Abstract
Statement of Purpose. IgG4-related disease (IgG4-RD) is usually associated to an increase of serum IgG4 levels. However other conditions have also been associated to high serum IgG4 levels. Methods. All IgG subclasses analyses performed in our hospital over a one-year period were analyzed. When IgG4 level were over 1.35 g/L, the patient's clinical observation was analyzed and both final diagnosis and reason leading to IgG subclasses analysis were recorded. Only polyclonal increases of IgG4 were considered. Summary of the Results. On 646 IgG subclass analysis performed, 59 patients had serum IgG4 over 1.35 g/L. The final diagnosis associated to serum IgG4 increase was very variable. Most patients (25%) presented with repeated infections, 13.5% with autoimmune diseases, and 10% with IgG4-RD. Other patients presented with cancer, primary immune deficiencies, idiopathic interstitial lung disease, cystic fibrosis, histiocytosis, or systemic vasculitis and 13.5% presented with various pathologies or no diagnosis. Mean IgG4 levels and IgG4/IgG ratio were higher in IgG4-RD than in other pathologies associated to elevated IgG4 levels. Conclusions. Our study confirms that elevation of serum IgG4 is not specific to IgG4-RD. Before retaining IgG4-RD diagnosis in cases of serum IgG4 above 1.35 g/L, several other pathological conditions should be excluded.
1. Introduction
Immunoglobulin G4 (IgG4) represents the less abundant of the four IgG subclasses in human serum accounting for 3 to 6% of the total IgG [1].
IgG4 has been associated with several pathological conditions. Most of these associations suggest a protective effect of IgG4, such as in allergen-specific immunotherapy [2] and protection from inflammatory manifestations during parasitosis [3]. In few situations, IgG4 is associated with a direct pathogenic effect, such as in pemphigus. During this blistering dermatosis, antidesmosome autoantibodies belong to the IgG4 subclass [4]. However, total IgG4 serum levels are not raised in these conditions.
In 2001, Hamano et al. report a quantitative serum IgG4 elevation during sclerosing (or “autoimmune”) pancreatitis above the cutoff value of 135 mg/dL in 95% of patients with autoimmune pancreatitis [1]. This entity was first described in 1961 by Sarles and colleagues in patients with lymphoplasmacytic infiltrate and fibrosis of the pancreas associated to polyclonal hypergammaglobulinemia [5]. Polyclonal hypergammaglobulinemia raised initially the issue of the possible auto-immune nature of the disease, but this hypothesis has not been confirmed to date. Indeed, no specific autoantibody has been associated with auto-immune pancreatitis. Serum IgG4 elevation becomes from this date a biological marker of sclerosing (or “auto-immune”) pancreatitis. Other fibroinflammatory organ involvements with similar histopathological characteristics have since been reported, associated or not with pancreatic involvement, in a context of serum IgG4 elevation [6], leading to the concept of an IgG4-related disease [7]. To date, serum IgG4 elevation is considered as a diagnosis criteria for IgG4-related disease [8–10]. However, serum IgG4 elevation is not necessary for the diagnosis, as proposed by the diagnosis criteria, and IgG4 elevation is not specific of the disease [11]. Serum IgG4 elevation has also been reported in various pathological situations: multicentric Castleman's disease [12], Wegener's granulomatosis [13], Churg-Strauss syndrome [14], or pancreatic adenocarcinoma [15].
Few works have systematically studied diagnosis associated with a serum IgG4 elevation [11, 16, 17]. In order to better know diagnosis associated with this biological situation, we studied retrospectively all IgG4 subclass measurements achieved during a one-year period at the University Hospital of Marseille, France.
2. Materials and Methods
All results for IgG subclasses evaluation performed from January 1st, 2009 to December 31th, 2009 at the laboratory of Immunology of our University Hospital of Marseille, France, were analyzed. Serum total IgG and IgG subclasses (1 to 4) levels were measured by immunonephelometry (Siemens Nephelometer Analyser II) with reagents from Siemens (NAS IgG1, NAS IgG2, N latex IgG3, and N Latex IgG4). All results with serum IgG4 polyclonal increase above the cutoff value of 1.35 g/L were considered for the study.
The patients' medical records were retrospectively analyzed and demographic, clinical, paraclinical, and evolutive characteristics were recorded. The reason leading the physician to the prescription of IgG subclass evaluation was also recorded. Patients were classified according to the disease associated to IgG4 elevation. Mean values in different groups were compared using the Mann-Whitney test. Results were considered significant for P < 0.05. Figures and statistics were realized using GraphPad Prism v. 4.0.
3. Results
3.1. Patients' Characteristics
A total of 646 IgG subclass analyses were recorded at the laboratory of Immunology during the one-year period. An IgG4 level above 1.35 g/L was found on 75 samples that corresponded to 60 different patients (some of them took 2 or more samples during the year). Among these 60 patients, data could be collected for 59 patients.
Among the 59 patients analyzed, 30 were men and 29 were women (sex ratio 1/1). Mean age at IgG subclass measurement was 47.2 years (range: 4–85 years). Mean serum IgG level was 18.50 g/L (range: 7.38–40.4 g/L). Thirty-eight patients (64%) presented an elevated serum IgG level (>14 g/L). By definition, a serum IgG4 level above 1.35 g/L was present in all 59 patients. Mean serum IgG4 level was 4.35 g/L (range: 1.37–20.6 g/L).
The reasons leading the physicians to perform IgG subclass analysis are presented in Table 1. Suspicion of immune deficiency (with or without hypogammaglobulinemia) was the first indication for IgG subclass measurement in 21 patients (35.6%). Polyclonal hypergammaglobulinemia was the reason leading to serum IgG subclass measurement in 15 patients (25.4%), IgG4-related disease (IgG4-RD) suspicion in 14 patients (23.7%) with one or more compatible organ involvement. In 9 patients (15.3%), the reason was unclear and classified as other.
Table 1.
Clinical reasons leading to IgG subclass measurement in patients with serum IgG4 elevation n = 59.
|
|
% (n) |
|---|---|
| Hypogammaglobulinemia or PID suspicion | 35.6% (21) |
| Hypergammaglobulinemia | 25.4% (15) |
| IgG4-RD suspicion | 23.7% (14) |
| Other | 15.3% (9) |
PID: primary immune deficiency; IgG4-RD: IgG4-related disease.
3.2. Diagnosis Associated with Serum IgG4 over 1.35 g/L
Final diagnosis in patients who presented with elevated levels of serum IgG4 was divided into different categories presented in Table 2. Most represented categories were repeated infections with 15 patients (25.4%). Site of infections (pneumonia, sinusitis, skin and soft tissues infections, osteitis, and pericarditis) and microorganisms implicated (community-acquired bacteria, Staphylococcus sp., herpes virus group (HPV, HSV, and EBV), Nocardia, Toxoplasma gondii, Enterobius vermicularis) were variable in this group. In 8 patients (13.6%), final diagnosis was an autoimmune disease: systemic lupus erythematosus (SLE) in 4 patients (with secondary antiphospholipid syndrome in 2), Sjögren Syndrome (SS) in 2 patients, Biermer disease in 1 patient, and systemic sclerosis in 1 patient. An IgG4-related disease (IgG4-RD) was the final diagnosis in 6 patients (10.1%). Histological documentation was available in all cases of IgG4-RD with characteristic histopathological features with lymphocytic and plasmacytic polyclonal inflammatory infiltrate (with predominant IgG4 positive plasma cells when immunohistological study where available, n = 5) and fibrosis. Organ involvements included sclerosing pancreatitis in 4 patients, tubulointerstitial nephritis in 5 patients, polyadenopathy in 5 patients, sialadenitis in 3 patients, sclerosing cholangitis in 2 patients, dacryoadenitis in 1 patient, hypophysitis in one patient, and inflammatory pseudotumors (hepatic in one case, orbital in another case). Four of these patients with IgG4-RD were under treatment (corticosteroids and/or immunosuppressive therapy) at the time of IgG subclass measurement. In 5 patients (8.5%), an IgG4-RD was considered as possible. These patients presented with one or more compatible organ involvements but without histopathological documentation. Sclerosing cholangitis was presented in 4 cases (with renal involvement in one of these patients), sclerosing pancreatitis in the other case. In 4 patients (6.8%) the final diagnosis was a cancer: ampullary carcinoma, angioimmunoblastic T-cell lymphoma, pancreatic carcinoma, and bronchopulmonary carcinoma. Primary immune deficiency (PID) was the final diagnosis in 4 cases (6.8%): Wiskott-Aldrich syndrome, chronic granulomatous disease, common variable immunodeficiency, and complex humoral immune deficiency. Hypogammaglobulinemia on blood electrophoresis was found in 3 of these patients. Idiopathic interstitial pneumonitis was the final diagnosis in 3 patients (5%) (with pulmonary fibrosis in 2 cases). Cystic fibrosis, Erdheim-Chester disease and vasculitis (hepatitis C-virus-associated type-II-mixed cryoglobulinemia in one case and microscopic polyangiitis in another case) were, respectively, diagnosed in 2 patients (3.4%). In 8 patients (13.6%), no final diagnosis could be retained.
Table 2.
Final diagnosis in patients with elevated serum IgG4 level (>1.35 g/L).
| n (%) | Mean IgG4 levels (g/L) |
|
|---|---|---|
| (extremes) | ||
| Repeated infections | 15 (25.4%) | 2.31 (1.37–4.3) |
| Autoimmune diseases | 8 (13.6%) | 3.62 (1.38–11.3) |
| No final diagnosis | 8 (13.6%) | 1.94 (1.37–2.97) |
| IgG4-RD | 6 (10.1%) | 12.64 (2.48−20.6) |
| Possible IgG4-RD | 5 (8.5%) | 2.23 (1.56–3.37) |
| Cancer | 4 (6.8%) | 2.00 (1.71–2.32) |
| Primary immune deficiency | 4 (6.8%) | 1.80 (1.44–2.24) |
| Interstitial pneumonitis | 3 (5%) | 5.54 (1.51–12.7) |
| Cystic fibrosis | 2 (3.4%) | 4.49 (3.36–5.62) |
| Erdheim Chester disease | 2 (3.4%) | 3.05 (2.15–3.94) |
| Vasculitis | 2 (3.4%) | 3.68 (3.06–4.30) |
IgG4-RD: IgG4-related disease.
Apart from the final diagnostic retained, allergic and atopic manifestations were found in 10 patients (16.9%).
Apart from the diagnosis category considered, allergic and atopic manifestations (allergic rhinoconjunctivitis, nasal polyps and allergic chronic rhinosinusitis, asthma and bronchial hyperreactivity, urticarial skin lesions, and angioedema, hypereosinophilia and/or IgE elevation) were found in the record of only 10 patients (16.9%).
3.3. Serum IgG4 Levels Are Significantly Higher in IgG4-RD Than in Other Pathologies
Serum IgG4 levels found in patients of each diagnosis category are presented in Figure 1. Serum IgG4 elevation observed in IgG4-RD group was significantly more important than in the groups “repeated infections” (P = 0.0021), “auto-immune diseases” (P = 0.0127), “absence of diagnosis” (P = 0.0027), “possible IgG4-RD” (P = 0.0173), “cancer” (P = 0.0095), and “primary immune deficiency” (P = 0.0095). Difference with the group “idiopathic interstitial pneumonitis” was not statistically significant (P = 0.1667). Statistical analysis with the groups “cystic fibrosis,” “Erdheim-Chester disease,” and “vasculitis” was not possible because of the too small size of these groups (n < 3).
Figure 1.
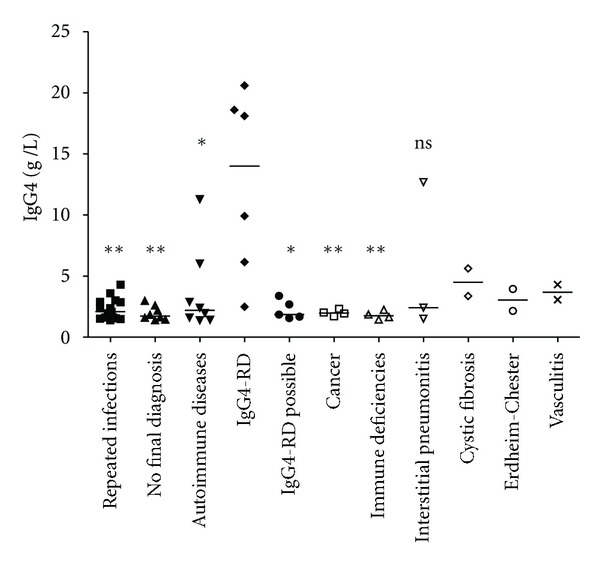
Serum IgG4 levels in different final diagnostic categories of patients with serum IgG4 elevation. IgG4-RD = IgG4-related disease. Horizontal bars represent median values observed in each group. Results obtained in each group were compared to results obtained in IgG4-RD group (Mann-Whitney test): **P < 0.005; *P < 0.05; ns: not significant.
An analysis of the serum IgG4/serum IgG ratio was performed. Serum IgG4/serum IgG ratios observed in patients of each diagnosis category are presented in Figure 2. Elevation of serum IgG4/serum IgG ratio observed in IgG4-RD group was significantly more important than in the groups “repeated infections” (P = 0.0217), “auto-immune diseases” (P = 0.0127), “absence of diagnosis” (P = 0.0426), and “cancer” (P = 0.0381). Differences with the groups “possible IgG4-RD” (P = 0.0519), “primary immune deficiency” (P = 0.1143), and “idiopathic interstitial pneumonitis” (P = 0.2619) were not statistically significant. Statistical analysis with the groups “cystic fibrosis,” “Erdheim-Chester disease,” and “vasculitis” was not possible because of the too small size of these groups (n < 3).
Figure 2.
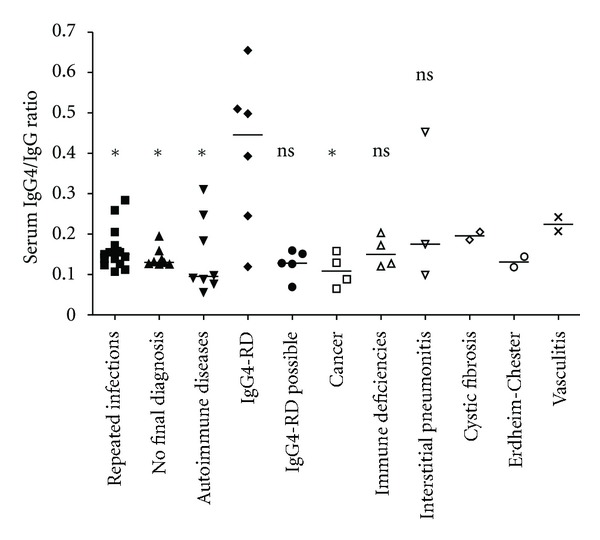
Serum IgG4/serum IgG ratios in different final diagnostic categories of patients with serum IgG4 elevation. IgG4-RD = IgG4-related disease. Horizontal bars represent median values observed in each group. Results obtained in each group were compared to results obtained in IgG4-RD group (Mann-Whitney test): *P < 0.05; ns: not significant.
4. Discussion
Serum IgG4 elevation above 1.35 g/L has been shown to be a predictive marker of type 1 AIP [1] and IgG4-related sclerosing cholangitis [39]. Usefulness of serum IgG4 in diagnosis of AIP has been evaluated for sensibility and specificity [40] and the cutoff value of 1.35 g/L (135 mg/dL) proposed by Hamano in 2001 is widely accepted in literature. To better distinguish AIP from pancreatic cancer more elevated IgG4 cutoff values (140 mg/dL [21] or 280 mg/dL [18]) have been proposed.
In IgG4-RD, the Japan G4 team has retained the elevation of serum IgG4 above 1.35 g/L as an individual diagnosis criteria, [10]. In fact, according to these criteria the presence of a clinical or radiological organ involvement with IgG4 above 1.35 g/L is sufficient to retain the diagnosis of possible IgG4-RD [10].
However, IgG4 elevation has been reported in several well-characterized pathologies as pancreatic carcinoma (10% for a cut-off of 140 mg/dL, 1% for a cut-off of 280 mg/dL [41]), allergic diseases [2], parasitic infections [3], and systemic diseases [11]. The different pathological situations associated to serum IgG4 elevation in literature are reported in Table 3.
Table 3.
Pathologies (excepted IgG4-RD organ involvements) associated to serum IgG4 elevation in medical literature.
| Number of cases and references | ||
|---|---|---|
| Cancer | Pancreatic adenocarcinoma | 13 cases [18], 5 cases [19], 1 case [20], 8 cases [16, 17], 2 cases [1], 11 cases [21], 1 case [22] |
| Bile duct cancer/cholangiocarcinoma | 3 cases [16, 17], 4 cases [22], 17 + 20 cases [23] | |
| Intraductal papillary mucinous neoplasm | 1 case [16, 17] | |
|
| ||
| Autoimmune diseases | Systemic lupus erythematosus | 1 case [11], 2 cases [16, 17], 4 cases [our study], 1 case [24] |
| Antiphospholipid syndrome | 1 case [11], 2 cases [our study] | |
| Autoimmune hepatitis | 1 case [16, 17] | |
| Rheumatoid arthritis | 5 cases [11], 2 cases [25] | |
| Systemic sclerosis | 3 cases [11], 1 case [our study] | |
| Sjögren's syndrome | 3 cases [11], 2 patients [our study] | |
| Polymyositis/dermatomyositis | 1 case [11] | |
|
| ||
| ANCA-related vasculitis | Churg-Strauss syndrome* | 4 cases [11], 4 cases [14] |
| Microscopic polyangiitis | 1 case [11], 1 case [our study] | |
| Nonspecified | 1 case [16, 17] | |
|
| ||
| Infections | Parasitic infections | 2 cases [our study], specific IgG4 antibody elevation [3, 26–29] |
| Bacterial infections | 10 cases [our study] | |
| Viral infections | 3 cases [our study] | |
|
| ||
| Others | Multicentric Castleman's disease* | 7 cases [11], 1 case [30], 1 case [16], 1 case [31], 5 cases [12] |
| Eosinophilic disorders (fasciitis, pneumonia, and hypereosinophilic syndrome) | 1 case each (fasciitis and pneumonia) [11], 2 cases of hypereosinophilic syndrome [16, 17] |
|
| Chronic hepatitis | 1 case [11], 2 cases (auto-immune) [32] | |
| Liver cirrhosis | 2 cases [11], frequent cause of serum IgG4 elevation [33] | |
| Bronchial asthma | 1 case [11], 3 cases [34] | |
| Idiopathic pulmonary fibrosis/interstitial pneumonia | 1 case [11], 4 cases [16, 17], 3 cases [our study] | |
| Primary sclerosing cholangitis | 1 case [16, 17], 3 cases [35], 12 cases [36], 33 cases [37] | |
| Chronic and idiopathic/acute pancreatitis | 1 and 2 cases, respectively [16, 17], 4 and 5 cases, respectively [18] | |
| Behcet's disease | 1 case [16, 17] | |
| Cystic fibrosis | 7 cases [38], 2 cases [our study] | |
Pathologies in bold with * represent pathologies with especially frequent and high serum IgG4 elevation.
Thus, exclusion criteria, including pathologies other than IgG4-RD, should be added to the diagnosis criteria as proposed by recent CDC criteria for IgG4-RD [10]. To better characterize pathologies associated with serum IgG4 above 1.35 g/L which could be discussed in the differential diagnosis of IgG4-RD, we retrospectively studied all IgG subclass measurements performed over a one-year period at our university hospital. Recruitment was largely including pediatric and adult patients without restricted specialized medical field.
Only 10% of 59 patients with elevated serum IgG4 (>1.35 g/L) were diagnosed with IgG4-RD. Other diagnoses associated were infections, auto-immune diseases, cancers, primary immune deficiencies, idiopathic interstitial pneumonitis, or vasculitis. Only patients with IgG subclass evaluation and IgG4 above 1.35 g/L were included in this retrospective study without any control group. Thus, the specificity or the sensitivity of serum IgG4 elevation for diagnosis of IgG4-RD could not be assessed.
Mean serum IgG4 value was found significantly more important in the IgG4-RD patients. However, IgG4 values observed were overlapping with values observed in some other groups (Figure 1).
As already reported, IgG4 elevation was found to be associated with cystic fibrosis [38], vasculitis [11, 14], and cancer [15]. More surprisingly, it was also associated with auto-immune diseases and patients with repeated infections or primary-immune deficiencies. We also show that IgG4 elevation can be associated to hypogammaglobulinemia in few patients with other IgG subclass deficiencies.
Cystic fibrosis has already been associated with serum IgG4 elevation during colonization and infection by Pseudomonas aeruginosa (present in our two patients) [42] and immediate-type hypersensitivity manifestations [38]. These observations, taken together with the observation of IgG4 elevation in a group of patients presenting with repeated infections, raise the question of the role of chronic infectious stimulation in IgG4 elevation. Cancer was associated with serum IgG4 elevation in 6.7% of patients in our study. Of note, a recent work found a more higher standardized incidence ratio for malignancies in IgG4-RD than in the general population [43]. However, none of our patients with cancer presented either clinical or histological evidence for IgG4-RD.
In 3 cases, a diagnosis of idiopathic interstitial lung disease was retained. Different intrathoracic involvements have been reported during IgG4-RD including interstitial lung disease [44]. Because of the absence of histological documentation obtained in these three patients, we cannot exclude an IgG4-RD with isolated or predominant lung involvement, especially in patient with highest serum IgG4 level.
Allergic manifestations were noted in only 10 patients (16.7%) in our study and could therefore not account for IgG4 elevation in this population.
Our retrospective study clearly confirms that serum IgG4 elevation above 1.35 g/L is not specific for the diagnosis of IgG4-RD and can be observed in several clinical situations. The serum IgG4 elevation is more important in IgG4-RD but associated with an important variability within this group. Further studies are needed to define the sensibility and specificity of IgG4 values for the diagnosis of IgG4-RD. Until more specific biomarkers for IgG4-RD are made available, it must be kept in mind that several pathologies should be evoked end excluded in case of IgG4 elevation, before IgG4-RD diagnosis is retained.
5. Conclusion
IgG4-related disease (IgG4-RD) is characterized by one or several fibroinflammatory organ involvements with typical pathological findings. A serum IgG4 elevation above 1.35 g/L is currently retained as an important biomarker of the disease, included in the diagnosis criteria. We confirm in this retrospective study, analyzing systematically the diagnosis associated with serum IgG4 above 1.35 g/L in a large unselected cohort of patients evaluated for IgG subclass, that IgG4 elevation is not specific of IgG4-RD. However, the patients with defined IgG4-RD presented with the most elevated serum IgG4 levels. Thus, several different pathologies should be excluded before IgG4-RD is retained in the context of serum IgG4 elevation.
References
- 1.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. New England Journal of Medicine. 2001;344(10):732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 2.Aalberse RC, Van Milligen F, Tan KY, Stapel SO. Allergen-specific IgG4 in atopic disease. Allergy. 1993;48(8):559–569. doi: 10.1111/j.1398-9995.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 3.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis: predominant localization of blocking antibody to the IgG4 subclass. Journal of Immunology. 1992;148(9):2731–2737. [PubMed] [Google Scholar]
- 4.Shirakata Y, Shiraishi S, Sayama K, Miki Y. Subclass characteristics of IgG autoantibodies in bullous pemphigoid and pemphigus. Journal of Dermatology. 1990;17(11):661–666. doi: 10.1111/j.1346-8138.1990.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease? The American Journal of Digestive Diseases. 1961;6(7):688–698. doi: 10.1007/BF02232341. [DOI] [PubMed] [Google Scholar]
- 6.Saeki T, Saito A, Hiura T, et al. Lymphoplasmacytic infiltration of multiple organs with immunoreactivity for IgG4: IgG4-related systemic disease. Internal Medicine. 2006;45(3):163–167. doi: 10.2169/internalmedicine.45.1431. [DOI] [PubMed] [Google Scholar]
- 7.Khosroshahi A, Stone JH. IgG4-related systemic disease: the age of discovery. Current Opinion in Rheumatology. 2011;23(1):72–73. doi: 10.1097/BOR.0b013e328341a229. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki K. Annual Reports of Research Committee of Intractable Diseases Supported By Ministry of Health, Labour and Welfare of Japan. 2009. Proposal of the concept and diagnostic criteria of IgG4-related disease. [Google Scholar]
- 9.Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. Journal of Gastroenterology. 2011;46(3):277–288. doi: 10.1007/s00535-011-0386-x. [DOI] [PubMed] [Google Scholar]
- 10.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD) Modern Rheumatology. 2012;22(1):21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Tabeya T, Naishiro Y, Yajima H, Ishigami K, Shimizu Y, et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Modern Rheumatology. 2012;22(3):419–425. doi: 10.1007/s10165-011-0532-6. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Kojima M, Takata K, et al. Multicentric Castleman’s disease with abundant IgG4-positive cells: a clinical and pathological analysis of six cases. Journal of Clinical Pathology. 2010;63(12):1084–1089. doi: 10.1136/jcp.2010.082958. [DOI] [PubMed] [Google Scholar]
- 13.Liu LJ, Chen M, Yu F, Zhao MH, Wang HY. IgG subclass distribution, affinity of anti-myeloperoxidase antibodies in sera from patients with Wegener’s granulomatosis and microscopic polyangiitis. Nephrology. 2008;13(7):629–635. doi: 10.1111/j.1440-1797.2008.00976.x. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Takahashi H, Suzuki C, et al. Analysis of serum IgG subclasses in churg-strauss syndrome-The meaning of elevated serum levels of IgG4. Internal Medicine. 2010;49(14):1365–1370. doi: 10.2169/internalmedicine.49.3532. [DOI] [PubMed] [Google Scholar]
- 15.Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Current Opinion in Rheumatology. 2011;23(1):108–113. doi: 10.1097/BOR.0b013e3283413469. [DOI] [PubMed] [Google Scholar]
- 16.Tabata T, Kamisawa T, Takuma K, et al. Serum IgG4 concentrations and IgG4-related sclerosing disease. Clinica Chimica Acta. 2009;408(1-2):25–28. doi: 10.1016/j.cca.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Tabata T, Kamisawa T, Takuma K, et al. Serial changes of elevated serum IgG4 levels in IgG4-related systemic disease. Internal Medicine. 2011;50(2):69–75. doi: 10.2169/internalmedicine.50.4321. [DOI] [PubMed] [Google Scholar]
- 18.Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. American Journal of Gastroenterology. 2007;102(8):1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 19.Raina A, Krasinskas AM, Greer JB, et al. Serum immunoglobulin G fraction 4 levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Archives of Pathology and Laboratory Medicine. 2008;132(1):48–53. doi: 10.5858/2008-132-48-SIGFLI. [DOI] [PubMed] [Google Scholar]
- 20.Choi EK, Kim MH, Lee TY, et al. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007;35(2):156–161. doi: 10.1097/MPA.0b013e318053eacc. [DOI] [PubMed] [Google Scholar]
- 21.Frulloni L, Lunardi C, Simone R, et al. Identification of a novel antibody associated with autoimmune pancreatitis. New England Journal of Medicine. 2009;361(22):2135–2142. doi: 10.1056/NEJMoa0903068. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa T, Naitoh I, Hayashi K, Okumura F, Miyabe K, Yoshida M, et al. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. Journal of Gastroenterology. 2012;47(1):79–87. doi: 10.1007/s00535-011-0465-z. [DOI] [PubMed] [Google Scholar]
- 23.Oseini AM, Chaiteerakij R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54:940–948. doi: 10.1002/hep.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin GG, Li JM. IgG subclass serum levels in systemic lupus erythematosus patients. Clinical Rheumatology. 2009;28(11):1315–1318. doi: 10.1007/s10067-009-1224-x. [DOI] [PubMed] [Google Scholar]
- 25.Lin G, Li J. Elevation of serum IgG subclass concentration in patients with rheumatoid arthritis. Rheumatology International. 2010;30(6):837–840. doi: 10.1007/s00296-009-1330-8. [DOI] [PubMed] [Google Scholar]
- 26.Satoh M, Toma H, Sato Y, et al. Production of a high level of specific IgG4 antibody associated with resistance to albendazole treatment in HLA-DRB1∗0901-positive patients with strongyloidiasis. American Journal of Tropical Medicine and Hygiene. 1999;61(4):668–671. doi: 10.4269/ajtmh.1999.61.668. [DOI] [PubMed] [Google Scholar]
- 27.Genta RM, Lillibridge JP. Prominence of IgG4 antibodies in the human response to Strongyloides stercoralis infection. Journal of Infectious Diseases. 1989;160(4):692–699. doi: 10.1093/infdis/160.4.692. [DOI] [PubMed] [Google Scholar]
- 28.Hagan P, Blumenthal UJ, Dunn D, Simpson AJG, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosomahaematobium . Nature. 1991;349(6306):243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 29.Kwan-Lim GE, Forsyth KP, Maizels RM. Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. Journal of Immunology. 1990;145(12):4298–4305. [PubMed] [Google Scholar]
- 30.Yamamoto M, Takahashi H, Hasebe K, et al. The analysis of interleukin-6 in patients with systemic IgG4-related plasmacytic syndrome—expansion of SIPS to the territory of Castleman’s disease. Rheumatology. 2009;48(7):860–862. doi: 10.1093/rheumatology/kep098. [DOI] [PubMed] [Google Scholar]
- 31.Ishida F, Kitano K, Kobayashi H, Saito H, Kiyosawa K. Elevated IgG4 levels in a case with multicentric Castleman’s disease. British Journal of Haematology. 1997;99(4):981–982. [PubMed] [Google Scholar]
- 32.Umemura T, Zen Y, Hamano H, et al. Clinical significance of immunoglobulin G4-associated autoimmune hepatitis. Journal of Gastroenterology. 2011;46(supplement 1):48–55. doi: 10.1007/s00535-010-0323-4. [DOI] [PubMed] [Google Scholar]
- 33.Dorn L, Finkenstedt A, Schranz M, Prokop W, Griesmacher A, Vogel W, et al. Immunoglobulin subclass 4 for the diagnosis of immunoglobulin subclass 4-associated diseases in an unselected liver and pancreas clinic population. HPB. 2012;14:122–125. doi: 10.1111/j.1477-2574.2011.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Moraes Lui C, Oliveira LC, Diogo CL, Kirschfink M, Grumach AS. Immunoglobulin G subclass concentrations and infections in children and adolescents with severe asthma. Pediatric Allergy and Immunology. 2002;13(3):195–202. doi: 10.1034/j.1399-3038.2002.00058.x. [DOI] [PubMed] [Google Scholar]
- 35.Koyabu M, Uchida K, Fukata N, et al. Primary sclerosing cholangitis with elevated serum IgG4 levels and/or infiltration of abundant IgG4-positive plasma cells. Journal of Gastroenterology. 2010;45(1):122–129. doi: 10.1007/s00535-009-0130-y. [DOI] [PubMed] [Google Scholar]
- 36.Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. American Journal of Gastroenterology. 2006;101(9):2070–2075. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 37.Björnsson E, Chari S, Silveira M, et al. Primary sclerosing cholangitis associated with elevated immunoglobulinG4: clinical characteristics and response to therapy. American Journal of Therapeutics. 2011;18(3):198–205. doi: 10.1097/MJT.0b013e3181c9dac6. [DOI] [PubMed] [Google Scholar]
- 38.Shakib F, Stanworth DR, Smalley CA, Brown GA. Elevated serum IgG4 levels in cystic fibrosis patients. Clinical Allergy. 1976;6(3):237–240. doi: 10.1111/j.1365-2222.1976.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 39.Alderlieste YA, Van Den Elzen BDJ, Rauws EAJ, Beuers U. Immunoglobulin G4-associated cholangitis: one variant of immunoglobulin G4-related systemic disease. Digestion. 2009;79(4):220–228. doi: 10.1159/000213364. [DOI] [PubMed] [Google Scholar]
- 40.Morselli-Labate AM, Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow up of autoimmune pancreatitis: a systematic literature review and meta-analysis. Journal of Gastroenterology and Hepatology. 2009;24(1):15–36. doi: 10.1111/j.1440-1746.2008.05676.x. [DOI] [PubMed] [Google Scholar]
- 41.Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Current Opinion in Rheumatology. 2011;23(1):108–113. doi: 10.1097/BOR.0b013e3283413469. [DOI] [PubMed] [Google Scholar]
- 42.Moss RB. The role of IgG subclass antibodies in chronic infection: the case of cystic fibrosis. New England and regional allergy proceedings. 1988;9(1):57–61. doi: 10.2500/108854188778984491. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto M, Takahashi H, Tabeya T, Suzuki C, Naishiro Y, Ishigami K, et al. Risk of malignancies in IgG4-related disease. Modern Rheumatology. 2012;22(3):414–418. doi: 10.1007/s10165-011-0520-x. [DOI] [PubMed] [Google Scholar]
- 44.Ryu JH, Sekiguchi H, Yi ES. Pulmonary manifestations of IgG4-related sclerosing disease. European Respiratory Journal. 2012;39(1):180–186. doi: 10.1183/09031936.00025211. [DOI] [PubMed] [Google Scholar]


