Abstract
Background
Lack of health insurance is a key barrier to accessing care for chronic conditions and cancer screening. We examined the influence of insurance type (private, public, none) on survivor-focused and general preventive health care in adult survivors of childhood cancer.
Methods
The Childhood Cancer Survivor Study is a retrospective cohort study of childhood cancer survivors diagnosed between 1970–1986. Among 8425 adult survivors, the Relative Risk (RR), 95% confidence interval (CI) of receiving survivor-focused and general preventive health care were estimated for uninsured (n=1390) and publicly insured (n=640), comparing to privately insured (n=6395).
Results
Uninsured survivors were less likely than privately insured to report a cancer-related (adjusted RR=0.83, 95% CI, 0.75–0.91) or a cancer center visit (adjusted RR=0.83, 95% CI, 0.71–0.98). Uninsured survivors had lower levels of utilization in all measures of care in comparison with privately insured. In contrast, publicly insured survivors were more likely to report a cancer-related (adjusted RR=1.22, 95% CI, 1.11–1.35) or a cancer center visit (adjusted RR=1.41, 95% CI, 1.18–1.70) than privately insured. While having a similar utilization level of general health examinations, publicly insured survivors were less likely to report Papanicolaou smear or dental examinations.
Conclusion
Among this large, socioeconomically diverse cohort, publicly insured survivors utilize survivor-focused health care at rates at least as high as survivors with private insurance. Uninsured survivors have lower utilization to both survivor-focused and general preventive health care.
Keywords: Childhood Cancer Survivors, Health Insurance, Health Care Access, Survivorship, Delivery of Health Care
There are in excess of 325,000 survivors of childhood cancer in the United States (U.S.).1 This number continues to grow as the 5-year survival rate for pediatric cancers approaches 80%.2 Despite improved survival rates, this population has an increased risk of premature mortality and diminished health status due to late effects or long term complications of cancer treatment, particularly in the adult years.3–6 Because the incidence and severity of many late effects may be reduced with prevention and early detection, the Institute of Medicine recommends lifetime follow-up for all childhood cancer survivors.7 Less than 50% of adult survivors of childhood cancer who reside in the U.S. and Canada are receiving cancer-related follow-up care.8 Only 32% are reporting survivor-focused care including risk reduction counseling or late effects screening.9
Lack of health insurance is a key barrier to accessing medical care in the U.S. for adults with chronic conditions.10 Lack of health insurance is associated with lower cancer screening utilization.11 Among childhood cancer survivors, risk factors for being uninsured, include younger age at cancer diagnosis, lower educational level, income less than $20,000, marital status (widowed, divorced, or separated), being a current or former smoker, and cranial radiation treatment.12 In addition to insurance status, ethnicity is associated with different rates of follow-up care for childhood cancer survivors. When adjusting for having health insurance, Hispanic female survivors are more likely to follow-up at a cancer center than white, non-Hispanic female survivors but are less likely to report standard cancer screening such as Papanicolaou (Pap) smears.13 These findings are in line with studies examining cancer screening practices and cancer care of the general U.S. population.14, 15 Studies conducted within health maintenance organizations or managed care practices have demonstrated lower utilization rates of preventive care by specific groups of ethnic minorities despite the same access to services.16, 17
We sought to better understand the complex relationship between health insurance status, racial/ethnic group status, and health care utilization among this high risk population. Using the large, geographically and socioeconomically diverse Childhood Cancer Survivor Study (CCSS) cohort, we examined the influence of insurance type (private, public, and no insurance) on survivor-focused and general preventive health care within three different racial/ethnic groups of adult survivors of childhood cancer.
METHODS
Childhood Cancer Survivor Study
The CCSS is a multi-institutional study of individuals who survived ≥ 5 years after treatment for childhood cancer. Childhood cancer diagnoses include: leukemia, brain tumor, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone tumors. The participants were diagnosed before 21 years of age and treated at one of 26 collaborating CCSS institutions between January 1, 1970 and December 31, 1986. A detailed description of the CCSS study-design and -conduct methodology has been reported.18–20 The study was approved by the institutional review board at each of the participating institutions and informed consent was obtained from each participant.
The current analysis included 8,425 survivors who completed the CCSS Baseline Questionnaire, were ≥18 years old, completed information for race/ethnicity and insurance information (questionnaire can be downloaded from http://ccss.stjude.org). Because of differences in health care systems, participants who lived in Canada were excluded (n= 735). Only survivors ≥18 years of age were included as this is the age period when insurance coverage is typically lost for adult survivors of childhood cancer due to aging out of parental or public insurance programs. As shown in Figure 1, of the 20,691 childhood cancer survivors included in the cohort, 3,058 (14.8%) were lost to follow-up because of unsuccessful tracing due to incorrect last known address provided by the treating institution. The participants and those lost to follow-up were similar with regard to sex, cancer type, treatment received, age at diagnosis, and age at study participation (or for those lost to follow-up, the age at which the cohort was assembled). Among the 17,633 subjects located, 14,357 (81.4%) completed the questionnaire, including 10,398 participants who were ≥18 years. The cohort examined here was based on subjects’ self report of race/ethnicity. Those patients (n=1,238) with missing race or insurance information were excluded from the analysis.
Figure 1.
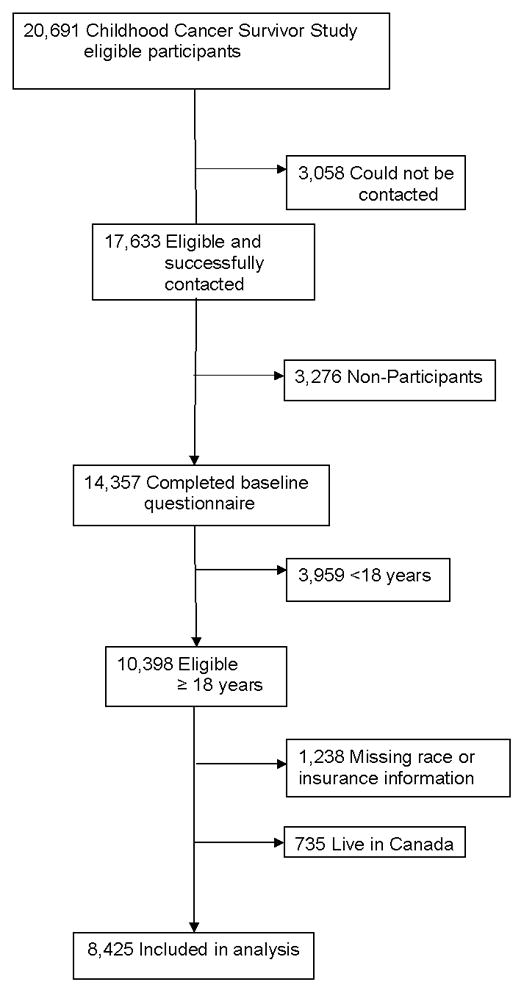
Flow Diagram of Participants
Primary Outcome Measures
Survivor-focused health care
Two measures defined survivor-focused health care received within the previous two years: cancer-related visit and cancer center visit. As previously described, participants were asked how many visits to a physician’s office were related to their previous cancer (cancer-related visit) and whether any of the visits were at an oncology center (cancer center visit).8 These outcomes were not mutually exclusive.
General preventive health care
Two measures defined general preventive health care for both men and women: having a general physical examination ≤ 2 years, and a dental examination ≤ 1 years. For women, general preventive health care included a clinical breast examination ≤ 1 years and a Pap smear ≤ 3 years.21
Independent variables
Health insurance was classified as private, public (Medicaid, Medicare, or other public assistance programs), or no health insurance. Based on self-reported race/ethnicity, participants were categorized into one of three groups: non-Hispanic white (NHW); black, non-Hispanic; and Hispanic. Other independent variables included gender, highest level of educational attainment, household income, and cancer diagnosis. To assess the influence of comorbid health conditions on health care utilization, we included the prevalence and severity of a chronic health condition. As previously described, the severity of chronic health conditions were based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) and classified as mild (grade 1), moderate (grade 2), severe (grade 3) and life-threatening or disabling (grade 4).4
Statistical analysis
Descriptive statistics were computed within each category of the three insurance categories and compared across insurance categories, using Chi-square test for categorical variables and ANOVA for continuous variables. The prevalence was estimated for sociodemographic variables, medical variables, and type of survivor-focused and general preventive health care received. Using log-binomial regression with an interaction term of race and insurance types, we estimated the relative risk (RR) of each outcome of interest (survivor-focused and general preventive health care) in survivors with no insurance and those with public insurance, relative to those with private insurance, within each ethnic group. These RR estimates were adjusted for age, gender, household income, highest level of education attainment, and having a severe, life threatening, or disabling chronic condition (grade 3 or 4). For each RR estimate, a corresponding 95% confidence interval (CI) was obtained using the standard large-sample inference method for generalized linear models. In the case where log-binomial regression did not converge, the COPY method was employed.22 Data were analyzed with SAS version 9.1 (SAS institute, Cary, NC).
RESULTS
The mean age at interview for the entire cohort was 28.1 years for privately insured, 27.2 years for publicly insured, and 26.3 years for the uninsured. The age range at interview for the entire cohort was 18 – 48.9 years. The mean interval from time of diagnosis for the entire cohort was 17.5 years for privately insured, 17.4 years for publicly insured, and 17.4 years for the uninsured.
TABLE 1 reports the additional characteristics of the 8,425 adult survivors of childhood cancer participants. There were more males who were uninsured (59.8%) when compared to the rates of males with private and public insurance within the entire cohort and for each ethnic group. In contrast, within the entire cohort, females were more likely to be publicly insured (59.8%) when compared to the rates of females with private insurance or who were uninsured. Publicly insured survivors had lower levels of educational attainment (63.4% were high school graduates or less) and lower household incomes (67.1% with income less than $20,000) than privately insured survivors or those without health insurance. There were higher rates of being uninsured among leukemia and non-Hodgkin lymphoma survivors. The publicly insured group was disproportionately represented by brain tumor survivors and survivors with a serious chronic health condition (grade 3 or 4). These trends were similar across all three ethnic groups.
Table 1.
Characteristics of adult survivors of childhood cancer by race/ethnicity and insurance type
| Insurance | Entire Cohort n=8425 | Black Non-Hispanic Survivors n=417 | Hispanic Survivors n=477 | White Non-Hispanic Survivors n=7531 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Private n=6395 | Public n=640 | None n=1390 | Private n=256 | Public n=67 | None n=94 | Private n=296 | Public n=72 | None n=109 | Private n=5843 | Public n=501 | None n=1187 | |
| Characteristics | % | % | % | % | % | % | % | % | % | % | % | % |
| Gendera b c d | ||||||||||||
| Male | 52.4 | 40.2 | 59.8 | 51.6 | 34.3 | 57.4 | 48.3 | 37.5 | 57.8 | 52.7 | 41.3 | 60.2 |
| Female | 47.6 | 59.8 | 40.2 | 48.4 | 65.7 | 42.6 | 51.7 | 62.5 | 42.2 | 47.3 | 58.7 | 39.8 |
| Educationa b c d | ||||||||||||
| HS or less | 23.8 | 63.4 | 47.8 | 33.5 | 56.7 | 50.0 | 35.5 | 65.2 | 54.8 | 22.8 | 64.1 | 47.0 |
| HS + some college | 76.2 | 36.6 | 52.2 | 66.5 | 43.3 | 50.0 | 64.5 | 34.8 | 45.2 | 77.2 | 35.9 | 53.0 |
| Household incomea b c d | ||||||||||||
| <$10,000 | 4.1 | 40.4 | 19.8 | 10.8 | 53.7 | 37.5 | 5.1 | 42.3 | 21 | 3.8 | 38.5 | 18.5 |
| $10,000–$19,999 | 9.6 | 26.7 | 27.3 | 18.6 | 20.4 | 25.0 | 14.9 | 28.8 | 27.2 | 9.0 | 27.2 | 27.4 |
| $20,000–$39,999 | 31.5 | 22.0 | 32.1 | 37.2 | 16.7 | 20.9 | 32.2 | 15.4 | 40.7 | 31.2 | 23.5 | 32.2 |
| $40,000–$59,999 | 25.2 | 7.3 | 12.7 | 21.6 | 7.4 | 9.7 | 23.9 | 7.7 | 7.4 | 25.4 | 7.2 | 13.3 |
| Over $60,000 | 29.6 | 3.6 | 8.1 | 11.8 | 1.8 | 6.9 | 23.9 | 5.8 | 3.7 | 30.6 | 3.6 | 8.6 |
| Cancer diagnosisa c d | ||||||||||||
| Leukemia | 29.0 | 26.2 | 34.9 | 25.0 | 22.4 | 22.3 | 38.5 | 31.9 | 44.9 | 28.7 | 25.9 | 35.1 |
| CNS | 10.2 | 26.9 | 9.9 | 8.6 | 20.9 | 6.4 | 5.7 | 25.0 | 6.4 | 10.5 | 27.9 | 10.4 |
| HL | 20.1 | 10.5 | 15.0 | 12.9 | 11.9 | 21.3 | 17.9 | 12.5 | 11.9 | 20.6 | 10.0 | 14.7 |
| NHL | 9.3 | 5.8 | 11.1 | 11.7 | 4.5 | 5.3 | 9.5 | 6.9 | 9.2 | 9.2 | 5.8 | 11.7 |
| Kidney (Wilms) | 6.3 | 5.3 | 7.1 | 12.9 | 10.4 | 10.6 | 4.7 | 4.2 | 9.2 | 6.1 | 4.8 | 6.7 |
| Neuroblastoma | 3.9 | 3.8 | 4.0 | 3.5 | 3.0 | 4.3 | 4.1 | 2.8 | 0.9 | 3.9 | 4.0 | 4.3 |
| Soft tissue sarcoma | 10.0 | 7.9 | 9.6 | 13.3 | 10.5 | 13.8 | 10.1 | 5.6 | 8.3 | 9.8 | 8.0 | 9.4 |
| Bone cancer | 11.2 | 13.6 | 8.4 | 12.1 | 16.4 | 16.0 | 9.5 | 11.1 | 9.2 | 11.2 | 13.6 | 7.7 |
| Age at interviewa c d | ||||||||||||
| 18 – 24 | 36.2 | 39.5 | 49.5 | 45.7 | 29.9 | 40.5 | 39.9 | 47.2 | 56.0 | 35.6 | 39.7 | 49.6 |
| 25 – 34 | 48.5 | 50.8 | 41.7 | 43.8 | 61.1 | 48.9 | 47.6 | 47.2 | 35.7 | 48.8 | 49.9 | 41.7 |
| 35 + | 15.3 | 9.7 | 8.8 | 10.5 | 9.0 | 10.6 | 12.5 | 5.6 | 8.3 | 15.6 | 10.4 | 8.7 |
| Grade 3/4 conditiona c d | ||||||||||||
| Yes | 26.5 | 40.9 | 22.9 | 23.0 | 32.8 | 29.8 | 26.4 | 37.5 | 15.6 | 26.7 | 42.5 | 23.0 |
| No | 73.5 | 59.1 | 77.1 | 77.0 | 67.2 | 70.2 | 73.6 | 62.5 | 84.4 | 73.3 | 57.5 | 77.0 |
Abbreviations: HS or less, some high school or high school graduate; HS + some college, high school graduate with either some college courses or other training; CNS, central nervous system tumor; HL, Hodgkin lymphoma; NHL, Non-Hodgkin lymphoma
Note: Percentages are based upon the total with available data for each variable
P<.05 from test of equality across the three insurance categories among all survivors.
P<.05 from test of equality across the three insurance categories among Black survivors.
P<.05 from test of equality across the three insurance categories among Hispanic survivors.
P<.05 from test of equality across the three insurance categories among White survivors.
The survivor-focused and general preventive health care reported by the entire cohort and across three ethnic groups is provided in TABLE 2. Uninsured survivors, for the entire cohort and, for the most part, across each ethnic group, demonstrated the lowest rates of survivor-focused and general preventive health care. The single exception was for uninsured black, non-Hispanic survivors whose rate of reporting survivor-focused care was not significantly different from their counterparts with private or public insurance. In contrast, publicly insured survivors reported the highest rates of survivor-focused health care (entire cohort and across each ethnic group except for black survivors). While the proportion of publicly insured survivors who reported a general physical examination in the previous two years (68.6%) was similar to that of privately insured survivors (67.1%), they reported lower rates of other types of general preventive care (dental care, clinical breast exam, Pap smears). Publicly insured Hispanic females had lower rates of reporting a Pap smear (68.9%) compared to those with private insurance (76.5%). Uninsured Hispanic females had even lower rates of reporting a Pap smear (53.3%). Among NHW females, both public and uninsured survivors had lower rates of Pap smear utilization (72.2% and 72.9% respectively) compared to privately insured females (81.9%). The proportion of uninsured black females reporting a Pap smear was similar to those who had private or public insurance.
Table 2.
Percentage of Black, non-Hispanic, Hispanic, and White, non-Hispanic adult survivors of childhood cancer who reported the following types of health care, by insurance type
| Entire Cohort n=8425 | Black Non-Hispanic Survivors n=417 | Hispanic Survivors n=477 | White Non-Hispanic Survivors n=7531 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insurance Outcome |
Private n=6395 | Public n=640 | None n=1390 | P Value | Private n=256 | Public n=67 | None n=94 | P Value | Private n=296 | Public n=72 | None n=109 | P Value | Private n=5843 | Public n=501 | None n=1187 | P Value |
| Survivor-Focused Care, w/in past 2 yrs | ||||||||||||||||
| Cancer-related visit | 41.6 | 55.3 | 33.5 | <.001 | 34.9 | 42.1 | 28.7 | .25 | 44.6 | 68.2 | 41.0 | <.001 | 41.7 | 55.1 | 33.1 | <.001 |
| Cancer center visit | 18.4 | 23.3 | 14.9 | <.001 | 13.7 | 19.4 | 16.0 | .49 | 23.3 | 40.3 | 22.0 | .008 | 18.4 | 21.4 | 14.2 | <.001 |
| General Preventive Care | ||||||||||||||||
| General physical exam ≤2 yrs | 67.1 | 68.6 | 48.8 | <.001 | 63.9 | 67.2 | 47.3 | .01 | 63.8 | 73.2 | 49.0 | .003 | 67.4 | 68.1 | 48.9 | <.001 |
| Dental exam ≤ 1 yr | 64.5 | 52.9 | 39.2 | <.001 | 53.9 | 45.5 | 31.5 | <.001 | 56.1 | 45.8 | 35.2 | <.001 | 65.4 | 54.9 | 40.2 | <.001 |
| Clinical breast exam ≤ 1 yra | 66.6 | 58.1 | 48.6 | <.001 | 70.2 | 72.1 | 50.0 | .05 | 63.2 | 62.2 | 31.1 | <.001 | 66.7 | 55.4 | 50.2 | <.001 |
| Pap smear ≤ 3 yrsa | 81.9 | 73.6 | 71.7 | <.001 | 86.9 | 88.1 | 78.9 | .42 | 76.5 | 68.9 | 53.3 | .01 | 81.9 | 72.2 | 72.9 | <.001 |
Females only
TABLE 3 reports the adjusted relative risks of reporting survivor-focused and general preventive health care among survivors with public insurance or no insurance in comparison to those with private insurance (referent). After adjustment for race/ethnicity, age at interview, gender, household income, highest level of educational attainment and presence of a serious (grade 3 or 4) chronic health condition, uninsured survivors were less likely to report a cancer-related visit (RR=0.83, 95% CI, 0.75–0.91) and also less likely to report a cancer center visit (RR=0.83, 95% CI, 0.71–0.98) than privately insured survivors. In contrast, publicly insured survivors were more likely to have had survivor-focused health care (both cancer-related and cancer center visit) than privately insured survivors (RR=1.22, 95% CI, 1.11–1.35; and RR=1.41, 95% CI, 1.18–1.70, respectively). Within the three ethnic groups, uninsured NHW survivors were less likely to report survivor focused-care (RR=0.82, 95% CI, 0.74–0.90 for a cancer-related visit; RR=0.79, 95% CI, 0.66–0.94 for a cancer center visit) compared to privately-insured NHW survivors. Both uninsured and publicly insured NHW female survivors were less likely to report Pap smear utilization (RR=0.93, 95% CI, 0.87–0.99 and RR=0.87, 95% CI, 0.80–0.95, respectively).
Table 3.
Relative Risk (RR) and 95% Confidence Intervals (CI) of the likelihood of reporting survivor-focused or general preventive health care among survivors with public insurance or no insurance in comparison to those with private insurance (reference)
| Insurance | Entire Cohorta | Black Non-Hispanic Survivorsb | Hispanic Survivorsb | White Non-Hispanic Survivorsb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference n=6395 | Reference n=256 | Reference n=296 | Reference n=5843 | |||||||||||||
| Public n=640 | None n=1390 | Public n=67 | None n=94 | Public n=72 | None n=109 | Public n=501 | None n=1187 | |||||||||
| Outcome | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Survivor-Focused Care, w/in past 2 yrs | ||||||||||||||||
| Cancer-related visit | 1.22 | 1.11–1.35 | 0.83 | 0.75–0.91 | 1.22 | 0.83–1.80 | 0.90 | 0.60–1.36 | 1.41 | 1.12–1.78 | 0.94 | 0.68–1.30 | 1.20 | 1.08–1.34 | 0.82 | 0.74–0.90 |
| Cancer center visit | 1.41 | 1.18–1.70 | 0.83 | 0.71–0.98 | 1.86 | 1.00–3.47 | 1.27 | 0.67–2.41 | 1.88 | 1.28–2.77 | 1.03 | 0.64–1.66 | 1.31 | 1.06–1.62 | 0.79 | 0.66–0.94 |
| General Preventive Care | ||||||||||||||||
| General physical exam ≤2 yrs | 1.07 | 1.00–1.14 | 0.79 | 0.74–0.85 | 1.13 | 0.90–1.42 | 0.81 | 0.62–1.05 | 1.20 | 0.99–1.45 | 0.82 | 0.64–1.04 | 1.06 | 0.98–1.14 | 0.79 | 0.74–0.85 |
| Dental exam ≤ 1 yr | 0.90 | 0.82–0.99 | 0.68 | 0.63–0.74 | 0.83 | 0.57–1.21 | 0.56 | 0.38–0.84 | 0.81 | 0.56–1.16 | 0.77 | 0.57–1.04 | 0.91 | 0.82–1.01 | 0.68 | 0.63–0.74 |
| Clinical breast exam ≤ 1 yrc | 0.94 | 0.84–1.05 | 0.77 | 0.70–0.86 | 1.18 | 0.94–1.46 | 0.71 | 0.48–1.04 | 1.02 | 0.74–1.40 | 0.50 | 0.31–0.81 | 0.90 | 0.79–1.02 | 0.82 | 0.73–0.91 |
| Pap smear ≤ 3 yrsc | 0.90 | 0.84–0.97 | 0.90 | 0.85–0.96 | 0.91 | 0.80–1.03 | 0.83 | 0.69–1.01 | 0.97 | 0.76–1.24 | 0.74 | 0.55–0.99 | 0.87 | 0.80–0.95 | 0.93 | 0.87–0.99 |
Adjusted for age, gender, household income, highest level of educational attainment, race/ethnicity, and grade 3 or 4 chronic condition.
Adjusted for age, gender, household income, highest level of educational attainment, and grade 3 or 4 chronic condition.
Females only
Uninsured survivors were less likely than privately insured survivors to report all four measures of general preventive health care. However, in contrast with the above described trends for survivor-focused health care, publicly insured survivors were not more likely to report general preventive care than privately insured survivors. While the likelihood of a general physical exam was similar between both groups, publicly insured survivors were less likely to report a dental exam (RR = 0.90, 95% CI, 0.82–0.99) and publicly insured female survivors were less likely to report a Pap smear (RR = 0.90, 95% CI 0.84–0.97) than privately insured females.
COMMENT
Adult survivors of childhood cancer are at-risk for a myriad of late effects related to their cancer treatment, including early onset cardiovascular disease, stroke and second malignancies.23–27 Much of morbidity and mortality related to the childhood cancer therapy received occurs during young adulthood with a long latency after the initial exposure.3–6 It is important for adult survivors of childhood cancer to have access to long-term follow-up care and cancer screening, with the intent to prevent or lessen future morbidity and mortality. Affordable health insurance plans and/or public programs are an important factor toward ensuring survivor-focused health care.
To our knowledge, this is the first large study of adult survivors of childhood cancer from across the U.S. to examine the influence of insurance type, by three ethnic groups, on survivor-focused and general preventive health care utilization. There were several notable findings. Despite being a significantly more disadvantaged group with high rates of poverty, survivors with public health insurance reported utilizing survivor-focused health care at rates higher than survivors with private health insurance. This suggests that Medicaid/Medicare services are providing much needed access to care for high risk survivors with serious health conditions related to their previous cancer treatment. Our findings are similar to other studies conducted among low-income populations which have found that having any type of health insurance coverage, including public, has a significant impact on access to needed health care services.28
In contrast, a substantial proportion of uninsured survivors with serious chronic diseases did not report utilization of survivor-focused or general preventive health care. Large population-based studies have demonstrated significant benefits of public health insurance programs on the receipt of quality health care, as well as, improvements in the continuity of care and receipt of preventive health services.29–32 Similar policy initiatives, in which both the federal and state governments finance a public insurance plan for low income, at-risk childhood cancer survivors, could provide a vital safety net to improve access to health services.
We found significant differences in the rates of utilization of general preventive health care within the different categories of insurance coverage. We analyzed the three ethnic groups separately because health care utilization can vary by ethnicity for various factors, including cultural influences such as acculturation and nativity.33–35 Similar to other studies, we found lower reported rates of utilization of general preventive health care for the uninsured across all three ethnic groups.36 In the general population, uninsured adults are more likely to be diagnosed with advanced stage cancers due to poor access to cancer screening.37–40
We found lower probabilities for reporting cancer screening practices (clinical breast exam and Pap smear) for both uninsured and publicly insured NHW females. This finding of lower utilization rates in uninsured and publicly insured NHW survivors has similarly been observed in other cancer control studies. For example, in a study examining the breast cancer screening practices of uninsured woman, they found that uninsured NHW females (even after controlling for SES factors) had lower reported utilization than Black and Hispanic woman.41 Community level factors, specifically county-level proportions of uninsured woman, impacted on breast cancer screening rates. Woman who lived in counties with higher rates of uninsured were less likely to be screened. This county level effect on screening rates, however, had little impact on those who had household income levels, between $25,000–$75,000. A second study, evaluating county level covariates (including residence in health professional shortage areas, urban/rural setting, racial/ethnic composition, and number of health centers/clinics) found that Black women were more likely than NHW women to report Pap smears. Among woman who resided in urban areas with lower primary care physician supply, there were lower rates of Pap smear use. Woman in rural areas were also less likely to report Pap smear use.42 Although the explanatory factors for the observed differences for NHW survivors in this CCSS study are not known, community level factors that may impact on healthcare utilization for diverse groups of survivors, should be explored in future research. 28, 43
We also found lower reported rates of dental care utilization for both the uninsured and publicly insured compared to privately insured survivors. In the general population, adult Medicaid beneficiaries have less utilization of dental services than privately insured adults.44 In states evaluating methods to enhance access to dental care services, improvements in utilization occurred when Medicaid programs reimbursed dental charges at rates comparable to private dental rates.45, 46 Given the significant risk for delayed and poor dental development in childhood cancer survivors, policy considerations to improve necessary dental care services for survivors are needed.47, 48
This study shows that public insurance programs result in high rates of reported utilization of survivor-focused health care. Specifically, we found that both Hispanic and NHW publicly insured survivors had a higher likelihood of reporting a cancer center visit compared to privately insured survivors. This finding was unanticipated as we hypothesized that privately insured survivors would have the highest utilization of survivor-focused health care. There are several possible explanations. First, having a cancer diagnosis reflects a “teachable moment” particularly for survivors with public insurance.49, 50 If a cancer patient with a lower socioeconomic status enters the public health care system for the first time due to the need for cancer treatment, they may be more motivated to continue the recommended follow-up. They are now able to utilize health care services that may have not been available prior to their diagnosis of cancer. In contrast, for those survivors with private insurance, they may have barriers to utilize survivor-focused health care because of time missed from work, large out-of-pocket spending, higher deductibles, co-payments, and/or lifetime caps in insurance coverage.51
A second explanation for our finding may be that the quality of care for publicly insured survivors is influenced by the hospital type where they receive care. In the Medicare population, black or poor patients are more likely to receive care in urban teaching hospitals which deliver higher quality of care.52 Although black and poor patients were found to receive lower quality of care, when adjusted for hospital type, the receipt of care in these urban teaching hospitals almost completely offsets the poor quality care they received within each hospital. The authors found, through the use of zip code data, that black or poor patients are almost two times more likely to receive care in urban teaching hospitals rather than in rural or non-teaching hospitals. It is also possible that adult childhood cancer survivors are more likely to receive their survivorship care in urban teaching hospitals as the vast majority of pediatric oncology care is delivered within academic centers.
We found lower reported utilization of survivor-focused and general preventive care for uninsured survivors. We hypothesize these findings may be due to lacking a usual source of care, being underinsured, and lapses in insurance coverage.11, 28, 53 Since the primary causes of late mortality among adult childhood cancer survivors include cancer recurrence or development of a second malignancy, affordable access to general preventive care, including cancer screening, is critical for survivors.3 It will be important for future research to evaluate the effect of the recent national policy changes including the Patient Protection and Affordable Care Act (passed in the Senate, December 2009) and the major expansion of Medicaid.54
When interpreting our study results, there are the following limitations. Although this is the largest national cohort of childhood cancer survivors, the insurance status and SES of non-participants is not known. As a result, there may be a selection bias in our sample, including a possible lower representation of uninsured survivors or those with a lower SES, which could select for those survivors who are more likely to utilize care thereby decreasing differences observed across insurance groups. Using self-report data for the measurement of utilization of survivor-focused and general preventive health care, can result in an overestimation of receipt of services because of recall bias.55, 56 Our findings demonstrate a statistically significant lower probability of reporting survivor-focused and general preventive health care for uninsured childhood cancer survivors. These findings further emphasize the need to develop targeted policy efforts to improve access to affordable health care options for adult survivors of childhood cancer. A third limitation is the use of current insurance status alone and lack of data regarding the continuity of insurance status for survivors in the sample. Previous population-based studies demonstrate that the uninsured, as well as those unstably insured (i.e. lapses in continuous insurance coverage), report nearly the same rates of poor access to care.43 Future research examining the effect of uninterrupted insurance coverage on access to survivor-focused and general preventive health care in young adult survivors is essential.
In summary, a significant proportion of uninsured adult survivors of childhood cancer, across all ethnicities, have lower utilization rates of survivor-focused and general preventive health care. Targeted policy changes directed at greater access to affordable health care for all adult survivors of childhood cancer is critical given the significant burden of chronic disease due to cancer treatment at a young age.
Acknowledgments
Funding/Support: This work was supported by Grant U24-CA-55727 (L.L. Robison, Principal Investigator) from the Department of Health and Human Services, funding to the University of Minnesota from the Children’s Cancer Research Fund, funding to St. Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities (ALSAC), the UCLA STOP Cancer Career Development Award and the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation.
Footnotes
Financial disclosures: None reported.
References
- 1.Mariotto AB, Rowland JH, Yabroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 2.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113(9):2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Geenen MM, Cardous-Ubbink MC, Kremer LCM, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 6.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2(1):61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(27):4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU. A national study of chronic disease prevalence and access to care in uninsured U.S. adults. Ann Intern Med. 2008;149:170–176. doi: 10.7326/0003-4819-149-3-200808050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Robinson J, Shavers V. The role of health insurance coverage in cancer screening utilization. J Health Care Poor Underserved. 2008;19(3):842–856. doi: 10.1353/hpu.0.0048. [DOI] [PubMed] [Google Scholar]
- 12.Park ER, Li FP, Liu Y, et al. Health insurance coverage in survivors of childhood cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(36):9187–9197. doi: 10.1200/JCO.2005.01.7418. [DOI] [PubMed] [Google Scholar]
- 13.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: a comparison of long-term outcomes, health care utilization, and health-related behaviors from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(27):6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 14.Wells K, Roetzheim R. Health disparities in receipt of screening mammography in Latinas: a critical review of recent literature. Cancer Control. 2007;14(4):369–379. doi: 10.1177/107327480701400407. [DOI] [PubMed] [Google Scholar]
- 15.Goss E, Lopez AM, Brown CL, Wollins DS, Brawley OW, Raghavan D. American Society of Clinical Oncology policy statement: disparities in cancer care. J Clin Oncol. 2009;27:2881–2885. doi: 10.1200/JCO.2008.21.1680. [DOI] [PubMed] [Google Scholar]
- 16.Kolb B, Wallace AM, Hill D, Royce M. Disparities in cancer care among racial and ethnic minorities. Oncology. 2006;20(10):1256–1261. [PubMed] [Google Scholar]
- 17.Haas J, Phillips K, Sonneborn D, McCulloch C, Liang S. Effect of managed care insurance on the use of preventive care for specific ethnic groups in the United States. Med Care. 2002;40(9):743–751. doi: 10.1097/00005650-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Mertens AC, Walls RS, Taylor L, et al. Characteristics of childhood cancer survivors predicted their successful tracing. J Clin Epidemiol. 2004;57(9):933–944. doi: 10.1016/j.jclinepi.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 20.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeazel MW, Oeffinger KC, Gurney JG, et al. The cancer screening practices of adult survivors of childhood cancer. Cancer. 2004;100(3):631–640. doi: 10.1002/cncr.20008. [DOI] [PubMed] [Google Scholar]
- 22.Deddens JA, Petersen MR. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2004;159(2):213–214. doi: 10.1093/aje/kwh022. [DOI] [PubMed] [Google Scholar]
- 23.Oeffinger K, Hudson M. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin. 2004;54(4):208–236. doi: 10.3322/canjclin.54.4.208. [DOI] [PubMed] [Google Scholar]
- 24.van der Pal HJ, van Dalen EC, Kremer LC, Bakker PJ, van Leeuwen FE. Risk of morbidity and mortality from cardiovascular disease following radiotherapy for childhood cancer: a systematic review. Cancer Treat Rev 2005. 2005;31(3):173–185. doi: 10.1016/j.ctrv.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Bowers DC, McNeil DE, Liu Y, et al. Stroke as a late treatment effect of Hodgkin’s Disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(27):6508–6515. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 26.Guibout C, Adjadj E, Rubino C, et al. Malignant breast tumors after radiotherapy for a first cancer during childhood. J Clin Oncol. 2005;23(1):197–204. doi: 10.1200/JCO.2005.06.225. [DOI] [PubMed] [Google Scholar]
- 27.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141(8):590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 28.Emmons KM, Lobb R, Puleo E, Bennett G, Stoffel E, Syngal S. Colorectal cancer screening: prevalence among low-income groups with health insurance. Health Aff. 2009;28(1):169–177. doi: 10.1377/hlthaff.28.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Medicare and Medicaid Services. [Accessed May 29, 2010];Overview: low cost health insurance for families and children [Internet] Available from: http://www.cms.hhs.gov/LowCostHealthInsFamChild/
- 30.Szilagyi PG, Dick AW, Klein JD, Shone LP, Zwanziger J, McInerny T. Improved access and quality of care after enrollment in the New York State Children’s Health Insurance Program (SCHIP) Pediatrics. 2004;113(5):e395–404. doi: 10.1542/peds.113.5.e395. [DOI] [PubMed] [Google Scholar]
- 31.Tu HT, Cunningham PJ. Public coverage provides a vital safety net for children with special health care needs. Issue Brief Cent Stud Health Syst Change. 2005;(98):1–7. [PubMed] [Google Scholar]
- 32.Brach C, Lewit EM, VanLandeghem K, et al. Who’s enrolled in the State Children’s Health Insurance Program (SCHIP)? An overview of findings from the Child Health Insurance Research Initiative (CHIRI) Pediatrics. 2003;112(6):e499. [PubMed] [Google Scholar]
- 33.Kandula NR, Wen M, Jacobs EA, Lauderdale DS. Low rates of colorectal, cervical, and breast cancer screening in Asian Americans compared with non-Hispanic whites. Cancer. 2006;107(1):184–192. doi: 10.1002/cncr.21968. [DOI] [PubMed] [Google Scholar]
- 34.Ponce NA, Chawla N, Babey SH, et al. Is there a language divide in PAP test use? Med Care. 2006;44(11):998–1004. doi: 10.1097/01.mlr.0000233676.61237.ef. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez LE, Morales A. Language and use of cancer screening services among border and non-border Hispanic Texas women. Ethn Health. 2007;12(3):245–263. doi: 10.1080/13557850701235150. [DOI] [PubMed] [Google Scholar]
- 36.Yeazel MW, Gurney JG, Oeffinger KC, et al. An examination of the dental utilization practices of adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Public Health Dent. 2004;64(1):50–54. doi: 10.1111/j.1752-7325.2004.tb02726.x. [DOI] [PubMed] [Google Scholar]
- 37.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91(16):1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 38.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 39.O’Malley CD, Shema SJ, Clarke LS, Clarke CA, Perkins CI. Medicaid status and stage at diagnosis of cervical cancer. Am J Public Health. 2006;96(12):2179–2185. doi: 10.2105/AJPH.2005.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley C, Given C, Roberts C. Late stage cancers in a Medicaid-insured population. Med Care. 2003;41(6):722–728. doi: 10.1097/01.MLR.0000065126.73750.D1. [DOI] [PubMed] [Google Scholar]
- 41.Schootman M, Walker MS, Jeffe DB, Rohrer JE, Baker EA. Breast cancer screening and incidence in communities with a high proportion of uninsured. Am J Prev Med. 2007;33(5):379–386. doi: 10.1016/j.amepre.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med. 2008;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Schoen C, DesRoches C. Uninsured and unstably insured: the importance of continuous insurance coverage. Health Serv Res. 2000;35(1):187–206. [PMC free article] [PubMed] [Google Scholar]
- 44.Ku L. Medical and dental care utilization and expenditures under Medicaid and private health insurance. Med Care Res Rev. 2009;66(4):456–471. doi: 10.1177/1077558709334896. [DOI] [PubMed] [Google Scholar]
- 45.Damiano P, Momany E, Carter K, Jones M, Askelson N. Time to first dental visit after initially enrolling in Medicaid and S-SCHIP. Med Care. 2008;46(12):1234–1239. doi: 10.1097/MLR.0b013e31817d92cd. [DOI] [PubMed] [Google Scholar]
- 46.Eklund S, Pittman J, Clark S. Michigan Medicaid’s Healthy Kids dental program: an assessment of the first 12 months. J Am Dent Assoc. 2003;134(11):1509–1515. doi: 10.14219/jada.archive.2003.0083. [DOI] [PubMed] [Google Scholar]
- 47.van der Pasvan Voskuilen I, Veerkamp J, Raber-Durlacher J, et al. Long-term adverse effects of hematopoietic stem cell transplantation on dental development in children. Support Care Cancer. 2009;17(9):1169–1175. doi: 10.1007/s00520-008-0567-1. [DOI] [PubMed] [Google Scholar]
- 48.Cubukçu C, Sevinir B. Dental health indices of long-term childhood cancer survivors who had oral supervision during treatment: a case-control study. Pediatr Hematol Oncol. 2008;25(7):638–646. doi: 10.1080/08880010802237849. [DOI] [PubMed] [Google Scholar]
- 49.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganz PA. A teachable moment for oncologists: cancer survivors, 10 million strong and growing! J Clin Oncol. 2005;23(24):5458–5460. doi: 10.1200/JCO.2005.04.916. [DOI] [PubMed] [Google Scholar]
- 51.American Cancer Society. [Accessed May 29, 2010];Insurance and cost-related barriers to cancer care: cancer facts and figures. 2008 Available from: http://www.cancer.org/downloads/accesstocare/CFF2008_Special_Section.pdf.
- 52.Kahn KL, Pearson ML, Harrison ER, et al. Health care for black and poor hospitalized Medicare patients. JAMA. 1994;271(15):1169–1174. [PubMed] [Google Scholar]
- 53.Schoen C, CD Uninsured and unstably insured: the importance of continuous insurance coverage. Health Serv Res. 2000;35:187–206. [PMC free article] [PubMed] [Google Scholar]
- 54.Democratic Policy Committee. [Accessed June 1, 2010];The Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act. doi: 10.1002/cncr.25725. Available from: http://dpc.senate.gov/dpcdoc-sen_health_care_bill.cfm. [DOI] [PubMed]
- 55.McGovern PG, Lurie N, Margolis KL, Slater JS. Accuracy of self-report of mammography and PAP smear in a low-income urban population. Am J Prev Med. 1998;14(3):201–208. doi: 10.1016/s0749-3797(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 56.Tumiel-Berhalter LM, Finney MF, Jaen CR. Self-report and primary care medical record documentation of mammography and PAP smear utilization among low-income women. J Natl Med Assoc. 2004;96(12):1632–1639. [PMC free article] [PubMed] [Google Scholar]


