SUMMARY
The Hippo pathway is crucial in organ size control and its dysregulation contributes to tumorigenesis. However, upstream signals that regulate the mammalian Hippo pathway have remained elusive. Here we report that the Hippo pathway is regulated by G-protein coupled receptor (GPCR) signaling. Serum-borne lysophosphatidic acid (LPA) and sphingosine 1-phosphophate (S1P) act through G12/13-coupled receptors to inhibit the Hippo pathway kinases Lats1/2 thereby activating YAP and TAZ transcription co-activators, which are oncoproteins repressed by Lats1/2. YAP and TAZ are involved in LPA-induced gene expression, cell migration, and proliferation. In contrast, stimulation of Gs-coupled receptors by glucagon or epinephrine activates Lats1/2 kinase activity, thereby inhibiting YAP function. Thus, GPCR signaling can either activate or inhibit the Hippo-YAP pathway depending on the coupled G-protein. Our study identifies extracellular diffusible signals that modulate the Hippo pathway and also establishes the Hippo-YAP pathway as a critical signaling branch downstream of GPCR.
INTRODUCTION
How organ size is controlled in multicellular organisms remains a fundamental biological question. The mammalian target of rapamycin (mTOR) pathway and the Hippo pathway have been proposed to control organ size by affecting cell size and cell number, respectively (reviewed in Lee et al., 2007; Zhao et al., 2010a). The Hippo pathway was initially defined by genetic studies in Drosophila, wherein mosaic mutations of Hippo pathway genes resulted in tissue overgrowth (reviewed in Pan, 2007). Genetically modified mouse models demonstrate that function of the Hippo pathway in organ size regulation is conserved in mammals (reviewed in Zhao et al., 2010a).
The kinase cascade of MST1/2 and Lats1/2 represents a core component of the mammalian Hippo pathway. MST1/2, in complex with a regulatory protein salvador (Sav1), phosphorylate and activate Lats1/2 kinases, which also form a complex with a regulatory protein Mob1 (Zhao et al., 2010a). The transcription co-activator Yes-associated protein (YAP) is a major downstream effector of the Hippo pathway (Dong et al., 2007). Lats1/2 inhibit YAP by direct phosphorylation at S127, which results in YAP binding to 14-3-3 and cytoplasmic sequestration (Dong et al., 2007; Hao et al., 2008; Zhao et al., 2007). YAP acts mainly through TEAD family transcription factors to stimulate expression of genes that promote proliferation and inhibit apoptosis (Zhao et al., 2008). Phosphorylation of YAP S381 by Lats1/2 kinases can also promote its ubiquitination-dependent degradation (Zhao et al., 2010b). TAZ is a YAP paralog in mammals similarly regulated by the Hippo pathway (Lei et al., 2008).
In transgenic mice YAP promotes liver enlargement in a reversible manner (Camargo et al., 2007; Dong et al., 2007), suggesting organ size control relies on tight regulation of Hippo pathway activity. Sustained YAP expression results in hyperplasia and eventual tumor development (Dong et al., 2007). Genetic ablation of Hippo pathway components in mice also leads to tumor formation (Benhamouche et al., 2010; Cai et al., 2010; Lee et al., 2010; Lu et al., 2010; Xu et al., 2009; Zhang et al., 2010; Zhou et al., 2009). Moreover, abnormal activation of YAP and TAZ has been associated with human cancers (Overholtzer et al., 2006; Steinhardt et al., 2008; Zender et al., 2006; Zhao et al., 2007), suggesting an important role for the Hippo pathway in tumorigenesis.
Despite extensive studies that have identified many upstream components of the Hippo pathway, extracellular ligands and cell surface receptors regulating the Hippo pathway have remained elusive. Although CD44 has been proposed to impact the Hippo pathway (Xu et al., 2010), further studies are required to demonstrate the physiological relevance of CD44 in Hippo pathway regulation. In this study, we report the identification of various GPCRs and their agonists as Hippo pathway regulators. Activation of Gs-coupled receptors, by epinephrine or glucagon stimulation, increases Lats1/2 kinase activity, thus resulting in inhibition of YAP function. In contrast, activation of G12/13- or Gq/11-coupled receptors, by lysophosphatidic acid (LPA) or sphingosine 1-phosphate (S1P), inhibits Lats1/2 kinases, resulting in YAP activation. Our study demonstrates an important role for the Hippo-YAP pathway in mediating the physiological functions of GPCRs and their corresponding extracellular ligands.
RESULTS
Serum induces dephosphorylation and nuclear localization of YAP
In search of signals that might regulate YAP phosphorylation, we found that in HEK293A cells YAP was highly phosphorylated following serum starvation and addition of serum resulted in a rapid decrease in YAP phosphorylation as determined by immunoblotting using a phospho-YAP antibody (S127) and differential migration on phos-tag-containing gels (Figure 1A). This phenomenon was observed in multiple cell lines, including Hela, RC3, SK-Mel-28, SF268, U2OS, and MCF10A (Figure S1A-D). The effect of serum on YAP phosphorylation was transient as YAP phosphorylation was partially recovered 4 h after serum stimulation (Figure 1A). Serum also caused a mobility shift of TAZ, suggesting TAZ was also dephosphorylated in response to serum (Figure 1A). Along with decreased phosphorylation, protein levels of both YAP and TAZ, especially TAZ, were increased by serum, consistent with previous observation that phosphorylation promotes YAP/TAZ degradation (Liu et al., 2010; Zhao et al., 2010b). In contrast, protein levels of the MST1 and Lats1 were unaffected by serum stimulation (Figure 1A).
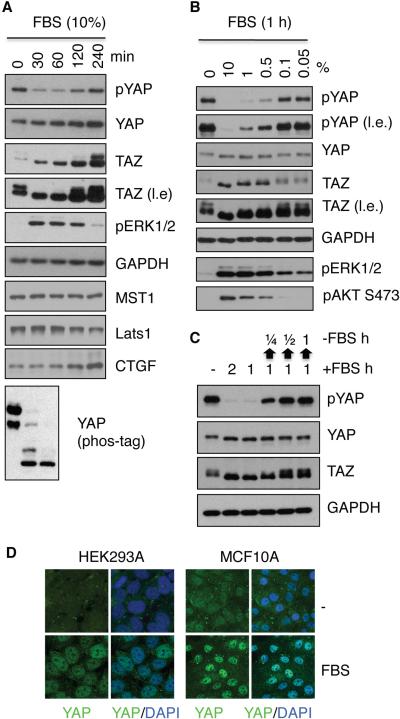
Figure 1. Serum induces dephosphorylation of YAP and TAZ.
(A, B) Serum induces YAP and TAZ dephosphorylation. HEK293A cells were starved in serum-free medium for 12 h and then stimulated with 10% FBS for the indicated times (A) or with different concentrations of FBS for 1 h (B). Cell lysates were subjected to immunoblotting with the indicated antibodies. Where indicated, gels containing phos-tag were employed for assessment of YAP phosphorylation status (A, the bottom panel). l.e. denotes long exposure. (C) Serum reversibly regulates YAP/TAZ phosphorylation. Serum-starved HEK293A cells were treated with 10% FBS for 1 or 2 h as indicated. In the last three lanes, after 1 h stimulation FBS was removed for ¼, ½ or 1 h as indicated by upwards arrows. (D) Serum induces YAP nuclear localization in HEK293A and MCF10A cells. YAP subcellular localization was determined by immunofluorescence staining for endogenous YAP (green) along with DAPI for DNA (blue). Serum stimulation (10% FBS, 1 h) is indicated. The data presented in this figure and all the subsequent figures are representative of at least three independent experiments. See also Figure S1.
The effect of serum on YAP/TAZ phosphorylation was dose-dependent. YAP dephosphorylation was evident when as little as 0.5% serum was added (Figure 1B). Moreover, serum-induced YAP dephosphorylation was rapid (visible at 5 min, Figure S1B) and reversible (Figure 1C), indicating that the effect of serum on YAP phosphorylation is likely a direct signaling event. Phosphorylation of YAP at S127 leads to YAP cytoplasmic localization (Zhao et al., 2007). Consistently, serum caused a significant nuclear accumulation of YAP in both HEK293A and MCF10A cells (Figure 1D). These data demonstrate that a component in serum could potently activate YAP by inducing dephosphorylation and nuclear localization.
Identification of LPA as a YAP-activating component in serum
To rule out the possibility that the YAP/TAZ activating component(s) was present in a particular batch of serum, we examined serum from different sources and found that all could induce YAP dephosphorylation (Figure 2A). In contrast, a defined embryonic stem cell culture medium (mTeSR1) that contains several growth factors showed no effect on YAP phosphorylation, although phosphorylation of extracellular-signal-regulated kinases (ERKs) was induced (Figure 2A), suggesting that growth factors present in mTesR1 do not regulate YAP phosphorylation. Moreover, we tested several growth factors including insulin, EGF, FGF, and PDGF, and found that their evoked signaling pathways were not involved in YAP/TAZ activation (Figure S1E, S1F), indicating that the active component(s) commonly present in serum is unlikely a general growth factor. Further, inhibition of MEK by U0126, PI3K by wortmannin, mTOR by torin, and p38 by SB253580 had no effect on the ability of FBS to induce dephosphorylation of YAP/TAZ (Figure S1G, S1H).
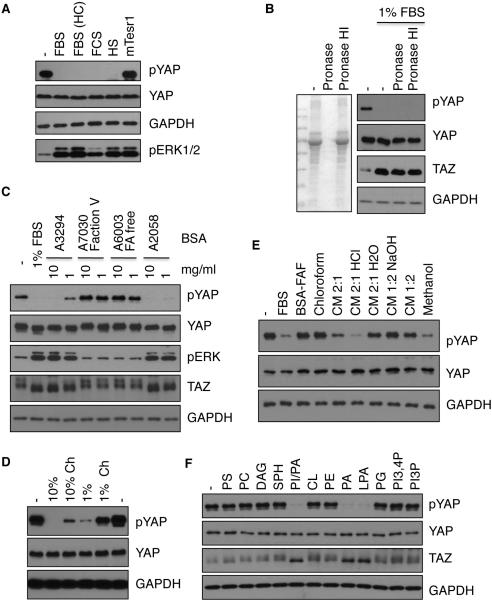
Figure 2. Characterization of serum factor(s) responsible for YAP/TAZ dephosphorylation.
(A) Serum contains a YAP-activating activity. HEK293A cells were treated with 10% of different brands of serum: FBS (from Omega Scientific or Hyclone (HC)), fetal calf serum (FCS), horse serum (HS), or 10% mTesr1. Total cell lysates were subjected to immunoblotting. (B) The YAP-activating activity in serum is protease-resistant. FBS that were pre-treated with pronase E or heat-inactivated pronase E (HI). The effectiveness of pronase E was demonstrated by Coomassie Blue staining (left panel). Cells were stimulated with control or pronase E treated FBS. (C) YAP-activating activity in BSA. Different BSA preparations (from Sigma Aldrich) were used to treat HEK293A cells. A3294 was prepared by heat shock, A7073 Fraction V (FV) and A6003 (fatty acid (FA)-free) were prepared by ethanol precipitation, and A2058 was prepared by chromatography. Protein contents of different BSA preparations were similar as indicated by Coomassie Blue staining (not shown). Serum-starved HEK293A cells were treated with 1 or 10 mg/ml BSA for 1 h before harvest. (D) Charcoal treatment depletes the YAP-activating activity in serum. 10% or 1% of regular or charcoal-stripped (Ch) FBS was used to stimulate serum-starved HEK293A cells for 1 h. (E) The YAP-activating activity in FBS is sensitive to organic extraction under acidic conditions. FBS was extracted using chloroform, methanol, or different ratios of chloroform and methanol mixture (CM, in the presence of HCl or NaOH); organic solvent was evaporated and materials extracted were dissolved in 2 mg/ml fatty acid-free BSA (FAF) and used to treat cells. (F) LPA induces YAP dephosphorylation. HEK293A cells were treated with 100 μM of various lipids. Full names of lipids used are shown in Extended Experimental Procedures. Also see Figure S2.
In order to determine if a protein component in serum is responsible for YAP/TAZ activation, we treated serum with pronase E, which effectively degraded serum proteins (Figure 2B). Interestingly, we found that the activity in serum that induces YAP/TAZ-dephosphorylation was largely unaffected by pronase treatment (Figure 2B). Moreover, the YAP/TAZ-dephosphorylating activity was resistant to heating and dialysis (data not shown). These observations indicate that the YAP/TAZ-activating factor(s) in serum is not a protein but likely a macromolecule or a small molecule tightly associated with a macromolecule.
Bovine serum albumin (BSA) was included as a control in our studies. Surprisingly, BSA also potently decreased YAP/TAZ phosphorylation (Figure 2C). BSA is a major serum component that functions as a carrier for many molecules. We therefore tested different BSA preparations on YAP/TAZ phosphorylation. While some BSA preparations induced YAP/TAZ dephosphorylation, fatty acid-free BSA and Fraction V BSA displayed no activity towards YAP/TAZ phosphorylation (Figure 2C). Similar to fatty acid-free BSA, fraction V BSA contains less lipids since it is prepared by ethanol precipitation. These observations suggest that a hydrophobic compound in BSA, possibly a lipid, is responsible for inducing YAP/TAZ dephosphorylation. In support of this hypothesis, charcoal-stripped FBS, which has reduced lipid content, had a markedly decreased ability to induce YAP dephosphorylation (Figure 2D).
To further characterize the YAP/TAZ-dephosphorylating activity in FBS, we performed a series of extraction experiments using different organic solvents (Quehenberger et al., 2010). Chloroform failed to extract the activity, whereas methanol or ethanol could extract the activity (Figure 2E and data not shown). Moreover, a chloroform/methanol mixture effectively extracted the activity only at low pH but not at high pH (Figure 2E). These results suggest that the active ingredient in serum is an amphiphilic molecule with an acidic group. At low pH the acidic group in the active component is not charged allowing it to be extracted by chloroform/methanol. In contrast, at high pH the acidic group in the active component is charged and thus could not partition into the organic solvents. Phospholipids, particularly lysophospholipids, which have hydrophobic tails with phosphate heads, represent the best known examples of amphiphilic signaling molecules. Thus, we tested if phospholipids might induce YAP dephosphorylation. Among the phospholipids tested, we found that phosphatidic acid (PA), LPA, and a mixture of PA and phosphoinositol strongly induced dephosphorylation of YAP/TAZ (Figure 2F).
LPA and S1P stimulate YAP/TAZ activity
LPA is a family of glycerophospholipid signaling molecules present in all tissues and serum (Choi et al., 2009). Low concentrations of LPA were effective in inducing YAP/TAZ dephosphorylation, with 0.01 μM and 0.1 μM inducing partial and complete YAP/TAZ dephosphorylation, respectively (Figure S2A), indicating that LPA could activate YAP/TAZ at physiological (sub-micromolar) concentrations (Choi et al., 2009). Next, we examined various LPA isoforms with different lengths and degrees of saturation of the fatty acid tails and found that all tested isoforms could induce YAP/TAZ dephosphorylation (Fig. S2B). We subsequently tested PA and found that a much higher concentration, 100 μM, was needed to induce YAP dephosphorylation (Figure S2C). Because PA can be converted to LPA by phospholipases and is significantly less potent than LPA, our data suggest that PA may not directly induce YAP dephosphorylation. Rather, the conversion of PA to LPA or residual LPA contamination in the PA preparation might contribute to the activity detected at high concentrations of PA (Figure 2F).
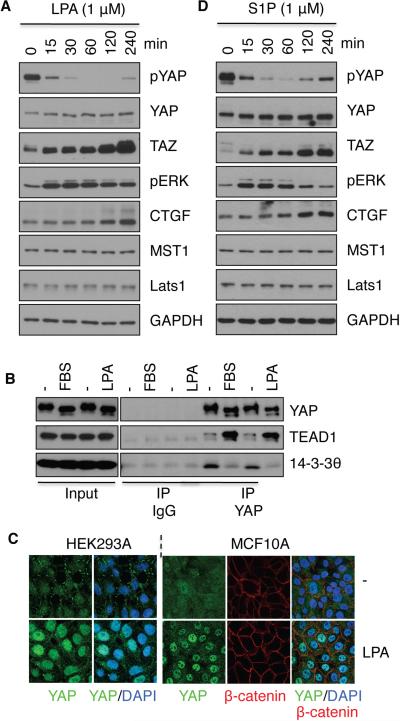
Similar to serum, LPA induced rapid YAP/TAZ dephosphorylation at S127 (Figure 3A). Lats can phosphorylate YAP on 5 serine residues including S381, with phosphorylation at S381 priming S384 phosphorylation by casein kinase (Zhao et al., 2010b). Indeed, we found that phosphorylation of S381/384 was also decreased in response to LPA treatment (Figure S3A). YAP S127 phosphorylation is required for 14-3-3 binding and cytoplasmic retention. Consistent with its ability to promote YAP dephosphorylation, LPA treatment attenuated YAP-14-3-3 interaction (Figure 3C) and induced YAP nuclear localization (Figure 3D). The subcellular localization of YAP was reversible as YAP protein redistributed into cytoplasm 30 min after LPA withdrawal (Figure S3B). LPA also enhanced the interaction between YAP and the nuclear-localized TEAD1, a transcription factor target of YAP/TAZ (Figure 3C).
Figure 3. LPA and S1P activate YAP/TAZ by dephosphorylation.
HEK293A cells were treated with 1 μM LPA (A) or S1P (B) for the indicated times. Cell lysates were subjected to immunoblotting with the indicated antibodies. (C) Serum and LPA stimulate YAP interaction with TEAD1 but inhibit YAP interaction with 14-3-3. Cells were treated with LPA or serum as indicated. Cell lysates were subjected to immunoprecipitation (IP) with control IgG or YAP antibody. The co-immunoprecipitated TEAD1 and 14-3-3 were detected by immunoblotting. (D) LPA treatment (1 μM, for 1 h) induces YAP nuclear localization in HEK293A and MCF10A cells. Also see Figures S2 and S3.
The S1P group of lysophospholipids has overlapping physiological functions with LPA (Rosen et al., 2009). Similar to LPA, S1P potently induced YAP/TAZ dephosphorylation (Figure 3B, S2D). Taken together, our data demonstrate that LPA and S1P are activators of YAP/TAZ.
YAP and TAZ are involved in LPA-induced gene expression, cell migration, and cell proliferation
As a transcription co-activator, the major function of YAP/TAZ is to stimulate gene expression. CTGF, Cyr61, and ANKRD1 are well-characterized YAP target genes. Indeed, LPA, S1P, and serum treatment induced the expression of CTGF (Figure 1A, 3A and 3B). Further mRNA and/or protein levels of CTGF, Cyr61, and ANKRD1 were also increased in cells stably expressing ectopic LPA receptor (LPA1) and autotaxin (ATX, an LPA producing enzyme; Figure S4A and S4B). To determine the function of YAP/TAZ in LPA-induced gene expression, YAP and TAZ were knocked down using shRNAs (Figure S4C). We found that knockdown of YAP/TAZ strongly repressed the mRNA induction of CTGF, Cyr61, ANKRD1, TAGLN, EDN1, and PPP1R3B by LPA (Figure 4A), supporting a role of YAP/TAZ in LPA-induced gene expression. However, the expression of two other LPA inducible genes, EGR3 and EGR4, was not dependent on YAP/TAZ (Figure 4A). LPA is known to activate multiple signaling pathways, including ERK (Figure 3A). Indeed both EGR3 and ERG4 are regulated by ERK activation (Li et al., 2007; Ludwig et al., 2011).
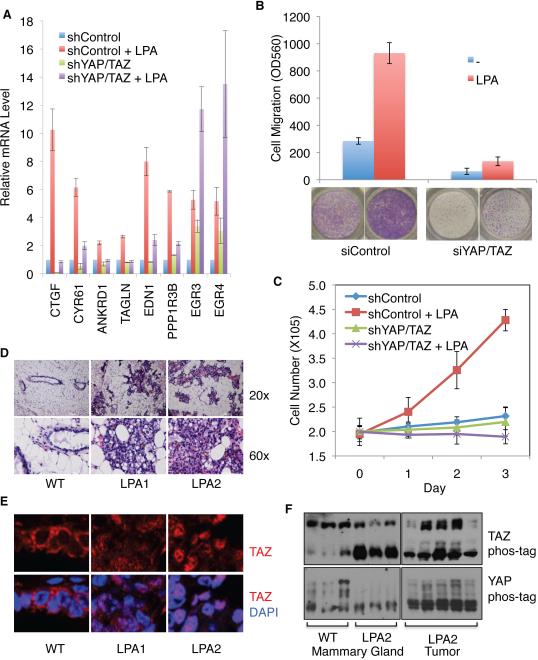
Figure 4. YAP/TAZ are required for LPA functions and are regulated by LPA signaling.
(A) YAP/TAZ are required for LPA to induce gene expression. mRNA levels of the indicated genes were measured by quantitative PCR. LPA (1 μM) treatment was for 1 h. HEK293A cells with stable knockdown of YAP/TAZ or control cells were used. (B) Knockdown of YAP/TAZ blocks LPA-induced cell migration. Migration of MCF10A cells transfected with control siRNA or YAP/TAZ siRNA was assessed by transwell cell migration assays. (C) YAP/TAZ is required for LPA to stimulate cell proliferation. Control and YAP/TAZ knockdown HEK293A cells were cultured in the absence of FBS and treated with or without 10 μM LPA for 0, 1, 2 or 3 day as indicated. LPA was replenished every day. Cell number was then counted. (D) Hyperplasia caused by transgenic LPA1 and LPA2 expression. H & E staining. (E) LPA receptor transgenic mouse tissues exhibit increased TAZ nuclear localization. Immunofluorescence staining for TAZ (red) and DNA (blue). (F) LPA receptor transgenic mouse tissues exhibit decreased YAP/TAZ phosphorylation. Sample in each lane was from an individual mouse. Mammary tissues were analyzed in (D-F). Also see Figure S4.
LPA is known to stimulate cell migration and has been implicated in tumor metastasis (Shida et al., 2003). We examined the effect of YAP/TAZ knockdown in MCF10A cells (Figure S4D) on cell migration using a transwell migration assay. We found that LPA-stimulated cell migration was strongly inhibited in YAP/TAZ double-knockdown cells (Figure 4B). In a wound-healing assay YAP/TAZ knockdown also blocked the effect of LPA on cell migration (Figure S4E). Another well-characterized function of LPA is to promote cell proliferation (van Corven et al., 1989). We found that HEK293A cells displayed little proliferation in the absence of serum, and addition of LPA induced cell proliferation in control cells but not in YAP/TAZ double-knockdown cells (Figure 4C). These data demonstrate important roles for YAP/TAZ in mediating physiological functions of LPA.
It has been shown that TAZ expression is elevated in invasive human breast cancers (Chan et al., 2008), and overexpression of the LPA receptor in mouse mammary glands causes hyperplasia and tumor formation (Liu et al., 2009). To determine whether LPA receptors regulate YAP/TAZ in vivo, we analyzed an LPA receptor transgenic mouse model. As expected, LPA1 and LPA2 transgenic mammary tissues exhibited massive overgrowth (Figure 4D). In contrast to the cytoplasmic localization of TAZ in control tissues, TAZ was enriched in the cell nucleus of LPA1 and LPA2 transgenic tissues (Figure 4E). Additionally, YAP/TAZ were dephosphorylated in LPA receptor transgenic mammary tissues and tumors (Figure 4F). Moreover, in LPA2 tumors the protein levels of YAP/TAZ and their target gene, CTGF, were significantly upregulated (Figure S4G). The above observations support a role of LPA signaling in promoting YAP/TAZ dephosphorylation and activation in vivo.
LPA inhibits Lats1/2 kinase activity
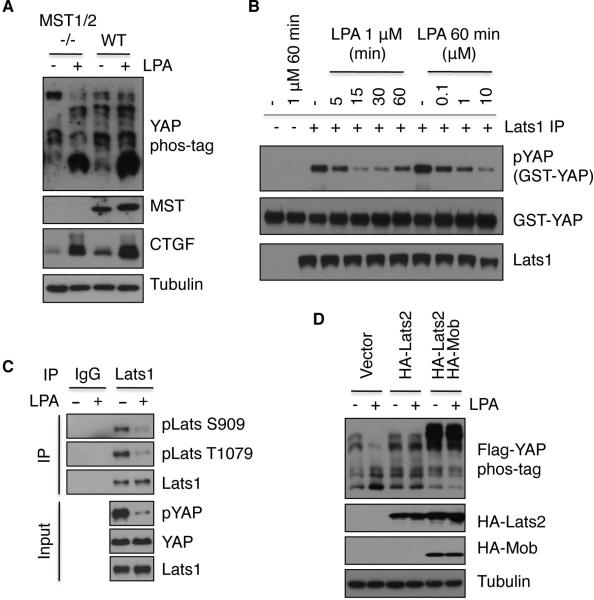
To determine whether LPA acts through the Hippo pathway core components MST and Lats kinases to regulate YAP phosphorylation, we examined the effect of LPA on MST1/2 and Lats1/2 kinase activities. We found that LPA and serum had no detectable effect on MST1 kinase activity as visualized by in vitro phosphorylation of Mob, a known MST1/2 substrate, and MST1 autophosphorylation (Figure S5A). Similarly, the phosphorylation of MST2 at T180 was not changed following LPA treatment (Figure S5B). In addition, LPA induced YAP dephosphorylation in MST1/2 double knockout MEF cells (Figure 5A), indicating that MST1/2 are not required for YAP regulation by LPA in MEF cells.
Figure 5. LPA and S1P repress Lats kinase activity.
(A) MST1/2 are not required for LPA-induced YAP dephosphorylation and CTGF induction in MEF cells. WT or knockout MEF cells at similar density were untreated or treated with 1 μM LPA for 1 h. YAP phosphorylation was assessed by immunoblotting in the presence of phos-tag. (B) Lats kinase activity is inhibited by LPA. Endogenous Lats1 was immunoprecipitated from HEK293A cells that had been treated with LPA at various times and doses of LPA, and Lats1 kinase activity was determined using GTS-YAP as a substrate. (C) Lats phosphorylation is repressed by LPA. Cell lysates from control or LPA-treated (1 μM for 1 h) cells were divided into two parts, one for IgG IP and the other for Lats1 IP. Endogenous Lats1 was immunoprecipitated and probed with phospho-specific antibodies. (D) Lats overexpression suppresses the effect of LPA on YAP phosphorylation. HEK293A cells were co-transfected with Flag-YAP and HA-Lats2 or HA-Mob. One day after transfection, cells were serum-starved for 24 h, and then treated with 1 μM LPA for 1 h. Also see also Figure S5.
Next, we measured Lats1 kinase activity and found that Lats1 kinase activity was rapidly inhibited by serum or LPA treatment (Figure 5B, S5C). The inhibition of Lats1 kinase activity by serum and LPA correlated with the repression of endogenous YAP phosphorylation in both dose- and time-dependent manners (Figure S5C), suggesting that LPA and serum decrease YAP phosphorylation by inhibiting Lats1/2 kinase activity. Consistent with the observed Lats inhibition, phosphorylation levels of Lats1 at activation loop (S909) and hydrophobic motif (T1079), both of which determine Lats activity, were decreased upon LPA treatment (Figure 5C). Moreover, the effect of LPA on YAP phosphorylation was abolished by overexpression of Lats2 (Figure 5D), reinforcing the role of Lats1/2 inhibition in LPA-induced YAP activation. Our data show that LPA signaling acts upstream of Lats1/2 and parallel to MST1/2.
LPA/S1P act through G12/13-coupled receptors and Rho to induce YAP/TAZ dephosphorylation
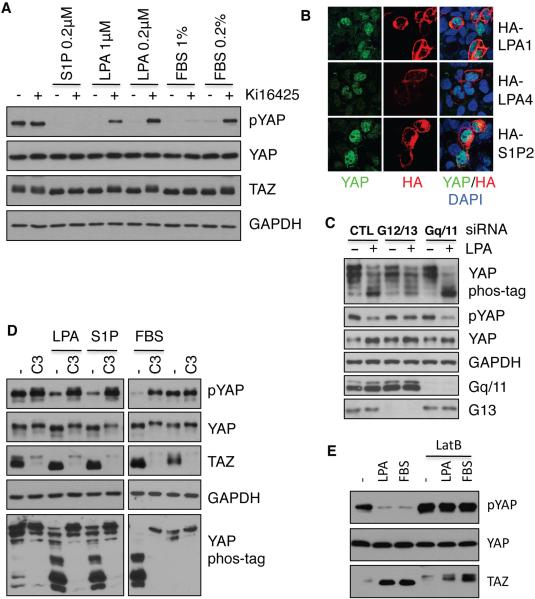
LPA binds to a family of GPCRs known as LPA receptors (LPA1-6) to initiate intracellular signaling (Choi et al., 2009). LPA1 was highly expressed and LPA3 was detectable in HEK293A cells compared to other LPA receptors (Figure S6A). To determine if LPA receptors were required for LPA-induced YAP/TAZ activation, we treated HEK293A cells with Ki16425, which preferentially inhibits LPA1 and LPA3 (Ohta et al., 2003). Ki16425 treatment blocked LPA- but not S1P-induced dephosphorylation of YAP/TAZ (Figure 6A), suggesting LPA1 and LPA3 mediate LPA-induced YAP dephosphorylation in HEK293A cells. Consistently, LPA-induced YAP dephosphorylation was significantly blocked by stable knockdown of LPA1 and LPA3 (Figure S6B). Furthermore, ectopic expression of LPA and S1P receptors was sufficient to induce YAP nuclear localization and dephosphorylation (Figure 6B, S6C). These data suggest that the effect of LPA or S1P on the Hippo-YAP pathway is mediated by their cognate transmembrane receptors. Notably, Ki16425 partially inhibited the ability of serum to repress YAP/TAZ phosphorylation, particularly at low serum concentrations (0.2%) (Figure 6A). The Ki16425-insensitive YAP-dephosphorylating activity in serum could be due to S1P or other factors.
Figure 6. LPA and S1P modulate YAP/TAZ through their membrane receptors and Rho GTPases.
(A) LPA1/3 antagonist Ki16425 completely blocks LPA and partially blocks serum effects on YAP/TAZ phosphorylation. HEK293A cells were treated with Ki16425 (10 μM) or DMSO control for 30 min as indicated, then cells were stimulated with S1P, LPA or FBS for 1 h. (B). LPA and S1P receptor overexpression promotes YAP nuclear localization. Cells were transfected with HA-tagged LPA1, LPA4, or S1P2 as indicated. The transfected receptors were detected by HA antibody (red) and endogenous YAP was detected by YAP antibody (green). Note that the receptor expressing red cells have higher nuclear YAP. (C) Knockdown of G12 and G13 blocks the effect of LPA on YAP phosphorylation. HEK293A cells were transfected with control siRNA, a pool of siRNAs for G12 and G13, or a pool of siRNAs for Gq and G11, serum was removed at 48 h. Following 16 h serum starvation, cells were treated with 1 μM LPA for 1 h. (D) Inactivation of Rho by C3 toxin prevents YAP/TAZ dephosphorylation by LPA, S1P, and serum. HEK293A cells were pretreated with 2 μg/ml C3 for 4 h, then stimulated with LPA, S1P, or FBS for 1 h. (E), Disruption of actin cytoskeleton prevents YAP/TAZ dephosphorylation by LPA or serum. HEK293A cells were pretreated with 1 μg/ml LatB for 30 min, then stimulated with LPA or serum for 1 h. Also see Figure S6.
Both LPA and S1P receptors activate several heterotrimeric G proteins to initiate intracellular signaling pathways. To determine if Gα proteins are involved in YAP regulation, we tested the effect of Gα overexpression on YAP phosphorylation. Our data indicate that overexpression of active G12/13 could induce YAP dephosphorylation (Figure S6D). Indeed, knockdown of both G12 and G13 largely blocked LPA-induced YAP dephosphorylation (Figure 6C), suggesting that G12/13 play a major role in mediating LPA signaling to the Hippo pathway.
Rho GTPases are known downstream mediators of G12/13 and LPA. We therefore expressed the RhoA-N19 dominant-negative mutant and found that it blocked serum-induced YAP dephosphorylation (Figure S6D). Conversely, expression of the constitutively active RhoA-L63 mutant induced a robust YAP dephosphorylation even in the absence of serum (Figure S6D). Likewise, botulinum toxin C3, a specific inhibitor of Rho GTPases, not only elevated basal phosphorylation of YAP/TAZ but also blocked LPA-, S1P-, and serum-induced YAP/TAZ dephosphorylation (Figure 6D). These data indicate a critical role for Rho in mediating the LPA/S1P signal to YAP. We also found that co-transfection of active G12, G13 and RhoA repressed Lats2 kinase activity (Figure S6E). Moreover, inhibition of LPA1 and LPA3 by Ki16425 and inactivation of Rho GTPases by C3 treatment effectively blocked Lats1 inhibition by LPA, S1P, and serum (Figure S6F, S6G). Taken together, these data support a model wherein both LPA and S1P act through membrane receptors, G12/13 and Rho GTPases to inhibit Lats1/2 activity and thereby promote YAP activation.
The major function of Rho GTPases is to regulate cellular actin dynamics. A role of actin cytoskeleton on the Hippo-YAP pathway has recently been suggested (Dupont et al., 2011; Fernandez et al., 2011; Rauskolb et al., 2011; Sansores-Garcia et al., 2011; Zhao et al., 2012). We therefore determined if changes in the actin cytoskeleton contribute to YAP activation by LPA. YAP nuclear localization under LPA or S1P treatment correlated with levels of cellular actin filaments (Figure S3B, S6H and S6I). When cells were treated with the actin-disrupting agent, latrunculin B (LatB), the effects of LPA or S1P on YAP were blocked (Figure 6E, S6I). These results indicate that LPA or S1P may regulate Lats kinase activity by modulating actin cytoskeleton dynamics.
Regulation of YAP phosphorylation by GPCRs
GPCRs represent one of the largest gene families in the human genome. There are approximately a thousand GPCRs that are coupled to fifteen different Gα proteins (Wettschureck and Offermanns, 2005). We asked whether other GPCRs, especially those that are not coupled to G12/13, could modulate YAP/TAZ activity. It is difficult to test the effect of many GPCR ligands because the expression of GPCRs is tissue-specific and only a limited number of receptors are expressed in any given cell line. However, overexpression of GPCRs often can activate signaling as we observed by the overexpression of LPA receptors (Figure 6B and S6C). We therefore tested the effect of representative members of different GPCR subgroups on YAP/TAZ activity by overexpression. YAP or TAZ phosphorylation was reduced upon overexpression of adrenergic receptor alpha 1B, LPA receptors, purinergic receptors, 5-hydroxytryptamine receptor 4, muscarinic acetylcholine receptor M1, adenosine receptor A1A, angiontensin II receptor, free fatty acid receptor 1, platelet-activating factor receptor, thromboxane A2, frizzled homolog D4, complement component 3a receptor 1, estrogen receptor 1, glutamate receptor metabotropic 2, opioid receptor delta 1, secretin receptor, thyroid stimulating hormone receptor, gastrin-releasing peptide receptor, melanocortin receptor 1, somatostatin receptor 1, prostaglandin E receptor 2, and bombesin like receptor 3 (Table S1). In contrast, YAP/TAZ phosphorylation was increased by adrenergic receptor beta 2, dopamine receptor D1 and glucagon receptor (Table S1). Our data indicate that GPCRs that activate G12/13-, Gq/11, or Gi/o could repress YAP/TAZ phosphorylation. On the other hand, GPCRs that mainly activate Gs signaling could induce YAP/TAZ phosphorylation.
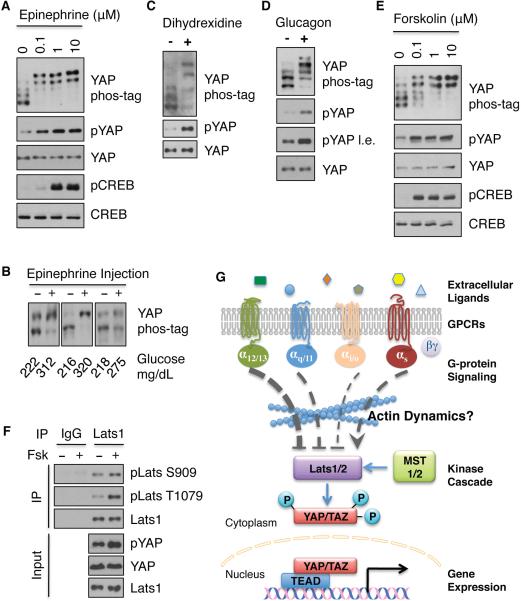
Overexpression of GPCRs could result in non-specific activation of Gα that might not occur under physiological conditions. To further establish the role of specific GPCRs in YAP regulation, we tested the effect of physiological hormones or GPCR agonists on YAP/TAZ phosphorylation using cell lines that are known to express their corresponding receptors. We were particularly interested in agonists that stimulate Gs-coupled receptors, as overexpression of Gs-coupled receptors induced YAP phosphorylation and their agonists may represent negative regulators for YAP/TAZ function. In MDA-MB-231 breast cancer cells, stimulation with epinephrine resulted in a dose-dependent phosphorylation of YAP (Figure 7A). As expected, epinephrine increased phosphorylation of the cAMP responsive element binding protein (CREB), indicating that Gs and cAMP production are stimulatd by epinephrine. In addition, when fed mice were injected with epinephrine YAP phosphorylation was significantly increased in the heart, a physiological target organ of epinephrine (Figure 7B), suggesting a role of epinephrine in YAP regulation in vivo.
Figure 7. Stimulation of Gs coupled GPCRs increases YAP phosphorylation.
(A) Epinephrine stimulates YAP phosphorylation. MDA-MB-231 cells were treated with indicated concentrations of epinephrine for 1 h. Phosphorylation of CREB was determined by immunoblotting with phospho-CREB-specific antibody (pCREB). B) Phosphorylation of YAP from the heart of mice injected with epinephrine is increased. Samples from three representative pairs (from strong to weak induction of YAP phosphorylation) of mice were shown. Epinephrine is known to increase blood glucose levels, which are indicated underneath each sample. (C) Dopamine agonist dihydrexidine stimulates YAP phosphorylation. U2OS cells were treated with 10 μM dihydrexidine for 1 h. YAP phosphorylation status was assessed. (D) Glucagon stimulates YAP phosphorylation. Primary mouse hepatocytes were treated with 2 μM glucagon for 1 h, and YAP phosphorylation status was determined. (E) Forskolin induces YAP phosphorylation. MDA-MB-231 cells were treated with different concentrations of Forskolin for 1 h. (F) Forskolin induces Lats1 phosphorylation. Endogenous Lats1 was immunoprecipitated from control cells and Forskolin (Fsk)-treated (10 μM for 1 h) HEK293A cells, and protein lysates were divided into two parts, one for IgG IP and the other for Lats1 IP. Proteins immunoprecipitated were probed with phospho-specific antibodies against S909 and T1079 of Lats1. (G) A proposed model for GPCRs and G-proteins in the regulation of Lats and YAP/TAZ activities. See discussion for details. Also see Figure S7 and Tables S1 and S2.
Overexpression of the dopamine receptor 1 or the glucagon receptor known to activate Gs also increased YAP phosphorylation (Table S1). To extend the notion that activation of Gs by other GPCR agonists also increases YAP phosphorylation, we examined the effect of dihydrexidine, an agonist for dopamine receptor 1 and 5. Dihydrexidine treatment strongly increased YAP phosphorylation in U2OS cells (Figure 7C). As the glucagon receptor is expressed in hepatocytes, we therefore isolated primary mouse hepatocytes and tested the effect of glucagon. As shown in Figure 7D, glucagon treatment also increased YAP phosphorylation. The above data support that activation of Gs-coupled receptors results in YAP hyperphosphorylation and inactivation under physiological conditions.
To further explore the role of cAMP signaling in the Hippo pathway, we treated cells with forskolin, an activator of adenylyl cyclase that results in cAMP production. We found that forskolin effectively increased YAP phosphorylation (Figure 7E). The cAMP signaling cascade can activate protein kinase A (PKA) or exchange protein activated by cAMP (Epac). We found that the PKA selective activator, 6-Bnz-cAMP, dramatically increased YAP phosphorylation whereas the effect of an Epac selective activator, 8-CPT-2’-O-Me-cAMP, on YAP phosphorylation was less dramatic (Figure S7B). Thus, Gs-coupled GPCR can induce YAP phosphorylation mainly via cAMP and PKA.
Consistent with the increase in YAP phosphorylation, immunofluorescence staining demonstrated that epinephrine and forskolin induced an accumulation of cytoplasmic YAP (Figure S7C). We hypothesized that Gs-coupled signals might compete with G12/13- and Gq/11-coupled signals. Indeed, epinephrine and LPA antagonized each other's effect on YAP phosphorylation (Figure S7D). Consistent with the pathways elucidated above, forskolin increased Lats1, but not MST2, phosphorylation (Figure 7F, S7E). Moreover, epinephrine increased Lats1 activity (Figure S7F). Our data suggest that Gs-initiated signaling stimulates Lats kinase activity, therefore increasing YAP/TAZ phosphorylation.
Differential functions of Gα in the regulation of YAP phosphorylation
Finally, we tested Gα subunits for their ability to modulate YAP phosphorylation by overexpression (Table S2). Because only the GTP-bound Gα is active and directly participates in signaling, we expressed constitutively active mutants (GTP-bound form) of Gα subunits. We found that active Gα mutants decreased YAP phosphorylation to varying degrees with the exception of Gs and Gz. Among the Gα subunits that decreased YAP phosphorylation, G12, G13, Gq, G11, G14, and G15 were more potent than Gi, Gt, and Go in repressing YAP phosphorylation. Moreover, expression of wild-type G12 or G13 but not wild-type G11 or Gq was sufficient to inhibit YAP phosphorylation. These results indicate that G12/13 is the most potent inhibitor of the Hippo pathway followed by Gq, G11, G14, and G15 (these four belong to Gq/11 subfamily), whereas Gi, Gt, and Go (all belong to Gi/o subfamily) are less potent. In contrast, expression of the constitutively active Gs mutant increased YAP phosphorylation. Together, these data further support differential roles of various Gα, hence GPCRs and their corresponding ligands in regulation of the Hippo-YAP pathway (Figure 7G).
DISCUSSION
In this report, we demonstrate that serum contains an activity that inhibits YAP/TAZ phosphorylation and increases YAP/TAZ activity. Based on biochemical characterizations, we identified LPA and S1P as potent serum-borne signals regulating the Hippo-YAP pathway. In addition, we have discovered that epinephrine, glucagon, and dihydrexidine can stimulate YAP phosphorylation. Therefore, the Hippo-YAP pathway can be both positively and negatively regulated by diverse extracellular signals.
Hippo pathway as a downstream branch of GPCR signaling
All signals that modulate the Hippo-YAP pathway identified in this study turn out to be agonists for GPCRs. GPCRs regulate a wide array of physiological functions and represent the major targets for therapeutic drugs. Our study places the Hippo-YAP pathway as a downstream branch of GPCR signaling. We propose that Lats1/2 kinases are inhibited by G12/13-, Gq/11-, and Gi/o-coupled receptors and activated by Gs-coupled receptors. Moreover, Rho GTPases and actin cytoskeleton organization appear to be located between Gα and Lats1/2 (Figure 7G). The precise mechanism by which Rho or actin cytoskeleton controls Lats1/2 phosphorylation and activity requires further investigation.
YAP and TAZ are transcription co-activators and their activation/inhibition may therefore play an important role in GPCR-mediated gene regulation. Consistent with this model, YAP/TAZ is required for the expression of some LPA-induced genes, indicating a direct role of YAP/TAZ in the transcriptional response of GPCR. YAP/TAZ play critical roles in cell proliferation and cell migration in response to LPA. Although the effects of GPCR agonists on YAP/TAZ phosphorylation are transient (Figure 1A, S3A, S3B), the transient dephosphorylation and nuclear localization effects are sufficient to induce gene expression (Figure 4A), which may generate long-term physiological effects, such as cell migration and proliferation (Figure 4B, 4C). GPCR activation has been linked to cell proliferation, and many mechanisms have been proposed (Dorsam and Gutkind, 2007). Gq/11-, G12/13-, and Gi/o-coupled receptors usually show stimulatory effects on cell proliferation. This is consistent with their function on YAP/TAZ activation. The role of Gs-coupled receptors in cell proliferation is rather complex although activation of Gs and PKA is generally associated with growth inhibition (Stork and Schmitt, 2002). Inhibition of YAP/TAZ activity by Gs-coupled receptor signaling may lead to growth inhibition in some types of cells. We noticed that basal YAP/TAZ activity varies significantly across different cell lines (unpublished data), and so YAP/TAZ may not respond to Gs-coupled signaling when basal activity is low (highly phosphorylated), and an alternative signaling may promote cell proliferation.
Complexity of Hippo-YAP regulation
The regulation of the Hippo-YAP pathway by multiple signals is not surprising given the important role of this pathway in cell proliferation and apoptosis, hence organ size control, and tumorigenesis. Multiple regulators may coordinate with each other to fine-tune physiological and pathological processes. This scenario is similar to MAP kinases or PI3 kinases, which are regulated by a large numbers of growth factors via receptor tyrosine kinases (RTK) and other receptors. It is worth noting that YAP phosphorylation is not affected by the RTK ligands tested (Figure S1).
Our results suggest that the upstream signals for the Hippo-YAP pathway are highly redundant (Figure 7G). GPCRs represent the largest class of cell surface receptors, and can couple to different G-proteins (Figure 7G, Table S1). It is likely that many ligands acting through these G-proteins will similarly modulate Lats1/2 kinases and YAP/TAZ activity.
Regulation of Hippo-YAP by GPCR can be rather complex due to the presence of multiple receptors for a single agonist. For example, LPA has at least 6 receptors, which can be coupled to different G-proteins. Therefore, it is possible that one ligand may increase YAP phosphorylation in one cell type but decrease YAP phosphorylation in another cell type depending on which receptor is dominantly expressed and which Gα is coupled to the receptor in that particular cell type. We reason that the high redundancy and complexity may hinder genetic efforts to isolate upstream signals and receptors for the Hippo-YAP pathway because knockout or knockdown of a single GPCR may not significantly affect the Hippo-YAP pathway.
Implication of GPCR-YAP signaling in organ size and cancer
Organ size control is a fundamental issue in biology and final organ size is determined both intrinsically and extrinsically. The identification of GPCR ligands as Hippo pathway regulators opens new possibilities to explore the role of the Hippo pathway in organ size control. It is possible that certain GPCR activating hormones play central roles in organ size control through the Hippo pathway. Depending on the distribution of ligands and receptors, the signaling from GPCR to Hippo pathway may regulate organ size in a tissue specific manner. Indeed, it has been shown that knockout of gprc6a in Leydig cells reduces testis size (Oury et al., 2011). Gprc6a is able to activate Gq (Kuang et al., 2005; Wellendorph et al., 2005), and it is possible that YAP/TAZ activity is compromised in gprc6a knockout cells and contributes to the small organ size phenotype. The Hippo-YAP pathway also plays an important function in the nervous system (Cao et al., 2008). The effect of a dopamine receptor agonist on YAP activity demonstrated in this study also indicates that the Hippo-YAP pathway could be dynamically regulated by neurotransmitters. Therefore, it is also possible that a neuroendocrine mechanism is involved in organ size control. Conversely, YAP may play a critical role in the nervous system and neuronal activity. Future studies are needed to address these important biological issues.
Elevated YAP/TAZ nuclear localization is observed in many types of human cancers (Chan et al., 2008; Overholtzer et al., 2006; Steinhardt et al., 2008; Zender et al., 2006; Zhao et al., 2007) but the mechanism behind YAP/TAZ activation in cancer is largely unknown. The connection between GPCR and the Hippo pathway revealed by this study may provide an explanation for YAP/TAZ activation in certain tumors. GPCR signaling plays important roles in cancer development as both familial and somatic activating mutations of GPCRs have been linked to human cancer (Dorsam and Gutkind, 2007). Recently, GPCR mutations have been identified in a wide range of human cancer specimens (Kan et al., 2010; Prickett et al., 2011). We have demonstrated here that transgenic expression of LPA receptors increases YAP/TAZ activity and that the oncogenic activity of YAP/TAZ may contribute to the hyperplasia and tumor phenotype in these mice. Mutations of G-proteins are also linked to cancer. For instance, activating mutations of Gq and G11 are frequently associated with uveal melanoma, the most common tumor in the eye (Van Raamsdonk et al., 2010). In fact, approximately 83% of uveal melanoma have activating mutations in either Gq or G11 in a mutually exclusive manner. Based on our study, one may predict that constitutive activation of Gq or G11 in uveal melanomas results in abnormal YAP activation, which then contributes to uveal melanoma development. Future investigation to determine the function of YAP/TAZ activation in the development of uveal melanoma or other GPCR mutation-containing cancers may provide new insights into the mechanism of tumorigenesis and possibly new therapeutic targets. We hypothesize that inhibition of YAP/TAZ will be a new approach to treat human cancers caused by dysregulated Rho GTPase, G-proteins, GPCRs or their agonists.
EXPERIMENTAL PROCEDURES
Cell culture
All cell lines were maintained at 37°C with 5% CO2. Detailed medium composition is shown in supplementary information. For serum starvation, cells were incubated in DMEM or DMEM/F12 without other supplements. Detailed culture conditions are described in Extended Experimental Procedures.
Transfection
Cells were transfected with plasmid DNA using PolyJet™ DNA In Vitro Tranfection Reagent (Signagen Laboratories) according to manufacturer's instructions. siRNAs were delivered into cells using RNAiMAX (Invitrogen) according to manufacturer's instructions. Sources of plasmids used are described in Extended Experimental Procedures.
Immunoprecipitation and Immunoblotting
Cells were lysed using mild lysis buffer. Cell lysates were centrifuged for 10 min at 4°C, and supernatants were used for immunoprecipitation. Immunoprecipitates were washed four times with lysis buffer, and proteins were eluted with SDS-PAGE sample buffer. Immunoblotting was performed using standard protocol. Information for antibodies and phos-tag-containing gels is shown in Extended Experimental Procedures.
Immunofluorescence staining
HEK293A or MCF10A cells were fixed with 4% paraformaldehyde-PBS for 15 min. Following permeabilization and blocking, cells were incubated with primary antibodies overnight at 4°C. Secondary antibodies used were Alexa Fluor 488 or 555 (Invitrogen, 1:1000 dilution). Samples were mounted using ProLong Gold antifade reagent with DAPI (Invitrogen) and immunofluorescence was detected using Olympus confocal microscopy. For paraffin-embedded tissues from control or LPAR transgenic mammary glands or tumors, sections were prepared and subjected for immunostaining following deparaffinization, hydration and antigen retrieval. Detailed methods are described in Extended Experimental Procedures.
Kinase assay
Immunoprecipitated MST1 was subjected to a kinase assay in the presence of 500 μM cold ATP, 10 μCi [γ-32P]ATP, and 1 μg of GST-Mob. The reaction mixtures were incubated for 30 min at 30°C, terminated with SDS sample buffer, and subjected to SDS-PAGE and autoradiography. Lats1 or HA-Lats2 kinase assays were performed similarly but using GST-YAP as substrates in the absence of [γ-32P]ATP. The phosphorylation of GST-YAP at S127 was determined by immunoblotting using pYAP antibody. Detailed methods are described in Extended Experimental Procedures.
RNA extraction, Reverse Transcription and Real-Time PCR
RNA samples were prepared using RNeasy Plus mini kit (Qiagen). Reverse transcription was performed using iScript reverse transcriptase (Bio-Rad). Real-Time PCR was performed using KAPA SYBR FAST qPCR master mix (Kapa Biosystems). Detailed methods and information for primers are described in Extended Experimental Procedures.
RNA interference
Lentiviral shRNAs were obtained from Sigma Aldrich. ShRNA plasmids together with pMD2.G and psPAX2 were used to produce virus in 293T cells. ON-TARGET plus SMARTpool siRNA were purchased from Dharmacon. Information for shRNAs is described in Extended Experimental Procedures.
Cell proliferation Assay
HEK293A cells (expressing control shRNA or YAP/TAZ shRNA, 2×105) in serum-free media were maintained in the presence or absence of 10 μM LPA for 1, 2 or 3 day. Cell numbers were determined daily using a cell counter (Bio-Rad). LPA was replenished everyday.
Cell migration Assay
Cell migration assay was performed using BD Falcon™ Cell culture inserts for 24-well plates with 8.0 μm pores filter according to manufacturer's instructions. Detailed methods are described in Extended Experimental Procedures.
Epinephrine Injection
A detailed protocol for epinephrine injection experiments is shown in Extended Experimental Procedures.
Lipid Extraction
A detailed protocol for lipid extraction from serum is shown in Extended Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
LPA and S1P activate YAP/TAZ activity by inhibiting Lats kinase
YAP/TAZ mediate physiological functions of LPA
G12/13, Gq/11 and Gi/o coupled receptors and agonists activate YAP/TAZ
Epinephrine and glucagon inhibit YAP/TAZ via Gs coupled GPCR signaling
ACKNOWLEDGEMENTS
We thank Drs. Jack Dixon, Edward Dennis, Joan Heller Brown and Jerold Chun for constructive comments, Dr. Rick Neubig for several G protein constructs, Drs. Ji Zhang, Richard Rivera, Zhongrui Zhou, Sunny Xiang, Andrew Markley, Zhang Dong and Richard Harkewicz for technical help, and Jean Guan and Drs. Yanhui Xu and Ryan Russell for critical reading of the manuscript. This work was supported by grants from NIH and CIRM to K.L.G.; J.L.J was supported by the National Cancer Institute Training Grant T32CA121938, and the cost of confocal imaging was partially supported by NIH Grant P30 CA23100.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTARY INFORMATION
Supplementary information contains Extended Experimental Procedures and supplementary figures and tables.
REFERENCES
- Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, McClatchey AI. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, et al. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2009;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem. 2005;93:383–391. doi: 10.1111/j.1471-4159.2005.03025.x. [DOI] [PubMed] [Google Scholar]
- Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol. 2007;47:443–467. doi: 10.1146/annurev.pharmtox.47.120505.105359. [DOI] [PubMed] [Google Scholar]
- Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, Tourtellotte WG. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol Cell Neurosci. 2007;35:76–88. doi: 10.1016/j.mcn.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Uvarov P, Soni S, Thomas-Crusells J, Airaksinen MS, Rivera C. Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J Neurosci. 2011;31:644–649. doi: 10.1523/JNEUROSCI.2006-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Prickett TD, Wei X, Cardenas-Navia I, Teer JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet. 2011;43:1119–1126. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–1711. [PubMed] [Google Scholar]
- Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Brauner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–2464. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010a;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010b;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.