Abstract
Neonatal herpes simplex virus (HSV) infection continues to cause significant morbidity and mortality despite advances in diagnosis and treatment. Prior to antiviral therapy, 85% of patients with disseminated HSV disease and 50% of patients with central nervous system disease died within 1 year. The advent of antiviral therapy has dramatically improved the prognosis of neonatal HSV with initially vidarabine and subsequently acyclovir increasing the survival rate of infected neonates and improving long-term developmental outcomes. More recently, polymerase chain reaction has allowed earlier identification of HSV infection and provided a quantitative guide to treatment. Current advances in the treatment of neonatal HSV infections are looking toward the role of prolonged oral suppression therapy in reducing the incidence of recurrent disease. Of concern, however, are increasing reports of acyclovir-resistant HSV isolates in patients following prolonged therapy.
1 Background
Neonatal herpes simplex virus (HSV) infection continues to cause significant morbidity and mortality despite significant advances in treatment [1]. The current estimated rate of occurrence of neonatal HSV disease in the United States is approximately 1 in 3,200 deliveries and an estimated 1,500 cases of neonatal HSV infection per year [2].The frequency of neonatal HSV disease varies from country to country; most nations have significantly lower incidences than the United States for reasons that are not clearly understood [3].
2 Epidemiology of Neonatal HSV
HSV disease of the newborn can be acquired during one of three time periods:in utero, perinatally, or postnatally. The most common mode of transmission is via direct contact of the baby with infected vaginal secretions during delivery [2]. The risk of transmission is influenced by several factors. The risk is greater with primary HSV infection acquired during pregnancy compared to reactivation of previous infection [2, 4, 5]. Among mothers with primary infection, acquisition near the time of labor is the major risk factor for transmission to the neonate [6]. The risk of transmission increases with the length of time the membranes are ruptured [7] and is increased if there is disruption of the mucocutaneous barriers (e.g., through the use of fetal scalp electrodes) [2, 8]. Transmission of infections is substantially reduced by caesarean section [2]. Maternal seroconversion prior to delivery is also associated with a decreased risk of neonatal HSV. This observation is probably related to a protective role of HSV-specific maternal antibodies [9].
3 Clinical Manifestations of Neonatal HSV
HSV infections in newborns can be classified into three patterns, which occur with roughly equal frequency [10]. These comprise disseminated disease involving multiple visceral organs, including lungs, liver, adrenal glands, skin, eyes, and the brain; central nervous system (CNS) disease, with or without skin lesions; and disease limited to the skin, eyes, and/or mouth (SEM disease). Patients with disseminated disease and SEM disease present earliest, generally at 10–12 days of life, whereas CNS disease presents during the second or third week of life [10].
The initial manifestations of CNS disease are frequently non-specific and include temperature instability, respiratory distress, poor feeding, and lethargy, which can then progress to hypotension, disseminated intravascular coagulation (DIC), apnoea, and shock. Between 60 and 70% of babies with CNS disease have associated skin vesicles [11]. In disseminated disease, involvement of the CNS is a common feature occurring in approximately 60–75% of infants. Severe coagulopathy, liver dysfunction, and pulmonary involvement are further serious complications. Twenty percent of neonates with disseminated HSV disease will not develop cutaneous vesicles during the course of infection [10].
4 Mortality and Morbidity
Since the advent of antiviral therapy the prognosis of neonatal HSV has improved [11]. Prior to antiviral therapy, 85% of patients with disseminated HSV disease and 50% of patients with CNS disease died within 1 year [12]. With the use of high-dose acyclovir (60 mg/kg/day for 21 days), 12-month mortality has reduced to 29% for disseminated neonatal HSV disease and to 4% for CNS HSV disease [13]. Improvements in morbidity rates in these disease categories have not been so dramatic with the advent of antiviral therapy. Morbidity figures show that in survivors with neonatal disseminated HSV disease, normal neurological development occurs in 83% [11], an increase from 50% in the pre-antiviral era [12]. In the case of CNS neonatal HSV disease, little change has occurred with 31% of patients today having normal neurological development [12, 13]. In contrast, the morbidity from SEM disease has improved dramatically with the advent of antivirals with fewer than 2% of patients today having developmental delay after SEM disease compared with 25% historically [11, 12].
5 Antiviral Therapy
In 1980, the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG) reported the first successful trial of vidarabine for treatment of neonatal HSV infections [12]. Fifty-six infants were enrolled in the trial of which 31 were randomized to receive vidarabine at a dose of 15 mg/kg/day for 10 days. This study showed a significant reduction in mortality at 6 months, irrespective of gestational age at delivery, in babies with CNS and disseminated disease from 74 to 38% with vidarabine therapy (P=0.014). In addition, morbidity was improved threefold, as 29% of treated infants compared to 11% of placebo recipients developed normally at 1 year of life.
Although treatment with vidarabine was significantly better than placebo, mortality and morbidity remained high, irrespective of the dose of vidarabine used [14]. Also of concern was the fact that disease progression, from a lesser to more severe form, occurred in 8 out of 29 patients (21%) treated with vidarabine, and in infants with SEM disease who survived, 86% had recurrent skin lesions during the first year of life after treatment [14].
In the 1980s acyclovir was developed as a selective and specific inhibitor of viral replication. Initial reports suggested that acyclovir was superior to vidarabine in treatment of biopsy-proven HSV encephalitis [15]. Whitley et al. [16] conducted a randomized controlled trial comparing vidarabine and acyclovir and their effect on treatment outcomes of neonatal HSV infection in 210 infants with virologically proven neonatal HSV infection [16]. Ninety-five infants received intravenous vidarabine (30 mg/kg/day) and 107 infants received acyclovir (30 mg/kg/day) for 10 days. These results were disappointing, showing no significant differences between the two agents in either morbidity (P=0.83) or mortality (P=0.27) [16]. Despite adjusting for the extent of disease, the statistical power was insufficient to determine whether sizeable differences existed within disease categories. However, the number of patients that continued to shed virus during treatment declined more rapidly in the acyclovir group (P<0.001). The disease progressed in four infants during therapy, specifically disease advanced from being localized to the skin to involve the central nervous system or from brain involvement to multiorgan disease [16]. In six infants with disseminated infection, four treated with vidarabine and two receiving acyclovir, the disease spread to an additional organ after the beginning of therapy [16]. Eight percent of babies relapsed after treatment, suggesting that not only a higher dosage but also a more prolonged course of intravenous therapy may be more effective.
6 Determining Dosage and Duration of Treatment
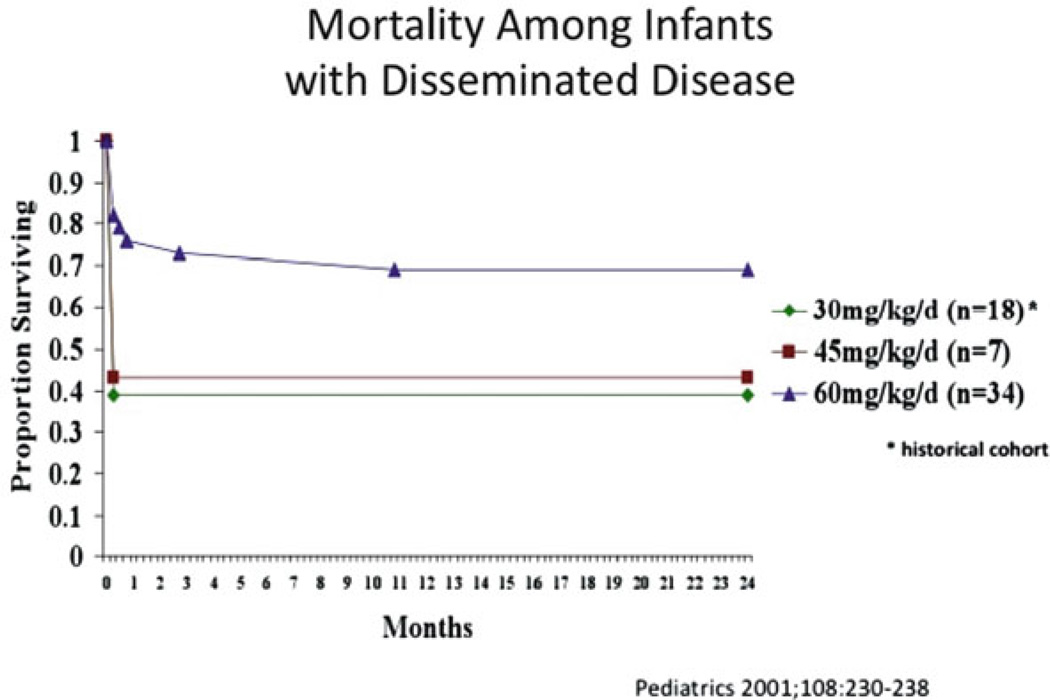
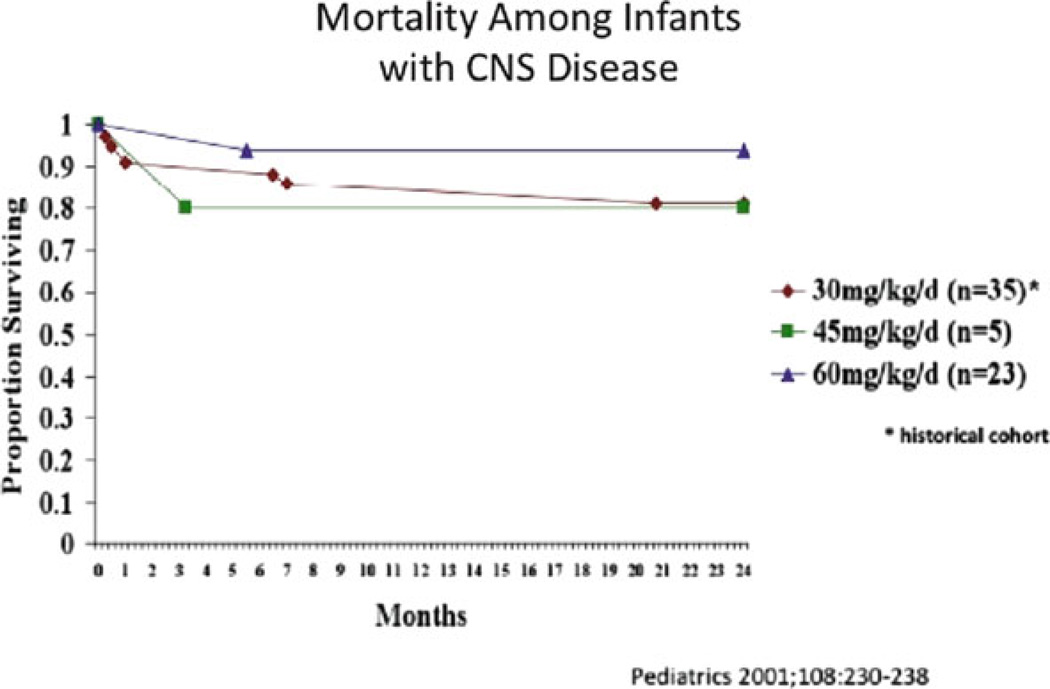
Kimberlin et al. [10] investigated the therapeutic efficacy and safety of high-dose acyclovir in the treatment of neonatal HSV disease [11]. In this study a more prolonged treatment course of 21 days was used. Sixteen patients received intermediate-dose acyclovir (45 mg/kg/day) and 72 patients received high-dose acyclovir (60 mg/kg/day). Data were also compared with those of a previous NIAID CASG trial in which 15 patients received standard-dose acyclovir (15 mg/kg/day) for 10 days [16]. Recipients of high-dose acyclovir had an increased survival rate compared to patients treated with standard-dose acyclovir (P=0.0035; OR=3.3; 95% CI: 1.5–7.3) (Figs. 15.1 and 15.2). A higher percentage of patients treated with high-dose acyclovir were developmentally normal at 12 months, but the difference was not significant (P=0.539; OR=1.5; 95% CI: 0.4–5.7). However, when controlled for the confounding factor of HSV type (HSV-1 vs. HSV-2) a borderline significant difference in the number of children returning to normal between high-dose and standard-dose acyclovir groups was found (P=0.051).
Figure 15.1.
Reduction in mortality from disseminated HSV disease with 60 mg/kg/day acyclovir
Figure 15.2.
Reduction in mortality from CNS HSV disease with 60 mg/kg/day acyclovir
As a result of these studies [11, 16] the current recommended antiviral regimen for neonatal HSV infection is intravenous acyclovir 60 mg/kg/day divided into three doses (i.e., every 8 h) for 21 days in patients with CNS or disseminated disease [11] and for 14 days in patients with SEM disease [11].
7 Polymerase Chain Reaction (PCR)-Guided Therapy
Despite these achievements the morbidity and the mortality from neonatal HSV infection remain unacceptably high [11]. The diagnosis of neonatal HSV infections has been revolutionized by the application of PCR to clinical specimens including CSF [17] and blood [18]. The PCR method has enabled the detection and implementation of treatment earlier in the illness, before widespread viral dissemination throughout the body or significant replication within the nervous system, and it has also allowed clinicians to study the response to treatment.
Serial examinations of CSF for viral DNA have shown that HSV DNA is present for a mean of 10.1 days after onset of neurological disease and has been shown to temporarily recur in the CSF in patients with recurrent illness [19]. Kimberlin et al. retrospectively evaluated 77 neonates with culture-proven HSV disease by PCR [17]. They found that HSV DNA was detected by PCR in the CSF of 7 of the 29 infants who had been categorized previously as having SEM disease, 13 of the 14 infants previously classified as having disseminated disease, and 26 of the 34 infants previously categorized as having CNS disease [20]. This gave an overall specificity of 71% and sensitivity of 80%. In comparison, other retrospective studies have reported higher specificities of 100% [21, 22] and greater sensitivities of 100 [22] and 75% [23].
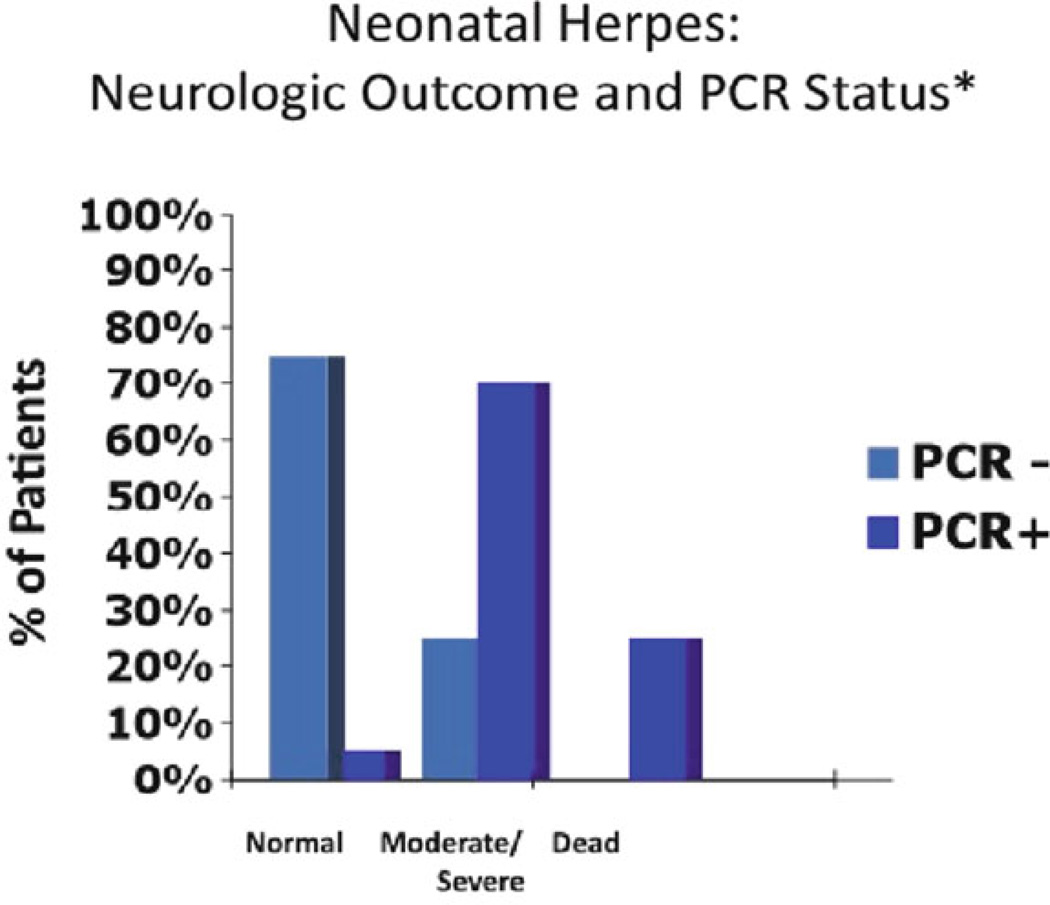
In addition to diagnosis, PCR has been used to guide treatment by establishing quantitative measures of assessing HSV DNA in the CSF of patients with HSV encephalitis to determine the effect of antiviral therapy on the clearance of viral DNA from the CSF [24]. This study suggested that patients with less than 100 copies of HSV DNA per microliter of CSF prior or within 4 days of initiation of treatment had more favorable outcomes (Fig. 15.3). Moreover, a retrospective study performed by Kimberlin et al. demonstrated that infants who had HSV DNA detected in the CSF by PCR after completing intravenous antiviral therapy had poorer outcomes compared to infants who were CSF PCR negative at the end of therapy [20]. The use of quantitative PCR measures of HSV DNA in combination with clinical observations and neuroimaging may help guide in defining the duration of therapy of CNS disease. Current recommendations are that all patients with CNS HSV disease should have a repeat lumbar puncture performed at the end of intravenous acyclovir treatment to ensure that the CSF PCR is negative [11]. Those patients who remain PCR positive continue to receive antiviral therapy until PCR negativity is achieved as a component of ongoing NIH studies in the United States [10].
Figure 15.3.
Neurological outcome following completion of intravenous acyclovir therapy depending on the status polymerase chain reaction result in the CSF. PCR positive was defined as greater than 50 copies of HSV DNA per 200 µL
PCR evaluation of serum from HSV-infected neonates is a promising diagnostic modality, since serum viral load correlates with classification of disease [25]. Neonates with disseminated disease have a statistically higher viral load than infants with CNS disease (P<0.0001) or infants with SEM disease (P<0.001) [25]. Serum viral load is also significantly higher in patients who die from neonatal HSV disease compared to those who survive and are neurologically normal (P=0.005) or who survive with neurological sequelae (P=0.0008). However, data on using serum PCR in HSV disease are insufficient to use this modality to determine the effectiveness of antiviral therapy or guide the appropriate time to discontinue therapy [26].
8 Oral Suppression Therapy
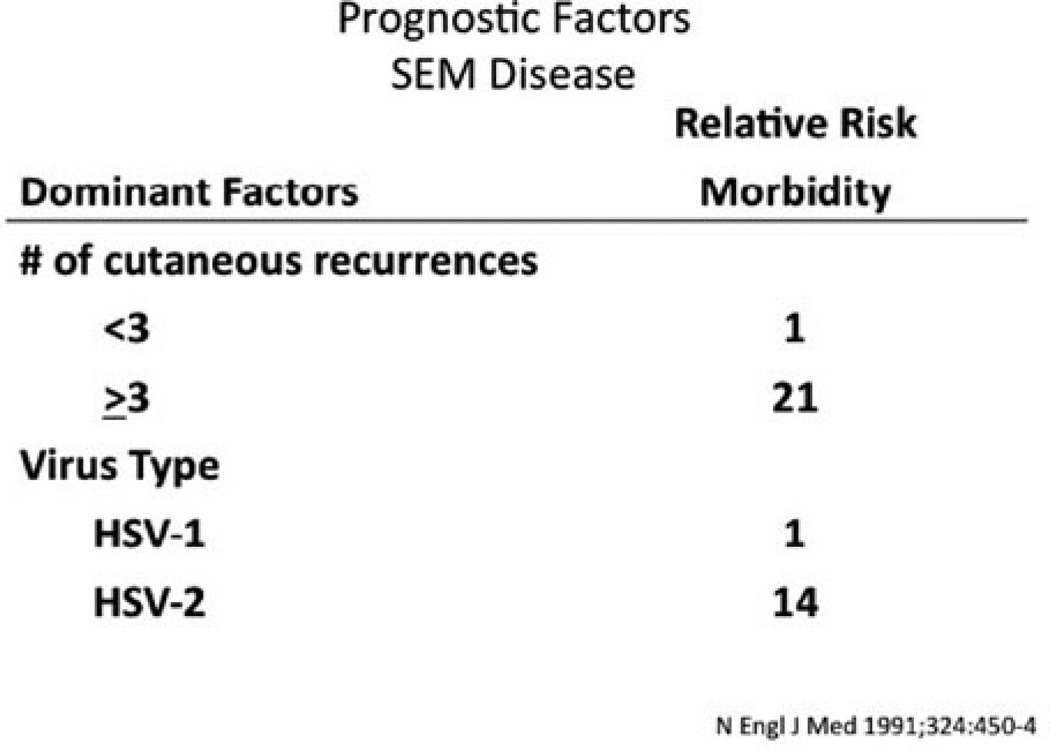
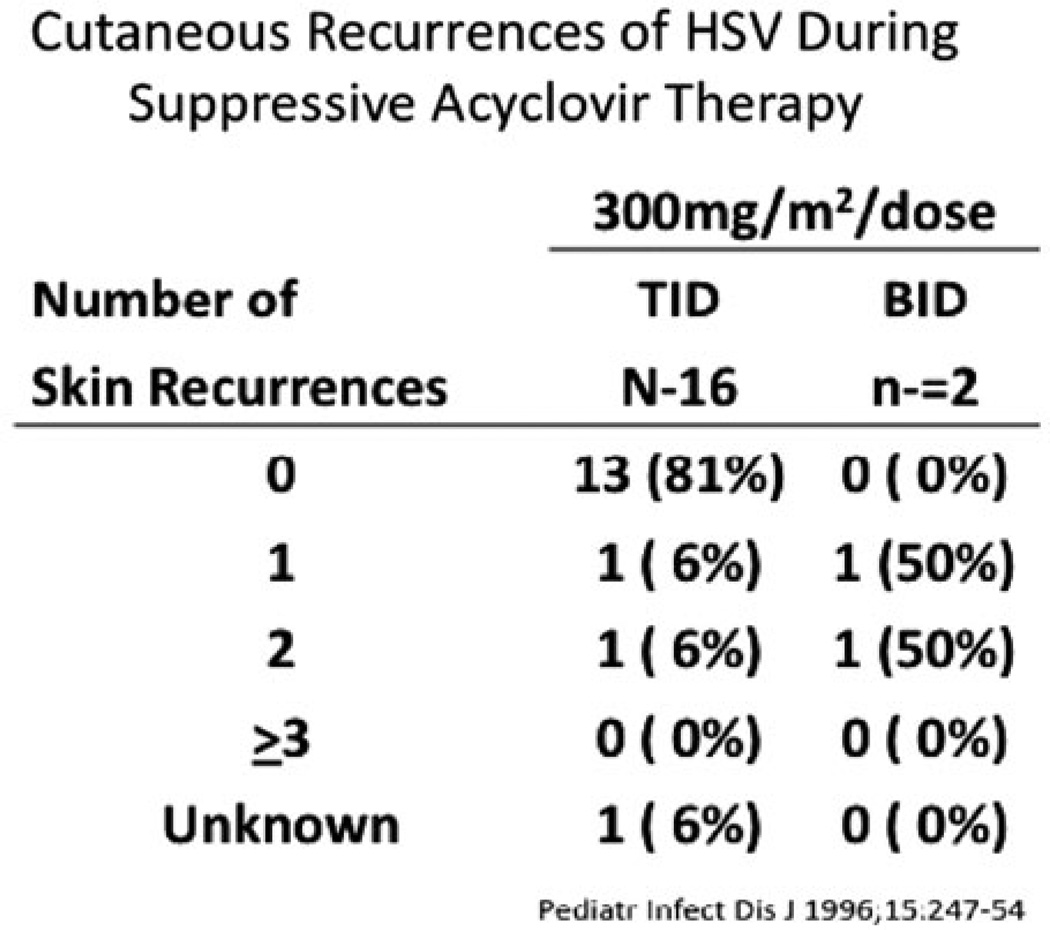
Current advances in the treatment of neonatal HSV have looked toward the role of oral suppression therapy. The studies performed by the NIAID CASG demonstrated that there was a direct correlation between the frequency of recurrent skin lesions after HSV-2 SEM disease and the development of adverse neurological complications [27]. Normal development was seen in all babies with SEM disease who had fewer than three episodes of recurrent skin lesions over a 6-month period compared to only 79% of babies with three or more recurrences of skin lesions [16] (Fig. 15.4). As a result of these observations, the CASG conducted a Phase I/II trial of oral acyclovir therapy for the suppression of cutaneous recurrences after SEM disease in 26 infants [28]. Suppressive oral acyclovir therapy (300 mg/m2 given either twice daily or three times per day) was administered for 6 months. Thirteen of the 16 infants (81%) who received acyclovir three times a day had no recurrent lesions while on therapy. However, in the 6 months after cessation of oral prophylaxis, 7 of the 16 patients on the three times a day regime experienced cutaneous recurrences of HSV disease. Interestingly, of those available for evaluation at 1 year of life (13 out of 18 on suppressive therapy), all were developing normally (Fig. 15.5).
Figure 15.4.
Factors affecting outcome in SEM HSV disease
Figure 15.5.
Cutaneous recurrences of HSV disease with twice daily vs. three times a day oral suppression therapy in 18 patients evaluated for safety, efficacy, and PK data
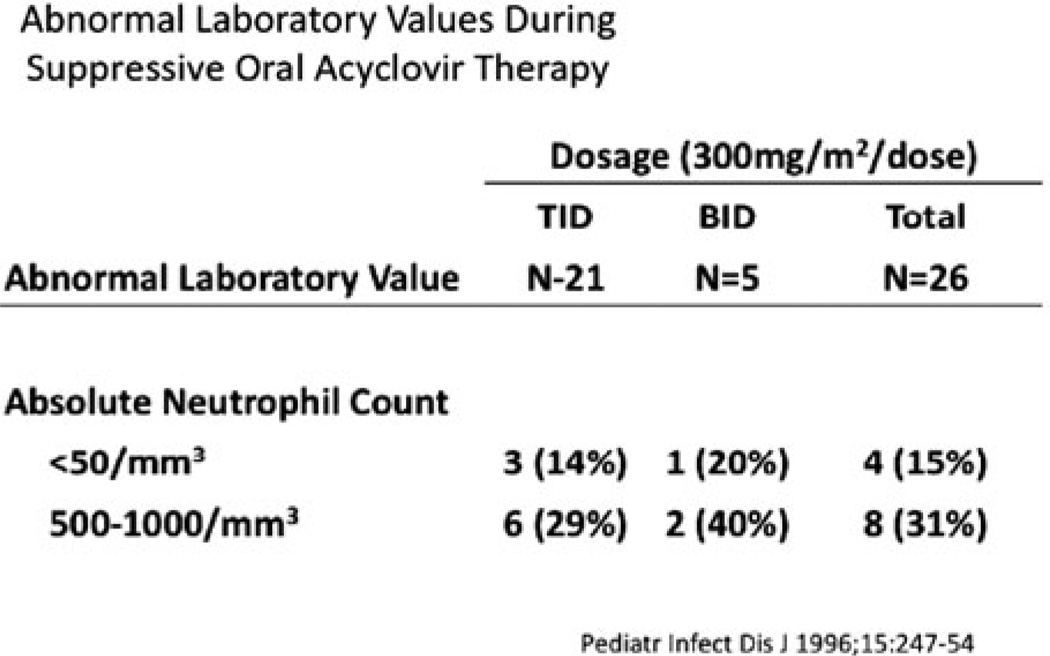
Twelve of the 26 patients developed neutropenia during treatment. Ten of the 12 infants continued taking acyclovir without interruption throughout the period of neutropenia and had spontaneous recovery in their absolute neutrophil counts. The other two infants’ neutropenia resolved after a dose reduction of 50% (Fig. 15.6).
Figure 15.6.
Abnormal absolute neutrophil counts during twice daily vs. three times a day oral suppression therapy in 26 patients evaluated for safety and PK data
The results of this study suggest that oral acyclovir could be given safely during the first 6 months of life if monitored closely for neutropenia. However, the study lacked adequate power to assess the effect of suppressive therapy on neurological outcome.
9 Antiviral Resistance
A concerning finding from this study was the identification of an isolate of HSV that was highly resistant to acyclovir [28]. This was identified by culture in a patient with a cutaneous recurrence that occurred 36 h after cessation of the 6-month suppressive course of acyclovir.
There have been two further case reports in the literature that have documented acyclovir-resistant HSV in treated infants. One was in a premature infant with SEM disease who, after completing 21 days of acyclovir, developed disseminated HSV with an acyclovir-resistant isolate and subsequently died [29]. Another identified an acyclovir-resistant HSV isolate from clinical specimens from a case of fatal disseminated neonatal HSV during the first 7 days of acyclovir therapy [30]. These case reports and the Phase I/II trial of suppression therapy illustrate that prolonged administration of oral acyclovir after neonatal HSV disease may have adverse virological consequences.
10 Conclusion
High-dose acyclovir (60 mg/kg/day) given in three divided doses over 21 days has been shown to improve both mortality and morbidity from neonatal HSV disease and is the current recommended treatment regime [11]. PCR has dramatically revolutionized the early diagnosis and implementation of treatment of neonatal HSV disease and is important in guiding the duration of treatment.
In the future the role of oral suppression therapy and its effect on neurological outcome needs to be assessed, as does the role of antiviral prophylaxis during pregnancy. Ultimately, elimination of neonatal HSV requires the development of an effective HSV vaccine that will protect against genital HSV-1 and HSV-2 infection and disease.
References
- 1.Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17(1):1–13. doi: 10.1128/CMR.17.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown ZA, Wald A, Morrow RM, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 3.Kimberlin DW. Neonatal HSV infections: the global picture. Herpes. 2004;11(2):31–32. [PubMed] [Google Scholar]
- 4.Brown ZA, Benedetti J, Ashley R, Burchett S, Selke S, Berry S, Vontver LA, Corey L. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–1252. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- 5.Brown ZA, Vontver LA, Benedetti J, Critchlow CW, Sells CJ, Berry S, Corey L. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. 1987;317:1246–1251. doi: 10.1056/NEJM198711123172002. [DOI] [PubMed] [Google Scholar]
- 6.Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337(8):509–515. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 7.Nahmias AJ, Josey WE, Naib ZM, Freeman MG, Fernandez RJ, Wheeler JH. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol. 1971;110(6):825–837. doi: 10.1016/0002-9378(71)90580-1. [DOI] [PubMed] [Google Scholar]
- 8.Parvey LS, Ch’ien LT. Neonatal herpes simplex virus infection introduced by fetal-monitor scalp electrodes. Pediatrics. 1980;65(6):1150–1153. [PubMed] [Google Scholar]
- 9.Johnston C, Magaret A, Selke S, Remington M, Corey L, Wald A. Herpes simplex virus viremia during primary genital infection. J Infect Dis. 2008;198(1):31–34. doi: 10.1086/588676. [DOI] [PubMed] [Google Scholar]
- 10.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Frenkel LM, Gruber WC, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin DW, Lin C-Y, Jacobs RF, Powell DA, Frenkel LM, Gruber W, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108:223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 12.Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics. 1980;66(4):495–501. [PubMed] [Google Scholar]
- 13.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108(2):230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 14.Whitley RJ, Yeager A, Kartus P, Bryson Y, Connor JD, Alford CA, et al. Neonatal herpes simplex virus infection: follow-up evaluation of vidarabine therapy. Pediatrics. 1983;72(6):778–785. [PubMed] [Google Scholar]
- 15.Whitley RJ, Alford CA, Jr, Hirsch MS, Schooley RT, Luby JP, Aoki FY, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- 16.Whitley R, Arvin A, Prober C, Burchett S, Corey L, Powell D, et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. infectious diseases collaborative antiviral study group. N Engl J Med. 1991;324(7):444–449. doi: 10.1056/NEJM199102143240703. [DOI] [PubMed] [Google Scholar]
- 17.Kimberlin DW, Lakeman FD, Arvin AM, Prober CG, Corey L, Powell DW, et al. Application of the polymerase chain reactin to the diagnosis and management of neonatal herpes simplex virus disease. J Infect Dis. 1996;174:1162–1167. doi: 10.1093/infdis/174.6.1162. [DOI] [PubMed] [Google Scholar]
- 18.Malm G, Forsgren M. Neonatal herpes simplex virus infections: HSV DNA in cerebrospinal fluid and serum. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F24–F29. doi: 10.1136/fn.81.1.f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura H, Aso K, Kuzushima K, Hanada N, Shibata M, Morishima T. Relapse of herpes simplex encephalitis in children. Pediatrics. 1992;89(5 Pt 1):891–894. [PubMed] [Google Scholar]
- 20.Kimberlin DW, Lakeman FD, Arvin AM, Prober CG, Corey L, Powell DA, et al. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1996;174(6):1162–1167. doi: 10.1093/infdis/174.6.1162. [DOI] [PubMed] [Google Scholar]
- 21.Troendle-Atkins J, Demmler GJ, Buffone GJ. Rapid diagnosis of herpes simplex virus encephalitis by using the polymerase chain reaction. J Pediatr. 1993;123:376–380. doi: 10.1016/s0022-3476(05)81735-4. [DOI] [PubMed] [Google Scholar]
- 22.Kimura H, Futamura M, Kito H, Ando T, Goto M, Kuzushima K, et al. Detection of viral DNA in neonatal herpes simplex virus infections: Frequent and prolonged presence in serum and cerebrospinal fluid. J Infect Dis. 1991;164:289–293. doi: 10.1093/infdis/164.2.289. [DOI] [PubMed] [Google Scholar]
- 23.Troendle-Atkins J, Demmler GJ, Buffone GJ. Rapid diagnosis of herpes simplex virus encephalitis by using the polymerase chain reaction. J Pediatr. 1993;123(3):376–380. doi: 10.1016/s0022-3476(05)81735-4. [DOI] [PubMed] [Google Scholar]
- 24.Domingues RB, Lakeman FD, Mayo MS, Whitley RJ. Application of competitive PCR to cerebrospinal fluid samples from patients with herpes simplex encephalitis. J Clin Microbiol. 1998;36:2229–2234. doi: 10.1128/jcm.36.8.2229-2234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura H, Ito Y, Futamura M, Ando Y, Yabuta Y, Hoshino Y, et al. Quantitation of viral load in neonatal herpes simplex virus infection and comparison between type 1 and type 2. J Med Virol. 2002;67(3):349–353. doi: 10.1002/jmv.10084. [DOI] [PubMed] [Google Scholar]
- 26.Kimberlin DW, Whitley RJ. Neonatal herpes: what have we learned. Semin Pediatr Infect Dis. 2005;16(1):7–16. doi: 10.1053/j.spid.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Whitley R, Arvin A, Prober C, Corey L, Burchett S, Plotkin S, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991;324(7):450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 28.Kimberlin D, Powell D, Gruber W, Diaz P, Arvin A, Kumar M, et al. Administration of oral acyclovir suppressive therapy following neonatal herpes simplex virus disease limited to the skin, eyes, and mouth: results of a Phase I/II tiral. Pediatr Infect Dis J. 1996;15:247–254. doi: 10.1097/00006454-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Oram RJ, Marcellino D, Strauss D, Gustafson E, Talarico CL, Root AK, et al. Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature neonate. J Infect Dis. 2000;181(4):1458–1461. doi: 10.1086/315387. [DOI] [PubMed] [Google Scholar]
- 30.Levin MJ, Weinberg A, Leary JJ, Sarisky RT. Development of acyclovir-resistant herpes simplex virus early during the treatment of herpes neonatorum. Pediatr Infect Dis J. 2001;20(11):1094–1097. doi: 10.1097/00006454-200111000-00021. [DOI] [PubMed] [Google Scholar]