Abstract
The healing activity of gallic acid enriched ethanolic extract (GAE) of Phyllanthus emblica fruits (amla) against the indomethacin-induced gastric ulceration in mice was investigated. The activity was correlated with the ability of GAE to alter the cyclooxygenase- (COX-) dependent healing pathways. Histology of the stomach tissues revealed maximum ulceration on the 3rd day after indomethacin (18 mg/kg, single dose) administration that was associated with significant increase in inflammatory factors, namely, mucosal myeloperoxidase (MPO) activity and inducible nitric oxide synthase (i-NOS) expression. Proangiogenic parameters such as the levels of prostaglandin (PG) E2, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), von Willebrand Factor VIII, and endothelial NOS (e-NOS) were downregulated by indomethacin. Treatment with GAE (5 mg/kg/day) and omeprazole (3 mg/kg/day) for 3 days led to effective healing of the acute ulceration, while GAE could reverse the indomethacin-induced proinflammatory changes of the designated biochemical parameters. The ulcer healing activity of GAE was, however, compromised by coadministration of the nonspecific NOS inhibitor, N-nitro-L-arginine methyl ester (L-NAME), but not the i-NOS-specific inhibitor, L-N6-(1-iminoethyl) lysine hydrochloride (L-NIL). Taken together, these results suggested that the GAE treatment accelerates ulcer healing by inducing PGE2 synthesis and augmenting e-NOS/i-NOS ratio.
1. Introduction
In Indian Ayurvedic system of medicine, Phyllanthus emblica (syn: Emblica officinalis, family: Euphorbiaceae), is valued for its remarkable therapeutic activity against different diseases. According to belief in ancient Indian mythology, P. emblica (family: Euphorbiaceae) is the first tree to be created in the universe [1]. Its fruits, commonly known as “amla” are rich sources of vitamin C, various hydrolysable tannins such as emblicanin A and B, punigluconin, pedunculagin, galloellagitannins, and flavones like rutin [2]. However, gallic acid is the major key bioactive component having excellent antioxidative [3], antimutagenic [4], anticancer, and antiviral activities [5, 6].
The nonsteroidal anti-inflammatory drugs (NSAIDs) are one of the most widely prescribed drugs in the world and are extensively used to alleviate clinical cases of pain and inflammation [7], prevention and treatment of ischemic heart disease [8], and neoplasia [9]. However, these drugs are well known for stomach ulceration and delayed ulcer healing properties [10]. Currently, the use of NSAIDs accounts for approximately 25% of gastric ulcer cases with an upward trend [11, 12]. Apart from the systemic activity which mainly involves inhibition of cyclooxygenases (COXs), reduced prostaglandin synthesis, and impaired prostaglandin-(PG-) mediated angiogenesis, the NSAIDs also affect the COX-independent mechanisms especially the nitrogen-metabolizing enzymes that are also key contributors in wound healing [13, 14]. Despite recent advances, adequate remedy for the NSAID-induced gastropathy remains elusive. The World Health Organization (WHO) has stressed the need to develop drugs from plant origin, which will be inexpensive, accessible particularly to the rural people in the developing countries, and show less/no side effects. Recently, we have shown that the ethanolic extract of amla has significant healing activity against indomethacin-induced gastric ulceration in mice [15] by its antioxidant action. In a separate study, we have also established gallic acid as the active principle of the amla extract and explained the healing action in terms of its immunomodulatory action [16]. Hence, in the present study we fractionated the ethanolic extract of amla to prepare the gallic acid-enriched extract (designated as GAE throughout the paper) and tested its gastric ulcer healing activity in mice. Because PG [17], endothelial NOS (e-NOS), and nitric oxide (NO) (but not inducible nitric oxide synthase (i-NOS)), derived (NO)) are crucial for gastric ulcer healing, we compared the status of the mucosal i-NOS/e-NOS ratio as well as the NO and PGE2 levels in the ulcerated and GAE-treated mice. In addition, the myeloperoxidase(MPO) activity, expression of growth factors (vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF)) in the gastrointestinal tract, and angiogenesis (in terms of von Willebrand Factor VIII) [14, 18] that facilitate tissue formation and tissue remodeling were investigated in the ulcerated and treatment groups. Finally the healing property of GAE was correlated with its ability to modulate the above parameters. However, we also assessed the ulcer healing in the pure gallic acid-treated mice by tissue histology to reaffirm that it is the active principle of the amla extract.
2. Material and Methods
2.1. Chemicals and Reagents
Indomethacin, omeprazole, 5-bromo-4-chloro-3-indolyl phosphate (BCIP), nitroblue tetrazolium (NBT), Tween-20, Bradford reagent, hexadecyltrimethylammonium bromide (HTAB), L-N6-(1-iminoethyl) lysine hydrochloride (L-NIL), N-nitro-L-arginine methyl ester (L-NAME), and gallic acid were purchased from Sigma Chemical Co, St. Louis, MO, USA. Other chemicals used were ethanol and methanol (E. Merck, Mumbai, India); 35% hydrogen peroxide (H2O2) (Lancaster, Morecambe, UK); disodium hydrogen phosphate and sodium dihydrogen phosphate (BDH, Poole Dorset, UK); bovine serum albumin (BSA), hematoxylin monohydrate and eosin yellowish (Merck, Darmstadt, Germany); horseradish peroxidase (HRPO, Sisco Research Laboratory, Mumbai, India), dimethylformamide (DMF), tetramethylbenzidine (TMB), von Willebrand Factor (rabbit anti-human, Chemicon, Temecula, CA, USA); rabbit polyclonal inducible NOS (iNOS) and endothelial NOS (eNOS) antibodies (Santacruz Biotechnology, Delaware, USA); peroxidase conjugated anti-rabbit IgG antibody, enhanced chemiluminescence detection kit (Roche, Mannheim, Germany), PGE2 EIA kit, nitrate/ nitrite fluorometric assay kit, VEGF and HGF ELISA kits (Cayman Chem., Ann Arbor, MI, USA). All other chemicals were of analytical grade.
2.2. Preparation of GAE
Fruits of P. emblica L. were collected from the local market and identified by the Botanical Survey of India (Ref. no. BSI/CNH/AD/Tech./2009). The dried fruits were chopped into fine pieces, soaked in 95% ethanol for seven days and the extract was filtered through a nylon mesh. The entire process was repeated three times. The combined ethanol extracts were evaporated in vacuo and finally dried in a lyophilizer to obtain an amorphous brown semisolid in 10% w/w yield. The semisolid extract was stored in a vacuum dessicator.
The above extract (20.0 g) was subjected to column chromatography over silica gel (350 g), eluted with 5-100% ethyl acetate (EtOAc)/hexane and twenty-seven fractions (each of 1.0 L) were collected. All fractions were concentrated in vacuo and each of the fractions was tested for the DPPH (Di phenyl picryl hydrazyl) scavenging activity. The best four fractions, designated as F1-F4 obtained in 0.043, 6.08, 19.31 and 1.6% yields respectively, were used for their anti-ulcerogenic activity. Preparative thin layer chromatography (silica gel, 15 : 1.5 : 1 ethyl acetate: methanol: water) of F3 furnished pure gallic acid (65% w/w in F3). Hence it was designated as GAE and used for all the experiments. The high performance liquid chromatography (HPLC) analysis of GAE was carried out using a Zorbax 5 μm C-18 column (150 × 4.6 mm) using methanol water (2 : 3 v/v; flow rate: 1.0 mL/min) as the eluent under ambient conditions. The peaks were detected at 237 nm. The major constituent was identified as gallic acid by comparing the HPLC profile of an authentic sample under the same conditions.
2.3. Animal
Male Swiss albino mice (6–8 weeks, 25 ± 2 g), bred in-house with free access to food and water were used for all the experiments. The mice were kept in 12-h light/dark cycles and housed at 25° ± 1°C. The animals were handled following the International Animal Ethics Committee Guidelines, ensuring minimum animal suffering. The experiments were conducted in accordance with the guidelines of the animal ethics committee of the Postgraduate Institute of Basic Medical Sciences, I.P.G.M.E&R, Kolkata (Animal Ethical Committee, Sanction No IAEC/SB-3/2008/UCM-64 Dated-15/05/08-2011).
2.4. Preparation of Test Samples
The test samples (GAE, gallic acid, and omeprazole) were prepared as aqueous suspensions in 2% gum acacia as the vehicle and administered to the mice orally. In some experiments, the mice were additionally treated intraperitoneally with L-NAME at the dose of 10 mg/kg, once daily for three days before drug treatment and/or L-NIL at the dose of 3 mg/kg, twice daily (first dose was administered 1 h before drug treatment and second dose was administered 15 min before drug treatment) [19].
2.5. Experimental Protocol for Ulceration and Assessment of Healing
The mice were divided into several groups (each containing eight mice) and each experiment was repeated four times. Except for the normal control, ulceration in the other mice was induced by indomethacin (18 mg/kg, p. o., single dose), dissolved in distilled water and suspended in 2% gum acacia as the vehicle. The animals were deprived of food but had free access to tap water 24 h, before ulcer induction. The sham treated and ulcerated untreated groups received the vehicle (0.2 ml) only throughout the course of the experiments. For the standardization of dose, GAE (3, 4, 5, 6, 7 mg/kg) was orally administered to the mice once daily up to seven days, starting the first dose 6 h after the indomethacin-administration. For comparison, treatment was also carried out with gallic acid and the positive control, omeprazole (each 3 mg/kg, p. o.). The doses of indomethacin and omeprazole were standardized in our earlier study [20, 21]. The dose of gallic acid (3 mg/kg) was decided based on its concentration in GAE. The mice were euthanized at 1st, 3rd and 7th days, four hours after the last dose of the test sample on the respective days. The stomachs from the normal and treated groups were removed rapidly, opened along the greater curvature and thoroughly rinsed with normal saline. The extent of healing was assessed from the microscopic damage scores and myeloperoxidase (MPO) activity. Additional experiments were also carried out by treating the mice with GAE or gallic acid only without indomethacin.
2.6. Histology and Assay of Damage Score
The fundic portion of stomach was sectioned for histological studies as well as damage score analysis. The tissue samples were fixed in 10% formalin and embedded in paraffin. The sections (5 μm) were cut using microtome, stained with hematoxylin and eosin and assessed under an Olympus microscope (BX41, Hamburg, Germany). From the histological slides, the damage scores were assessed [22] by grading the gastric injury on a 0−4 scale, based on the severity of hyperemia and hemorrhagic erosions: 0—almost normal mucosa, 0.5—hyperemia, 1—one or two lesions, 2—severe lesions, 3—very severe lesions and 4—mucosa full of lesions (lesions—hemorrhagic erosions, hyperemia—vascular congestions). The sum of the total scores divided by the mean damage score is expressed as the damage score. The experiments were performed by two investigators blinded to the groups and the treatment of animals.
2.7. MPO Assay
Following a reported method [23] with slight modifications, the MPO activity was determined immediately after sacrificing the animals. The whole process was carried out at 4°C. The gastric glandular portions of the stomach (100–150 mg) tissues were homogenized for 30 s in a 50 mM phosphate buffer (pH 6.0) containing 0.5% HTAB and 10 mM EDTA, followed by freezing and thawing three times. The homogenate was centrifuged at 12000 × g for 20 min at 4°C. The supernatant was collected, and the protein content was determined [24] prior to MPO assay. Then the supernatant (50 μL) was added to 80 mM phosphate buffer, pH 5.4 (250 μL), 0.03 M tetra methyl benzidine (TMB) (150 μL) and 0.3 M hydrogen peroxide (H2O2) (50 μL). After incubating the mixture at 25°C for 25 min, the reaction was terminated by adding 0.5 M sulphuric acid (H2SO4) (2.5 ml). The MPO activity was calculated from the absorbance of the mixture at 450 nm, using horseradish peroxidase (HRPO), as the standard. The MPO activity is expressed as μM of H2O2 consumed per min per mg protein at 25°C and pH 5.4.
2.8. Western Blot Analysis
The glandular part of the gastric mucosa after being washed with PBS containing protease inhibitors was minced and homogenized in a lysis buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Triton X-100) containing aprotinin (2 μg/ml), leupeptin (2 μg/ml) PMSF (0.4 μM), and type II phosphatase inhibitor. Following centrifugation at 15,000 × g for 30 min at 4°C, the supernatant was collected, aliquoted and kept at −70°C prior to use for the western blots. The protein concentration was measured [24]. The proteins (40 μg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. The membrane was blocked for 2 h in TBST buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.02% Tween 20) containing 99% fat-free milk powder (5%) and incubated overnight at 4°C with rabbit polyclonal iNOS or eNOS antibody (1 : 2000 dilution). The membrane was washed over a period of 2 h with TBST and incubated with peroxidase conjugated anti-rabbit IgG (1 : 2500 dilution). The bands were detected using an enhanced chemiluminescence detection kit and quantified with respect to that of bands of a suitable loading control, using the Kodak Gelquant software.
2.9. Protein Content Assay
The protein content was determined by the Bradford method using BSA as the standard [24].
2.10. PGE2 Assay
The PGE2 level in tissue homogenate was estimated using commercially available ELISA kit (Cayman Chemical, Ann Arbor, MI, USA), following manufacturer's protocol. The stomach was excised, weighed (100 mg) and suspended in 10 mM sodium phosphate buffer, pH 7.4 (1 mL). The tissues were finely minced and incubated at 37°C for 20 min. After centrifugation (9000 × g), the PGE2 level in the supernatant was measured by ELISA, following manufacturer's instructions. The samples along with the standards were seeded to each well at an appropriate dilution and PGE2 express AChE tracer and PGE2 express monoclonal antibody (both AChE tracer and monoclonal antibody supplied in kit) were added. The plate was covered and incubated for 60 min at room temperature on an orbital shaker. The wells were washed (5 times), Ellman's reagent to each well was added, and the mixture was incubated further for 60–90 min at dark. Next, absorbance was read at wavelength 405 nm.
2.11. Tissue NO Assay
In aqueous medium, cellular NO is rapidly converted to nitrite and nitrate. However, their ratio varies substantially depending on the environment. Hence, for this assay, we used a nitrate/nitrite fluorometric assay kit (Cayman Chem., Ann Arbor, MI). In brief, tissue samples were homogenized in PBS (pH 7.4) and centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was filtered through a 0.45 micron filter (Millipore, Leiden, the Netherlands) and the filtrate was filtered through a 10 kDa molecular weight cutoff ultrafiltration unit (Millipore, Leiden, the Netherlands). The filtrate was assayed spectrofluorimetrically using the fluorescent dye 2, 3-diaminonaphthalene (DAN, excitation wavelength = 365 nm and emission wavelength = 430 nm), following manufacturer's protocol [25].
2.12. Quantification of von Willebrand Factor VIII
The number of microvessels were assessed from von Willebrand Factor VIII, following a reported procedure [19] with slight modifications. In brief, after deparaffinization and rehydration, the endogenous peroxidase activity in the tissue was blocked with 0.3% hydrogen peroxide in methanol. The tissue sections were incubated with the polyclonal rabbit anti-human von Willebrand Factor VIII antibody for 2 h at room temperature and the bound primary antibody was detected using the cell and tissue staining kit. Any positive-staining endothelial cell or endothelial cell cluster that was clearly separated from adjacent microvessels was considered as an angiogenic microvessel. The vascular areas immediately adjacent to the normal tissue of the stomach served as the internal control. The microvessels (under 10X magnification) in five randomly selected microscopic fields of mucosal erosions were counted in a blinded manner and the data were averaged.
2.13. Estimation of Tissue Growth Factors
The tissue VEGF and HGF levels were estimated using commercially available ELISA kits (Cayman Chemical, Ann Arbor, MI).
2.14. Statistical Analysis
Data are expressed as mean ± S.E. unless mentioned otherwise. Values of the band intensity of the immunoblots (arbitrary unit, mean ± S.E.M.) are the density scanning results of three independent experiments, considering that of normal mice as 1. Comparisons were made between different treatments using one-way analysis of variance (ANOVA) followed by an error protecting multiple comparison procedure, namely, Tukey-Kramer post hoc test by Graph Pad InStat (GraphPad Software Inc., San Diego, USA) software for the analysis of significance of all the data.
3. Results
3.1. Assessment of Ulcer Healing
Indomethacin (18 mg/kg, p.o., single dose) administration produced acute time-dependent mucosal lesions in the mice stomach, as evident from histology. Quantification of the damage scores on the respective days revealed maximum ulcerative damage on the 3rd day of indomethacin administration. However, the ulcerative damage reduced on the 7th day. Amongst the chosen doses of GAE, best ulcer healing was observed at a dose of 5 mg/kg, irrespective of the day of ulceration (Figure 1). The healing capacities of GAE at its optimized dose (5 mg/kg daily × 3 days, p.o.) and pure gallic acid (3 mg/kg daily × 3 days, p.o.) are shown in stomach histology (Figure 2(b)) and damage scores (Figure 2(a)). Compared to the untreated group, the damage scores in the GAE, gallic acid and omeprazole-treated groups were reduced by 79.6%, 71.2% and 55.9%, respectively. Mice receiving only vehicle did not produce any gastric lesion. On their own, GAE and gallic acid were found to be nonulcerogenic.
Figure 1.
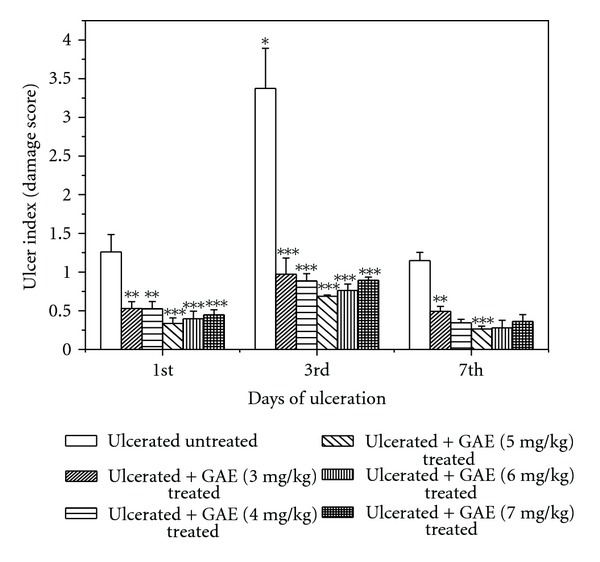
Healing capacities of GAE under various treatment regimes against indomethacin-induced acute gastric mucosal injury in mice. Ulceration in the mice was induced by indomethacin (18 mg /kg, single dose, p.o.). Treatment was carried out with GAE (3, 4, 5, 6, 7 mg/kg, single dose daily up to 7 days, p. o.) after indomethacin administration. The section of mice stomachs were dissected on the 1st, 3rd, and 7th days of ulceration, 4 h after the last dose of the test sample, and the damage scores of different mice groups were measured. The values are mean ± S.E. of four independent experiments, each with 8 mice/group. *P < 0.001, compared to 1st day ulcerated mice; **P < 0.01, ***P < 0.001, compared to untreated mice of the same day.
Figure 2.
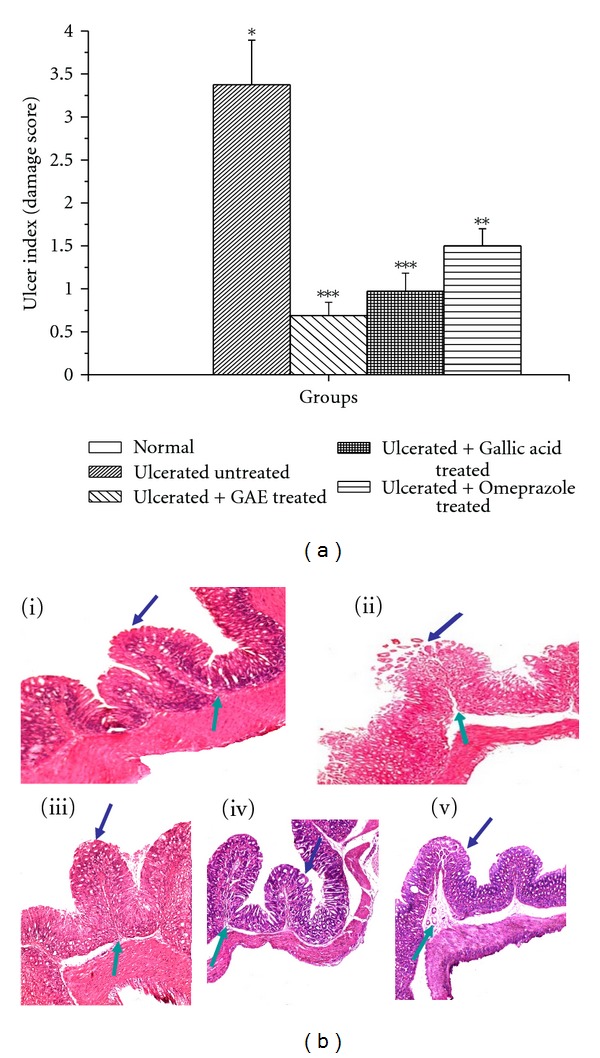
Healing capacities of GAE, gallic acid and omeprazole under the optimized treatment regime. (a) ulcer indices; (b) histology. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose, p.o.). Treatment was carried out with GAE (5 mg/kg daily, p.o.), gallic acid (3 mg/kg daily, p.o.), and omeprazole (3 mg/kg daily, p.o.) for 3 days, starting the first dose 6 h postulcer induction. The sections of mice stomachs were processed for capturing the images. Representative histology of gastric tissue sections are shown at 10x magnification. (i) normal, (ii) Ulcerated untreated, (iii) Ulcerated + GAE treated, (iv) Ulcerated + Gallic acid treated, (v) Ulcerated + Omeprazole treated, mucosal, and submucosal layers are shown by blue and green arrows, respectively. The ulcer indiceswere calculated from the damage scores.The values are mean ± S.E. of four independent experiments, each with 8 mice/group. *P < 0.001, compared to normal mice; **P < 0.01,***P < 0.001, compared to untreated mice.
3.2. Regulation of Mucosal MPO Activity
The mucosal MPO activity of the indomethacin-administered mice increased immediately, reaching the peak value on the 3rd day, and thereafter declining on the 7th day of ulceration (Figure 3(a)). The results were consistent with the damage score data. Treatment with GAE (5 mg/kg daily, p.o.) and omeprazole (3 mg/kg daily, p.o.) for 3 days reduced the MPO activity by 78.8% and 51.8%, respectively, compared to that of the untreated group (Figure 3(b)).
Figure 3.
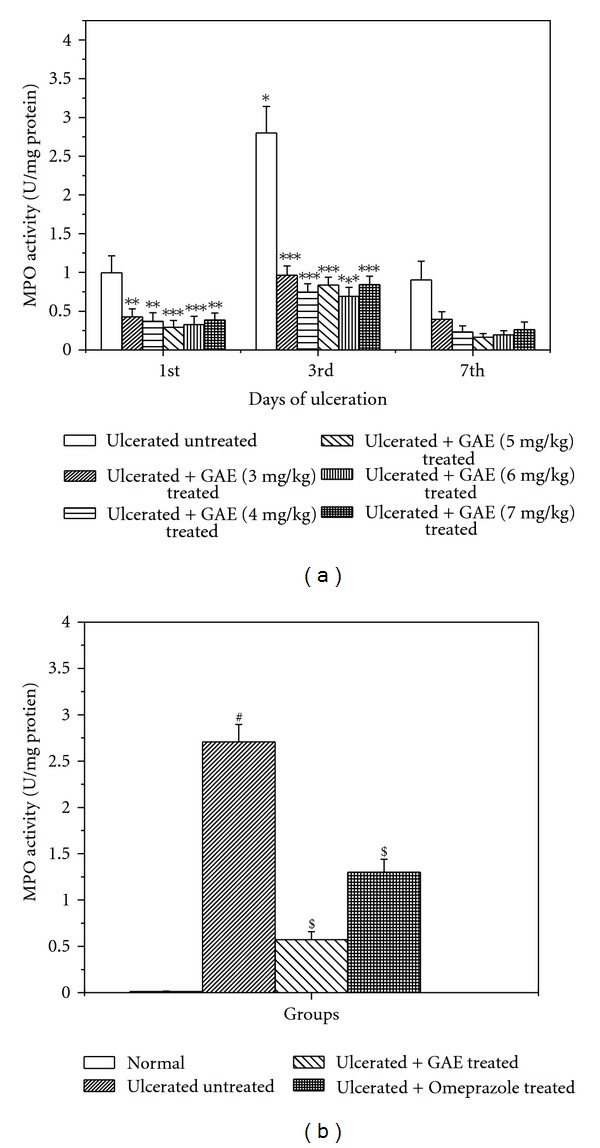
Reduction of the mucosal MPO activity in ulcerated mice by GAE. (a) under various treatment regimes; (b) optimized treatment regime. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose, p.o.). Treatment was carried out with different doses of GAE upto 7 days after indomethacin (18 mg/kg, single dose, p.o.) administration. The section of mice stomachs were dissected on the 1st, 3rd, and 7th days of ulceration, 4 h after the last dose of the test sample, and the MPO activities of different groups of mice were measured. Omeprazole (3 mg/kg × 3 days, p. o.) was used as the positive control. The values are mean ± S.E. of four independent experiments, each with 8 mice/group. *P < 0.001, compared to 1st day ulcerated mice; **P < 0.01, ***P < 0.001, compared to untreated mice of the same day. # P < 0.001, compared to 3rd day normal mice; $ P < 0.001, compared to 3rd day untreated mice.
3.3. Regulation of PGE2 Synthesis
Compared to the normal control, the mucosal PGE2 level was markedly suppressed (2.1 fold) in the untreated mice. Treatment with GAE and omeprazole for three days upregulated the mucosal PGE2 level by 88.2% and 65% respectively, compared to the untreated group (Figure 4).
Figure 4.
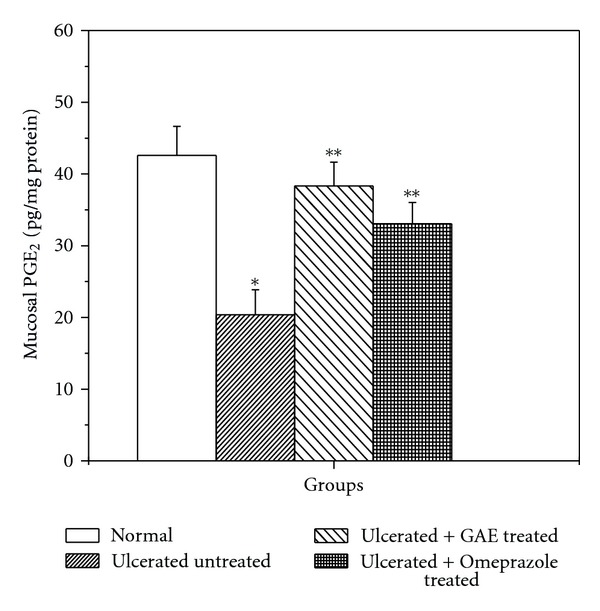
Effect of GAE on mucosal PGE2 synthesis in indomethacin-induced ulcerated mice. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose, p.o.). Treatment was carried out for 3 days with GAE (5 mg/kg, daily, p. o.) or omeprazole (3 mg/kg, p. o.) after ulcer induction. The mucosal PGE2 levels of the ulcerated untreated and treated mice were measured. The values are mean ± S.E. of four independent experiments, each with 8 mice/group. *P < 0.001, compared to normal mice; **P < 0.001, compared to untreated mice.
3.4. Modulation of NOS Expression
The Western blots of e-NOS and i-NOS expressions in the gastric mucosa of the control, ulcerated and drug (GAE or omeprazole)-treated mice are shown in Figure 5. The e-NOS expression was detected in both normal and ulcerated gastric tissues. In contrast, the i-NOS expression was very high in the ulcerated tissues, but much less in normal gastric tissues. Our western blot data revealed that three-day treatment with GAE significantly induced e-NOS expression, while reducing i-NOS expression, compared to that in the untreated group. Although omeprazole also made similar changes in the expressions of the enzymes, however, the effect was much less.
Figure 5.
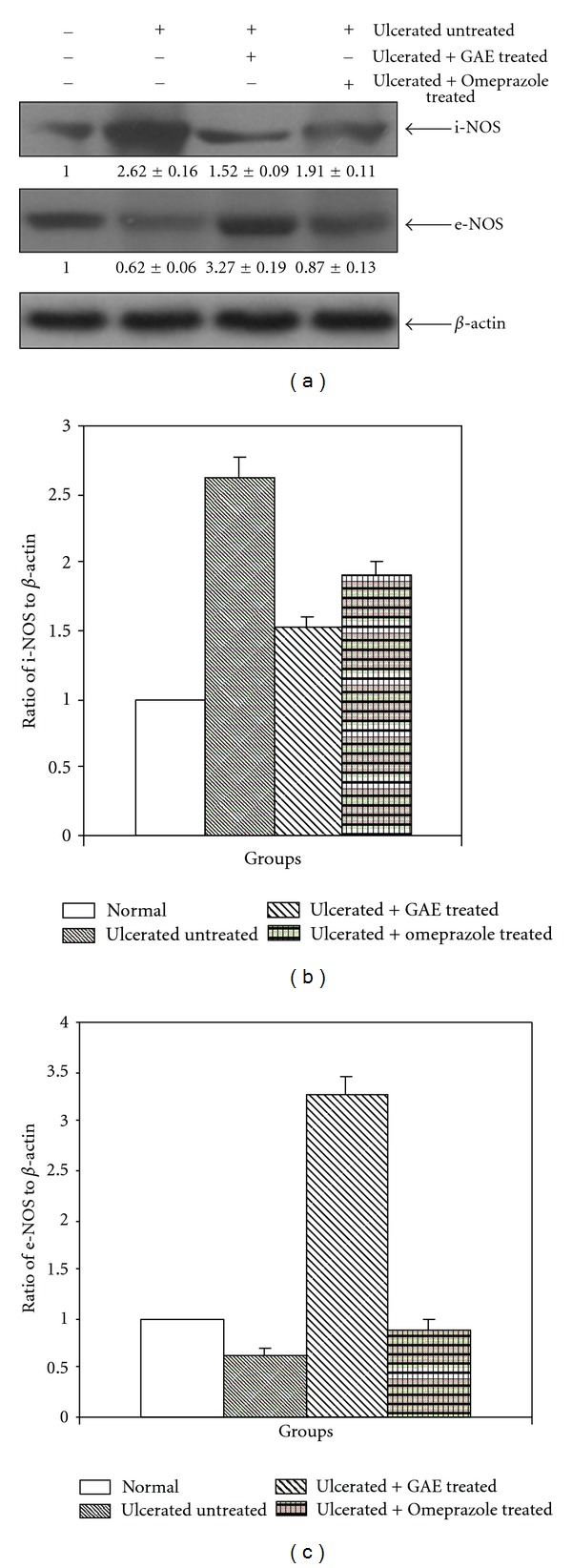
The e-NOS and i-NOS expressions in normal, ulcerated and GAE (5 mg/kg, single dose daily × 3 days, p. o.) or omeprazole (3 mg/kg, p. o.) treated gastric tissues of mice, and their quantifications. Western blots of the expressions of the enzymes (A). Ratios of the intensities of i-NOS (B) and e-NOS (C) bands to that of the respective β-actin bands as quantified from the western blot images, using Kodak Gelquant software. The values (arbitrary unit, mean ± S.E.M.) are the density scanning results of three independent experiments, considering that of normal mice as 1.
3.5. Modulation of Tissue NO Level
Compared to the normal control group, the tissue NO level in the ulcerated untreated mice was suppressed by 69.4% (Figure 6). Compared to the untreated mice, the tissue NO level was markedly increased (2.2 fold) in the GAE-treated group, while omeprazole did not significantly alter this.
Figure 6.
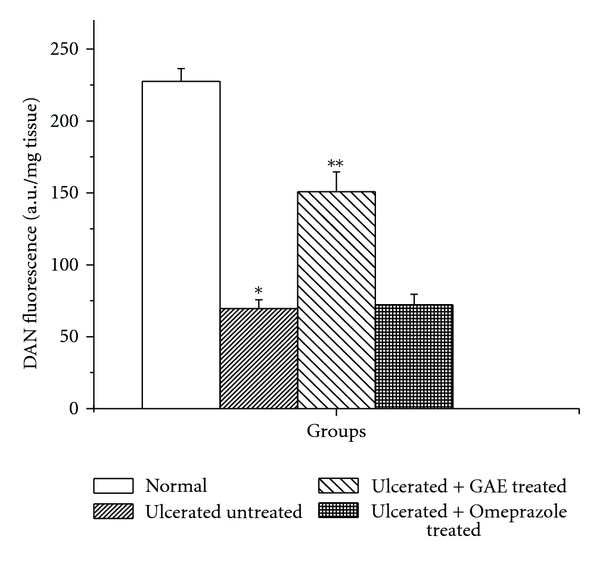
Effect of GAE on mucosal NO level in indomethacin-induced ulcerated mice. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose, p. o.). Treatment was carried out for 3 days with GAE (5 mg/kg, single dose daily × 3 days, p. o.) or omeprazole (3 mg/kg, p. o.) after ulcer induction. The mucosal NO levels of the ulcerated untreated and treated mice were measured. The values are mean ± S.E. of four independent experiments, each with 8 mice/group. *P < 0.001, compared to normal mice; **P < 0.001, compared to untreated mice.
3.6. Quantification of von Willebrand Factor (vWF) VIII
The microscopic results using immunostaining of the von Willebrand Factor VIII revealed the presence of 19.6 ± 1.15 microvessels/mm2 in submucosa of control mice. This increased to 24.9 ± 1.42 in the ulcerated untreated mice (Figure 7(a)). Treatment with GAE and omeprazole-enhanced the microvessel number by 51.6% and 27.3% respectively compared to that in the untreated mice.
Figure 7.
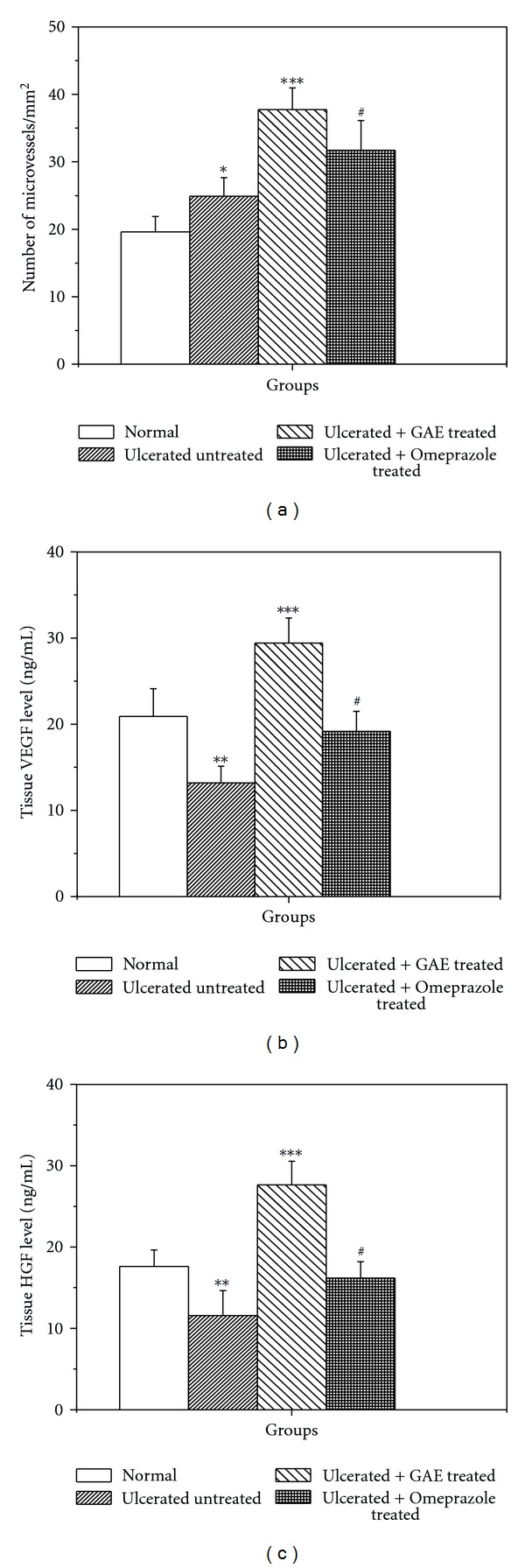
Effect of GAE on different angiogenic parameters in indomethacin-induced ulcerated mice. (a) von Willebrand Factor VIII; (b) mucosal VEGF level; (c) mucosal HGF level. Ulceration in the mice was induced by indomethacin (18 mg /kg, single dose, p. o.). Treatment was carried out for 3 days with GAE (5 mg/kg, single dose daily × 3 days, p. o.) or omeprazole (3 mg/kg, p. o.) after ulcer induction. The mucosal von Willebrand Factor VIII (expressed as number of microvessels/mm2) and growth factors (expressed as ng/ml tissue extract) were measured by immunohistochemistry and colorimetry, respectively. *P < 0.05, **P < 0.01, compared to normal mice; # P < 0.01, ***P < 0.001, compared to untreated mice.
3.7. Regulation of Growth Factors
Indomethacin administration downregulated the VEGF and HGF levels by 36.8% and 34.2%, respectively, compared to sham-treated mice. The VEGF and HGF levels in the GAE-treated group were increased 2.2-fold and 2.4-fold, respectively, compared to the untreated mice (Figures 7(b) and 7(c)). Omeprazole increased the VEGF and HGF levels by 45.1% and 39.6%, respectively, compared to the untreated mice.
3.8. Effect of NOS Inhibitors on the Healing Property of GAE
Treatment with L-NAME in the GAE-treated group significantly increased the damage score (50.7%) (Figure 8(a)) and MPO activity (78.9%) (Figure 8(b)), but reduced the mucosal NO level by 1.1-fold (Figure 8(c)) and microvessels numbers by 1.2-fold (Figure 8(d)) compared to the sole GAE-treated group. But none of these parameters changed significantly in the GAE + L-NIL group, compared to the GAE-treated group.
Figure 8.
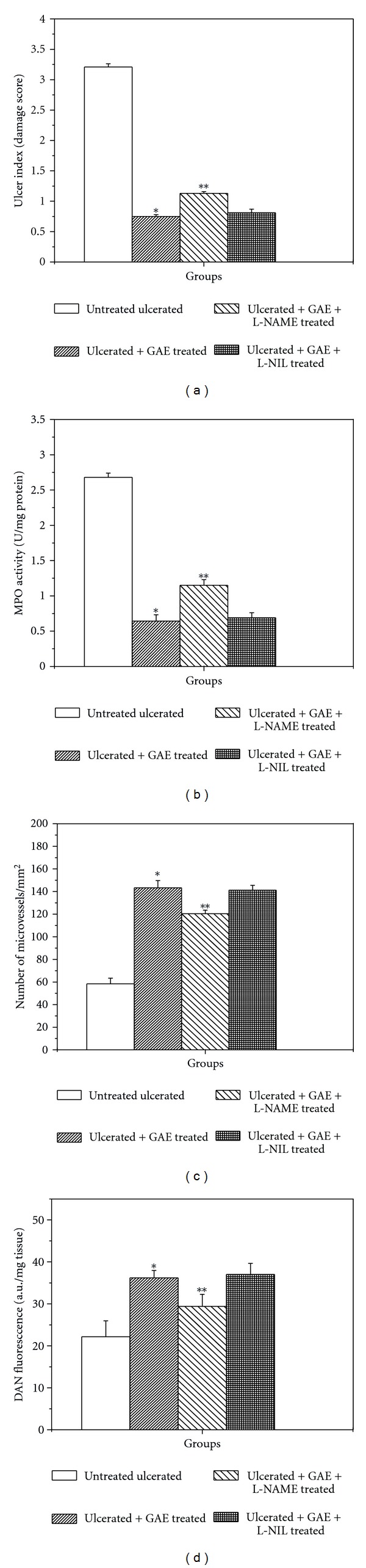
Effect of NOS inhibitors on the healing activity of GAE in indomethacin-induced ulcerated mice. (a) damage score, (b) MPO activity, (c) von Willebrand Factor VIII, (d) NO level. Ulceration in the mice was induced by indomethacin (18 mg /kg, single dose, p.o.). After ulcer induction, treatment was carried out with GAE (5 mg/kg, single dose daily, p. o.) alone or in conjunction with L-NAME (15 mg/kg, once daily) or L-NIL (3 mg/kg, twice daily) for 3 days. The parameters of the ulcerated untreated and treated mice were measured. *P < 0.001 compared to normal mice; **P < 0.01, compared to GAE-treatment.
4. Discussion
Several factors such as oxidative stress, neutrophils activation, as well as modulation of various enzymes, cytokines and soluble mediators play crucial roles in the indomethacin-mediated gastric ulceration and delayed ulcer healing [26]. Controlling these factors provides an opportunity to develop improved antiulcer medications. The gastrotoxicity of indomethacin is generally explained in terms of COX inhibition, reduced PG synthesis and the impaired PG-mediated angiogenesis. However, the process also involves alternate COX-independent mechanisms, wherein other contributors such as the nitrogen-metabolizing enzymes [13, 14] and neutrophil infiltration [27] determine the healing process.
The impressive healing capacity of the ethanolic extract of amla against the indomethacin-induced gastric ulcer [15] encouraged us to investigate the probable modulatory effect of the extract on the COX-dependent [28] and independent pathways [29] of wound healing. For this purpose, we used the gallic acid-enriched fraction (GAE) of the amla extract. In the present study, indomethacin administration led to mucosal damage and augmented the MPO activity in the ulcerated area of the gastric wall. Because MPO activity is increased by the activated neutrophils, the above results suggested the involvement of neutrophils infiltration in gastric ulceration. The MPO activity is known to increase under the ulcerated conditions, and reduced during the healing process [30]. It is often used as a risk marker and diagnostic tool for assessing severity of gastric ulcer [31]. Treatment with GAE could sufficiently restore the normal gastric mucosal integrity, while reducing the MPO activity. Earlier, the crude ethanolic extract of amla (60 mg/kg) showed similar healing activity as that of GAE (5 mg/kg). However, the GAE content of the crude extract (60 mg/kg) would be ~11 mg. Further, the extract was marginally more potent than pure gallic acid at the concentrations present in the effective dose of GAE. Taken together, these results established that gallic acid is the active constituent of GAE, but some other constituents of GAE may play synergistic roles in healing activity of GAE. These results also suggested a close relationship between the state of the gastric inflammation and MPO activity. Earlier pretreatment with an antibody against neutrophils was found to prevent the indomethacin-induced gastric ulceration [32]. Based on these, it is tempting to propose that indomethacin first stimulates the neutrophils to release substances which are related to inflammation. However, further studies are needed to clarify the sequence of events.
Besides indicating ulcer initiation and progression, neutrophils infiltration is also reported to delay gastric ulcer healing [33] and its reduction accelerates ulcer healing [34]. Oxygen-free radicals derived from the activated neutrophils delay gastric ulcer healing in rats [35]. Furthermore, neutrophils infiltration induces microcirculatory abnormalities [36] and its suppression promotes healing [37]. Hence, we used it as an oxidative marker in the present study.
The NSAIDs exert both therapeutic and toxic effects, mainly through reduction of the levels of circulating PGE2 at the gastric mucosa. Besides stimulating mucus and bicarbonate secretion and mucosal blood flow, PGs also contribute to ulcer healing by inducing angiogenesis [17]. The reduced PGE2 causes gastric ulceration and also exacerbates preexisting gastric ulcers in rodents and human [25]. Our data showed that indomethacin treatment depleted the tissue PGE2 level that was increased significantly in the drug-treated groups (Figure 4). The effect of GAE was better than that of omeprazole. Enhanced PG synthesis is known to inhibit neutrophils-mediated free radical generation [38]. Therefore, stimulation of PGE2 level by GAE might contribute to its antioxidative property, observed in the previous study.
The physiologically important NO, produced during arginine catabolism by the NOSs plays dual roles in gastric mucosal defense and injury. The low concentration of NO, produced by e-NOS, one of the constitutive NOS isoforms helps in wound healing by increasing blood flow [39] and angiogenesis [19, 40] in the damaged gastric mucosa. However, its enhanced generation by i-NOS may contribute to the pathogenesis of various gastroduodenal disorders including peptic ulcer [30]. An increase in i-NOS activity and a decrease in e-NOS activity in the gastric mucosa are closely related to the development of gastric mucosal lesions. Currently we confirmed that the indomethacin-induced gastric ulceration increased the mucosal i-NOS expression, but reduced the e-NOS expression in mice. Piotrowski et al. [41] showed a 12-fold increase in gastric epithelial expression of iNOS activity in the indomethacin-administered animals, compared to controls and the increase correlated positively with the epithelium damage. Our results showed only 1.3-fold increased i-NOS expression after ulceration. This may possibly be due to the fact that we assayed it on the 3rd day of ulceration. Despite the increased i-NOS expression, the tissue NO level was significantly reduced in the indomethacin group. The apparent discrepancy may be due the fact that i-NOS expression itself may not match with its activity. Also, the generated NO may be scavenged through NADPH oxidase or MPO catalyzed reactions [42]. The reduction of the beneficial vasodilatory NO would delay the ulcer healing. Treatment with GAE raised the e-NOS/i-NOS ratio to a level favourable for efficient ulcer healing. The associated increase in the tissue NO level must be derived through the e-NOS-catalyzed reaction, because GAE increased e-NOS, but not i-NOS expressions. Earlier, using e-NOS deficient mice, the importance of e-NOS and e-NOS-derived NO in regulating microvascular structure during acute inflammation has been demonstrated [43].
Gastric ulcer healing entails several distinct repair mechanisms. The epithelial cell proliferation and migration from the ulcer edge across the ulcer bed is accompanied by maturation of granulation tissue beneath the ulcer base. Within this tissue vascular endothelial cells form new capillaries to restore the microvasculature, while fibroblasts restore the lamina propria. The degree of neovascularisation (angiogenesis), assessed by specific endothelial markers including von Willebrand Factor VIII, CD31, and CD34 in experimental ulcer models correlates well with the extent and speed of ulcer healing. Among these markers, von Willebrand Factor VIII acts as a cofactor for platelet binding to expose extracellular matrix in injured vessel walls. A large number of factors including several growth factors regulate angiogenic wound healing at its various stages [18, 44, 45]. Amongst these, VEGF triggers endothelial proliferation and migration and accelerate ulcer healing by promoting angiogenesis [14, 46]. Likewise HGF, expressed at the ulcer margin to act as trophic factors for the gastric mucosa helps angiogenesis by multiple mechanisms including COX activation [47]. Hence, we focused on these growth factors for the present studies.
Our result on the increased number of mucosal von Willebrand Factor VIII in the ulcerated mice over that of normal control mice is consistent with the requirement of more microvessels for ulcer healing. The increased number of microvessels would assist better blood flow and transport of oxygen and nutrients to the site of inflammation for quicker healing. Treatment with GAE increased the von Willebrand Factor VIII further. This explains the accelerated ulcer healing by GAE, compared to natural healing. The results are consistent with our previous finding with a resveratrol-analogue that also increased the e-NOS/i-NOS ratio to provide better angiogenesis [25]. Indomethacin inhibits ADP-induced platelet aggregation and release of the α-granule, which stores VEGF. Consequently, indomethacin treatment would reduce VEGF release. We also found that indomethacin administration suppressed the levels of VEGF and HGF. Both these parameters were increased significantly beyond the respective normal values by GAE treatment (Figures 7(b) and 7(c)).
Enhanced synthesis of mucosal PGE2 and e-NOS-derived NO by GAE might be instrumental in their ulcer-healing action. On the other hand, omeprazole did not show any significant effect on NO synthesis (data not shown).
To substantiate our hypothesis that modulation of e-NOS may primarily account for the excellent ulcer healing capacity of GAE, we studied the effects of L-NIL, a specific i-NOS inhibitor and L-NAME, a nonspecific NOS inhibitor on the healing capacities of GAE. For this, we assessed four different parameters, namely, (i) ulcer index, (ii) MPO activity, (iii) von Willebrand Factor VIII, and (iv) tissue NO level of the GAE-treated mice in the absence and presence of the above inhibitors. Since i-NOS expression was effectively inhibited by GAE alone (Figure 5), addition of L-NIL did not alter any of these parameters significantly. However, addition of L-NAME would suppress both e-NOS and i-NOS expressions, negating the augmented e-NOS expression, caused by GAE. Consistent with this, treatment with GAE in conjunction with L-NAME led to increased ulcer index and MPO activity with associated decrease in von Willebrand Factor VIII and tissue NO level, compared to that with the only GAE-treated mice (Figure 8). Taken together our results established that the e-NOS-derived NO contributed maximum to the ulcer healing property of GAE, although a role for neuronal NOS-derived NO cannot be excluded.
5. Conclusion
Overall, gallic acid was the active principle of the gallic acid enriched ethanolic amla extract (GAE) that promoted healing of indomethacin-induced gastric ulcers in mice. The beneficial effect of GAE was due to its ability to reduce neutrophils infiltration and increase mucosal PGE2 as well as NO levels that were downregulated by indomethacin. GAE increased the mucosal NO by augmenting the e-NOS/i-NOS ratio. All these factors, especially the modulation of the NOS-pathway helped in upregulating mucosal VEGF and HGF levels to promote angiogenesis and accelerate ulcer healing (Figure 9). Our present and previous results with amla extract, coupled with its nontoxic nature, suggest GAE as a promising antiulcerogenic formulation and opened the way for further evaluation.
Figure 9.
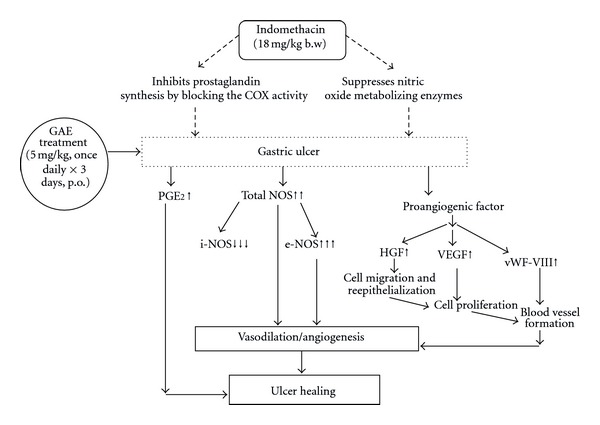
Schematic representation of the plausible mechanism of the ulcer healing by GAE.
Supplementary Material
Supplementary Figure 1: Effect of GAE on total antioxidant status (A) and lipid peroxidation (B) in indomethacininduced ulcerated mice. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose). Treatment was carried out for 3 days with GAE (5 mg/kg, daily) or omeprazole (3 mg/kg) after ulcer induction.
Supplementary Figure 2: Effect of L-NAME in indomethacin -induced ulcerated mice. A: damage score, B: MPO activity, C: von Willebrand Factor VIII, D: NO level. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose). After ulcer induction, treatment was carried out with L-NAME (15 mg/kg, once daily) for 3 days.
Acknowledgments
A. Chatterjee is thankful to Board of Research in Nuclear Science (BRNS), Department of Atomic Energy (DAE), GOI for providing Fellowship. Authors would like to acknowledge Director, IPGME&R, Kolkata, and Director, KPC Medical College and Hospital, Kolkata, for providing infrastructural facilities, encouragement and kind support; Dr. Subrata Nag (Department of Physiology, IPGME&R, Kolkata) for analysis of the histological data; Dr. Biplab Adhikary for providing support in western blot development by chemiluminescence method. The authors also thank the editor and reviewers for their valuable comments and suggestions. The work was financially supported by Board of Research in Nuclear Science (BRNS), Department of Atomic Energy, Government of India.
Abbreviations
- GAE:
Gallic acid enriched fraction
- NSAID:
Nonsteroidal anti-inflammatory drug
- PG:
Prostaglandin
- VEGF:
Vascular endothelial growth factor
- HGF:
Hepatocyte growth factor
- L-NIL:
L-N6-(1-iminoethyl) lysine hydrochloride
- L-NAME:
N-nitro-L-arginine methyl ester.
References
- 1.Khan KH. Roles of emblica officinalis in medicine—a review. Botany Research International. 2009;2(4):218–228. [Google Scholar]
- 2.Anonymous. Case study on Amla-related patent, technology information. Forecasting & Assessment Council (TIFAC) Bulletin. 2001;7:6–6. [Google Scholar]
- 3.Soong Y-Y, Barlow PJ. Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chemistry. 2006;97(3):524–530. [Google Scholar]
- 4.Gentile JM, Rahimi S, Zwiesler J, Gentile GJ, Ferguson LR. Effect of selected antimutagens on the genotoxicity of antitumor agents. Mutation Research. 1998;402(1-2):289–298. doi: 10.1016/s0027-5107(97)00308-4. [DOI] [PubMed] [Google Scholar]
- 5.Indap MA, Radhika S, Motiwale L, Rao KVK. Anticancer activity of phenolic antioxidants against breast cancer cells and a spontaneous mammary tumor. Indian Journal of Pharmaceutical Sciences. 2006;68(4):470–474. [Google Scholar]
- 6.Kratz JM, Andrighetti-Fröhner CR, Leal PC, et al. Evaluation of anti-HSV-2 activity of gallic acid and pentyl gallate. Biological and Pharmaceutical Bulletin. 2008;31(5):903–907. doi: 10.1248/bpb.31.903. [DOI] [PubMed] [Google Scholar]
- 7.Brooks P, Emery P, Evans JF, et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology. 1999;38(8):779–788. doi: 10.1093/rheumatology/38.8.779. [DOI] [PubMed] [Google Scholar]
- 8.Gladding PA, Webster MWI, Farrell HB, Zeng ISL, Park R, Ruijne N. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. American Journal of Cardiology. 2008;101(7):1060–1063. doi: 10.1016/j.amjcard.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 9.Piazza GA, Keeton AB, Tinsley HN, et al. NSAIDs: old drugs reveal new anticancer targets. Pharmaceuticals. 2010;3(5):1652–1667. doi: 10.3390/ph3051652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanas A, Perez-Aisa MA, Feu F, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. American Journal of Gastroenterology. 2005;100(8):1685–1693. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 11.Tarnawski AS, Jones MK. Inhibition of angiogenesis by NSAIDs: molecular mechanisms and clinical implications. Journal of Molecular Medicine. 2003;81(10):627–636. doi: 10.1007/s00109-003-0479-y. [DOI] [PubMed] [Google Scholar]
- 12.Chan FKL. Primer: managing NSAID-induced ulcer complications—balancing gastrointestinal and cardiovascular risks. Nature Clinical Practice Gastroenterology and Hepatology. 2006;3(10):563–573. doi: 10.1038/ncpgasthep0610. [DOI] [PubMed] [Google Scholar]
- 13.Isenberg JI, McQuaid KR, Laine L, Rubin W. Acid-peptic disorders. In: Yamada T, Alpers DH, Owyng C, Powell DW, Silverstein FE, editors. Textbook of Gastroenterology. Vol. 1. 1991. p. p. 1253. [Google Scholar]
- 14.Tarnawski A, Szabo IL, Husain SS, Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. Journal of Physiology Paris. 2001;95(1-6):337–344. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee A, Chattopadhyay S, Bandyopadhyay SK. Biphasic effect of Phyllanthus emblica L. extract on NSAID-induced ulcer: an antioxidative trail weaved with immunomodulatory effect. Evidence-Based Complementary and Alternative Medicine. 2011;2011:13 pages. doi: 10.1155/2011/146808.146808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharya S, Chatterjee S, Bauri A, et al. Immunopharmacological basis of the healing of indomethacin-induced gastric mucosal damage in rats by the constituents of Phyllanthus emblica . Current Science. 2007;93(1):47–53. [Google Scholar]
- 17.Jones MK, Wang H, Peskar BM, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nature Medicine. 1999;5(12):1418–1423. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87(7):1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. American Journal of Physiology. 2000;279(2):G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee D, Maity B, Bauri AK, Bandyopadhyay SK, Chattopadhyay S. Gastroprotective properties of Myristica malabarica against indometacin-induced stomach ulceration: a mechanistic exploration. Journal of Pharmacy and Pharmacology. 2007;59(11):1555–1565. doi: 10.1211/jpp.59.11.0014. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee D, Bauri AK, Guha RK, Bandyopadhyay SK, Chattopadhyay S. Healing properties of malabaricone B and malabaricone C, against indomethacin-induced gastric ulceration and mechanism of action. European Journal of Pharmacology. 2008;578(2-3):300–312. doi: 10.1016/j.ejphar.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Dokmeci D, Akpolat M, Aydogu N, Doganay L, Turan FN. L-Carnitine inhibits ethanol-induced gastric mucosal injury in rats. Pharmacological Reports. 2005;57:481–488. [PubMed] [Google Scholar]
- 23.Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Analytical Biochemistry. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Guha P, Dey A, Chatterjee A, Chattopadhyay S, Bandyopadhyay SK. Pro-ulcer effects of resveratrol in mice with indomethacin-induced gastric ulcers are reversed by L-arginine. British Journal of Pharmacology. 2010;159:726–734. doi: 10.1111/j.1476-5381.2009.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorucci S, Antonelli E, Morelli A. Mechanism of non-steroidal anti-inflammatory drug-gastropathy. Digestive and Liver Disease. 2001;33(2):S35–S43. doi: 10.1016/s1590-8658(01)80157-2. [DOI] [PubMed] [Google Scholar]
- 27.Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- 28.Langenbach R, Morham SG, Tiano HF, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid- induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83(3):483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 29.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB Journal. 2001;15(12):2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 30.Souza MHLP, Paula Lemos H, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour factor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53(6):791–796. doi: 10.1136/gut.2002.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87(6):1344–1350. [PubMed] [Google Scholar]
- 32.Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. American Journal of Physiology. 1990;259(3):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- 33.Fujita H, Takahashi S, Okabe S. Mechanism by which indomethacin delays the healing of acetic acid- induced ulcers in rats. Role of neutrophil antichemotactic and chemotactic activities. Journal of Physiology and Pharmacology. 1998;49(1):71–82. [PubMed] [Google Scholar]
- 34.Shimizu N, Watanabe T, Arakawa T, Fujiwara Y, Higuchi K, Kuroki T. Pentoxifylline accelerates gastric ulcer healing in rats: roles of tumor necrosis factor alpha and neutrophils during the early phase of ulcer healing. Digestion. 2000;61(3):157–164. doi: 10.1159/000007752. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki Y, Ishihara M, Segami T, Ito M. Anti-ulcer effects of antioxidants, quercetin, α-tocopherol, nifedipine and tetracycline in rats. Japanese Journal of Pharmacology. 1998;78(4):435–441. doi: 10.1254/jjp.78.435. [DOI] [PubMed] [Google Scholar]
- 36.Bou-Abboud CF, Wayland H, Paulsen G, Guth PH. Microcirculatory stasis precedes tissue necrosis in ethanol-induced gastric mucosal injury in the rat. Digestive Diseases and Sciences. 1988;33(7):872–877. doi: 10.1007/BF01550978. [DOI] [PubMed] [Google Scholar]
- 37.Tsukimi Y, Nozue C, Okabe S. Effects of leminoprazole, omeprazole and sucralfate on indomethacin-induced delayed healing of kissing gastric ulcers in rats. Journal of Gastroenterology and Hepatology. 1996;11(4):335–340. doi: 10.1111/j.1440-1746.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 38.Gryglewski RJ, Szczeklik A, Wandzilak M. The effect of six prostaglandins, prostacyclin and iloprost on generation of superoxide anions by human polymorphonuclear leukocytes stimulated by zymosan or formyl-methionyl-leucyl-phenylalanine. Biochemistry Pharmacology. 1987;36:4209–4213. doi: 10.1016/0006-2952(87)90660-5. [DOI] [PubMed] [Google Scholar]
- 39.Whittle JR. Nitric oxide in gastrointestinal physiology and pathology. In: Johnson LR, editor. The Physiology of the Gastrointestinal Tract. New York, NY, USA: Raven; 1994. pp. 267–294. [Google Scholar]
- 40.Ziche M, Morbidelli L, Masini E, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. Journal of Clinical Investigation. 1994;94(5):2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piotrowski J, Slomiany A, Slomiany BL. Activation of apoptotic caspase-3 and nitric oxide synthase-2 in gastric mucosal injury induced by indomethacin. Scandinavian Journal of Gastroenterology. 1999;34(2):129–134. doi: 10.1080/00365529950172961. [DOI] [PubMed] [Google Scholar]
- 42.Morton J, Coles B, Wright K, et al. Circulating neutrophils maintain physiological blood pressure by suppressing bacteria and IFNγ-dependent iNOS expression in the vasculature of healthy mice. Blood. 2008;111(10):5187–5194. doi: 10.1182/blood-2007-10-117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo JC, Shin VY, Liu ESL, et al. Non-ulcerogenic dose of dexamethasone delays gastric ulcer healing in rats. Journal of Pharmacology and Experimental Therapeutics. 2003;307(2):692–698. doi: 10.1124/jpet.103.055202. [DOI] [PubMed] [Google Scholar]
- 44.Folkman J, Shin Y. Angiogenesis. Journal of Biological Chemistry. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 45.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–673. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 46.Takahashia M, Oguraa K, Maedaa S, et al. Promoters of epithelialization induce expression of vascular endothelial growth factor in human gastric epithelial cells in primary culture. FEBS Letter. 1997;418:115–118. doi: 10.1016/s0014-5793(97)01354-9. [DOI] [PubMed] [Google Scholar]
- 47.Brzozowski T, Konturek PC, Konturek SJ, et al. Involvement of cyclooxygenase (COX)-2 products in acceleration of ulcer healing by gastrin and hepatocyte growth factor. Journal of Physiology and Pharmacology. 2000;51(4):751–773. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Effect of GAE on total antioxidant status (A) and lipid peroxidation (B) in indomethacininduced ulcerated mice. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose). Treatment was carried out for 3 days with GAE (5 mg/kg, daily) or omeprazole (3 mg/kg) after ulcer induction.
Supplementary Figure 2: Effect of L-NAME in indomethacin -induced ulcerated mice. A: damage score, B: MPO activity, C: von Willebrand Factor VIII, D: NO level. Ulceration in the mice was induced by indomethacin (18 mg/kg, single dose). After ulcer induction, treatment was carried out with L-NAME (15 mg/kg, once daily) for 3 days.


