory epithelial cells to transmural pressures activates fibrotic pathways35,38 and that over-distension of epithelial cells activates inflammatory pathways,29,41 little information is available on how alveolar epithelial cells sense and transduce transmural pressures into inflammatory signals. Therefore, the goal of this study was to determine how static and dynamic pressures are sensed by the epithelium and transduced into altered inflammatory signaling. We used an in vitro cell culture system to test the hypothesis that exposing alveolar epithelial cells to static and dynamic pressure can activate the NF-κB transcription factor which is known to regulate a variety of inflammatory pathways. We also tested the hypothesis that alterations in the actin cytoskeleton can be used to modulate the mechanotransduction of pressure forces into NF-κB activation.
Figures 5 and 6 demonstrate that applying oscillatory or static pressures to the apical side of polarized alveolar epithelial cells activates NF-κB. For static pressure experiments, positive and negative 14 cmH2O pressure, relative to atmospheric conditions, activates NF-κB to the same level (Fig. 6a). Note that in this study we applied transmural pressures since the basal compartment was exposed to atmospheric pressure. Additional experiments indicate that changes in the magnitude of static pressure did not significantly influence the amount of NF-κB activation (data not shown). In contrast, when applying oscillatory pressures at a frequency of 0.125 Hz, the amount of NF-κB activation was magnitude dependent with maximum activation occurring at ±12 cmH2O (Fig. 5). In addition, at a frequency of 0.18 Hz increased activation was observed only at ±12 cmH2O. We note that in this study we applied temporal gradients in pressure to the alveolar epithelial cells while previous studies have focused on the damaging effects of spatial gradients in pressure applied to the epithelium during airway reopening.3,44 In order to provide a comparative analysis, the spatial pressure gradients (dp/dx) reported in previous studies can be converted into a temporal gradient by multiplying by the reopening velocity (dp/dt = dp/dx × U). For example, Bilek et al.3 reported significant cell necrosis at a pressure gradient of dp/dx = 7 dyn/cm2/μm and reopening velocity of U = 2.7 mm/s. This translates into a temporal pressure gradient of 19 cmH2O/s which is larger than the temporal pressure gradients used in this study where dp/dt = 5 cmH2O/s for the ±10 cmH2O, 0.125 Hz condition and dp/dt = 10.1 cmH2O/s for the ±14 cmH2O, 0.18 Hz condition. However, Bilek et al.3 also reported minimal cell necrosis at a pressure gradient of dp/dx = 1.8 dyn/cm2/μm and a reopening velocity of U = 2.7 mm/s. This translates into a temporal pressure gradient of 4.9 cmH2O/s which is comparable to the temporal pressure gradients used in this study. These calculations indicate that although the oscillatory pressure magnitudes used in this study are not large enough to cause significant cell necrosis, as confirmed by direct measurements of cell death in this study, they are large enough to be sensed by the epithelial cells and transduced into NF-κB activation and inflammatory signaling.
Given the important role of the actin cytoskeleton in mechanotransduction,1 we were interested in how changes in the actin cytoskeleton during Latrunculin A and Jasplakinolide treatment influence the transduction of static and oscillatory pressure into NF-κB activation. We first evaluated how treatment with Latrunculin A or Jasplakinolide alone influenced the cell’s cytoskeletal structure (Fig. 2), tight-junction structure/organization (Fig. 3) and activation of NF-κB (Fig. 4). In this study, polarized epithelial cells grown on transwell inserts had functional tight junctions (Fig. 3a) and paracellular resistance to molecular diffusion (Fig. 3b). In addition, untreated cells contained actin stress fibers and a peripheral distribution of actin (Fig. 2a). Latrunculin A is a marine toxin which inhibits the polymerization of actin subunits (G-actin) into filamentous form (F-actin) and this promotes the depolymerization of F-actin.34 Cells treated with Latrunculin A exhibited a disrupted cytoskeleton and loss of actin mass (Fig. 2b), thus confirming the expected de-polymerization effects. In addition, treatment with Latrunculin A resulted in a loss of tight junction organization (Fig. 3a) and an increase in paracellular permeability (Fig. 3b). However, these changes in the cell’s cytoskeletal structure and cell–cell adhesions only resulted in a small decrease in NF-κB activation that was not statistically significant (Fig. 4). We note that these results in alveolar epithelial cells are in contrast to a minor increase in NF-κB activation observed in monocytes treated with 0.5 μM Latrunculin B.22 Jasplakinolide is a toxin which can either promote polymerization of purified actin and stabilize actin filaments at moderate concentrations or result in an increase in actin mass in the perinuclear region at higher concentrations.34 For the conditions used in this study, Jasplakinolide treatment did not result in a significant change in actin mass (Fig. 2b) but did result in a redistribution of actin to the perinuclear region (Fig. 2a). We note that this type of change in the actin cytoskeleton during treatment with 0.5 μM Jasplakinolide has also been reported in kidney epithelial cells.34 Treatment with 0.5 μM Jasplakinolide also resulted in a loss of tight junction organization (Fig. 3a) but was not accompanied by a change in paracellular permeability (Fig. 3b). These changes in the cell’s cytoskeletal structure and cell–cell adhesions resulted in a statistically significant increase in NF-κB activation (Fig. 4). Similar increases in NF-κB activation during Jasplakinolide treatment were also observed in monocytes.22
Since the cytoskeletal drugs themselves had either minor or significant effects on NF-κB activation, we normalized all data obtained during static and oscillatory pressure loading in the Latrunculin A treated and Jasplakinolide treated groups with the amount of activation in an unloaded but drug treated control sample. Therefore, increases or decreases in normalized activation indicate that the changes in actin cytoskeletal structure either enhanced or mitigated the mechanotransduction of pressure into NF-κB activation. We note that previous studies also implemented similar normalization procedures to investigate the effect of cytoskeletal agents on mechanotransduction processes.28
For static pressure, Latrunculin A treated cells exhibited significantly more normalized NF-κB activation at the +14 and −14 cmH2O pressure level compared to unloaded controls (Fig. 6a). In contrast, Jasplakinolide-treated cells did not exhibit any statistically significant change in normalized NF-κB activation compared to unloaded controls. In addition, at the +14 cmH2O pressure level, Jasplakinolide-treated cells exhibited less normalized NF-κB activation than untreated cells while Latrunculin-treated cells exhibited more activation. Previous studies23,45 indicate that depolymerization of actin during Latrunculin treatment results in a less rigid cell with a lower elastic modulus while Jasplakinolide treatment can result in a stiffer cell with a larger elastic modulus. Thus, for the same level of static pressure, Latrunculin treated cells would be expected to deform more than untreated cells while Jasplakinolide cells would deform less. These differences in the amount of cell deformation would be consistent with the hypothesis that the amount of mechanotransduction is directly related to the amount of cell deformation. Specifically, more cellular deformation in the less rigid Latrunculin treated cells leads to more NF-κB activation while less deformation in the more rigid Jasplakinolide treated cells leads to no additional NF-κB activation. We note that since Latrunculin treated cells exhibited disrupted tight junctions (Fig. 3), it is not likely that cellular deformation is “sensed” by these cell–cell adhesion molecules in this system. However, cell deformation could result in the release of important signaling molecules and we have therefore investigated the potential role of calcium signaling in pressure-induced NF-κB activation.
Previous studies indicate that the release of calcium from intracellular stores is important in fluid-shear stress induced NF-κB activation in osteoblasts6 and nickel-induced inflammation in A549 epithelial cells.10 In contrast, other studies indicate that stretch-induced activation of inflammation in epithelial cells is dependent on the influx of extracellular calcium.9 As shown Fig. 8, we have used extracellular and intracellular calcium chelators (EGTA and BAPTA-AM, respectively) to determine if pressure-induced activation of NF-κB in A549 cells depends on extracellular calcium, intracellular calcium or both. Treatment with BAPTA resulted in a complete block of the pressure-induced activation of NF-κB while treatment with EGTA resulted in no statistically significant change in pressure- induced NFκB activation. As a result, we conclude that the release of calcium into the intracellular space is important in pressure-induced NFκB activation but that this activation is not dependent on the influx of extracellular calcium into the cell. This implies that pressure loading does not open or activate stretch-induced or other types of ion channels in the plasma membrane but rather stimulates the release of calcium from intracellular stores such as the smooth endoplasmic reticulum.
In addition to calcium signaling, we used western blotting to further identify the mechanisms by which pressure induces NF-κB activation. NF-κB activation may occur via a canonical pathway in which degradation of the IκBα inhibitory protein, which normally binds to and sequesters NFκB in the cytoplasm, allows for the nuclear translocation of NFκB or by a non-canonical pathway which does not depend on IκBα degradation. As shown in Fig. 7, application of +14 cmH2O static pressure resulted in IκBα degradation and NF-κB nuclear translocation for all treatment conditions. For untreated and Latrunculin treated cells, NFκB in the nucleus also binds to DNA as indicated by the Trans-AM assay data reported in Fig. 6a. It is interesting to note that treatment with Japlakinolide alone (i.e. no pressure stimulus) results in only a minor increase in nuclear NF-κB while our Trans-AM binding assay (Fig. 4) indicates that treatment with Jasplakinolide results in a significant increase in DNA binding of NF-κB. Since DNA binding is required for transcription, the Trans-AM binding assay is more relevant when investigating if a given stimulus results in inflammatory activation. Nonetheless, the western blot results do indicate that pressure-induced activation of NF-κB occurs via the canonical signaling pathway and is dependent on IκBα degradation.
For oscillatory pressures, at a frequency of 0.125 Hz, Latrunculin A treated cells exhibited significantly more normalized NF-κB activation at the ±12 and ±14 cmH2O pressure level compared to unloaded controls (Fig. 6b). In contrast, Jasplakinolide-treated cells did not exhibit any statistically significant increase in normalized NF-κB activation compared to unloaded controls at either ±12 and ±14 cmH2O. In addition, at the ±12 cmH2O pressure level, Jasplakinolide-treated cells exhibited less normalized NF-κB activation than untreated cells while Latrunculin-treated cells exhibited more activation than untreated cells. Therefore, the results at this lower frequency are very similar to the results obtained under static conditions. However, at a higher frequency of 0.18 Hz, both the Latrunculin-treated and Jasplakinolide-treated cells did not exhibit any statistically significant change in normalized NF-κB activation compared to unloaded controls at either ±12 or ±14 cmH2O (Fig. 6c). Previous studies23,45 indicate that in addition to exhibiting a lower elastic modulus (i.e. a reduction in stiffness), Latrunculin-treated cells also exhibit an increase in fluid-like properties or an increase in viscosity. Increased viscosity would result in cells that deform slower in response to an applied load. In addition, for oscillatory loads, the slower deformation response of a more viscous cell would result in less deformation at high loading frequencies. As a result, we would expect the Latrunculin treated cells to deform less at higher loading frequencies compared with lower frequencies (or static loads) due to their increased viscosity. This reduced cell deformation would be consistent with the observed reduction in mechanotransduction in these cells at higher frequency (Fig. 6c). Note that at a lower frequency of 0.125 Hz, the increased NF-κB activation in Latrunculin treated cells, indicates that at this frequency, even though the Latrunculin treated cells are more viscous, they have enough time to deform and that the reduced stiffness of these cells allows them to undergo more deformation- induced mechanotransduction.
We also note that the viscoelastic nature of the epithelial cells may also explain the lower NF-κB activation at the ±14 cmH2O level compared to the ±12 cmH2O level shown in Fig. 5. Specifically, at a constant frequency (i.e. period of oscillation) the use of a triangle waveform results in a larger pressure loading rate (i.e. dp/dt) at the ±14 cmH2O level. For a viscoelastic cell, less deformation would be expected at higher loading rates. Therefore, even though the pressure magnitude is higher, a viscous cell might not deform as much when dp/dt is larger thus resulting in a lower amount of NF-κB activation.
We note that our results for NF-κB activation in Latrunculin treated cells under static loading (Fig. 6a) is consistent with previous studies in which shear stress induced NF-κB activation and nuclear translocation in osteoblasts with a disrupted actin cytoskeleton.6 In contrast, other studies showed that disruption of the actin cytoskeleton inhibits shear stress induced NF-κB activation in vascular endothelial cells.42 However, the current results were obtained for different loading conditions (i.e. transmural pressure) and in a different cell type (i.e. alveolar epithelial cells). Therefore, mechanotransduction of NF-κB activation likely depends on both loading conditions and cell type.
In this discussion, we have assumed that increased cell deformation correlates with increased transduction of static and oscillatory pressures into NF-κB activation. However, we have not directly measured the amount of cell deformation during static/oscillatory pressure and future studies could utilize computational techniques to estimate these deformations.11,12 Although we have investigated the role of calcium signaling in this study, we did not attempt to identify all of the mechanosensing mechanisms by which static/oscillatory pressure alters biochemical signaling. Future studies could investigate these mechanisms by utilizing specific inhibitors, such as focal adhesion kinase inhibitors,42 to identify the role of integrins in pressure induced inflammation. Although the use of Latrunculin or Jasplakinolide is clearly not a clinically relevant approach, there are clinically approved compounds (i.e. simvastatin) that have distinct effects on the cytoskeleton including attenuation of stress fiber formation and peripheral distribution of actin.19 If these cytoskeletal changes result in a “stiffer” or “more viscous” cell, the current study would suggest that these cells would experience less deformation and less activation of inflammatory pathways. Therefore, future studies that characterize how clinically relevant compounds alter cell mechanics, cytoskeletal structure and the mechanotransduction of pressure forces are clearly warranted. Finally, we note that the current study focused on the rapid response of cells to ½ h or 1 h of applied load. This short duration of exposure to pressure loading was clearly sufficient to cause nuclear translocation and activation of NF-κB but future studies may need to utilize longer durations to study the effect on inflammatory protein expression and secretion.
In conclusion, the results of this study clearly demonstrate that static and oscillatory pressures can activate NF-κB pathways in alveolar epithelial cells. In addition, we have demonstrated that changes in the actin cytoskeleton can be used to mitigate or modify these mechanotransduction events. Specifically, the current results suggest that changes in cell rheology, due to changes in the actin cytoskeleton, may be useful in minimizing the amount of cell deformation that occurs during transient pressure loading. In addition to minimizing cell injury, the current study indicates that minimizing cell deformation may also be an effective way to prevent the mechanically induced activation of inflammatory pathways during respiratory disorders such as acute lung injury and ventilation induced lung injury. Additional studies are required to identify the specific mechanisms responsible for pressure-induced activation of NF-κB and to identify clinically relevant pharmaceutical agents that can mitigate these mechanotransduction events.
Acknowledgments
This work was supported in part by NIH/NIDCD grants DC007230 and DC007667 and NSF CAREER grant 0852417.
References
- 1.Alenghat FJ, et al. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res. 2004;301(1):23–30. doi: 10.1016/j.yexcr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Arold SP, Bartolak-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol. 2009;296(4):L574–L581. doi: 10.1152/ajplung.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilek AM, Dee KC, Gaver DP., 3rd Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol. 2003;94(2):770–783. doi: 10.1152/japplphysiol.00764.2002. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone CD, Stool SE, Kenna MA. Otitis Media, atelectasis, and eustachian tube dysfunction. In: Bluestone CD, Klein JO, editors. Pediatric Otolaryngology. Philadelphia: W.B. Saunders Company; 1996. pp. 388–582. [Google Scholar]
- 5.Brower RG, et al. Higher versus lower positive endexpiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 6.Chen NX, et al. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33(3):399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 7.Copland IB, Post M. Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. J Cell Physiol. 2007;210(1):133–143. doi: 10.1002/jcp.20840. [DOI] [PubMed] [Google Scholar]
- 8.Copland IB, et al. Early changes in lung gene expression due to high tidal volume. Am J Respir Crit Care Med. 2003;168(9):1051–1059. doi: 10.1164/rccm.200208-964OC. [DOI] [PubMed] [Google Scholar]
- 9.Copland IB, et al. Mechanotransduction of stretchinduced prostanoid release by fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L487–L495. doi: 10.1152/ajplung.00510.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cortijo J, et al. Nickel induces intracellular calcium mobilization and pathophysiological responses in human cultured airway epithelial cells. Chem Biol Interact. 2010;183(1):25–33. doi: 10.1016/j.cbi.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Dailey HL, Ghadiali SN. Influence of power-law rheology on cell injury during microbubble flows. Biomech Model Mechanobiol. 2010;9(3):263–279. doi: 10.1007/s10237-009-0175-0. [DOI] [PubMed] [Google Scholar]
- 12.Dailey HL, et al. Image-based finite element modeling of alveolar epithelial cell injury during airway reopening. J Appl Physiol. 2009;106(1):221–232. doi: 10.1152/japplphysiol.90688.2008. [DOI] [PubMed] [Google Scholar]
- 13.Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L958–L965. doi: 10.1152/ajplung.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle WJ. Middle ear pressure regulation. In: Rosowski JJ, Merchant SN, editors. The Function and Mechanics of Normal, Diseased and Reconstructed Middle Ears. The Hague, Netherlands: Kugler Publications; 2000. pp. 3–21. [Google Scholar]
- 15.Even-Tzur N, et al. Mucus secretion and cytoskeletal modifications in cultured nasal epithelial cells exposed to wall shear stresses. Biophys J. 2008;95(6):2998–3008. doi: 10.1529/biophysj.107.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol. 2008;163(1–3):232–243. doi: 10.1016/j.resp.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han B, et al. Conversion of mechanical force into biochemical signaling. J Biol Chem. 2004;279(52):54793–54801. doi: 10.1074/jbc.M406880200. [DOI] [PubMed] [Google Scholar]
- 18.Hu S, et al. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am J Physiol Cell Physiol. 2003;285(5):C1082–C1090. doi: 10.1152/ajpcell.00159.2003. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson JR, et al. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol. 2004;30(5):662–670. doi: 10.1165/rcmb.2003-0267OC. [DOI] [PubMed] [Google Scholar]
- 20.Jafari B, et al. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology. 2004;9(1):43–53. doi: 10.1111/j.1440-1843.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 21.Kay SS, et al. Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol. 2004;97(1):269–276. doi: 10.1152/japplphysiol.01288.2003. [DOI] [PubMed] [Google Scholar]
- 22.Kustermans G, et al. Perturbation of actin dynamics induces NF-kappa B activation in myelomonocytic cells through an NADPH oxidase-dependent pathway. Biochem J. 2005;387:531–540. doi: 10.1042/BJ20041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laudadio RE, et al. Rat airway smooth muscle cell during actin modulation: rheology and glassy dynamics. Am J Physiol Cell Physiol. 2005;289(6):C1388–C1395. doi: 10.1152/ajpcell.00060.2005. [DOI] [PubMed] [Google Scholar]
- 24.Lazaro-Dieguez F, Egea G. Formatex. Comparative study of the impact of the actin cytoskeleton on cell shape and membrane surface in mammalian cells in response to actin toxins. In: Mendez-Vilas A, Diaz J, editors. Modern Research and Educational Topics in Microscopy. 2007. [Google Scholar]
- 25.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11(1):82–86. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 27.Liu WF, et al. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res. 2007;101(5):e44–e52. doi: 10.1161/CIRCRESAHA.107.158329. [DOI] [PubMed] [Google Scholar]
- 28.Myers KA, et al. Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem Biophys Res Commun. 2007;364(2):214–219. doi: 10.1016/j.bbrc.2007.09.109. [DOI] [PubMed] [Google Scholar]
- 29.Ning QM, Wang XR. Response of alveolar type II epithelial cells to mechanical stretch and lipopolysaccharide. Respiration. 2007;74(5):579–585. doi: 10.1159/000101724. [DOI] [PubMed] [Google Scholar]
- 30.Ressler B, et al. Molecular responses of rat tracheal epithelial cells to transmembrane pressure. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1264–L1272. doi: 10.1152/ajplung.2000.278.6.L1264. [DOI] [PubMed] [Google Scholar]
- 31.Ridge KM, et al. Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J Biol Chem. 2005;280(34):30400–30405. doi: 10.1074/jbc.M504239200. [DOI] [PubMed] [Google Scholar]
- 32.Sidhaye VK, et al. Shear stress regulates aquaporin-5 and airway epithelial barrier function. Proc Natl Acad Sci USA. 2008;105(9):3345–3350. doi: 10.1073/pnas.0712287105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smirnova MG, Birchall JP, Pearson JP. The immunoregulatory and allergy-associated cytokines in the aetiology of the Otitis Media with effusion. Mediators Inflamm. 2004;13(2):75–88. doi: 10.1080/09629350410001688477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spector I, et al. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc Res Tech. 1999;47(1):18–37. doi: 10.1002/(SICI)1097-0029(19991001)47:1<18::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 35.Tschumperlin DJ, Boudreault F, Liu F. Recent advances and new opportunities in lung mechanobiology. J Biomech. 2010;43(1):99–107. doi: 10.1016/j.jbiomech.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tschumperlin DJ, Drazen JM. Mechanical stimuli to airway remodeling. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S90–S94. doi: 10.1164/ajrccm.164.supplement_2.2106060. [DOI] [PubMed] [Google Scholar]
- 37.Tschumperlin DJ, et al. Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L904–L911. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- 38.Tschumperlin DJ, et al. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol. 2003;28(2):142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 39.Tschumperlin DJ, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429(6987):83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med. 2005;171(12):1328–1342. doi: 10.1164/rccm.200408-1036SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlahakis NE, et al. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277(1 Pt 1):L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 42.Wang YX, et al. Shear stress regulates the Flk-1/Cbl/PI3 K/NF-kappa B pathway via actin and tyrosine kinases. Cel Mol Bioeng. 2009;2(3):341–350. doi: 10.1007/s12195-009-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 44.Yalcin HC, Perry SF, Ghadiali SN. Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol. 2007;103(5):1796–1807. doi: 10.1152/japplphysiol.00164.2007. [DOI] [PubMed] [Google Scholar]
- 45.Yalcin HC, et al. Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol. 2009;297:L881–L891. doi: 10.1152/ajplung.90562.2008. [DOI] [PubMed] [Google Scholar]