Abstract
Placental P-glycoprotein (P-gp) acts to protect the developing fetus from exogenous compounds. This protection declines with advancing gestation leaving the fetus and fetal brain vulnerable to these compounds and potential teratogens in maternal circulation. This vulnerability may be more pronounced in pregnancies complicated by infection, which is common during pregnancy. Pro-inflammatory cytokines (released during infection) have been shown to be potent inhibitors of P-gp, but nothing is known regarding their effects at the developing blood-brain barrier (BBB). We hypothesized that P-gp function and expression in endothelial cells of the developing BBB will be inhibited by pro-inflammatory cytokines. We have derived brain endothelial cell (BEC) cultures from various stages of development of the guinea pig: gestational day (GD) 50, 65 (term ∼68 days) and postnatal day (PND) 14. Once these cultures reached confluence, BECs were treated with various doses (100–104 pg/mL) of pro-inflammatory cytokines: interleukin-1β (IL-1β), interleukin-6 (IL-6) or tumor necrosis factor- α (TNF-α). P-gp function or abcb1 mRNA (encodes P-gp) expression was assessed following treatment. Incubation of GD50 BECs with IL-1β, IL-6 or TNF-α resulted in no change in P-gp function. GD65 BECs displayed a dose-dependent decrease in function with all cytokines tested; maximal effects at 42%, 65% and 34% with IL-1β, IL-6 and TNF-α treatment, respectively (P<0.01). Inhibition of P-gp function by IL-1β, IL-6 and TNF-α was even greater in PND14 BECs; maximal effects at 36% (P<0.01), 84% (P<0.05) and 55% (P<0.01), respectively. Cytokine-induced reductions in P-gp function were associated with decreased abcb1 mRNA expression. These data suggest that BBB P-gp function is increasingly responsive to the inhibitory effects of pro-inflammatory cytokines, with increasing developmental age. Thus, women who experience infection and take prescription medication during pregnancy may expose the developing fetal brain to greater amounts of exogenous compounds – many of which are considered potentially teratogenic.
Introduction
The developing fetus is protected from potentially teratogenic compounds in the maternal circulation by multidrug resistance transporter proteins, such as P-glycoprotein (P-gp). At the placenta, P-gp acts to extrude a wide range of xenobiotics from the syncytiotrophoblast and away from the fetal compartment. Placental expression of P-gp has been shown in many mammalian species, including humans, to be high early in pregnancy before decreasing with advancing gestation [1]–[4]. Functionally, this decreased expression leads to increased accumulation of P-gp substrates in the fetus and amniotic fluid [5]. The decline in global fetal protection provided by placental P-gp leaves the fetus susceptible to teratogens that may be in maternal circulation. Epidemiological studies in various countries have estimated that 64–96% of women take prescription medication during pregnancy; 5–10% of which are considered to be potentially teratogenic [6]–[11]. Many of these drugs are P-gp substrates [12]–[14]. Exposure to environmental pollutants (including pesticides and herbicides) is also increasing, leading to increased incidence of teratogenesis, especially in developing industrialized countries [15]–[17]. Again many of these substances are substrates for P-gp [12].
We (and others) have shown the developing fetal blood-brain barrier (BBB) compensates for the declining placental protection by dramatically increasing the expression of BBB P-gp, near-term; increasing local brain protection [18]–[20]. However, as this chemical barrier develops at the fetal BBB, the developing fetal brain remains vulnerable to circulating xenobiotics that can pass through the placenta as a result of the decline in placental P-gp.
Fetal brain vulnerability may be even more pronounced in pregnancies complicated by maternal and/or intra-amniotic infection. Bacterial and viral infection is common during pregnancy [21], [22] and accounts for approximately 40% of preterm births [23]. Further, in developing countries, parasitic infections such as malaria [24] and toxoplasmosis [25] have detrimental effects on the developing fetus. Rodent models of infection involving lipopolysaccharide (LPS; a model of bacterial infection) or polyinosinic-polycytidylic acid (a model of viral infection) injections during pregnancy have demonstrated reductions in placental abcb1a and abcb1b mRNA (translated into P-gp) expression, suggesting reduced fetal protection against xenobiotics [2], [26], [27]. During an infection pro-inflammatory cytokines are released from endothelial cells and various cells of the immune system [27]–[34]. Pro-inflammatory cytokines have been shown to potently inhibit P-gp. In vivo studies involving the injection of adult mice with pro-inflammatory cytokines, interleukin-1β (IL-1β), interleukin-6 (IL-6) or tumor necrosis factor-α (TNF-α), results in significant decreases in P-gp mRNA expression in the liver [35]. Similar inhibition of P-gp expression and function has also been reported in adult rat hepatocytes and brain endothelial cells (BECs) following exposure to IL-1β, IL-6 or TNF-α, in vitro [36]–[41]. However, nothing is known as to how infection and pro-inflammatory cytokines can alter P-gp expression at the developing fetal BBB.
In this study, we examined the effects of pro-inflammatory cytokines on P-gp function in BECs derived at various stages of fetal development. We have previously established and characterized a robust BEC culture system in which we have derived BECs from various stages of development – both fetal and postnatal [19]. These BECs have clearly been demonstrated to maintain a distinct P-gp functional phenotype, which corresponds to their developmental age [19]. We hypothesized that treatment with IL-1β, IL-6 or TNF-α will result in decreased P-gp function in BECs, and that this inhibitory effect will increase in magnitude with increasing developmental age. These data are critical to increasing our understanding of how infection-complicated pregnancies can potentially harm the developing fetal brain.
Results
Developmental Changes in Baseline P-gp Function
BECs derived from male guinea pigs were utilized in this study; we have previously identified no sex-differences in the developmental expression of P-gp protein [19]. Compared to BECs derived from GD40, GD50 BECs exhibited approximately 28% more P-gp function (P<0.05; Fig. 1). Likewise, BECs derived from GD65 and PND14 male guinea pigs displayed significantly increased P-gp function compared to GD40 BECs (59% and 66% increases, respectively; P<0.001) and GD50 BECs (P<0.05). There was no significant difference in P-gp function between BECs derived from GD65 and PND14.
Figure 1. Baseline P-gp Activity in BECs increases with developmental age.
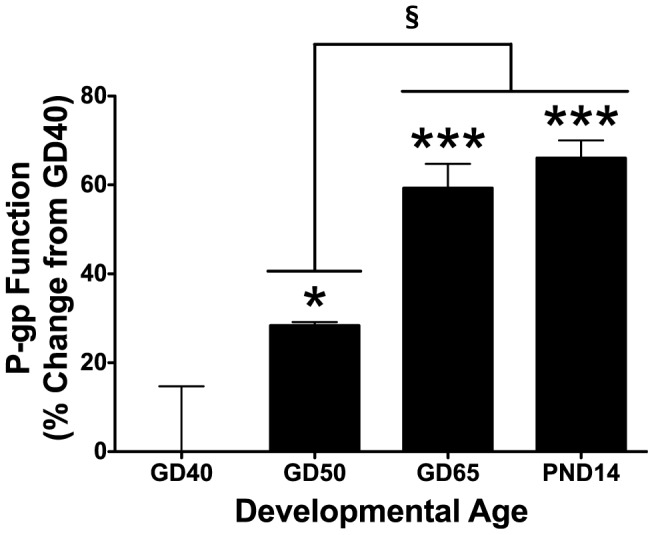
P-glycoprotein (P-gp) activity in brain endothelial cell (BEC) cultures derived from gestational day (GD) 40, 50, 65 and postnatal day (PND) 14 male guinea pigs (N = 4). P-gp activity is displayed as percent change from GD40 BECs (zero line). P-gp function was calculated over relative cell count. Values displayed as mean ± S.E.M. A significant difference from GD40 indicated by (*) P<0.05; (***) P<0.001.
Developmental Changes in Cytokine-Responsiveness
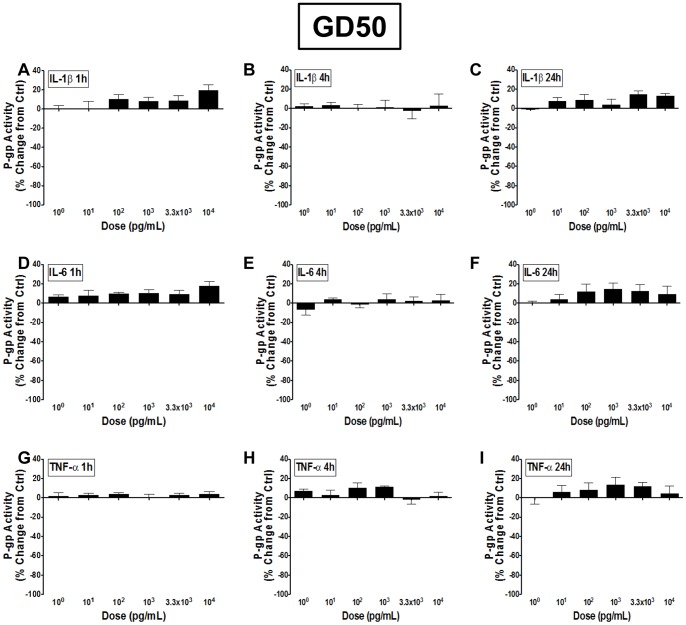
Male guinea pig BECs derived from GD50 exhibited little to no change in P-gp function to any dose of IL-1β, IL-6 or TNF-α, when treated for 1, 4 or 24 hours, in vitro (Fig. 2). There was a slight tendency for an increase in P-gp function, though this did not reach significance for any of the cytokines tested.
Figure 2. P-gp function in GD50 BECs is unresponsive to pro-inflammatory cytokines.
P-glycoprotein (P-gp) activity in brain endothelial cell (BEC) cultures derived from gestational day (GD) 50 male guinea pigs following treatment with 100–104 pg/mL A–C) interleukin-1β (IL-1β), D–F) interleukin-6 (IL-6) or G–I) tumor necrosis factor-α (TNF-α) for 1, 4 or 24 hours (N = 4). P-gp activity is displayed as percent change from untreated control cells (zero line). Values displayed as mean ± S.E.M.
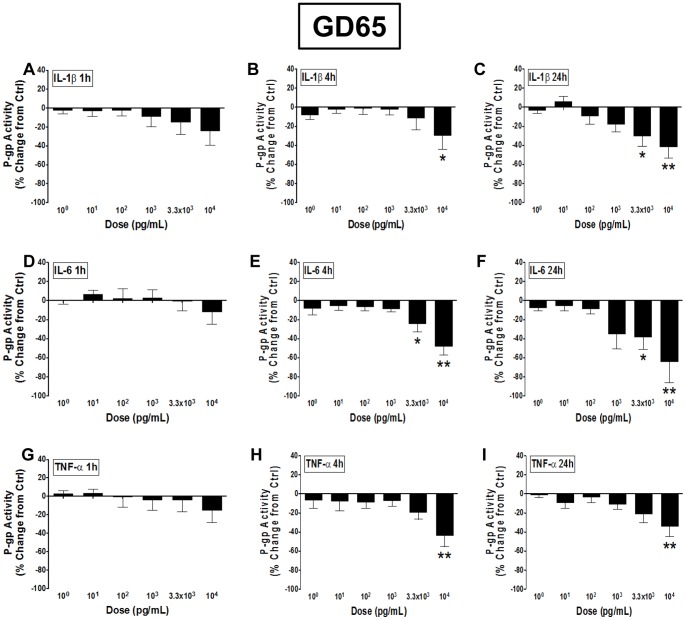
When treated with IL-1β for 1 hour, BECs derived from GD65 male guinea pigs displayed a trend towards a decrease in function, particularly with higher doses (Fig. 3A). Following 4 hours of treatment the 104 pg/mL dose of IL-1β resulted in a significant decrease in P-gp function (approximately 30% inhibition; P<0.05; Fig. 3B). This IL-1β-induced inhibition was even greater following 24 hours of treatment, displaying a dose-dependent decrease in P-gp function with 3.3×103 (∼30%; P<0.05) and 104 (∼42%; P<0.01) pg/mL doses (Fig. 3C). Incubation with IL-6 for 1 hour resulted in no change in P-gp function in GD65 BECs (Fig. 3D), however 4-hour treatment resulted in a dose-dependent decrease in function at 3.3×103 (∼25%; P<0.05) and 104 (∼48%; P<0.01) pg/mL doses (Fig. 3E). At these same doses, inhibition was even greater following 24 hours of IL-6 treatment of GD65 BECs –38% (P<0.05) and 65% (P<0.01), respectively (Fig. 3F). Only the highest dose of TNF-α (104 pg/mL) treatment of GD65 BECs only resulted in inhibition of P-gp function at 4 (∼44%; P<0.01; Fig. 3H) and 24 hours (∼34%; P<0.01; Fig. 3I).
Figure 3. Pro-inflammatory cytokines inhibit P-gp function in GD65 BECs in a dose-dependent manner.
P-glycoprotein (P-gp) activity in brain endothelial cell (BEC) cultures derived from gestational day (GD) 65 male guinea pigs following treatment with 100–104 pg/mL A–C) interleukin-1β (IL-1β), D–F) interleukin-6 (IL-6) or G–I) tumor necrosis factor-α (TNF-α) for 1, 4 or 24 hours (N = 4). P-gp activity is displayed as percent change from untreated control cells (zero line). Values displayed as mean ± S.E.M. A significant difference from control indicated by (*) P<0.05; (**) P<0.01.
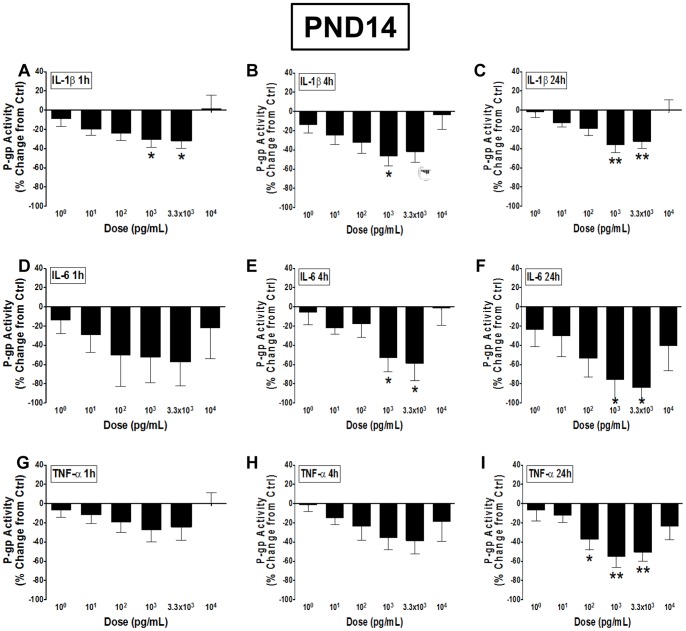
The inhibitory effects of cytokines on P-gp function were even greater on BECs derived from PND14. Treatment with 103 and 3.3×103 pg/mL IL-1β for 1 hour resulted in 31% and 32% decrease in function, respectively (Fig. 4A). This trend continued following 4 (P<0.05) and 24 (P<0.01) hour incubations with IL-1β (Fig. 4B–C). PND14 BECs following treatment with IL-6 exhibited the strongest inhibition of P-gp function. IL-6 treatment for 1 hour resulted in a trend towards decreased P-gp function, though this did not reach significance due to high variability (Fig. 4D). Following 4 hours of incubation, P-gp function decreased 53% and 59% with the 103 (P<0.05) and 3.3×104 (P<0.05) pg/mL dose of IL-6, respectively (Fig. 4E). The inhibition reached 76% and 84% at these same respective doses of IL-6, when incubated for 24 hours (P<0.05; Fig. 4F). Treatment of PND14 BECs with TNF-α for 1 and 4 hours resulted in a trend towards a dose-dependent inhibition of P-gp function, although these did not reach significance (Fig. 4G–H). Following 24 hours incubation of 102, 103 and 3.3×103 pg/mL doses of TNF-α, P-gp function was decreased by 38% (P<0.05), 55% (P<0.01) and 50% (P<0.01), respectively (Fig. 4I). There was little to no change in P-gp function following treatment with the highest (104 pg/mL) dose of IL-1β, IL-6 or TNF-α.
Figure 4. The inhibitory effects of pro-inflammatory cytokines on P-gp function are greatest in PND14 BECs.
P-glycoprotein (P-gp) activity in brain endothelial cell (BEC) cultures derived from postnatal day (PND) 14 male guinea pigs following treatment with 100–104 pg/mL A–C) interleukin-1β (IL-1β), D–F) interleukin-6 (IL-6) or G–I) tumor necrosis factor-α (TNF-α) for 1, 4 or 24 hours (N = 8). P-gp activity is displayed as percent change from untreated control cells (zero line). Values displayed as mean ± S.E.M. A significant difference from control indicated by (*) P<0.05; (**) P<0.01.
Specificity of Pro-inflammatory Cytokine Effects on P-gp Activity
The alternative P-gp substrate, Rho123, was used to confirm that the cytokine-induced effects on P-gp function were in fact P-gp specific. IL-1β, IL-6 or TNF-α treatments resulted in decreased Rho123 accumulation (and thus increased P-gp function; P<0.01; Fig. 5A) – similar to the inhibition of P-gp function seen in experiments using calcein-AM.
Figure 5. The inhibitory effects of pro-inflammatory cytokines are specific to P-gp and do not alter non-specific esterase activity.
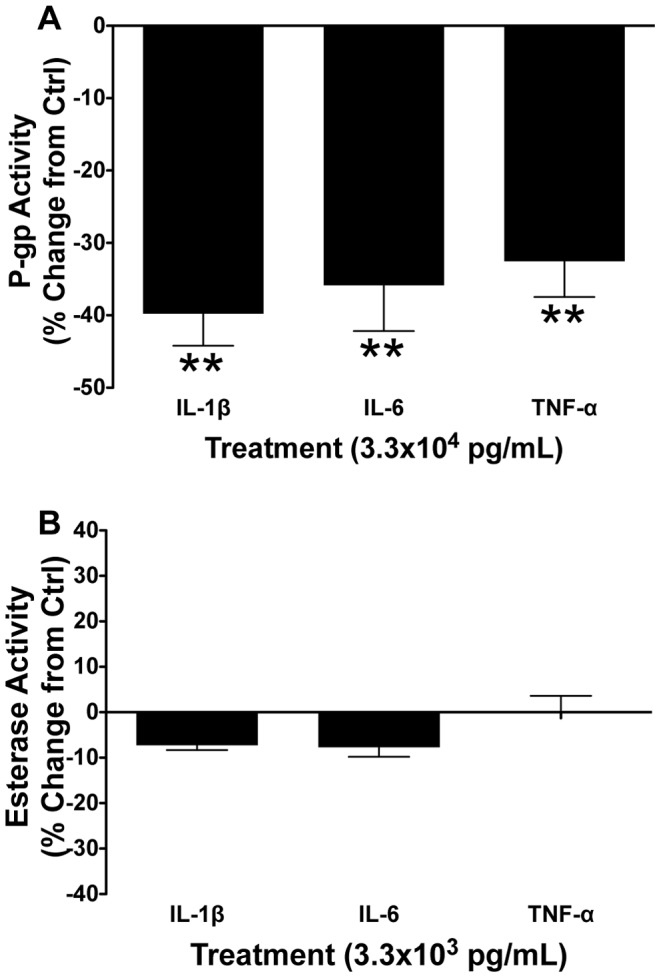
Postnatal day 14 male guinea pig BEC A) P-gp activity (using Rhodamine 123 as a P-gp substrate) following 24 hour treatment with 3.3×103 pg/mL interleukin-1β (IL-1β), interleukin-6 (IL-6) or tumor necrosis factor-α (TNF-α) (N = 6); and B) non-specific esterase activity following 24 hour treatment with 3.3×103 pg/mL IL-1β, IL-6 or TNF-α (N = 6). P-gp activity is displayed as percent change from untreated control cells (zero line). Values displayed as mean ± S.E.M. A significant difference from control indicated by (**) P<0.01.
Calcein-AM is converted to fluorescent calcein by cytoplasmic non-specific esterases, in addition to extrusion by P-gp. To determine if the conversion of calcein-AM to calcein was affected by cytokine treatment, changes in esterase activity were assessed. Treatment of BECs derived from PND14 guinea pigs with 3.3×103 pg/mL of IL-1β, IL-6 or TNF-α for 24 hours resulted in no change in esterase activity, compared to controls (P>0.05; Fig. 5B).
Changes in Abcb1 mRNA Expression Following Cytokine Treatment
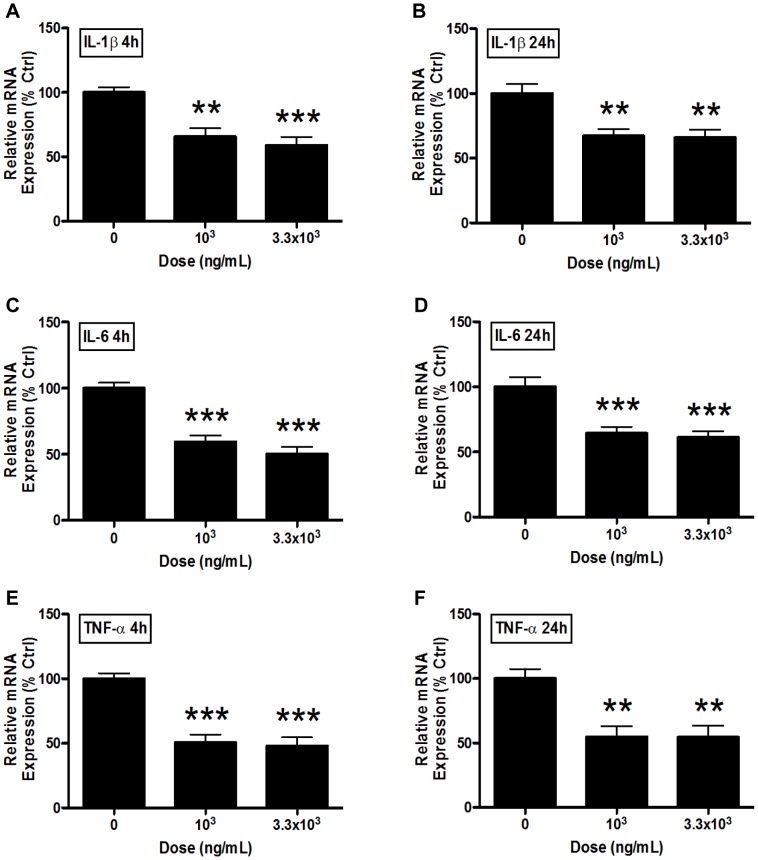
Treatment of PND14 BECS with 103 and 3.3×103 pg/ml of IL-1β for 4 hours resulted in a 34% (P<0.01) and 41% (P<0.001) decrease in abcb1 mRNA, respectively (Fig. 6A). This decreased expression is present following 24 hours of treatment as well, at roughly 33% (103 pg/mL; P<0.01) and 34% (3.3×103 pg/mL; P<0.01) (Fig. 6B). Similar decreases in abcb1 mRNA expression occur following IL-6 and TNF-α treatment. IL-6 displayed strong inhibition that ranged from 41–50% and 36–39% decreases in expression at 4 and 24 hours, respectively (P<0.001; Fig. 6C–D). TNF-α treatment for 4 and 24 hours resulted in 49–52% (P<0.001) and 45–46% inhibition (P<0.01), respectively (Fig. 6E–F).
Figure 6. Pro-inflammatory cytokines inhibit abcb1 mRNA expression.
Abcb1 mRNA expression in brain endothelial cell (BEC) cultures derived from postnatal day (PND) 14 male guinea pigs following treatment with 103 and 3.3×103 pg/mL A–B) interleukin-1β (IL-1β), C–D) interleukin-6 (IL-6) or E–F) tumor necrosis factor-α (TNF-α) for 4 (A,C,E) or 24 hours (B,D,F) (N = 7–8). Expression is displayed as percent of untreated control cell expression (i.e. 100% line) and taken over the reference gene, beta-actin. Values displayed as mean ± S.E.M. A significant difference from control indicated by (**) P<0.01; (***) P<0.001.
Discussion
We have demonstrated for the first time that pro-inflammatory cytokines exhibit an inhibitory effect on P-gp function in endothelial cells derived from the developing BBB. Our results indicate that the magnitude of this inhibition increases substantially with advancing age.
We have previously shown that P-gp protein expression in the developing BBB of the guinea pig increases approximately 7 to 8-fold between GD50 and GD65, and 14 to 17-fold between GD50 and PND14 [19]. As found in this current study, these changes in P-gp protein correspond to a 31% (at GD65) and 38% (at PND14) increase in in vitro BEC P-gp function, respectively when compared to GD50 BECs. This change in P-gp expression and function correspond with previous studies in mice and rats, which displayed increased developmental expression of P-gp in the whole brain [18], [20], as well as decreased in vivo radiolabelled P-gp substrate accumulation in the fetal brain with advancing gestation [20]. Unfortunately, it is not possible to assess in P-gp function in guinea pigs, in vivo, due to their large size (i.e. pregnant guinea pigs can be 20–25X greater in size than a pregnant mouse) and therefore the prohibitive costs of undertaking radiolabelled substrate accumulation studies. Functional studies of fetal human BBB activity are also not possible due to unavailability of tissues. However, human studies have shown increases in fetal brain P-gp expression with advancing gestation [42]–[44], suggesting that brain protection increases with developmental age, as we find in our and other rodent studies.
Our data suggest that while P-gp protection is developing at the fetal BBB, infection may render the developing brain susceptible to circulating xenobiotics and teratogens. Our experiments in BECs derived from PND14 clearly indicate that pro-inflammatory cytokines, IL-1β, IL-6 and TNF-α, have strong inhibitory effects on P-gp function. These results are in accordance with previous studies conducted in adult rodent hepatocytes and brain endothelial cells [36]–[40]. However, we have identified, for the first time that the inhibitory effects of pro-inflammatory cytokines on BBB P-gp function extend into fetal and neonatal life. In the fetal guinea pig, responsiveness to these inhibitory effects appears to develop between GD50 and term (∼GD68). At least at the level of the BBB, it appears that prior to GD50 pro-inflammatory cytokine release due to infection does not act to inhibit P-gp function. A lack of fetal BBB P-gp inhibition by pro-inflammatory cytokines up to 75% through gestation may bode well for pregnant women who experience infection earlier in pregnancy. At the same time, BBB P-gp expression and function is relatively low prior to GD50 (compared to term) and therefore may be much less adequate at protecting the fetal brain – with or without inhibition by pro-inflammatory cytokines. It is important to note that placental P-gp is also inhibited by infection [2], [26], [27], creating the potential for even greater exposure of the developing fetal brain to xenobiotics during maternal infection. Further in vivo studies are critical to elucidating the protective capacity of the fetal brain at various gestational time points, and how infection and/or pro-inflammatory cytokine exposure can alter fetal brain protection. It also remains unknown whether pro-inflammatory cytokine inhibition of P-gp function continues in brain endothelial cells past PND14. However, previous studies have found similar inhibitory effects of pro-inflammatory cytokines on adult (rodent and human) brain endothelial cell multidrug resistance [40], [41], suggesting that this responsiveness persists into adult life. As such, an infection in postnatal life through to adulthood may well reduce the protective capacity of the blood-brain barrier.
The intracellular mechanisms involved in the pro-inflammatory cytokine-induced reductions in P-gp function and expression remain unknown. Many regulatory pathways typically activated by pro-inflammatory cytokines, such as nuclear factor kappa B (NF-κB) [45], [46], activator protein-1 (AP-1) [47] and signal transducer and activator of transcription (STAT) [48], have been associated with the modulation of P-gp function and expression [45], [47]–[52]. The human ABCB1 promoter contains binding elements for NF-κB, AP-1 and STAT, suggesting the direct involvement of these regulatory proteins in the modulation of ABCB1 expression [53]–[56]. These regulatory protein-gene promoter interactions need to be further investigated in future studies, particularly whether or not they are involved in the alteration of multidrug resistance of guinea pig brain endothelial cells.
The increase in fetal BEC responsiveness to the inhibitory effects of pro-inflammatory cytokines (at the level of P-gp function) in late gestation suggests that fetal brain protection is potentially most vulnerable to infection at this time. Baseline P-gp function increases 31% between GD50 and GD65, but pro-inflammatory cytokine treatment of GD65 BECs results in a 30–50% decrease in function – essentially negating (and further inhibiting) the rise in baseline P-gp activity. This finding must be confirmed with in vivo models of infection near term, but certainly raises concerns for mothers experiencing infection in late gestation. Clinically, a pregnant women presenting with maternal or intra-amniotic infection is at risk of preterm labor (PTL) [23], and these women are often administered pharmacological treatments. Approximately 10% of women receive synthetic glucocorticoid treatment for being at risk of PTL (whether they present with infection or not) to help mature the fetal lungs and reduce the incidence of infant respiratory distress syndrome [57], [58]. Though this treatment has greatly improved neonatal outcomes, it has been associated with many long-term health consequences [59] and we have shown synthetic glucocorticoids to be potent modulators of fetal BEC P-gp activity [19]. Synthetic glucocorticoids such as dexamethasone and betamethasone are also substrates of P-gp, thus inhibition of fetal BBB P-gp by pro-inflammatory cytokines can potentially allow more synthetic glucocorticoid into the brain. Clearly, the clinical situation of preterm labor and synthetic glucocorticoid administration appears to be multi-faceted and thus it is critical to understand how this common clinical practice can alter fetal BBB multidrug resistance development, particularly in conjunction with infection. Often hormones such as progesterone, antibiotics or tocolytics are given to mothers at risk of PTL in order to prevent or delay labor [60]–[62]. However, many of these compounds are P-gp substrates [12], [63]–[65] and little to nothing is known regarding their long-term (potentially teratogenic) neurological effects in the fetus and newborn. Our data suggest that greater caution may be necessary when administering treatments or prescription medication to pregnant women with infections, but further study is critical to understand the effects on BBB multidrug resistance development and long-term consequence on the health of the newborn.
The rise in P-gp expression [19] and function, as well the increase in BEC responsiveness to cytokines may be attributed to epigenetic changes at the level of the abcb1 gene promoter as well as the endogenous late gestation surge in maternal and fetal plasma glucocorticoids. Changes in promoter methylation of the abcb1 gene at these developmental time points may explain the increased P-gp expression/function that we have identified, as well as help explain the increased ability of pro-inflammatory cytokine pathways to modulate transcription – though this remains speculative. Our previous data suggest that the late gestation glucocorticoid surge may represent an important signal for the upregulation of P-gp protein, function and responsiveness to pro-inflammatory cytokines at the fetal BBB, as this surge is critical to the maturation of many fetal organs including the lungs and brain [59], [66], and likely the BBB. Future studies are necessary to investigate whether glucocorticoids and/or epigenetic processes play a role in the increased protective capacity and responsiveness to pro-inflammatory cytokines at the developing BBB.
In conclusion, this is the first study to identify the effects of pro-inflammatory cytokines on multidrug resistance in the developing BBB. Our results clearly indicate that cytokine-induced inhibition of P-gp function increase in magnitude with advancing gestation. These changes in fetal BEC responsiveness to pro-inflammatory cytokines certainly warrant further mechanistic investigation. Indeed, these studies are critical given the high incidence of maternal and intra-amniotic infection and the large number of pregnant women on medication during pregnancy. Understanding how events, such as infection, during gestation can negatively affect the drug susceptibility of the offspring will be critical in the development of future therapies to counteract these effects.
Materials and Methods
Animals and Breeding
The guinea pig was chosen as the model organism due to its comparably longer gestation (approximately 68 days, compared to 19 and 21 days for the mouse and rat, respectively), which allowed for a wider range of developmental time points from which BECs could be derived. Guinea pigs give birth to neuroanatomically mature young, and undergo a pattern of fetal brain development that closely resembles humans and primates [67]. Placentation in the guinea pig is also similar to humans. Twelve week-old nulliparous female and postnatal day (PND) 14 male Dunkin-Hartley-strain guinea pigs were obtained from Charles River Canada Inc. (St. Constant, Quebec, Canada). Twelve week-old females were bred (as described previously [19]), and left untreated during pregnancy. All studies were carried out in accordance with protocols approved by the Animal Care Committee at the University of Toronto and in accordance with the Canadian Council on Animal Care.
Primary Guinea Pig Microvessel Extraction and BEC Culture
Pregnant guinea pigs were anaesthetized using isoflurane (Baxter Corp., Mississauga, Ontario, Canada) and euthanized by decapitation on gestational day GD40, 50 or 65 (term approximately 68 days; n = 4–6 for each gestational age). From each litter, fetal brains were collected as described previously [19]. Sex of each fetus was determined by the presence of uterine horns or testes. PND14 male guinea pig brains were also collected. Brains were placed on ice-cold Medium 199 (Invitrogen, Carlsbad, California, USA) supplemented with antibiotics-antimycotics (Invitrogen), immediately transferred to a biological safety cabinet and into ice-cold sterile Medium 199 supplemented with antibiotics-antimycotics. All remaining steps took place under sterile conditions. Brains were homogenized, and the homogenate was centrifuged (1000 g, 5 min, 4°C). The pellet was resuspended in dextran (Sigma, St. Louis, Missouri, USA) solution (17.5% w/v dextran in Hank’s Balanced salt solution [Invitrogen]) and centrifuged (4200 g, 15 min, 4°C). Vessel fractions were digested in type 1 collagenase (1 mg/mL; Sigma) dissolved in Dulbecco’s Modified Eagle Medium (DMEM, 15 min, 37°C; Wisent Inc., St. Bruno, Quebec, Canada). Following digestion, the mixture was centrifuged (500 g, 10 min, 4°C), and the cells (pellet) were resuspended in warm DMEM supplemented with 20% fetal bovine serum (Wisent) and ingredients modified from Zhang et al. (2003) [68]. Cells were plated on 0.5% gelatin (Sigma)-coated tissue culture flasks (Becton-Dickinson Biosciences, Franklin Lakes, New Jersey, USA) and grown in a 37°C/5% CO2-incubator. Following the extraction process, cells were determined to be more than 99% viable by trypan blue staining. We have previously shown that this extraction results in highly pure cultures of BECs (>99%) [19]. BECs derived from male guinea pigs were utilized in this study; we have previously identified no sex-differences in the developmental expression of P-gp protein [19].
Assessment of Baseline P-gp Function
BECs derived from GD40, 50, 65 and PND14 male guinea pigs were transferred to 0.5% gelatin-coated 96-well plates (Becton-Dickinson; 10,000 cells/well). At confluence, culture medium was removed and cells were washed with warm Tyrode salts’ (Tyrode) solution (Sigma) supplemented with sodium bicarbonate (1 g/L; Sigma). Cells were then incubated for 1 h with calcein-acetoxymethyl ester (calcein-AM; 10−6 M; Sigma, St. Louis, Missouri, USA) and C12-resazurin (10−5 M; Invitrogen) in Tyrode solution. Calcein (cleaved from calcein-AM by endogenous esterases inside the cell) accumulation is commonly used for the assessment of P-gp function in cell-based assays [69], [70]. Resazurin (metabolized into resorufin in live cells) has been used previously for determining relative cell counts [71] – which was used in this study to normalize calcein accumulation measurements in order to compare BEC P-gp function between developmental ages. Resazurin and resorufin are not P-gp substrates, and have previously been used in conjunction with the assessment of P-gp function [72], [73]. Cells were immediately placed on ice following calcein-AM/C12-resazurin incubation, washed with ice-cold Tyrode solution and lysed using ice-cold 1% Triton X-100 lysis buffer. Accumulation of fluorescent calcein was determined using a fluorescent plate reader (Calcein: Ex/Em = 485/510 nm; Resorufin: Ex/Em = 540/585 nm; Biotek, Winooski, Vermont, USA). Mean background fluorescence from each plate was subtracted from each control and treated well, prior to analysis, for all experiments measuring P-gp activity.
Pro-inflammatory Cytokine Effects on P-gp Function
BECs derived from GD50, 65 and PND14 male guinea pigs were transferred to 0.5% gelatin-coated 96-well plates (Becton-Dickinson; 10,000 cells/well). GD40 BECs were not used for cytokine treatment experiments, as we have previously shown that GD40 cells have very low P-gp protein levels and displayed no responsiveness to potent regulators of P-gp (i.e. corticosteroids) [19]. At confluence, culture medium was removed and replaced with phenol red-free DMEM (Wisent) supplemented with 20% charcoal-stripped fetal bovine serum (Wisent). Twenty-four hours following media change, cells were treated with various doses (100–104 pg/mL) of pro-inflammatory cytokines: IL-1β (Invitrogen), IL-6 (Invitrogen) and TNF-α (Invitrogen) for 1, 4 or 24 h at 37°C. These cytokines have previously been shown to alter P-gp expression and function in rat hepatocyte and rat/porcine BEC cultures [36]–[38], [40], as well as P-gp expression in mouse livers when injected, in vivo [35]. Dose ranges correspond to those used in previous studies [36]–[38], [40], as well as levels of circulating cytokines found in human and rodent infection studies [74], [75]. For instance, LPS treatment of post-labor maternal and umbilical cord blood has been shown to result in large elevations in IL-1β (control: ∼1 pg/mL; LPS: ∼150 pg/mL), IL-6 (control: ∼10 pg/mL; LPS: ∼2300 pg/mL) and TNF-α (control: ∼4 pg/mL; LPS: 250 pg/mL) [74]. This study did not investigate the additional cytokine contributions from white blood cells in lymph, as well as from endothelial cells Treatment of BECs derived from PND14 and fetal ages with IL-1β, IL-6 or TNF-α did not result in significant cell death (>99% viability). Control cells were re-incubated with media containing charcoal-stripped fetal bovine serum. Following treatment, cells were washed with warm Tyrode solution and then incubated for 1 h with calcein-AM (10−6 M in Tyrode solution). Cells were immediately placed on ice following calcein-AM incubation, washed with ice-cold Tyrode solution and lysed using ice-cold 1% Triton X-100 lysis buffer. Accumulation of fluorescent calcein was determined using a fluorescent plate reader, as described above. Mean background fluorescence from each plate was subtracted from each control and treated well, prior to analysis, for all experiments measuring P-gp activity.
Pro-inflammatory Cytokine Treatment and P-gp Specificity
BECs derived from PND14 male guinea pigs were grown to confluence in 96-well plates, as described above. At confluence, culture medium was removed and replaced with phenol red-free DMEM supplemented with 20% charcoal-stripped fetal bovine serum. Twenty-four hours later BECs were treated for 24 h with either phenol red-free medium containing stripped fetal bovine serum alone (controls), or with 3.3×103 pg/mL IL-1β, IL-6 or TNF-α. Following Tyrode wash, cells were incubated with an alternative P-gp substrate Rhodamine 123 (Rho123; 10−5 M; Sigma) for 30 minutes. Rho123 was utilized to further validate the use of calcein-AM as an indicator of P-gp activity. Following lysis, Rho123 accumulation was measured (Ex/Em: 485/528 nm).
Cytokine effects on esterase activity were also assessed as we have described previously [19]. Calcein-AM is a non-fluorescing P-gp substrate, which in addition to extrusion by P-gp, is actively converted to fluorescent calcein by cytoplasmic non-specific esterases. To confirm that esterase activity was not affected by cytokines, BECs were treated for 24 h with either phenol red-free medium containing stripped fetal bovine serum alone (controls), or with 3.3×103 pg/mL IL-1β, IL-6 or TNF-α. Cells were washed before the addition of warm lysis buffer containing 10−6 M calcein-AM. Conversion of calcein-AM to calcein, following treatment, was assessed immediately after 1-hour incubation with lysis buffer, as described above.
Pro-inflammatory Cytokine Effects on Abcb1 mRNA Expression
Abcb1 mRNA expression following cytokine treatment was only assessed in PND14 BECs as these cells exhibited the largest change in P-gp function. BECs derived from PND14 male guinea pigs were transferred to 0.5% gelatin-coated 10-cm culture dishes (Becton-Dickinson). At confluence, culture medium was removed and replaced with phenol red-free DMEM supplemented with 20% charcoal-stripped fetal bovine serum. Twenty-four hours following media change, cells were treated with 103 and 3.3×103 pg/mL of pro-inflammatory cytokines: IL-1β, IL-6 and TNF-α for 4 or 24 h at 37°C. These doses were chosen as they exhibited the largest change in P-gp function in PND14 BECs. Control cells were re-incubated with media containing charcoal-stripped fetal bovine serum. Following treatment, cells were placed on ice and washed twice with ice-cold Hank’s Balanced salt solution. RNA extraction was undertaken using TRIzol (Invitrogen), as previously described [5] and in accordance with the manufacturer’s instructions. Contaminating genomic DNA was removed by treating RNA samples with DNA-free deoxyribonuclease treatment (Ambion, Austin, Texas, USA). RNA purity and concentration were assessed using spectrophotometric analysis, and RNA integrity was verified using gel electrophoresis. RNA was stored at -80°C until further use. Total RNA was reverse-transcribed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, California, USA). Samples were incubated at 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min using the C1000 Thermal Cycler (Bio-Rad, Hercules, California, USA).
Real-time PCR was performed using the C1000 Thermal Cycler and quantified using the CFX96 Real-Time System (Bio-Rad). Samples were prepared using SsoFast EvaGreen Supermix (Bio-Rad), primer-probes for abcb1, beta-actin (abcb1: Forward – CAATCTGGGCAAAGATACTG, Reverse – CAAGTTCTTTGCTTTGTCCTC [Ensembl ID: ENSCPOT00000012540]; beta-actin: Forward – TTTACAATGAATTGCGTGTG, Reverse – ACATGATCTGGGTCATCTTC [Ensembl ID: ENSCPOT00000013600]), and cDNA template (100 ng) using ratios according to manufactures instructions. Data analysis was undertaken using CFX Manager Software (Bio-Rad). For each primer probe set, a standard curve was generated by serial dilution of a pooled reference sample with a minimum efficiency more than or equal to 90%. Samples were run in triplicate. Relative mRNA expression was calculated as gene of interest expression normalized [ΔΔc(t)] to reference gene expression (beta-actin). Beta-actin was not altered by pro-inflammatory cytokine treatment. For each plate, a non-template control (containing H2O in place of template cDNA) and non-amplification control (containing H2O in place of template RNA) was run to verify amplification and RT specificity.
Statistical Analysis
All statistical analyses were performed using Prism (GraphPad Software Inc., San Diego, California, USA). Functional P-gp data as well as abcb1 expression was analyzed using one-way ANOVA, followed by Dunnett’s (for comparisons against the control group). To assess baseline P-gp function across developmental ages, functional P-gp data was normalized against relative cell count and then expressed as percent change from GD40 BECs. For cytokine-treated BEC experiments, functional P-gp data is displayed as percent change in activity from controls. Significance was set at P<0.05.
Funding Statement
This study was funded by the Canadian Institutes for Health Research, FRN-57746; to WG and SGM and Doctoral Research Award to MI. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, et al. (2006) Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta 27: 602–609. [DOI] [PubMed] [Google Scholar]
- 2. Chen YH, Wang JP, Wang H, Sun MF, Wei LZ, et al. (2005) Lipopolysaccharide treatment downregulates the expression of the pregnane X receptor, cyp3a11 and mdr1a genes in mouse placenta. Toxicology 211: 242–252. [DOI] [PubMed] [Google Scholar]
- 3. Kalabis GM, Petropoulos S, Gibb W, Matthews SG (2009) Multidrug resistance phosphoglycoprotein (ABCB1) expression in the guinea pig placenta: developmental changes and regulation by betamethasone. Can J Physiol Pharmacol 87: 973–978. [DOI] [PubMed] [Google Scholar]
- 4. Kalabis GM, Kostaki A, Andrews MH, Petropoulos S, Gibb W, et al. (2005) Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: fetal protection. Biol Reprod 73: 591–597. [DOI] [PubMed] [Google Scholar]
- 5. Petropoulos S, Kalabis GM, Gibb W, Matthews SG (2007) Functional changes of mouse placental multidrug resistance phosphoglycoprotein (ABCB1) with advancing gestation and regulation by progesterone. Reprod Sci 14: 321–328. [DOI] [PubMed] [Google Scholar]
- 6. Irvine L, Flynn RW, Libby G, Crombie IK, Evans JM (2010) Drugs dispensed in primary care during pregnancy: a record-linkage analysis in Tayside, Scotland. Drug Saf 33: 593–604. [DOI] [PubMed] [Google Scholar]
- 7. Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, et al. (2004) Prescription drug use in pregnancy. Am J Obstet Gynecol 191: 398–407. [DOI] [PubMed] [Google Scholar]
- 8. Andrade SE, Raebel MA, Morse AN, Davis RL, Chan KA, et al. (2006) Use of prescription medications with a potential for fetal harm among pregnant women. Pharmacoepidemiol Drug Saf 15: 546–554. [DOI] [PubMed] [Google Scholar]
- 9. Lee E, Maneno MK, Smith L, Weiss SR, Zuckerman IH, et al. (2006) National patterns of medication use during pregnancy. Pharmacoepidemiol Drug Saf 15: 537–545. [DOI] [PubMed] [Google Scholar]
- 10. Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc JL (2000) Prescription of drugs during pregnancy in France. Lancet 356: 1735–1736. [DOI] [PubMed] [Google Scholar]
- 11. Malm H, Martikainen J, Klaukka T, Neuvonen PJ (2004) Prescription of hazardous drugs during pregnancy. Drug Saf 27: 899–908. [DOI] [PubMed] [Google Scholar]
- 12. Zhou SF (2008) Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 38: 802–832. [DOI] [PubMed] [Google Scholar]
- 13. Lagas JS, Vlaming ML, Schinkel AH (2009) Pharmacokinetic assessment of multiple ATP-binding cassette transporters: the power of combination knockout mice. Mol Interv 9: 136–145. [DOI] [PubMed] [Google Scholar]
- 14. Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG (2012) Placental drug transporters and their role in fetal protection. Placenta 33: 137–142. [DOI] [PubMed] [Google Scholar]
- 15. Garcia AM, Fletcher T, Benavides FG, Orts E (1999) Parental agricultural work and selected congenital malformations. Am J Epidemiol 149: 64–74. [DOI] [PubMed] [Google Scholar]
- 16. Loffredo CA, Silbergeld EK, Ferencz C, Zhang J (2001) Association of transposition of the great arteries in infants with maternal exposures to herbicides and rodenticides. Am J Epidemiol 153: 529–536. [DOI] [PubMed] [Google Scholar]
- 17. Regidor E, Ronda E, Garcia AM, Dominguez V (2004) Paternal exposure to agricultural pesticides and cause specific fetal death. Occup Environ Med 61: 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ek CJ, Wong A, Liddelow SA, Johansson PA, Dziegielewska KM, et al. (2010) Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol Lett 197: 51–59. [DOI] [PubMed] [Google Scholar]
- 19. Iqbal M, Gibb W, Matthews SG (2011) Corticosteroid regulation of P-glycoprotein in the developing blood-brain barrier. Endocrinology 152: 1067–1079. [DOI] [PubMed] [Google Scholar]
- 20. Petropoulos S, Gibb W, Matthews SG (2010) Developmental expression of multidrug resistance phosphoglycoprotein (P-gp) in the mouse fetal brain and glucocorticoid regulation. Brain Res 1357: 9–18. [DOI] [PubMed] [Google Scholar]
- 21.Yun HC, Hamza H, Berkowitz LB (2010) Bacterial infections and pregnancy. Medscape website. Available: http://emedicinemedscapecom/article/235054-overview. Accessed 2012 Jul 24.
- 22.Marino T, Laartz B, Smith SE, Gompf SG, Allaboun K, et al. (2010) Viral infections and pregnancy. Medscape website. Available: http://emedicinemedscapecom/article/235213-overview. Accessed 2012 Jul 24.
- 23. Romero R, Espinoza J, Chaiworapongsa T, Kalache K (2002) Infection and prematurity and the role of preventive strategies. Semin Neonatol 7: 259–274. [DOI] [PubMed] [Google Scholar]
- 24. Davis TM, Mueller I, Rogerson SJ (2010) Prevention and treatment of malaria in pregnancy. Future Microbiol 5: 1599–1613. [DOI] [PubMed] [Google Scholar]
- 25. Gao XJ, Zhao ZJ, He ZH, Wang T, Yang TB, et al. (2012) Toxoplasma gondii infection in pregnant women in China. Parasitology 139: 139–147. [DOI] [PubMed] [Google Scholar]
- 26. Wang JH, Scollard DA, Teng S, Reilly RM, Piquette-Miller M (2005) Detection of P-glycoprotein activity in endotoxemic rats by 99mTc-sestamibi imaging. J Nucl Med 46: 1537–1545. [PubMed] [Google Scholar]
- 27. Petrovic V, Piquette-Miller M (2010) Impact of polyinosinic/polycytidylic acid on placental and hepatobiliary drug transporters in pregnant rats. Drug Metab Dispos 38: 1760–1766. [DOI] [PubMed] [Google Scholar]
- 28. Piquette-Miller M, Pak A, Kim H, Anari R, Shahzamani A (1998) Decreased expression and activity of P-glycoprotein in rat liver during acute inflammation. Pharm Res 15: 706–711. [DOI] [PubMed] [Google Scholar]
- 29. Kalitsky-Szirtes J, Shayeganpour A, Brocks DR, Piquette-Miller M (2004) Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endotoxin-treated rats. Drug Metab Dispos 32: 20–27. [DOI] [PubMed] [Google Scholar]
- 30. Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL (2010) LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-kappaB. Cell Stress Chaperones 15: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nezic L, Skrbic R, Dobric S, Stojiljkovic MP, Satara SS, et al. (2009) Effect of simvastatin on proinflammatory cytokines production during lipopolysaccharide-induced inflammation in rats. Gen Physiol Biophys 28 Spec No: 119–126. [PubMed]
- 32. Agren J, Thiemermann C, Foster SJ, Wang JE, Aasen AO (2006) Cytokine responses to CpG DNA in human leukocytes. Scand J Immunol 64: 61–68. [DOI] [PubMed] [Google Scholar]
- 33. McRae MP, Brouwer KL, Kashuba AD (2003) Cytokine regulation of P-glycoprotein. Drug Metab Rev 35: 19–33. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Rousset CI, Hagberg H, Mallard C (2006) Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med 11: 343–353. [DOI] [PubMed] [Google Scholar]
- 35. Hartmann G, Kim H, Piquette-Miller M (2001) Regulation of the hepatic multidrug resistance gene expression by endotoxin and inflammatory cytokines in mice. Int Immunopharmacol 1: 189–199. [DOI] [PubMed] [Google Scholar]
- 36. Sukhai M, Yong A, Kalitsky J, Piquette-Miller M (2000) Inflammation and interleukin-6 mediate reductions in the hepatic expression and transcription of the mdr1a and mdr1b Genes. Mol Cell Biol Res Commun 4: 248–256. [DOI] [PubMed] [Google Scholar]
- 37. Sukhai M, Yong A, Pak A, Piquette-Miller M (2001) Decreased expression of P-glycoprotein in interleukin-1beta and interleukin-6 treated rat hepatocytes. Inflamm Res 50: 362–370. [DOI] [PubMed] [Google Scholar]
- 38. Hartz AM, Bauer B, Fricker G, Miller DS (2006) Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol 69: 462–470. [DOI] [PubMed] [Google Scholar]
- 39. Bauer B, Hartz AM, Miller DS (2007) Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71: 667–675. [DOI] [PubMed] [Google Scholar]
- 40. von Wedel-Parlow M, Wolte P, Galla HJ (2009) Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem 111: 111–118. [DOI] [PubMed] [Google Scholar]
- 41. Poller B, Drewe J, Krahenbuhl S, Huwyler J, Gutmann H (2010) Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol 30: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Virgintino D, Errede M, Girolamo F, Capobianco C, Robertson D, et al. (2008) Fetal blood-brain barrier P-glycoprotein contributes to brain protection during human development. J Neuropathol Exp Neurol 67: 50–61. [DOI] [PubMed] [Google Scholar]
- 43. van Kalken CK, Giaccone G, van der Valk P, Kuiper CM, Hadisaputro MM, et al. (1992) Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am J Pathol 141: 1063–1072. [PMC free article] [PubMed] [Google Scholar]
- 44. Schumacher U, Mollgard K (1997) The multidrug-resistance P-glycoprotein (Pgp, MDR1) is an early marker of blood-brain barrier development in the microvessels of the developing human brain. Histochem Cell Biol 108: 179–182. [DOI] [PubMed] [Google Scholar]
- 45. Nwaozuzu OM, Sellers LA, Barrand MA (2003) Signalling pathways influencing basal and H(2)O(2)-induced P-glycoprotein expression in endothelial cells derived from the blood-brain barrier. J Neurochem 87: 1043–1051. [DOI] [PubMed] [Google Scholar]
- 46. Trickler WJ, Mayhan WG, Miller DW (2005) Brain microvessel endothelial cell responses to tumor necrosis factor-alpha involve a nuclear factor kappa B (NF-kappaB) signal transduction pathway. Brain Res 1048: 24–31. [DOI] [PubMed] [Google Scholar]
- 47. Higa JK, Panee J (2011) Bamboo extract reduces interleukin 6 (IL-6) overproduction under lipotoxic conditions through inhibiting the activation of NF-kappaB and AP-1 pathways. Cytokine 55: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weidler M, Rether J, Anke T, Erkel G (2000) Inhibition of interleukin-6 signaling and Stat3 activation by a new class of bioactive cyclopentenone derivatives. Biochem Biophys Res Commun 276: 447–453. [DOI] [PubMed] [Google Scholar]
- 49. Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, et al. (2003) NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 22: 90–97. [DOI] [PubMed] [Google Scholar]
- 50. Thevenod F, Friedmann JM, Katsen AD, Hauser IA (2000) Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem 275: 1887–1896. [DOI] [PubMed] [Google Scholar]
- 51. Pedroza M, Schneider DJ, Karmouty-Quintana H, Coote J, Shaw S, et al. (2011) Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS One 6: e22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khalaf H, Jass J, Olsson PE (2010) Differential cytokine regulation by NF-kappaB and AP-1 in Jurkat T-cells. BMC Immunol 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scotto KW (2003) Transcriptional regulation of ABC drug transporters. Oncogene 22: 7496–7511. [DOI] [PubMed] [Google Scholar]
- 54. Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, et al. (2002) Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene 21: 1945–1954. [DOI] [PubMed] [Google Scholar]
- 55. Ikeguchi M, Teeter LD, Eckersberg T, Ganapathi R, Kuo MT (1991) Structural and functional analyses of the promoter of the murine multidrug resistance gene mdr3/mdr1a reveal a negative element containing the AP-1 binding site. DNA Cell Biol 10: 639–649. [DOI] [PubMed] [Google Scholar]
- 56. Bourguignon LY, Peyrollier K, Xia W, Gilad E (2008) Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem 283: 17635–17651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Slattery MM, Morrison JJ (2002) Preterm delivery. Lancet 360: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 58. Liggins GC, Howie RN (1972) A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 50: 515–525. [PubMed] [Google Scholar]
- 59. Kapoor A, Petropoulos S, Matthews SG (2008) Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res Rev 57: 586–595. [DOI] [PubMed] [Google Scholar]
- 60. Lamont RF (2005) Can antibiotics prevent preterm birth–the pro and con debate. BJOG 112 Suppl 1: 67–73. [DOI] [PubMed] [Google Scholar]
- 61. Mackenzie R, Walker M, Armson A, Hannah ME (2006) Progesterone for the prevention of preterm birth among women at increased risk: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol 194: 1234–1242. [DOI] [PubMed] [Google Scholar]
- 62. Simhan HN, Caritis SN (2007) Prevention of preterm delivery. N Engl J Med 357: 477–487. [DOI] [PubMed] [Google Scholar]
- 63. Hilgendorf C, Spahn-Langguth H, Regardh CG, Lipka E, Amidon GL, et al. (2000) Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci 89: 63–75. [DOI] [PubMed] [Google Scholar]
- 64. Viale M, Cordazzo C, de Totero D, Budriesi R, Rosano C, et al. (2011) Inhibition of MDR1 activity and induction of apoptosis by analogues of nifedipine and diltiazem: an in vitro analysis. Invest New Drugs 29: 98–109. [DOI] [PubMed] [Google Scholar]
- 65. Babic Z, Kucisec-Tepes N, Troskot R, Dorosulic Z, Svoboda-Beusan I (2009) The importance of P-glycoprotein multidrug transporter activity measurement in patients with Helicobacter pylori infection. Coll Antropol 33: 1145–1150. [PubMed] [Google Scholar]
- 66. Challis JRG, Matthews SG, Gibb W, Lye SJ (2000) Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 21: 514–550. [DOI] [PubMed] [Google Scholar]
- 67. Dobbing J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3: 79–83. [DOI] [PubMed] [Google Scholar]
- 68. Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, et al. (2003) The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J 17: 2085–2087. [DOI] [PubMed] [Google Scholar]
- 69. Weiss J, Dormann SM, Martin-Facklam M, Kerpen CJ, Ketabi-Kiyanvash N, et al. (2003) Inhibition of P-glycoprotein by newer antidepressants. J Pharmacol Exp Ther 305: 197–204. [DOI] [PubMed] [Google Scholar]
- 70. Feng B, Mills JB, Davidson RE, Mireles RJ, Janiszewski JS, et al. (2008) In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos 36: 268–275. [DOI] [PubMed] [Google Scholar]
- 71. Shaw SY, Westly EC, Pittet MJ, Subramanian A, Schreiber SL, et al. (2008) Perturbational profiling of nanomaterial biologic activity. Proc Natl Acad Sci U S A 105: 7387–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rahmat D, Sakloetsakun D, Shahnaz G, Sarti F, Laffleur F, et al. (2012) HEC-cysteamine conjugates: influence of degree of thiolation on efflux pump inhibitory and permeation enhancing properties. Int J Pharm 422: 40–46. [DOI] [PubMed] [Google Scholar]
- 73. Hartkoorn RC, Chandler B, Owen A, Ward SA, Bertel Squire S, et al. (2007) Differential drug susceptibility of intracellular and extracellular tuberculosis, and the impact of P-glycoprotein. Tuberculosis (Edinb) 87: 248–255. [DOI] [PubMed] [Google Scholar]
- 74.Kim-Fine S, Regnault TR, Lee JS, Gimbel SA, Greenspoon JA, et al. (2012) Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J Matern Fetal Neonatal Med (Epub ahead of print). [DOI] [PubMed]
- 75. Finney SJ, Leaver SK, Evans TW, Burke-Gaffney A (2012) Differences in lipopolysaccharide- and lipoteichoic acid-induced cytokine/chemokine expression. Intensive Care Med 38: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]