Scientific progress is punctuated with revolutions of insight and/or technology that at least temporarily cast aside valid frames of thought. Like the Titanic losers to the Olympian gods in Keats' unfinished epic Hyperion (1) from whence the title of this commentary comes, “vanquished” ideas or approaches frequently offer important alternatives to prevailing model systems. The successes of molecular genetics in biological circadian clocks are an excellent example of this dynamic, and the paper by Brandstätter et al. (2) in this issue of PNAS is a testament that systems analyses of clock function still have much to offer the circadian clocks field. It demonstrates that the clock within the avian pineal gland “remembers” the time of year, as well as the time of day, important characteristics for the adaptive function of clocks, which cannot be explained by current, more popular preparations. Further, the paper shows that the ability of the pineal gland to faithfully reflect photoperiod depends at least in part on its integration with other components of a larger, more precise circadian system (3, 4).
The avian pineal gland once held very high office in the Pantheon of circadian model systems. Thirty years ago, Gaston and Menaker (5) showed that pinealectomy of house sparrows, Passer domesticus, abolishes circadian locomotor rhythms in constant darkness (DD), demonstrating the pineal was critical for rhythmic behavior. They also showed that the gland is only part of a circadian system; pinealectomized sparrows entrain to light dark (LD) cycles and, on transfer from LD to DD, only gradually become arrhythmic.
The role(s) played by the pineal gland in rhythms and the mechanism of its rhythmic regulation of behavior became more apparent when Zimmerman and Menaker (6) demonstrated that pineal transplantation into the anterior chamber of the eye to pinealectomized, arrhythmic sparrows conferred rhythmic locomotion to the recipient birds. These data indicated the pineal confers its message via humoral signals, because rhythmicity returned to the recipients within a day of their transplant. Importantly, the study showed that the pineal gland carried within it a physiological correlate of time of day. Recipient birds expressed an earlier time of activity if they received pineal glands from donors entrained to an early LD cycle, whereas recipients who received pineal glands from donors entrained to a later LD cycle expressed later activity cycles.
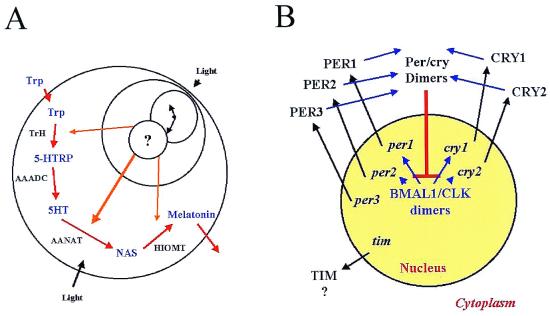
After the discovery and characterization of the pineal hormone melatonin and its biosynthetic pathway (cf. ref. 7), several groups showed the pineal's humoral signal to be melatonin. Further, the avian pineal gland was found to express circadian rhythms of melatonin biosynthesis, content and release in vitro (8–11), that could be entrained to LD in vitro (8–11). Thus, the avian pineal is important for overt rhythmicity, has physiologically relevant input (light), an endogenous circadian oscillator and an easily measured rhythmic output, melatonin (Fig. 1A).
Figure 1.
Although we know much about the biosynthesis of melatonin by the avian pineal gland, we know nothing of the clock that generates its rhythm. (A) The biosynthetic mechanism is regulated transcriptionally by a circadian clock that presumably resides in the nucleus. Rhythms of TrH, AANAT, and HIOMT activity are driven by rhythms in their mRNA levels. (B) A generalized schematic of the interactions of the putative components of the mammalian molecular clock. Positive elements BMAL1 and CLK drive transcription of the negative elements per1, per2, per3, cry1, and cry2. They then are translated, dimerize and, on transport back into the nucleus, disrupt positive regulation of clock gene transcription. Does a similar mechanism regulate avian melatonin rhythms?
Molecular Regulation of Avian Melatonin Rhythms
Although the biochemical and molecular mechanisms by which the avian pineal gland synthesizes melatonin are largely understood (cf. ref. 7), the molecular mechanism(s) by which endogenous rhythmicity is generated are completely unknown (Fig. 1A). No cellular or molecular component of the avian pineal gland has been identified that affects the phase or period of the circadian oscillation that produces melatonin. What do we know about melatonin?
Melatonin is synthesized from the amino acid tryptophan, which is taken up from the bloodstream. It is converted to 5-hydroxytryptophan (5-HTRP) by tryptophan hydroxylase (TrH) and then to 5-hydroxytryptamine (5HT) by aromatic amino acid decarboxylase (AAADC). Then, during the night, 5HT is N-acetylated by arylalkylamine N-acetyltransferase (AANAT) to form N-acetylserotonin (NAS), a substrate for the final enzyme in the pathway, hydroxyindole-O-methyltransferase (HIOMT). The circadian clock within each chick pinealocyte regulates melatonin biosynthesis transcriptionally in at least three of the enzymatic steps in this process, because mRNA of TrH, AANAT, and HIOMT are expressed rhythmically in both LD and DD in vivo and in vitro (7, 12). AANAT activity parallels the presence and absence of AANAT mRNA under these conditions, so it is presumed the clock regulates rhythmicity transcriptionally in birds (7), although posttranscriptional regulation occurs as well (13).
Molecular Regulation of Biological Clocks
At about the same time as the discovery of the circadian importance of the avian pineal gland, another revolution was brewing, when Konopka and Benzer (14) identified the period (per) mutation in Drosophila melanogaster that altered or abolished circadian rhythms of eclosion. In the 30 years since that seminal observation, a growing army of molecular geneticists, particularly the groups of Hall, Rosbash, and Young (15, 16), has pieced together the Drosophila biological clockworks from an ever-growing list of gears and escape mechanisms. Those authors, their students, and postdocs clearly showed the central importance to circadian clock function of six genes: per, timeless (tim), cryptochrome (cry), clock (clk), bmal1, and double-time (dbt). Further, they have proposed and successfully tested a plausible model by which these genes' products interact to produce overt rhythmicity. These, of course, have been reviewed extensively elsewhere (15, 16) and will not be belabored here.
However, through a variety of modern molecular techniques, similar, perhaps homologous, genes have now been isolated, cloned, and sequenced from rodents (17), and a few have been isolated in birds (18, 19). The remarkable similarity of the sequence of these genes with those in Drosophila and the remarkable similarity in the nature of their interactions have led many researchers to propose that these clock mechanisms, schematically represented in Fig. 1B, represent a phylogenetically ancient molecular clock loop whose core is conserved among all animals.
Memories of Seasons Past in the House Sparrow Pineal
What do these ruminations have to do with the paper by Brandstätter et al. (2)? Brandstätter et al. show that, in DD, the circadian pattern of activity and pineal melatonin retain an impression of the photoperiod in which the bird was entrained for at least 6 days. Thus, if birds were entrained in a long photoperiod, similar to the long days of summer, the duration of activity (α) remained long in both LD and DD, whereas the duration of pineal melatonin content remained short. Conversely, if birds were entrained to a short photoperiod, indicative of the short days of winter, α remained short in LD and DD, whereas the duration of melatonin remained as long as the winter nights. Perhaps, more remarkable is the fact that if they removed the pineal glands from these birds and measured melatonin efflux, the memory of the season in which the bird lived was retained for two of those days in vitro. These data show that, in addition to encoding a time of day, the sparrow pineal gland encodes the time of year and “remembers” that time of year in DD for at least 6 days in vivo and 2 days in vitro. This capacity presents a challenge to molecular biologists who seek to understand the molecular mechanisms of biological clock function, because the current models do not incorporate this capacity.
Recent studies in Siberian hamsters, Phodopus sungorus, suggest a similar capacity in the site of mammalian circadian clock function, the hypothalamic suprachiasmatic nucleus (SCN) and the pars tuberalis of the pituitary gland (20, 21). In these studies, the expression of per1 (21) and per2 (20) was determined by in situ hybridization under long days or short days. The authors discovered that the rhythm of these “clock genes”' mRNA was high during the day in LD and subjective day in DD, but that the duration of this molecular rhythm reflected the duration of the photoperiod. Interestingly, these seasonal patterns of per expression are partially influenced by administration of exogenous melatonin (21).
Revenge of the Circadian System
Another important feature presented by Brandstätter et al. (2) is the fact that although the pineal gland “remembered” the photoperiod for at least 6 days in vivo, the effect of photoperiod on melatonin duration in vitro was lost after 2 days. Although this is pretty good, it is clear that the pineal's capacity to reflect photoperiod at least partially depends on its connection to the rest of the circadian system.
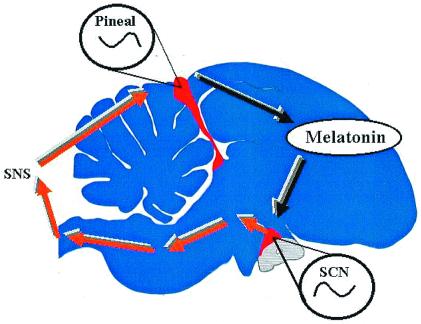
Two complementary models for avian circadian organization (3, 4) have been used to explain the system level properties of birds' clocks. These models posit that there are at least two circadian pacemakers that form the core of the avian circadian clock, the pineal gland and the avian homologue of the SCN (Fig. 2). In some species, but not the house sparrow, the retinae also may be incorporated into this system, but for simplicity's sake, I will ignore them. These models state that both the SCN and the pineal gland are damped circadian oscillators whose mutual interactions maintain self-sustainment. The SCN is active during subjective day and, via a multisynaptic pathway including the sympathetic nervous system, inhibits melatonin biosynthesis in the pineal gland and thereby restricts its output to the night. Conversely, the pineal gland is active during the night, secreting melatonin into the bloodstream, and, among other targets, inhibits activity within the SCN via specific melatonin receptors and restricting the SCN′s output to the subjective day.
Figure 2.
The neuroendocrine loop (or internal resonance) model of avian circadian organization. This schematic of a sparrow brain shows the locations of the pineal gland and suprachiasmatic nucleus (SCN) (in red), each of which are damped circadian oscillators that rely on their mutual interactions to maintain rhythm stability and amplitude.
Recent work from Gwinner's group (4, 22) has established that, in many migratory birds, amplitude modification of these pacemaker components can alter the amplitude of the entire system. This modification of system amplitude can have broad effects in the natural history of birds, many of whom must migrate great distances at certain times of year. By decreasing clock amplitude during migration, birds can rapidly adapt to new time zones in their new locale. They would, therefore, experience no “jet lag,” which may be uncomfortable for casual tourists but deadly to small passerine birds. Further, nocturnal migrants, normally active during the day, may be enabled to fly during the night by the “down-regulation” of their clock. Perhaps the seasonal change in the rhythm of melatonin encoded in the clock's memory described by Brandstätter et al. (2) is part of a global system that modifies clock properties depending on the time of year, ensuring that clock function is adaptable to prevailing environmental conditions.
I began this commentary with a somewhat cryptic quotation from John Keats' Hyperion. It is worth recalling that Hyperion was a Titan, a god overthrown with Saturn and the other Titans by Zeus, Apollo, and the other Olympian gods. In Keats' unfinished epic, we find Hyperion, the Titanic Father of Dawn, still in full control of his powers but made anxious by Saturn's cataclysmic fall. He therefore seeks Saturn and the other Titans out to rally them. We do not know whether he succeeds; the poem is unfinished. Still, Hyperion, the dawn, persists in diminished form throughout mythology. For his part, Apollo, the sun, is wracked by the battle as well and finds another Titan, Mnemosyne, goddess of memory, who returns to him the painful knowledge and power that go with immortality. In the end, “Apollo shriek'd; -and lo! From all his limbs Celestial …” and the poem ends. Thus, memory gives to the sun, perhaps the clock, too, its ascendancy. Interesting, eh?
Acknowledgments
I thank the members of the Texas A&M Biological Clocks program for useful discussion, particularly Deb Bell-Pedersen, Susan Golden, David Earnest, and Arjun Natesan. Research in my lab is supported by National Institute of Neurological Disorders and Stroke Grants RO1 NS 35822 and PO1 NS39546. I am a founding member of the Non-Human Texans.
Footnotes
See companion article on page 12324.
References
- 1.Keats J. Hyperion in The Poetical Works of John Keats. London: Bell; 1899. [Google Scholar]
- 2.Brandstätter R, Kumar V, Abraham U, Gwinner E. Proc Natl Acad Sci USA. 2000;97:12324–12328. doi: 10.1073/pnas.200354997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassone V M, Menaker M. J Exp Zool. 1984;232:539–549. doi: 10.1002/jez.1402320321. [DOI] [PubMed] [Google Scholar]
- 4.Gwinner E. In: Circadian Clocks in Ecology. Hiroshige T, Honma K, editors. Sapporo: Hokkaido Univ. Press; 1989. pp. 127–153. [Google Scholar]
- 5.Gaston S, Menaker M. Science. 1968;160:1125–1127. doi: 10.1126/science.160.3832.1125. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman N, Menaker M. Proc Natl Acad Sci USA. 1979;76:999–1003. doi: 10.1073/pnas.76.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein D C, Baler R, Roseboom P H, Weller J L, Bernard M, Gastel J A, Zatz M, Iuvone P M, Bégay V, Falcón J, et al. Handbook of Behavioral State Control. Boca Raton, FL: CRC; 1999. pp. 45–59. [Google Scholar]
- 8.Binkley S, Riebman J B, Reilly K B. Science. 1978;202:1198–1201. doi: 10.1126/science.214852. [DOI] [PubMed] [Google Scholar]
- 9.Deguchi T. Science. 1979;203:1245–1247. doi: 10.1126/science.424750. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi J S, Hamm H, Menaker M. Proc Natl Acad Sci USA. 1980;77:2319–2322. doi: 10.1073/pnas.77.4.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zatz M. Cell Dev Biol. 1996;7:811–820. [Google Scholar]
- 12.Bernard M, Klein D C, Zatz M. Proc Natl Acad Sci USA. 1997;94:304–306. doi: 10.1073/pnas.94.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard M, Iuvone P M, Cassone V M, Roseboom P H, Coon S L, Klein D C. J Neurochem. 1997;68:213–224. doi: 10.1046/j.1471-4159.1997.68010213.x. [DOI] [PubMed] [Google Scholar]
- 14.Konopka R J, Benzer S. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall J C, Rosbash M. Proc Natl Acad Sci USA. 1993;90:5382–5385. doi: 10.1073/pnas.90.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young M W. Science. 2000;288:451–453. doi: 10.1126/science.288.5465.451. [DOI] [PubMed] [Google Scholar]
- 17.Reppert S M. Neuron. 1998;21:1–4. doi: 10.1016/s0896-6273(00)80234-2. [DOI] [PubMed] [Google Scholar]
- 18.Larkin P, Baehr W, Semple-Rowland S. Mol Brain Res. 1999;70:253–263. doi: 10.1016/s0169-328x(99)00154-0. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, Matsuda Y, Namikawa T, Ebihara S. Mol Brain Res. 2000;78:207–215. doi: 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 20.Nuesslein-Hildesheim B, O'Brien J A, Ebling F J, Maywood E S, Hastings M H. Eur J Neurosci. 2000;12:2856–2864. doi: 10.1046/j.1460-9568.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 21.Messager S, Hazlerrigg D G, Mercer J G, Morgan P J. Eur J Neurosci. 2000;12:2865–2870. doi: 10.1046/j.1460-9568.2000.00174.x. [DOI] [PubMed] [Google Scholar]
- 22.Gwinner E. J Exp Biol. 1996;199:39–48. doi: 10.1242/jeb.199.1.39. [DOI] [PubMed] [Google Scholar]