Abstract
Objective
To examine the association between nocturnal sleep duration and weight and caloric intake outcomes among preschool-aged children who are obese and enrolled in a family-based weight management program.
Methods
Forty-one preschool-aged children who were obese (BMI ≥95th percentile) and enrolled in a weight management program completed pre- and posttreatment assessments of body mass, caloric intake, and sleep. Separate linear regression analyses examined the relationship between nocturnal sleep duration and posttreatment body mass index relative to ageand sex-linked norms (BMIz) and caloric intake.
Results
After controlling for pretreatment BMIz, longer posttreatment nocturnal sleep was significantly associated with lower posttreatment BMIz (β=-0.21, p=0.02) and explained a significant proportion of unique variance in posttreatment BMIz (ΔR2=0.04). Similarly, after controlling for pretreatment caloric intake, longer nocturnal sleep duration at posttreatment was significantly associated with lower caloric intake at posttreatment (β=-0.45, p=0.003) and explained a significant proportion of unique variance in posttreatment caloric intake (ΔR2=0.19).
Conclusions
These findings extend the literature on the sleep and weight relationship and suggest that adequate sleep may be an important element in interventions for preschoolers with obesity.
Keywords: obesity, weight management, sleep, pediatrics, diet, children, behavior, intervention
Obesity affects 12.1% of preschool-aged children (2-5 years old) (1) and is associated with negative health consequences (2). Preschoolers who are obese are more likely to remain overweight and obese in adolescence (3) and adulthood (4) than preschoolers who are at a healthy weight. Obesity interventions for preschoolers have generally targeted diet and activity and have had positive but modest outcomes, suggesting a need to further explore potentially complementary or synergistic targets for treatment (5). Sleep duration may be one such target. Short sleep has been associated with obesity across age ranges (6) and has been linked to increased caloric intake in adolescents (7) and adults (8).
In preschool-aged children, shortened sleep is a correlate and prospective predictor of obesity (9), with the likelihood of obesity 1.19-2.25 times greater among children who sleep <10 hours compared to peers sleeping ≥10 hours nightly (10). No published studies have assessed both sleep and caloric intake in preschool-aged children, and the few studies of the sleep-weight relationship in these children have relied heavily on retrospective, global caregiver reports of typical sleep behaviors (10-13) rather than prospective sleep diaries. Moreover, no published research has examined sleep in the context of obesity interventions for preschool-aged children, so the potential benefits of adding a sleep target to such interventions remain speculative.
To begin to fill this knowledge gap, this study capitalizes on secondary sleep data collected during a family-based obesity intervention for preschool-aged children that did not explicitly target sleep (14). Based on prior findings, we hypothesized that, at posttreatment, longer child sleep would be associated with better treatment outcomes, as indexed in lower ageand gender-corrected body mass index (BMI z-score) and lower caloric intake after controlling for pretreatment BMI z-score and caloric intake.
Methods
Participants
Participants who were two to five years of age (mean=4.7 years), had a BMI ≥95th percentile for age and sex, and had one parent with a BMI ≥25 enrolled in a larger study to develop and pilot test behavioral interventions for weight management. The larger study was an iterative process, the first iteration of which compared a home and clinic behavioral intervention to a one-time pediatrician counseling, and has been published (n=18) (14). Later iterations (n=42) included a third arm of clinic and behavioral intervention and are unpublished data. Because of research constraints of the larger study (14), families were excluded if they were non-English speaking, lived >50 miles (>80 kilometers) from the medical center, or if the child had a medical condition or was taking medication known to affect appetite/weight, had a disability limiting physical activity, or was enrolled in another weight-control program. Of the 60 enrolled, 51 attended at least one treatment session from which analyses were conducted on 41 (80%); six dropped out mid-study, and four had incomplete or missing sleep data.
Procedure
This study was approved by the Institutional Review Board at Cincinnati Children's Hospital Medical Center and informed consent was obtained from parents. Participating families completed pre- and posttreatment (six-months) weight, sleep/activity, and dietary assessments. Following the baseline assessment, families were randomized to one of three treatment arms, none of which targeted parent or child sleep behaviors: Clinic+Home (18 sessions), Clinic Only (10 sessions), or Pediatrician treatment (1 session). All parents were educated on the American Academy of Pediatrics (AAP) guidelines for diet and physical activity. The Pediatrician condition consisted of one 45-minute individual education session with a pediatrician discussing diet and activity. The Clinic+Home and the Clinic Only conditions included a behavioral management element delivered in 90-minute clinic group sessions and the Clinic+Home condition included home visits between group sessions. To maximize statistical power, data was collapsed across all three treatment conditions for current analyses.
Measures
Each child's sleep at posttreatment was assessed via sleep/activity diaries completed by parents that queried sleep onset and offset daily for one week. Nocturnal sleep duration (offset minus onset) was averaged across a mean 6.98 (sd=0.12) nights of available data on each child. Each child's caloric intake was averaged over three 24-hour diet recalls (two weekdays and one weekend day) completed by parents over a two-week period using the previously-validated multiple-pass method (15) and analyzed via the Minnesota Nutrient Data System for Research (NDSR) software, version 5.0 (University of Minnesota, Minneapolis, MN, USA). Child weight and height were measured in triplicate following standard anthropometric procedures and BMI was transformed into age- and sex-adjusted z-score (BMIz) and percentiles (16). Parents provided socio-demographic information (gender, race/ethnicity, age, parent education and occupation). Parent socioeconomic status (SES) was assessed with the Hollingshead Four-Factor Index of Social Status (17) and classified into five categories ranging from I=unskilled laborers and service workers to V=major business and professional.
Results
Participants were 58% female, 88% Caucasian, primarily from middle to higher SES families, 4.66±0.96 years old (mean±sd), and obese (median BMI percentile=99th; mean BMIz=2.36±0.53) pretreatment with a modest overall decline in BMIz from pre- to posttreatment (Table 1). Nocturnal sleep duration at pre- and posttreatment ranged from approximately 9-12 hours, similar to same-aged children in the US population (18).
Table 1.
Sample characteristics (n = 41)
| Age at baseline (months) | 55.90 ± 11.48 |
| Female Sex | 24 (58.54%) |
| Baseline BMIz | 2.36 ± 0.53 |
| Posttreatment BMIz | 2.17 ± 0.55 |
| Baseline Nocturnal Sleep (hour) | 10.11 ± 0.62 |
| Posttreatment Nocturnal Sleep (hour) | 10.02 ± 0.79 |
| Baseline Caloric Intake (kcal) | 1437.03 ± 289.20 |
| Posttreatment Caloric Intake (kcal) | 1402.40 ± 327.90 |
| Ethnicity | |
| White | 36 (87.80%) |
| Black | 3 (7.32%) |
| Multi-racial /Other | 2 (4.88%) |
| Participating Parent, Mothers | 41 (100%) |
| Hollingshead Classification | |
| I (8-19) | 1 (2.44%) |
| II (20-29) | 2 (4.88%) |
| III (30-39) | 6 (14.63%) |
| IV (40-54) | 21 (51.22%) |
| V (55-66) | 11(26.83%) |
Note. Frequency (%) shown for categorical variables; mean ± SD shown for continuous variables; BMIz = body mass index for age- and sex-linked norms; kcal = kilocalories
Preliminary bivariate tests indicated that none of the potential demographic confounds (child age, sex, ethnicity, SES) was significantly associated with any of the primary outcome measures: posttreatment BMIz, caloric intake, and nocturnal sleep duration (p >0.10). To maximize statistical power, demographics were consequently trimmed from final, linear regression analyses, which regressed posttreatment BMIz and caloric intake (in separate analyses) on pretreatment BMIz or caloric intake, followed in each case by posttreatment sleep duration. Controlling for pretreatment BMIz, posttreatment nocturnal sleep duration significantly predicted posttreatment BMIz, β=-0.21, t(40)=-2.52, p=0.02, explaining a small but unique proportion of variance, ΔR2=0.04. Each hour of nocturnal sleep was associated with a 0.14 unit decrease in posttreatment BMIz. Controlling for pretreatment caloric intake, posttreatment nocturnal sleep duration significantly predicted posttreatment caloric intake, β=-0.45, t(40)=-3.14, p=0.003, explaining a moderate unique proportion of variance, ΔR2=0.18. Each hour of nocturnal sleep was associated with 186 fewer calories consumed daily at posttreatment. All data were analyzed using SAS version 9.2 (SAS Institute, Cary North Carolina).
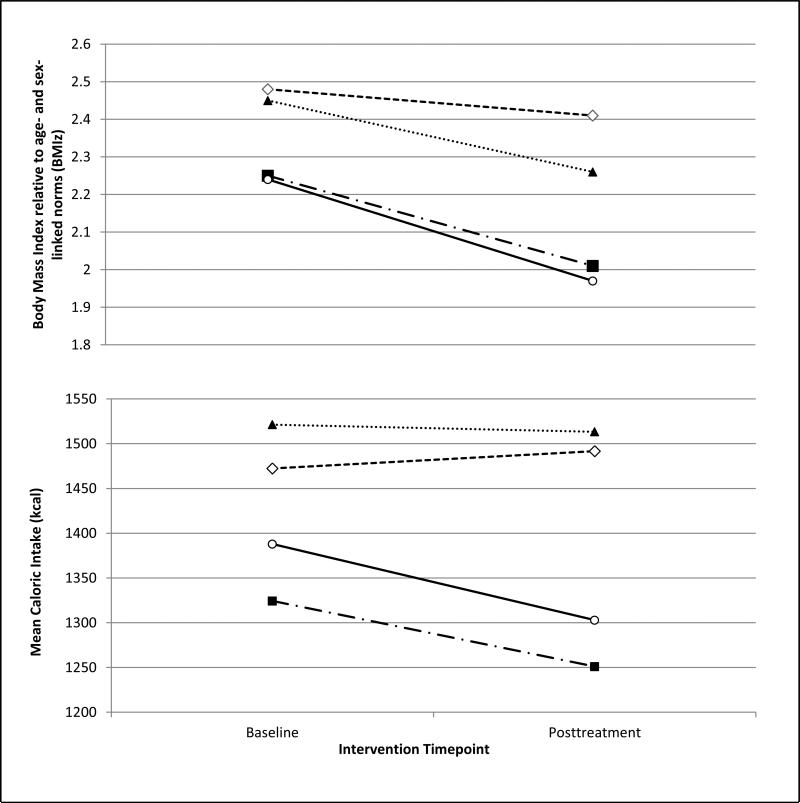
Finally, we explored whether these significant primary effects were related to changes in sleep across treatment (e.g., inadvertent impact of treatment on sleep). Using the previously established 10-hour benchmark (10, 12, 19), participants were categorized into four groups based on nocturnal sleep duration: ”Stable Longer Sleep” (≥10-hours at pre- and posttreatment), ”Stable Shorter Sleep” (≥10-hours at pre- and posttreatment), ”Increased Sleep” (pretreatment<10-hours; posttreatment≥10-hours), and ”Decreased Sleep” (pretreatment ≥10-hours; posttreatment <10-hours). Although the small sample precluded formal statistical analyses, Figure 1 provides a visual display of the relationship between ≥10 hours of sleep at posttreatment and BMIz and caloric intake outcomes.
Figure 1.
Trajectories of BMIz scores and caloric intake over the course of intervention based on nocturnal sleep patterns over time: Stable Shorter Sleep (<10-hours at both baseline and posttreatment; n=11, 27%), Decreased Sleep (≥10-hours baseline but <10-hours posttreatment n=11, 27%); Increased Sleep (sleep <10-hours baseline but >10-hours posttreatment; n=6, 15%); and Stable Longer Sleep (sleep ≥10-hours at both baseline and posttreatment; n=13, 32%).
Discussion
In our sample of preschool children being treated for obesity, longer sleep duration at posttreatment was associated with better posttreatment weight outcomes. This finding, although preliminary, is consistent with prior general-population studies and suggests that interventions to improve sleep duration may compliment or facilitate obesity interventions that focus on diet and activity. At posttreatment each hour of nocturnal sleep was associated with a 0.14 unit lower BMI z-score in preschool children who underwent a short-term intervention for obesity. This is strikingly similar to findings reported by Snell and colleagues who noted that each hour of sleep in the general population of three to eight-year old children was associated with a 0.15 unit decrease in standardized BMI over the subsequent five-years (19). Sleep-related differences in caloric intake may be a key mechanism; at posttreatment, we found that each hour of sleep was associated with the intake of approximately 186 less calories a day, which could quickly accumulate overtime and result in extra weight.
While causal inferences cannot be drawn from correlational data, a convergence of observational and experimental findings with adults suggests an association between shortened sleep on appetite and dietary intake (20). Both behavioral and hormonal mechanisms for this association appear plausible: sleep deprivation is known to reduce inhibitory control, induce negative mood, and impact appetite--regulating hormones (20, 21). With children, the mechanisms may be more complex because sleep deprivation may impact behavior and physiology differentially across development, and parents, who heavily influence children's eating context may be independently affected by child sleep problems (22).
The findings of this study should be considered in the context of its limitations. First, the sample size for the current study was modest and may have obscured significance in some analyses, particularly analyses examining whether change in sleep duration was predictive beyond posttreatment sleep duration. Second, the restricted demographic variability of the families in our study limit the generalizability of findings. Diversity in race/ethnicity and SES should be considered in future research (23). Third, the current sleep assessment was limited to nocturnal sleep duration. Prior work has linked nocturnal sleep, not daytime napping, to obesity in young children (10, 24), but we recommend that future studies involve more comprehensive sleep assessments (e.g., objective assessments of sleep quality and quantity across the full 24-hour span). Finally, we caution that present findings were post-hoc and observational and, consequently, open to multiple interpretations. For example, rather than being causally related to calorie intake or weight, child sleep duration may be a marker of a family's ability to modify lifestyle behaviors for better health more broadly.
Given limitations, present findings are best viewed as promising, but preliminary. Even so, they clearly support the need for further research with improved methods, including a prospective evaluation on the relationship between sleep and changes in BMIz with a larger sample that include other variables that may also contribute to changes in BMIz, including physical activity and screen time.
Acknowledgments
Funding
This study was supported by the National Institutes of Health (K24 DK 059492 to L.J.S., T32 DK063929 to S.W.P.), the National Center for Research Resources at the National Institute of Health (UL1RR026314), and the National Center for Advancing Translational Sciences (8 UL1 TR000077-04).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Lisa M. Clifford, Ph.D., Department of Behavioral Medicine and Clinical Psychology, Cincinnati Children's Hospital Medical Center.
Methods for this study are based on a study first reported in Stark et al., 2011
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012 Feb 1;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Q, Ding ZY, Fong DY, Karlberg J. Blood pressure is associated with body mass index in both normal and obese children. Hypertension. 2000 Aug;36(2):165–70. doi: 10.1161/01.hyp.36.2.165. [DOI] [PubMed] [Google Scholar]
- 3.Nader PR, O'Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006 Sep;118(3):e594–601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 4.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002 Sep;76(3):653–8. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl ES, Clifford LM, Stark LJ. Obesity in preschoolers: behavioral correlates and directions for treatment. Obesity (Silver Spring) 2012 Jan;20(1):3–29. doi: 10.1038/oby.2011.201. [DOI] [PubMed] [Google Scholar]
- 6.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008 May;31(5):619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010 Sep;33(9):1201–9. doi: 10.1093/sleep/33.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011 Jul;19(7):1374–81. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 9.Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Arch Pediatr Adolesc Med. 2010 Sep;164(9):840–5. doi: 10.1001/archpediatrics.2010.143. [DOI] [PubMed] [Google Scholar]
- 10.Jiang F, Zhu S, Yan C, Jin X, Bandla H, Shen X. Sleep and obesity in preschool children. J Pediatr. 2009 Jun;154(6):814–8. doi: 10.1016/j.jpeds.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Locard E, Mamelle N, Billette A, Miginiac M, Munoz F, Rey S. Risk factors of obesity in a five year old population. Parental versus environmental factors. Int J Obes Relat Metab Disord. 1992 Oct;16(10):721–9. [PubMed] [Google Scholar]
- 12.von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep--a cross-sectional study. Int J Obes Relat Metab Disord. 2002 May;26(5):710–6. doi: 10.1038/sj.ijo.0801980. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe E, Lee JS, Kawakubo K. Associations of maternal employment and three-generation families with pre-school children's overweight and obesity in Japan. Int J Obes (Lond) 2011 Jul;35(7):945–52. doi: 10.1038/ijo.2011.82. [DOI] [PubMed] [Google Scholar]
- 14.Stark LJ, Spear S, Boles R, Kuhl E, Ratcliff M, Scharf C, et al. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity (Silver Spring) 2011 Jan;19(1):134–41. doi: 10.1038/oby.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenther PM, Dodd KW, Reedy J, Krebs-Smith SM. Most Americans eat much less than recommended amounts of fruits and vegetables. J Am Diet Assoc. 2006 Sep;106(9):1371–9. doi: 10.1016/j.jada.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000 Jun;8(314):1–27. [PubMed] [Google Scholar]
- 17.Hollingshead AB. Four factor index of social status. 1975. Unpublished Manuscript.
- 18.Mindell JA, Meltzer LJ, Carskadon MA, Chervin RD. Developmental aspects of sleep hygiene: findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Med. 2009 Aug;10(7):771–9. doi: 10.1016/j.sleep.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007 Jan-Feb;78(1):309–23. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 20.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010 Oct;24(5):687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004 Dec;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meltzer LJ, Mindell JA. Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: a pilot study. J Fam Psychol. 2007 Mar;21(1):67–73. doi: 10.1037/0893-3200.21.1.67. [DOI] [PubMed] [Google Scholar]
- 23.Milan S, Snow S, Belay S. The context of preschool children's sleep: racial/ethnic differences in sleep locations, routines, and concerns. J Fam Psychol. 2007 Mar;21(1):20–8. doi: 10.1037/0893-3200.21.1.20. [DOI] [PubMed] [Google Scholar]
- 24.Sekine M, Yamagami T, Hamanishi S, Handa K, Saito T, Nanri S, et al. Parental obesity, lifestyle factors and obesity in preschool children: results of the Toyama Birth Cohort study. J Epidemiol. 2002 Jan;12(1):33–9. doi: 10.2188/jea.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]