Abstract
Dental fluorosis occurs as a result of excess fluoride ingestion during tooth formation. Enamel fluorosis and primary dentin fluorosis can only occur when teeth are forming, and therefore fluoride exposure (as it relates to dental fluorosis) occurs during childhood. In the permanent dentition, this would begin with the lower incisors, which complete mineralization at approximately 2–3 years of age, and end after mineralization of the third molars. The white opaque appearance of fluorosed enamel is caused by a hypomineralized enamel subsurface; with more severe dental fluorosis, pitting and a loss of the enamel surface occurs, leading to secondary staining (appearing as a brown color). Many of the changes caused by fluoride are related to cell/matrix/mineral interactions as the teeth are forming. At the early maturation stage, the relative quantity of amelogenin protein is increased in fluorosed enamel in a dose-related manner. This appears to result from a delay in the removal of amelogenins as the enamel matures. In vitro, when fluoride is incorporated into the mineral, more protein binds to the forming mineral, and protein removal by proteinases is delayed. This suggests that altered protein/mineral interactions are in part responsible for retention of amelogenins and the resultant hypomineralization that occurs in fluorosed enamel. Fluoride also appears to enhance mineral precipitation in forming teeth, resulting in hypermineralized bands of enamel, which are then followed by hypomineralized bands. Enhanced mineral precipitation with local increases in matrix acidity may affect maturation stage ameloblast modulation, potentially explaining the doserelated decrease in cycles of ameloblast modulation from ruffleended to smooth-ended cells that occur with fluoride exposure in rodents. Specific cellular effects of fluoride have been implicated, but more research is needed to determine which of these changes are relevant to the formation of fluorosed teeth. As further studies are done, we will better understand the mechanisms responsible for dental fluorosis.
Excess fluoride ingestion results in dental fluorosis. The mechanisms affected by longterm chronic exposure to low levels of fluoride are likely to differ from those affected by acute exposures to high levels of fluoride [1–3]. Some mechanisms affected by lower chronic fluoride levels, resulting in enamel fluorosis, are likely to be specific to this uniquely mineralizing tissue, while others may also affect other cells and tissues.
Enamel fluorosis refers to fluoride-related al-terations in enamel, which occur duringenamel development. These alterations become more severe with increasing fluoride intake, and time of exposure. The severity of fluorosis is related to the concentration of fluoride in the plasma, considered to be in equilibrium with the tissue fluid that bathes the enamel organ [4, 5]. Plasma fluoride levels are influenced by many factors, including total fluoride intake, type of intake (i.e. ingested vs. inhaled), renal function, rate of bone metabolism, metabolic activity, etc. [6]. In addition to these variables, genetic factors have been shown to dictate the severity of enamel fluorosis in mice [7].
In humans, plasma fluoride concentrations resulting from long-term ingestion of 1–10 ppm fluoride in the drinking water range from 1 to 10 μmol/l. Fluorotic changes can be obtained in incisors of rodents drinking water containing 25–100 ppm fluoride; these doses also elevate plasma fluoride levels to 3-10 μmol/l, similar to those found to cause fluorosis in humans. A complicating factor in assessing the exact dose, or determining the stages of enamel formation most sensitive to fluoride, is that fluoride incorporated into bone is gradually released by continuous bone remodeling [5, 8]. Levels of plasma fluoride as low as 1.5 μmol/l (resulting from fluoride release from bone) are still capable of inducing mild enamel fluorosis in the rat incisor after the initial exposure ends [4, 8].
The effects of chronic fluoride exposure have also been linked to effects on other tissues and systems [9]. However, in this chapter, we will focus primarily on the effects of fluoride on tooth development. The largest body of research has investigated the effects of fluoride on enamel formation, with much less known about the potential effects of fluoride on dentin formation. Therefore, most of the focus will be on enamel fluorosis. The sections of this chapter comprise:
Clinical manifestation, treatment and prevention of dental fluorosis
Etiology and prevalence of dental fluorosis
Pathology, pathogenesis and mechanism of dental fluorosis
Clinical Manifestation, Treatment and Prevention of Dental Fluorosis
Clinical Manifestations of Dental Fluorosis
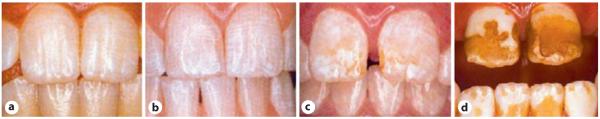
Clinically, mild cases of dental fluorosis are characterized by a white opaque appearance of the enamel, caused by increased subsurface porosity (fig. 1). The earliest sign is a change in color, showing many thin white horizontal lines running across the surfaces of the teeth, with white opacities at the newly erupted incisal end. The white lines run along the ‘perikymata’, a term referring to transverse ridges on the surface of the tooth, which correspond to the incremental lines in the enamel known as Striae of Retzius [10, 11].
Fig. 1.
Dental fluorosis. a Mild with slight accentuation of the perikymata. b Moderate, showing a white opaque appearance. c Moderate, white opaque enamel with some discoloration and pitting. d Severe.
At higher levels of fluoride exposure, the white lines in the enamel become more and more defined and thicker. Some patchy cloudy areas and thick opaque bands also appear on the involved teeth. With increased dental fluorosis, the entire tooth can be chalky white and lose transparency [10, 12]. With higher fluoride doses or prolonged exposure, deeper layers of enamel are affected; the enamel becomes less well mineralized. Damage to the enamel surface occurs in patients with moderate-to-severe degrees of enamel fluorosis. Teeth can erupt with pits, with additional pitting occurring with posteruptive enamel fracture.
In the individuals with moderate dental fluorosis, yellow to light brown staining is observed in the areas of enamel damage. In very severe cases, the enamel is porous, poorly mineralized, stains brown, and contains relatively less mineral and more proteins than sound enamel. Severely fluorosed enamel can easily chip posteruptively during normal mechanical use [13, 14]. Although teeth with mild dental fluorosis may be more resistant to dental decay because of the higher levels of fluoride contained in the enamel surface, severely fluorosed teeth are more susceptible to decay, most likely because of the uneven surface or loss of the outer protective layer [15].
Fluorosis Indices
In 1942, H.T. Dean developed an index to describe and diagnosis enamel fluorosis [16, 17]. He scored the fluorotic teeth into 6 categories according to their clinical manifestations, including normal teeth, which were given a score of 0 (table 1). Using this index, Dean [17, 18] determined the ‘optimal’ concentration of fluoride in drinking water (1 ppm), where caries incidence decreased and with a minimal level of dental fluorosis.
Table 1.
Fluorosis index of H.T. Dean (1942)
| Score | Criteria |
|---|---|
| Normal (0) | The enamel represents the usual translucent semivitriform type of structure. The surface is smooth, glossy, and usually of a pale creamy white color. |
| Questionable (0.5) |
The enamel discloses slight aberrations from the translucency of normal enamel, ranging from a few white flecks to occasional white spots. This classification is utilized in those instances where a definite diagnosis of the mildest form of fluorosis is not warranted and a classification of ‘normal’ is not justified. |
| Very mild (1) | Small opaque, paper white areas scattered irregularly over the tooth but not involving as much as 25% of the tooth surface. Frequently included in this classification are teeth showing no more than about 1–2 mm of white opacity at the tip of the summit of the cusps of the bicuspids or second molars. |
| Mild (2) | The white opaque areas in the enamel of the teeth are more extensive but do not involve as much as 50% of the tooth. |
| Moderate (3) | All enamel surfaces of the teeth are affected, and the surfaces subject to attrition show wear. Brown stain is frequently a disfiguring feature. |
| Severe (4) | Includes teeth formerly classified as ‘moderately severe and severe.’ All enamel surfaces are affected and hypoplasia is so marked that the general form of the tooth may be affected. The major diagnostic sign of this classification is discrete or confluent pitting. Brown stains are widespread and teeth often present a corroded-like appearance. |
As reproduced in National Academy of Sciences [p.169, 16].
This classification is still the ‘gold standard’, though other indices have been developed–including the widely used Thylstrup and Fejerskov Fluorosis Index (TFI) [19], which has an expanded range for the more severe forms of dental fluorosis. This index is a 10-point classification system to characterize dental fluorosis affecting bucal/lingual and occlusal surfaces and correlates visual assessment with polarized and light microscopic analysis [19]. Dean’s index is expanded to include: mild (TFI = 1–3), moderate (TFI = 4–5) and severe (TFI = 6–9) [19].
Treatment of Dental Fluorosis
The treatments for fluorotic teeth are limited. For the mildest forms of fluorosis (TFI 1, 2) bleaching can be recommended. Treatments for moderate dental fluorosis include microabrasion, where the outer affected layer of enamel is abraded from the tooth surface in an acidic environment. Composite restorations combined with microabrasion or application of aesthetic veneers can be used for the patients with TFI ≥5, while for the cases with TFI 8–9, prosthetic crowns may be necessary [19].
Prevention of Dental Fluorosis
Dental fluorosis can be limited or prevented by following the ‘recommended limits for fluoride exposure’, suggested by US Environmental Protection Agency (USEPA) [20]. The reference dose suggested by USEPA is 0.06 mg fluoride/kg/ day, which is the estimate of daily exposure that is likely to be without any appreciable risk of deleterious effects (any degrees of dental fluorosis) during a lifetime [20].
Specific guidelines for different ages (table 2) were published by the US Food and Nutrition Board of the Institute of Medicine in 1997, recommending total daily fluoride intakes [21]. In this guideline, the suggested total daily exposure dosage for infants younger than 6 months of age of 0.01 mg fluoride/day in all drinks and food is lower than the USEPA recommended reference dose. These guidelines suggest greater attention should be given to the total fluoride intake of infants from water used to dilute infant formulas, foods and other supplement sources.
Table 2.
Dietary reference intakes for fluoride
| Age groups | Reference weight, kg (lb) |
Adequate intake, mg/day |
Tolerable upper intake, mg/day |
|---|---|---|---|
| Infants 0–6 months | 7 (16) | 0.01 | 0.7 |
| Infants 7–12 months | 9 (20) | 0.5 | 0.9 |
| Children 1–3 years | 13 (29) | 0.7 | 1.3 |
| Children 4–8 years | 22(48) | 1.0 | 2.2 |
| Children 9–13 years | 40 (88) | 2.0 | 10 |
| Boys 14–18 years | 64 (142) | 3.0 | 10 |
| Girls 14–18 years | 57 (125) | 3.0 | 10 |
| Males ≥19 years | 76 (166) | 4.0 | 10 |
| Females ≥19 years | 61 (133) | 3.0 | 10 |
US National Academy of Sciences. Institute of Medicine. Food and Nutrition Board
Etiology and Prevalence of Dental Fluorosis
There are multiple sources of fluoride and all have the potential to cause dental fluorosis – including natural fluoride, artificial or added fluoride in drinking water and dental products, as well as occupation-related exposures.
Natural Sources of Fluoride Causing Dental Fluorosis
Dental fluorosis resulting from high fluoride levels in underground water is an issue in specific regions of the world. Fluoride can exist in an ionized form in ground waters, and in areas where the soil lacks calcium – such as occurs in areas with high levels of granite or gneiss – relatively high fluoride levels are detected in groundwater. When the level of fluoride is above 1.5 mg/l (1.5 ppm) in drinking water, dental fluorosis can occur. In some parts of Africa, China, the Middle East and southern Asia (India, Sri Lanka), as well as some areas in the Americas and Japan, high concentrations of ionic fluoride have been found in ground waters, vegetables, fruit, tea and other crops, although drinking water is usually the major source of the daily fluoride intake [22]. The atmosphere in these areas may have high levels of fluoride from dust in areas with fluoride-containing soils and gas, released from industries, underground coal fires and volcanic activities [22].
In the USA, approximately 10 million people are exposed to naturally fluoridated public water. In 1993, it was reported that 6.7 million people drank water with fluoride concentrations ≤1.2 mg/l, 1.4 million drank water with 1.3–1.9 mg/l fluoride, 1.4 million drank water with fluoride between 2.0 and 3.9 mg/l and 200,000 people ingested water with fluoride concentrations ≥4.0 mg/l [16]. Some areas have extremely high concentrations of fluoride in drinking water – such as in Colorado (11.2 mg/l), Oklahoma (12.0 mg/l), New Mexico (13.0 mg/l) and Idaho (15.9 mg/l) [9] – though water with levels higher that those recommended by the USEPA are monitored and are not used for human consumption.
Additional Sources of Fluoride Associated with Dental Fluorosis
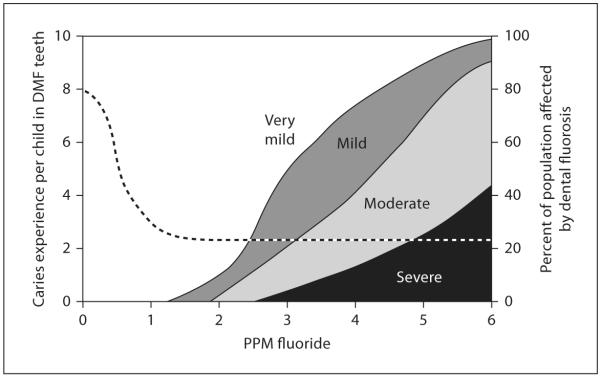
Two primary sources have been identified as being potentially responsible for the prevalence of dental fluorosis: fluoride in drinking water and fluoride-containing dental products (including fluoride supplements). Since 1945, fluoride has been used as a supplement in many public drinking water systems to control dental decay [23]. In 2000, approximately 162 million people (65.8% of the population served by public water systems) received water that contained fluoride ranging from 0.7 to 1.2 mg/l (usually 1 mg/l), depending on the local climate. The level of fluoridation is lower in high-temperature areas as people usually drink more water. The fluoridation of public drinking water has significantly decreased the incidence of dental decay at a relatively low cost. In the studies by Dean and colleagues completed in the 1930s, the risk of dental fluorosis at 1 ppm fluoride in drinking water was extremely low, particularly in relation to the impact of fluoride on dental caries (fig. 2) [24]. Following these studies, water fluoridation was considered by the US Centers for Disease Control to be 1 of the 10 great public health achievements in the 20th century [25].
Fig. 2.
Concentrations of fluoride in drinking water are related to caries incidence in children and severity of dental fluorosis. Adapted from a report of the Department of Health and Human Services of US (1991) [24].
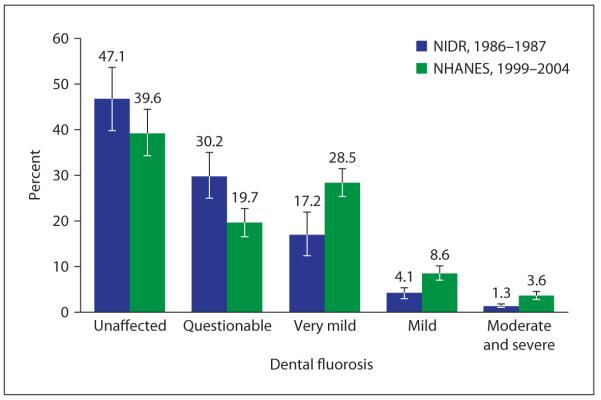
However, as fluoride has become more widely used in dental products (toothpastes, mouth rinses, fluoride supplements) and been incorporated into food sources (via fluoridated water), multiple sources of fluoride exposure are now related to the reported increase in the incidence of dental fluorosis. Even a small ‘pea-sized’ amount of toothpaste containing 1,450 ppm fluoride, would contain approximately 0.36–0.72 mg fluoride, which if consumed twice a day could contribute to fluoride levels that would increase the risk of dental fluorosis in children [26]. In the USA, the prevalence of dental fluorosis appears to be increasing. In children aged 15–17 years, the 1999–2004 National Health and Nutrition Examination Survey (NHANES) found 40.6% had very mild or greater enamel fluorosis, up from 22.6% in the 1986–1987 study (fig. 3) [27].
Fig. 3.
Change in dental fluorosis prevalence among children aged 12–15 years participating in 2 national surveys in the USA (1986–1987 and 1999–2004). Dental fluorosis (based on Dean’s fluorosis index) is defined as: very mild, mild, moderate or severe. Percentages do not sum to 100 due to rounding. Error bars = 95% CI. Sources: National Health and Nutrition Examination Survey (1999–2004) [27] and National Survey of Oral Health in U.S. School Children (1986–1987) [27].
The incidence of very mild and greater fluorosis in persons aged 6–39 years was 19.79% in white non-Hispanics, 32.88% in black non-Hispanics, and 25.8% in Hispanics (table 3). The increased prevalence of fluorosis in black non-Hispanics may suggest a genetic influence on fluorosis susceptibility.
Table 3.
Enamel fluorosis among persons aged 6–39 years by selected characteristics
| Unaffected |
Questionable | Very mild | Mild | Moderate/ Severe |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | SE | % | SE | % | SE | % | SE | % | SE | |
| Age group (years) | ||||||||||
| 6–11 | 59.81 | 4.07 | 11.80 | 2.50 | 19.85 | 2.12 | 5.83 | 0.73 | 2.71 | 0.59 |
| 12–15 | 51.46 | 3.51 | 11.96 | 1.84 | 25.33 | 1.98 | 7.68 | 0.93 | 3.56 | 0.59 |
| 16–19 | 58.32 | 3.30 | 10.21 | 1.70 | 20.79 | 1.78 | 6.65 | 0.67 | 4.03 | 0.77 |
| 20–39 | 74.86 | 2.28 | 8.83 | 1.23 | 11.15 | 1.22 | 3.34 | 0.58 | 1.81 | 0.39 |
|
| ||||||||||
| Sex | ||||||||||
| Male | 67.65 | 2.63 | 9.99 | 1.45 | 15.65 | 1.52 | 4.58 | 0.54 | 2.12 | 0.39 |
| Female | 66.97 | 2.84 | 9.83 | 1.34 | 15.58 | 1.36 | 4.84 | 0.61 | 2.78 | 0.49 |
|
| ||||||||||
| Race/ethnicity1 | ||||||||||
| White, non- Hispanic | 69.69 | 3.13 | 10.43 | 1.62 | 14.09 | 1.56 | 3.87 | 0.60 | 1.92 | 0.48 |
| Black, non- Hispanic | 56.72 | 3.30 | 10.40 | 2.16 | 21.21 | 2.16 | 8.24 | 0.82 | 3.43 | 0.54 |
| Mexican- American | 65.25 | 3.89 | 8.95 | 1.29 | 15.93 | 2.24 | 5.05 | 0.72 | 4.822 | 1.81 |
|
| ||||||||||
| Poverty Status3 | ||||||||||
| <100% FPL | 68.02 | 3.21 | 10.67 | 1.64 | 14.28 | 1.73 | 4.07 | 0.69 | 2.97 | 0.66 |
| 100–199% FPL | 66.92 | 2.91 | 9.11 | 1.79 | 16.11 | 1.46 | 5.21 | 0.78 | 2.65 | 0.56 |
| ≥200% FPL | 66.88 | 2.75 | 10.73 | 1.33 | 15.56 | 1.56 | 4.83 | 0.50 | 2.00 | 0.37 |
|
| ||||||||||
| Total | 67.40 | 2.65 | 9.91 | 1.35 | 15.55 | 1.37 | 4.69 | 0.49 | 2.45 | 0.40 |
Data from National Health and Nutrition Examination Survey (1999–2002) [27] and calculated using Dean’s index. All estimates are adjusted by age (single years) and sex to the USA 2000 standard population, except sex, which is adjusted only by age.
Calculated using ‘other race/ethnicity’ and ‘other Hispanic’ in the denominator.
Unreliable estimate: the standard error is 30% the value of the point estimate, or greater.
Percentage of the federal poverty level (FPL), which varies by income and number of persons living in the household.
Pathology, Pathogenesis and Mechanism of Dental Fluorosis
The primary pathological finding of fluorosed enamel is a subsurface porosity, along with hyper and hypomineralized bands within the forming enamel (fig. 4) [28–34]. Fluoride can also result in mineralization-related effects on dentin formation.
Fig. 4.
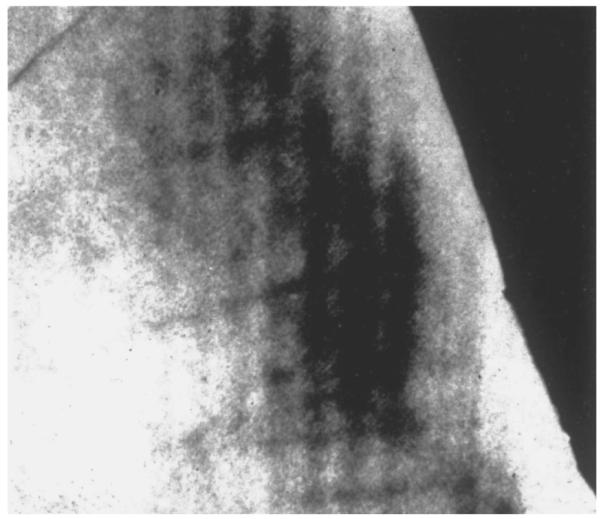
Microradiograph of fluorosed enamel from Colorado Springs. Note the radiolucent outer third of the enamel with a well-calcified surface layer. From Newbrun [97], reprinted with permission.
Severely fluorosed human dentin is characterized by a highly mineralized sclerotic background pattern, scattered with hypomineralized porous lesions primarily in the subsurface area. Scanning electron microscope images show dentin tubules with an irregular distribution and narrow and disrupted lumina, rather than the regular appearing lumina seen in normal dentin [35].
The pathogenesis of dental fluorosis is related to physiological conditions, including body weight, rate of skeletal growth and remodeling, nutrition, and renal function [36–38]. Bone is a reservoir of fluoride, as fluoride is incorporated in the forming apatite crystals, and this ion can also be released from these crystals as bone remodels. Therefore, rapid bone growth, as occurs in the growing child, will remove fluoride from the blood stream, possibly reducing the risk of dental fluorosis by lowering serum fluoride levels [8, 39]. Nutrition is also important for controlling the serum level of fluoride, as ions such as calcium, magnesium and aluminum can reduce the bioavailability of fluoride. A deficiency in these ions in food can also affect (enhance) fluoride up take [40].
Genetic background appears to have role in the pathogenesis of dental fluorosis. This may be the reason why in human populations, individuals drinking water with similar fluoride contents have a wide range of severity of dental fluorosis (fig. 2). Evidence for a genetic component to fluoride susceptibility comes from work by Everett et al. [7], which tested 12 different inbred mouse strains to compare their susceptibility to fluoride. Mouse teeth have been found to be an excellent model for human tooth formation, and in Everett’s study, they found that some mouse strains were highly susceptible to fluoride related dental fluorosis, while other strains were highly fluorosis resistant. They concluded that there is a genetic component to dental fluorosis susceptibility [41, 42].
Stages of Tooth Formation and Stage-Specific Effects of Chronic Fluoride Exposure
Fluoride is a single highly electronegative ion that interacts with the cells and matrix at the different stages of enamel formation in relation to fluoride dose and time of exposure. Tooth enamel development can be divided into 4 major stages: pre-secretory, secretory, transition and maturation stages, all with unique properties that affect fluoride susceptibility. Most of the studies of the mechanisms of fluoride in forming fluorosed enamel have used the rodent incisor or molars as a model, as it is not possible to do similar studies using human teeth. The rodent incisor is a continuously erupting tooth, with all stages of enamel formation present in each tooth, whereas the molar is a rooted tooth, which begins formation in utero. As previously mentioned, though rodents require the ingestion of much higher levels of fluoride in the drinking water (10–20 times) as compared to humans, the serum levels at which fluorosis is formed in rodents and humans is similar.
Pre-secretory ameloblasts differentiate into secretory ameloblasts after the dentin matrix begins to mineralize. The pre-secretory ameloblasts and overlying cells of the enamel, including the enamel knot, are thought to influence the tooth morphogenesis. However, there is no evidence that exposure of developing teeth to physiological levels of fluoride in vivo [43] and in organ culture [44–47] affects tooth morphogenesis. Even in teeth with severe fluorosis, the size and form of the teeth are not changed [48].
As the pre-ameloblasts differentiate to secretory ameloblasts, they begin to secrete enamel matrix proteins, and lay down a thin layer of aprismatic enamel deposited against mantle dentin. As the secretory ameloblast Tomes’ processes form, the inner enamel layer, which constitutes the bulk of enamel, begins to be laid down. This enamel matrix consists of prismatic enamel with rod (or prisms) and interrod structures (interprismatic enamel) formed by the Tomes’ processes of fully differentiated secretory ameloblasts. These cells secrete matrix protein (predominantly amelogenins) into the enamel space through which thin but long enamel crystals grow preferentially in length in the wake of the retreating cells.
Secretory stage ameloblasts exposed to high chronic levels of fluoride have a somewhat disrupted morphology and increased numbers of vacuoles at the apical border. Chronic exposure to fluoride in drinking water or repeated injections of moderate fluoride doses reduces the thickness of enamel by about 10% [43, 49]. Although this suggests that chronic exposure to fluoride reduces biosynthesis of matrix by secretory ameloblasts, there is no evidence to support this [1, 50, 51]. Instead, the small reduction in enamel thickness may be attributed to a limited disruption of vesicular transport in fluorotic secretory ameloblasts and subsequent intracellular degradation of a minor portion of the matrix by the lysosomal system [52–54].
At the end of secretion, the ameloblasts lose their Tomes’ process and deposit a final layer of aprismatic enamel with small crystals. The cells transform via a short transitional stage, where enamel matrix proteins undergo rapid proteolysis, leaving the porous enamel matrix characteristic of this transition stage.
The late secretory-transitional cell stage ameloblasts appear to be more sensitive to fluoride than early and fully secretory ameloblasts. In hamster molar tooth germs, a dose of 4.5 mg/kg fluoride induces the late secretory to transitional cells, but not early secretory ameloblasts to detach occasionally from the surface and form subameloblastic cysts. The enamel below the cysts under late secretory ameloblasts will give rise to the shallow occlusal pits, often seen in severely fluorosed teeth in various species [48, 55–61]. This stage of development is likely also to be associated with the formation of accentuated perikymata that is clinically the first sign of enamel fluorosis.
In the maturation stage, the ameloblasts modulate cyclically from cells with a smooth-ended to a ruffleended distal membrane, the latter with characteristics of resorbing cells. During this modulation, matrix proteins continue to be removed from the extracellular space, and mineralization increases to form a fully mineralized enamel matrix. Amelogenin proteins are retained in the fluorosed rat enamel matrix at this stage of enamel formation [51, 62].
Maturation ameloblasts of adult rat incisors [43] are shorter, and fluorotic enamel organs have a disrupted maturation ameloblast modulation [43, 63, 64]. The first modulation bands that disappear during fluoride exposure are the most incisal smoothended ameloblasts. At prolonged exposure other smooth-ended bands disappear one by one in an incisal to apical direction [63]. In addition to changes in modulation, fluoride also reduces the cyclic uptake of 45Ca labeling in a similar pattern [63]. When fluoride exposure is discontinued, smooth-ended bands reappear starting from the youngest most apical part towards older more incisal bands. This suggests that the fluoride effects on ameloblast modulation are reversible, and that the young modulating cells recover more rapidly than older ameloblasts. After eruption, the enamel is exposed to mineral ions of the oral fluids, including fluoride, which can influence the composition of the outer layers of enamel.
Direct Effects of Fluoride on Ameloblasts
Ameloblasts and tooth organs exposed to high (millimolar) levels of fluoride in vitro, which would be much greater than the micromolar levels of fluoride found in the plasma carrying fluoride ions to tooth organs in vivo, show many alterations. These include changes in the structure of early secretory ameloblasts, reduced protein synthesis, altered cell proliferation, apoptosis, stress-related protein upregulation and elevation of F-actin [65–68]. However, some of these same changes are not readily apparent in vivo, and therefore, the effects of fluoride when examined in culture, must be carefully analyzed for biological relevance.
However, there are in vitro data indicating that ameloblasts can be sensitive to low levels of fluoride. Human primary enamel organ epithelial cells grown in culture show that exposure to fluoride levels as low as 5 μmol/l results in reduced expression of the secretory stage matrix metalloproteinase 20 (MMP-20) [69], mediated by JNK/ c-Jun signaling [70]. These results suggest that fluoride may have specific effects on ameloblast differentiation mediated through MAP-kinase signaling.
Rodent studies have shown that ingestion of fluoride alters the number of bands of smooth ended ameloblasts and their rate of modulation in the maturation stage ameloblasts [43, 63]. However, there is currently no evidence to determine whether these changes in maturation stage ameloblast modulation are a direct effect of fluoride, or more likely, in response to matrixmediated alterations related to fluoride exposure to the developing enamel matrix.
At extremely high levels of ingested fluoride (150 ppm) in the drinking water, ameloblasts have been shown to exhibit apoptosis and endoplasmic reticulum stress responses [66]; however, at lower levels (75 ppm) these effects were not noted. Further studies at lower fluoride levels will need to be done to determine whether this is a potential mechanism relevant to chronic fluoride toxicity in humans.
Fluoride-Related Alterations of the Forming Enamel Matrix May Indirectly Affect Ameloblast Function
The extracellular enamel matrix proteins include amelogenins, ameloblastin and enamelin, all of which support and modulate enamel crystal formation [71]. Amelogenin is the chief structural protein constituting 90–95% of total proteins in the enamel protein matrix [72]. Amelogenin and the other matrix proteins are hydrolyzed by matrix proteinases as enamel forms, allowing replacement of the protein matrix with an organized hydroxyapatite structure. MMP-20 is the proteinase primarily responsible for the initial hydrolysis of amelogenins in the secretory enamel matrix, while kallikrein 4 (KLK4) is the predominant proteinase in the transition/maturation stage [73, 74].
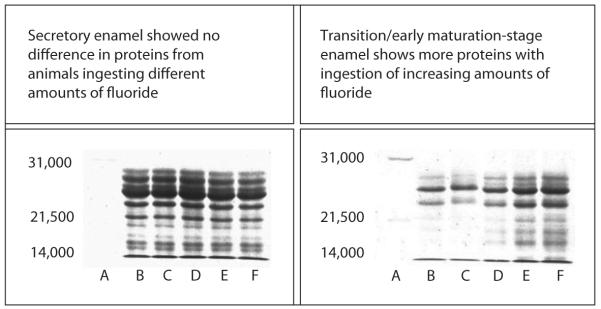
An analysis of proteolytic activity in enamel matrix, isolated from secretory and maturation stage rat enamel, showed a significantly reduced activity in early maturation stage enamel isolated from rats ingesting 100 ppm fluoride (5–10 μm serum fluoride), as compared to control maturation enamel [75]. This effect of fluoride ingestion in decreasing matrix proteinase activity correlates to an increased retention of amelogenin proteins in maturation stage fluorosed enamel in a dosedependent manner (fig. 5). Matrix proteins disappear from nonfluorosed enamel in the maturation stage, but are retained in fluorosed enamel, with increased retention at higher levels of ingested fluoride [49, 51].
Fig. 5.
SDS PAGE separation of proteins in secretory and maturation stages of enamel matrix of fluoride-treated and untreated rat tooth. A = Standard; B = 0 ppm; C = 10 ppm; D = 25 ppm; E = 50 ppm; F = 100 ppm. From DenBesten [51], reprinted with permission.
This retention of amelogenin proteins could delay final mineralization of the enamel matrix, contributing to subsurface hypomineralization characteristic of fluorosed enamel. The reason for this retention of amelogenins is most likely related to altered proteolytic activity in the fluorosed enamel matrix.
Reduced Proteolytic Activity May Be due to the Effects of Fluoride Incorporation into Growing Enamel Crystals
Crystals in sound enamel are long, and the dynamics of enamel crystal growth, size of the crystals and their shape are well controlled by matrix proteins during enamel formation [76–78]. Some studies report that crystals isolated from fluorosed enamel have a significantly greater diameter than crystals in sound enamel, as determined by high-resolution electron microscopy [79], X-ray diffraction of powdered enamel samples [80] or scanning microscopy of fractured inner enamel specimens [81]. Some organ culture studies have shown large flattened hexagonal crystals mixed with many small irregularly shaped crystals in hypermineralized areas [82, 83]. However, other studies reported no differences between fluorotic and normal human crystals [28, 84].
There is, however, no doubt that the fluoride content of crystals in fluorosed enamel is greater than that of normal enamel. Fluoride substitutes for hydroxyl groups in enamel carbonated hydroxyapatite crystals, altering the crystalline structures and surface characteristics. To determine whether an increased fluoride content of the apatite crystals could affect matrix/proteinase interactions, we measured the binding of recombinant human amelogenin to synthetic carbonated hydroxyapatite crystals.
The initial rate of amelogenin binding and the total amount of amelogenin bound to fluoride-containing carbonated hydroxyapatite was greater than that in the control carbonated hydroxyapatite [85]. These results suggest that fluoride incorporation into the crystal lattice alters the crystal surface to enhance amelogenin binding, potentially contributing to the increased amount of amelogenin and the inhibition of crystal growth in fluorosed enamel.
In further investigation of the role of fluoride incorporation into apatite on amelogenin processing, we characterized hydrolysis of amelogenins bound to fluoride-containing apatites by recombinant MMP-20 or KLK-4. When fluoride was in solution, amelogenin hydrolysis by MMP-20 was reduced only at 1,000 ppm (52 mm, which is far higher than physiological levels of fluoride in enamel fluids). However, incorporation of fluoride into apatite significantly delayed MMP-20 hydrolysis of the adsorbed amelogenin in a dose-dependent manner (fig. 6) even at the lowest level of fluoride-containing apatite (100 ppm F). This same effect of reduced amelogenin hydrolysis was found when amelogenins were hydrolyzed from fluoride-containing apatites with recombinant KLK-4 (unpublished results).
Fig. 6.
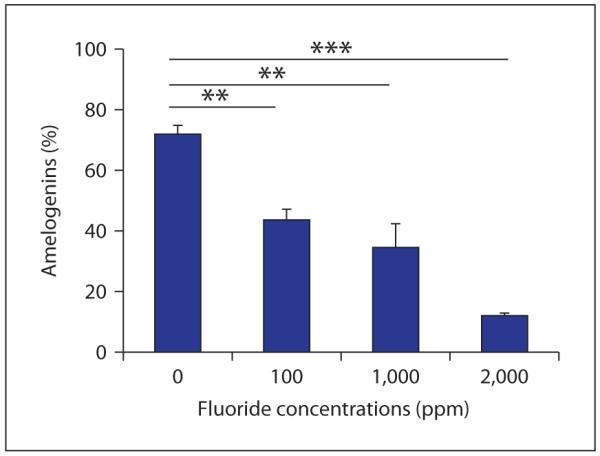
Degradation of amelogenin adsorbed on apatite crystals by MMP-20. Amelogenins were pre-bound to carbonated hydroxyapatite crystals containing different amounts of fluoride (X-axis) and then degraded by MMP-20. Y-axis indicates the percentages of amelogenins degraded by MMP-20 from apatite crystals as compared to the amount of amelogenin initially bound. Note the decreased degradation of amelogenin from the apatite crystal surface as the concentration of fluoride in the apatite increases.
The levels of fluoride incorporated into the apatite crystals in these in vitro studies are biologically relevant. Although the enamel fluid surrounding the ameloblasts is likely to contain no more than 10 μm (0.19 ppm) fluoride, fluoride is incorporated into the growing crystals in concentrations ranging from 10 ppm near the dentalenamel junction to several thousand ppm at the enamel surface [86]. Fluoride-containing apatite with fluoride concentrations of 100 ppm are found in the inner enamel (300 μm from the surface) of human teeth with minimal (mild) fluorosis [86]. The higher fluoride-containing apatite (approximately 2,000 ppm F) is similar to that found in the midlayer of enamel (150 μm from the surface) of severely fluorosed human teeth. Therefore, these studies indicate that the reduced hydrolysis of amelogenin found in fluorosed maturation stage enamel [1, 52] may be due to the reduction in the rate of hydrolysis of amelogenins bound to fluoride-containing enamel crystals.
These effects of fluoride incorporation on hydrolysis of apatite-bound amelogenins is consistent with the observation that fluoride-induced subsurface hypomineralization can independently occur in the maturation stage only [60, 63, 87]. Mineralization defects in rat incisor maturation stage enamel are characterized by the development of a generalized hypomineralized porous subsurface area along the entire crown enamel [4, 88–91]. This type of defect correlates to the porous white opacities seen clinically.
Potential Effects of Matrix pH on Fluoride-Related Changes in Enamel Formation
Matrix protein removal may also be influenced by fluoride-mediated changes in pH during apatite crystal formation. Formation of apatite results in the formation of a substantial number of protons [10Ca2+ + 6 HPO 2− 4+ 2H2O → Ca10(PO4)6(OH)2 + 8H+] that need to be neutralized. Amelogenins bind as many as 12 protons per molecule [92]. However, if this amelogenin buffering system is either not available, or is saturated, it is conceivable that a fluoride-induced pH drop could alter the amelogenin tertiary structure and affect its function [93].
Abundant amelogenins generated by secretory ameloblasts may be a potent contributor to controlling pH at the secretory stage, where the pH is maintained at neutral [77, 94]. At the end of the secretory stage, enamel matrix proteinases are activated, and at the transition stage, enamel matrix proteins are rapidly lost. At this stage, the cell junctions between the ameloblasts are open, allowing fluoride to readily move from the serum to the enamel matrix. The presence of increased amounts of fluoride could promote enhanced enamel matrix mineralization with potentially increased amelogenin retention in the presence of a reduced matrix pH.
At the maturation stage, the pH in the enamel matrix changes periodically between acidic (pH 5.8) and neutral as ameloblasts modulate (pH 7.2) [95, 96], and additional pH regulation is required. If we assume that the acidification of the enamel matrix promotes modulation from ruffle-ended to smooth ended ameloblasts during amelogenesis, in dental fluorosis, changes in matrix pH could contribute to a delay in the transition from ruffle-ended to smooth ended ameloblasts, resulting in fewer ameloblast modulations. This delay in ameloblast modulation (which is a characteristic of fluorosed maturation ameloblasts) could possibly contribute to the delay in removal of amelogenins.
Particularly at this final stage of enamel mineralization, Bronckers et al. [94] have hypothesized that fluoride in the enamel matrix may enhance mineralization resulting in localized hypermineralization, requiring the ameloblasts to pump additional bicarbonate into the extracellular enamel matrix. This hypermineralization would deplete the local reservoir or free calcium ions, resulting in a subsequent band of hypomineralized enamel. This hypothesis is supported by a recent study showing an upregulation of mRNA for the pH regulator NBCe1 in fluorosed maturation stage ameloblasts as compared to control maturation ameloblasts [93].
In summary, the mechanisms by which fluoride alters enamel maturation are multi-factorial. We propose a multi-stage model for the formation of fluorosed enamel, as follows:
Crystals forming in the secretory stage of enamel have an increased fluoride content and therefore bind more amelogenin.
Hydrolysis of amelogenins by proteinases is delayed by altered amelogenin interactions with the fluoride-containing hydroxyapatite crystals.
At the transition stage, fluoride is rapidly deposited into the porous enamel matrix between the open cell junctions, resulting in increased formation of fluoride-containing apatite, and a delay in protein hydrolysis secondary altered mineral/ matrix interactions.
The net result of these fluoride-related effects in the secretory and transition stages is retention of amelogenins in the maturation stage. This delay in removal of amelogenins increases the relative pH in the maturation stage under ruffle-ended ameloblasts as amelogenins buffer the increased protons resulting from mineral formation.
The reduced acidification of the matrix under ruffle-ended ameloblasts further delays modulation to smooth-ended ameloblasts, resulting in fewer bands of modulating ameloblasts.
In late maturation, when amelogenins are finally removed (or in mild dental fluorosis with minimal amelogenin retention), fluoride-mediated hypermineralization may increase the local acidification affecting ameloblast function, such as ion transport activities. Although porous subsurface enamel is the major phenotype of fluorosed enamel, successive layers of hypo-mineralized and hypermineralized enamel are also a characteristic of the fluorosed enamel matrix [4, 90].
It is likely that there are additional effects of fluoride, including other indirect effects on cells at different stages of formation, and that in the course of our and others’ studies this model and our understanding of the mechanisms (including more potential direct cellular effects) will be expanded.
References
- 1.Aoba T, Moreno EC, Tanabe T, Fukae M. Effects of fluoride on matrix proteins and their properties in rat secretory enamel. J Dent Res. 1990;69:1248–1250. doi: 10.1177/00220345900690060501. [DOI] [PubMed] [Google Scholar]
- 2.Richards A. Nature and mechanisms of dental fluorosis in animals. J Dent Res. 1990;69(spec No):701–705. doi: 10.1177/00220345900690S136. discussion 721. [DOI] [PubMed] [Google Scholar]
- 3.Giambro NJ, Prostak K, DenBesten PK. Characterization of fluorosed human enamel by color reflectance, ultrastructure, and elemental composition. Caries Res. 1995;29:251–257. doi: 10.1159/000262077. [DOI] [PubMed] [Google Scholar]
- 4.Angmar-Mansson B, Ericsson Y, Ekberg O. Plasma fluoride and enamel fluorosis. Calcif Tissue Res. 1976;22:77–84. doi: 10.1007/BF02010348. [DOI] [PubMed] [Google Scholar]
- 5.Angmar-Mansson B, Whitford GM. Enamel fluorosis related to plasma F levels in the rat. Caries Res. 1984;18:25–32. doi: 10.1159/000260743. [DOI] [PubMed] [Google Scholar]
- 6.Angmar-Mansson B, Lindh U, Whitford GM. Enamel and dentin fluoride levels and fluorosis following single fluoride doses: a nuclear microprobe study. Caries Res. 1990;24:258–262. doi: 10.1159/000261279. [DOI] [PubMed] [Google Scholar]
- 7.Everett ET, McHenry MA, Reynolds N, et al. Dental fluorosis: variability among different inbred mouse strains. J Dent Res. 2002;81:794–798. doi: 10.1177/0810794. [DOI] [PubMed] [Google Scholar]
- 8.Angmar-Mansson B, Whitford GM. Environmental and physiological factors affecting dental fluorosis. J Dent Res. 1990:706–713. doi: 10.1177/00220345900690S137. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council . Fluoride in Drinking Water.: A Scientific Review of EPA’s Standards. National Academies Press; Washington: 2006. p. 530. [Google Scholar]
- 10.Moller IJ. Fluorides and dental fluorosis. Int Dent J. 1982;32:135–147. [PubMed] [Google Scholar]
- 11.Kroncke A. Perikymata (in German) Dtsch Zahnarztl Z. 1966;21:1397–1401. [PubMed] [Google Scholar]
- 12.Smith GE. Fluoride, teeth and bone. Med J Aust. 1985;143:283–286. doi: 10.5694/j.1326-5377.1985.tb123008.x. [DOI] [PubMed] [Google Scholar]
- 13.McKay FS. The study of mottled enamel (dental fluorosis) J Am Dent Assoc. 1952;44:133–137. doi: 10.14219/jada.archive.1952.0057. [DOI] [PubMed] [Google Scholar]
- 14.Mottled enamel. Am J Public Health Nations Health. 1933;23:47–48. doi: 10.2105/ajph.23.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ockerse T, Wasserstein B. Stain in mottled enamel. J Am Dent Assoc. 1955;50:536–538. doi: 10.14219/jada.archive.1955.0095. [DOI] [PubMed] [Google Scholar]
- 16.Subcommittee on Health Effects of Ingested Fluoride (National Research Council) Health Effects of Ingested Fluoride. National Academy of Sciences; Washington: 1993. [Google Scholar]
- 17.Dean HT. Fluorine in the control of dental caries. J Am Dent Assoc. 1956;52:1–8. doi: 10.14219/jada.archive.1956.0011. [DOI] [PubMed] [Google Scholar]
- 18.Dean HT. Endemic fluorosis and its relation to dental caries: 1938. Public Health Rep. 2006;121(suppl 1):213–219. discussion 212. [PubMed] [Google Scholar]
- 19.Thylstrup A, Fejerskov O, Mosha HJ. A polarized light and microradiographic study of enamel in human primary teeth from a high fluoride area. Arch Oral Biol. 1978;23:373–380. doi: 10.1016/0003-9969(78)90095-x. [DOI] [PubMed] [Google Scholar]
- 20.USEPA . Fluorine (soluble fluoride) (CASRN 7782- 41- 4) Integrated Risk Information System; Washington: 1987. [Google Scholar]
- 21.Levy SM, Guha-Chowdhury N. Total fluoride intake and implications for dietary fluoride supplementation. J Public Health Dent. 1999;59:211–223. doi: 10.1111/j.1752-7325.1999.tb03272.x. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . Water for Life: Making It Happen. WHO; Geneva: 2005. [Google Scholar]
- 23.Lennon MA. One in a million: the first community trial of water fluoridation. National Institutes of Health; Washington: 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Service PH. Review of Fluoride Benefits and Risks, Report of the ad hoc Submommittee on Fluoride of the Committee to Coordinate Environmental Health and Related Programs. Department of Health and Human Services; Washington: 1991. [Google Scholar]
- 25.Centers for Disease Control . Ten Great Public Health Achievements in the 20th Century. CDC; Washington: 2008. [Google Scholar]
- 26.Institute of Medicine . Dietary reference intakes: for calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academies Press; Washington: 1997. [PubMed] [Google Scholar]
- 27.Beltran-Aguilar ED, Barker LK, Canto MT, et al. Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis–United States, 1988–1994 and 1999–2002. MMWR Surveill Summ. 2005;54:1–43. [PubMed] [Google Scholar]
- 28.Fejerskov O, Johnson NW, Silverstone LM. The ultrastructure of fluorosed human dental enamel. Scand J Dent Res. 1974;82:357–372. doi: 10.1111/j.1600-0722.1974.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 29.Fejerskov O, Larsen MJ, Josephsen K, Thylstrup A. Effect of longterm administration of fluoride on plasma fluoride and calcium in relation to forming enamel and dentin in rats. Scand J Dent Res. 1979;87:98–104. doi: 10.1111/j.1600-0722.1979.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 30.Fejerskov O, Silverstone LM, Melsen B, Moller IJ. Histological features of fluorosed human dental enamel. Caries Res. 1975;9:190–210. doi: 10.1159/000260157. [DOI] [PubMed] [Google Scholar]
- 31.Fejerskov O, Thylstrup A, Larsen MJ. Clinical and structural features and possible pathogenic mechanisms of dental fluorosis. Scand J Dent Res. 1977;85:510–534. doi: 10.1111/j.1600-0722.1977.tb02110.x. [DOI] [PubMed] [Google Scholar]
- 32.Fejerskov O, Yanagisawa T, Tohda H, et al. Posteruptive changes in human dental fluorosis–a histological and ultrastructural study. Proc Finn Dent Soc. 1991;87:607–619. [PubMed] [Google Scholar]
- 33.Kidd EA, Thylstrup A, Fejerskov O. The histopathology of enamel caries in fluorosed deciduous teeth. Caries Res. 1981;15:346–352. doi: 10.1159/000260537. [DOI] [PubMed] [Google Scholar]
- 34.Kierdorf U, Kierdorf H, Fejerskov O. Fluoride-induced developmental changes in enamel and dentine of European roe deer (Capreolus capreolus L.) as a result of environmental pollution. Arch Oral Biol. 1993;38:1071–1081. doi: 10.1016/0003-9969(93)90169-m. [DOI] [PubMed] [Google Scholar]
- 35.Rojas-Sanchez F, Alaminos M, Campos A, Rivera H, Sanchez-Quevedo MC. Dentin in severe fluorosis: a quantitative histochemical study. J Dent Res. 2007;86:857–861. doi: 10.1177/154405910708600910. [DOI] [PubMed] [Google Scholar]
- 36.Ekstrand J, Spak CJ, Ehrnebo M. Renal clearance of fluoride in a steady state condition in man: influence of urinary flow and pH changes by diet. Acta Pharmacol Toxicol (Copenh) 1982;50:321–325. doi: 10.1111/j.1600-0773.1982.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 37.Jarnberg PO, Ekstrand J, Irestedt L, Santesson J. Renal fluoride excretion during and after enflurane anaesthesia: dependency on spontaneous urinary pH-variations. Acta Anaesthesiol Scand. 1980;24:129–134. doi: 10.1111/j.1399-6576.1980.tb01520.x. [DOI] [PubMed] [Google Scholar]
- 38.Spak CJ, Berg U, Ekstrand J. Renal clear-ance of fluoride in children and adolescents. Pediatrics. 1985;75:575–579. [PubMed] [Google Scholar]
- 39.Pendrys DG, Stamm JW. Relationship of total fluoride intake to beneficial effects and enamel fluorosis. J Dent Res. 1990;69(spec No):529–538. doi: 10.1177/00220345900690S107. discussion 556–527. [DOI] [PubMed] [Google Scholar]
- 40.Taves DR. Dietary intake of fluoride ashed (total fluoride) v. unashed (inorganic fluoride) analysis of individual foods. Br J Nutr. 1983;49:295–301. doi: 10.1079/bjn19830038. [DOI] [PubMed] [Google Scholar]
- 41.Everett ET, Yan D, Weaver M, Liu L, Foroud T, Martinez-Mier EA. Detection of dental fluorosis-associated quantitative trait loci on mouse chromosomes 2 and 11. Cells Tissues Organs. 2009;189:212–218. doi: 10.1159/000151383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everett ET, Yan D, Weaver M, Liu L, Foroud T, Martinez-Mier EA. Detection of dental fluorosis-associated quantitative trait Loci on mouse chromosomes 2 and 11. Cells Tissues Organs. 2009;189:212–218. doi: 10.1159/000151383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith CE, Nanci A, Denbesten PK. Effects of chronic fluoride exposure on morphometric parameters defining the stages of amelogenesis and ameloblast modulation in rat incisors. Anat Rec. 1993;237:243–258. doi: 10.1002/ar.1092370212. [DOI] [PubMed] [Google Scholar]
- 44.Bronckers AL, Jansen LL, Woltgens JH. A histological study of the short-term effects of fluoride on enamel and dentine formation in hamster tooth-germs in organ culture in vitro. Arch Oral Biol. 1984;29:803–810. doi: 10.1016/0003-9969(84)90010-4. [DOI] [PubMed] [Google Scholar]
- 45.Bronckers AL, Jansen LL, Woltgens JH. Long-term (8 days) effects of exposure to low concentrations of fluoride on enamel formation in hamster tooth-germs in organ culture in vitro. Arch Oral Biol. 1984;29:811–819. doi: 10.1016/0003-9969(84)90011-6. [DOI] [PubMed] [Google Scholar]
- 46.Kerley MA, Kollar EJ. Regeneration of tooth development in vitro following sodium fluoride treatment. Am J Anat. 1977;149:181–195. doi: 10.1002/aja.1001490205. [DOI] [PubMed] [Google Scholar]
- 47.Levenson GE. The effect of fluoride on ameloblasts of mouse molar tooth germs ‘in vitro’. J Biol Buccale. 1980;8:255–263. [PubMed] [Google Scholar]
- 48.Kierdorf H, Kierdorf U. Disturbances of the secretory stage of amelogenesis in fluorosed deer teeth: a scanning electron-microscopic study. Cell Tissue Res. 1997;289:125–135. doi: 10.1007/s004410050858. [DOI] [PubMed] [Google Scholar]
- 49.Zhou R, Zaki AE, Eisenmann DR. Morphometry and autoradiography of altered rat enamel protein processing due to chronic exposure to fluoride. Arch Oral Biol. 1996;41:739–747. doi: 10.1016/s0003-9969(96)00078-7. [DOI] [PubMed] [Google Scholar]
- 50.Bronckers ALJJ, Woltgens JHM. Short term effects of fluoride on biosynthesis of enamel-matrix proteins and dentin collagens and on mineralization during hamster tooth-germ development in organ culture. Archs Oral Biol. 1985;39:181–185. doi: 10.1016/0003-9969(85)90113-x. [DOI] [PubMed] [Google Scholar]
- 51.DenBesten PK. Effects of fluoride on protein secretion and removal during enamel development in the rat. J Dent Res. 1986;65:1272–1277. doi: 10.1177/00220345860650101401. [DOI] [PubMed] [Google Scholar]
- 52.Bronckers AL, Lyaruu DM, Bervoets TJ, Woltgens JH. Fluoride enhances intracellular degradation of amelogenins during secretory phase of amelogenesis of hamster teeth in organ culture. Connect Tissue Res. 2002;43:456–465. doi: 10.1080/03008200290001113. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo S, Inai T, Kurisu K, Kiyomiya K, Kurebe M. Influence of fluoride on secretory pathway of the secretory ameloblast in rat incisor tooth germs exposed to sodium fluoride. Arch Toxi-col. 1996;70:420–429. doi: 10.1007/s002040050294. [DOI] [PubMed] [Google Scholar]
- 54.Monsour P, Harbrow J, Warshawsky H. Effects of acute doses of sodium fluoride on the morphology and the detectable calcium associated with secretory ameloblasts in rat incisors. J Histchem Cytochem. 1989;37:463–471. doi: 10.1177/37.4.2926125. [DOI] [PubMed] [Google Scholar]
- 55.Fejerskov O, Larsen MJ, Richards A, Baelum V. Dental tissue effects of fluoride. Adv Dent Res. 1994;8:15–31. doi: 10.1177/08959374940080010601. [DOI] [PubMed] [Google Scholar]
- 56.Kardos TB, Hunter AR, Hubbard MJ. Scanning electron microscopy of trypsin-treated enamel from fluorosed rat molars. Adv Dent Res. 1989;3:183–187. doi: 10.1177/08959374890030021801. [DOI] [PubMed] [Google Scholar]
- 57.Milhaud GE, Charles E, Loubiere ML, Kolf-Clauw M, Joubert C. Effects of fluoride on secretory and postsecretory phases of enamel formation in sheep molars. Am J Vet Res. 1992;53:1241–1247. [PubMed] [Google Scholar]
- 58.Nelson DG, Coote GE, Vickridge IC, Suckling G. Proton microprobe determination of fluorine profiles in the enamel and dentine of erupting incisors from sheep given low and high daily doses of fluoride. Arch Oral Biol. 1989;34:419–429. doi: 10.1016/0003-9969(89)90120-9. [DOI] [PubMed] [Google Scholar]
- 59.Richards A, Kragstrup J, Josephsen K, Fejerskov O. Dental fluorosis developed in post-secretory enamel. J Dent Res. 1986;65:1406–1409. doi: 10.1177/00220345860650120501. [DOI] [PubMed] [Google Scholar]
- 60.Suckling G, Thurley DC, Nelson DG. The macroscopic and scanning electronmicroscopic appearance and microhardness of the enamel, and the related histological changes in the enamel organ of erupting sheep incisors resulting from a prolonged low daily dose of fluoride. Arch Oral Biol. 1988;33:361–373. doi: 10.1016/0003-9969(88)90070-2. [DOI] [PubMed] [Google Scholar]
- 61.Susheela AK, Bhatnagar M. Fluoride toxicity: a biochemical and scanning electron microscopic study of enamel surface of rabbit teeth. Arch Toxicol. 1993;67:573–579. doi: 10.1007/BF01969271. [DOI] [PubMed] [Google Scholar]
- 62.DenBesten PK, Crenshaw MA. The effects of chronic high fluoride levels on forming enamel in the rat. Arch Oral Biol. 1984;29:675–679. doi: 10.1016/0003-9969(84)90171-7. [DOI] [PubMed] [Google Scholar]
- 63.DenBesten PK, Crenshaw MA, Wilson MH. Changes in the fluoride-induced modulation of maturation stage ameloblasts of rats. J Dent Res. 1985;64:1365–1370. doi: 10.1177/00220345850640120701. [DOI] [PubMed] [Google Scholar]
- 64.Nishikawa S, Josephsen K. Cyclic localization of actin and its relationship to junctional complexes in maturation ameloblasts of the rat incisor. Anat Rec. 1987;219:21–31. doi: 10.1002/ar.1092190106. [DOI] [PubMed] [Google Scholar]
- 65.Bartlett JD, Dwyer SE, Beniash E, Skobe Z, Payne-Ferreira TL. Fluorosis: a new model and new insights. J Dent Res. 2005;84:832–836. doi: 10.1177/154405910508400910. [DOI] [PubMed] [Google Scholar]
- 66.Kubota K, Lee DH, Tsuchiya M, et al. Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J Biol Chem. 2005;280:23194–23202. doi: 10.1074/jbc.M503288200. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Decker S, Yuan ZA, et al. Effects of sodium fluoride on the actin cytoskeleton of murine ameloblasts. Arch Oral Biol. 2005;50:681–688. doi: 10.1016/j.archoralbio.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 68.Yan Q, Zhang Y, Li W, Denbesten PK. Micromolar fluoride alters ameloblast lineage cells in vitro. J Dent Res. 2007;86:336–340. doi: 10.1177/154405910708600407. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Yan Q, Li W, DenBesten PK. Fluoride down-regulates the expression of matrix metalloproteinase-20 in human fetal tooth ameloblast-lineage cells in vitro. Eur J Oral Sci. 2006;114(suppl 1):105–110. doi: 10.1111/j.1600-0722.2006.00303.x. discussion 127–109, 380. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Li W, Chi HS, Chen J, Den-besten PK. JNK/c-Jun signaling pathway mediates the fluoride-induced downregulation of MMP-20 in vitro. Matrix Biol. 2007;26:633–641. doi: 10.1016/j.matbio.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robinson C, Brookes SJ, Shore RC, Kirkham J. The developing enamel matrix: nature and function. Eur J Oral Sci. 1998;106(suppl 1):282–291. doi: 10.1111/j.1600-0722.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- 72.Fincham AG, Moradian-Oldak J. Recent advances in amelogenin biochemistry. Connect Tissue Res. 1995;32:119–124. doi: 10.3109/03008209509013713. [DOI] [PubMed] [Google Scholar]
- 73.Hu JC, Ryu OH, Chen JJ, et al. Localization of EMSP1 expression during tooth formation and cloning of mouse cDNA. J Dent Res. 2000;79:70–76. doi: 10.1177/00220345000790011301. [DOI] [PubMed] [Google Scholar]
- 74.Hu JC, Sun X, Zhang C, et al. Enamelysin and kallikrein-4 mRNA expression in developing mouse molars. Eur J Oral Sci. 2002;110:307–315. doi: 10.1034/j.1600-0722.2002.21301.x. [DOI] [PubMed] [Google Scholar]
- 75.DenBesten PK, Yan Y, Featherstone JD, et al. Effects of fluoride on rat dental enamel matrix proteinases. Arch Oral Biol. 2002;47:763–770. doi: 10.1016/s0003-9969(02)00117-6. [DOI] [PubMed] [Google Scholar]
- 76.Moradian-Oldak J, Jimenez I, Maltby D, Fincham AG. Controlled proteolysis of amelogenins reveals exposure of both carboxy- and amino-terminal regions. Biopolymers. 2001;58:606–616. doi: 10.1002/1097-0282(200106)58:7<606::AID-BIP1034>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 77.Simmer JP, Fincham AG. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 1995;6:84–108. doi: 10.1177/10454411950060020701. [DOI] [PubMed] [Google Scholar]
- 78.Smith CE, Nanci A. Protein dynamics of amelogenesis. Anat Rec. 1996;245:186–207. doi: 10.1002/(SICI)1097-0185(199606)245:2<186::AID-AR7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 79.Kerebel B, Daculsi G. Ultrastructural and crystallographic study of human enamel in endemic fluorosis (in French) J Biol Buccale. 1976;4:143–154. [PubMed] [Google Scholar]
- 80.Vieira A, Hancock R, Dumitriu M, et al. How does fluoride affect dentin microhardness and mineralization? J Dent Res. 2005;84:951–957. doi: 10.1177/154405910508401015. [DOI] [PubMed] [Google Scholar]
- 81.Sundstrom B, Jongebloed WL, Arends J. Fluorosed human enamel. A SEM investigation of the anatomical surface and outer and inner regions of mildly fluorosed enamel. Caries Res. 1978;12:329–338. doi: 10.1159/000260352. [DOI] [PubMed] [Google Scholar]
- 82.Yanagisawa T, Takuma S, Fejerskov O. Ultrastructure and composition of enamel in human dental fluorosis. Adv Dent Res. 1989;3:203–210. doi: 10.1177/08959374890030022101. [DOI] [PubMed] [Google Scholar]
- 83.Yanagisawa T, Takuma S, Tohda H, Fejerskov O, Fearnhead RW. High resolution electron microscopy of enamel crystals in cases of human dental fluorosis. J Electron Microsc (Tokyo) 1989;38:441–448. [PubMed] [Google Scholar]
- 84.Robinson C, Yamamoto K, Connell SD, et al. The effects of fluoride on the nanostructure and surface pK of enamel crystals: an atomic force microscopy study of human and rat enamel. Eur J Oral Sci. 2006;114(suppl 1):99–104. doi: 10.1111/j.1600-0722.2006.00275.x. discussion 127–109, 380. [DOI] [PubMed] [Google Scholar]
- 85.Tanimoto K, Le T, Zhu L, et al. Effects of fluoride on the interactions between amelogenin and apatite crystals. J Dent Res. 2008;87:39–44. doi: 10.1177/154405910808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richards A, Fejerskov O, Baelum V. Enamel fluoride in relation to severity of human dental fluorosis. Adv Dent Res. 1989;3:147–153. doi: 10.1177/08959374890030021301. [DOI] [PubMed] [Google Scholar]
- 87.Richards A, Kragstrup J, Nielsen-Kudsk F. Pharmacokinetics of chronic fluoride ingestion in growing pigs. J Dent Res. 1985;64:425–430. doi: 10.1177/00220345850640030601. [DOI] [PubMed] [Google Scholar]
- 88.Angmar-Mansson B, Whitford GM. Plasma fluoride levels and enamel fluorosis in the rat. Caries Res. 1982;16:334–339. doi: 10.1159/000260617. [DOI] [PubMed] [Google Scholar]
- 89.Kierdorf H, Kierdorf U, Richards A, Josephsen K. Fluoride-induced alterations of enamel structure: an experimental study in the miniature pig. Anat Embryol (Berl) 2004;207:463–474. doi: 10.1007/s00429-003-0368-8. [DOI] [PubMed] [Google Scholar]
- 90.Richards A, Likimani S, Baelum V, Fejer-skov O. Fluoride concentrations in unerupted fluorotic human enamel. Caries Res. 1992;26:328–332. doi: 10.1159/000261463. [DOI] [PubMed] [Google Scholar]
- 91.Shinoda H. Effect of long-term administration of fluoride on physico-chemical properties of the rat incisor enamel. Calcif Tissue Res. 1975;18:91–100. doi: 10.1007/BF02546229. [DOI] [PubMed] [Google Scholar]
- 92.Ryu OH, Hu CC, Simmer JP. Biochemical characterization of recombinant mouse amelogenins: protein quantitation, proton absorption, and relative affinity for enamel crystals. Connect Tissue Res. 1998;38:207–214. doi: 10.3109/03008209809017038. discussion 241–206. [DOI] [PubMed] [Google Scholar]
- 93.Zheng L, Zhang Y, He P, et al. NBCe1 differs in mouse and human ameloblasts, and its expression is enhanced by fluoride in vivo. J Dent Res. 2011 doi: 10.1177/0022034511398273. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bronckers AL, Lyaruu DM, DenBesten PK. The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. J Dent Res. 2009;88:877–893. doi: 10.1177/0022034509343280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sasaki S, Takagi T, Suzuki M. Cyclical changes in pH in bovine developing enamel as sequential bands. Arch Oral Biol. 1991;36:227–231. doi: 10.1016/0003-9969(91)90090-h. [DOI] [PubMed] [Google Scholar]
- 96.Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 97.Newbrun E. Studies on the physical properties of fluorosed enamel. II. Microhardness. Arch Oral Biol. 1960;12:21–27. doi: 10.1016/0003-9969(60)90033-9. [DOI] [PubMed] [Google Scholar]