Abstract
Marine microalga, Scenedesmus sp., which is known to be suitable for biodiesel production because of its high lipid content, was subjected to the conventional Folch method of lipid extraction combined with high-pressure homogenization pretreatment process at 1200 psi and 35°C. Algal lipid yield was about 24.9% through this process, whereas only 19.8% lipid can be obtained by following a conventional lipid extraction procedure using the solvent, chloroform : methanol (2 : 1, v/v). Present approach requires 30 min process time and a moderate working temperature of 35°C as compared to the conventional extraction method which usually requires >5 hrs and 65°C temperature. It was found that this combined extraction process followed second-order reaction kinetics, which means most of the cellular lipids were extracted during initial periods of extraction, mostly within 30 min. In contrast, during the conventional extraction process, the cellular lipids were slowly and continuously extracted for >5 hrs by following first-order kinetics. Confocal and scanning electron microscopy revealed altered texture of algal biomass pretreated with high-pressure homogenization. These results clearly demonstrate that the Folch method coupled with high-pressure homogenization pretreatment can easily destruct the rigid cell walls of microalgae and release the intact lipids, with minimized extraction time and temperature, both of which are essential for maintaining good quality of the lipids for biodiesel production.
1. Introduction
Biodiesel production from microalgae mainly consists of biomass production, lipid extraction, and transesterification of extracted lipids [1, 2]. Among these processes, lipid extraction is the most costly, and can create a bottleneck of biodiesel production. Among the many types of microalga, Scenedesmus sp., marine green alga is known to be an excellent source of biodiesel due to its high lipid content (16~40%) [3]. However, research on lipid extractions from Scenedesmus sp. is insufficient compared to those from other microalgae, even though expeller/press method, solvent oil method, supercritical fluid extraction method, enzyme preparation, osmotic shock, ultrasonic assisted extraction, and others have been used thus far for lipid extraction from various biomass resources [1].
In general, solvent extraction method is most commonly used as it has been well studied. However, it can be used without any safety hazard and is most often used for the lipid extraction of animal tissues [4–6]. Microalgae have rigid cell walls, which work against the rate controlling step of solvent extraction resulting in difficulties in effective extraction [7]. Generally, it is known that the study on extraction dynamics and phase equilibrium is essential to the economic design of extraction devices and application of bioprocesses [8]. Specifically in the case of having a strong cell wall causing a slow extraction rate, it is necessary to study the extraction dynamic behavior that would provide information on the extraction mechanism in order to improve extraction efficiency. Therefore, the aim of this study was to introduce an effective extraction solvent method combined with a pretreatment step, which would enhance the extraction yield and rate of neutral lipids for biodiesel production from Scenedesmus sp. by considering their combination and kinetic analysis.
2. Materials and Methods
2.1. Sample Preparation
Scenedesmus sp. (Korea Ocean Research and Development Institute, Korea) was grown in IMK medium consisting of 200 mg NaNO3, 1.4 mg Na2HPO4, 5 mg K2HPO4, 2.68 mg NH4Cl, 5.2 mg Fe-EDTA, 0.332 mg Mn-EDTA, 37.2 mg Na2-EDTA, 0.023 mg ZnSO4·7H2O, 0.014 mg CoSO4·7H2O, 7.3 μg Na2MoO4·2H2O, 2.5 μg CuSO4·5H2O, 0.2 mg Thiamine-HCl, 1.5 μg biotin, 1.5 μg vitamin B12, and 0.18 mg MnCl2·4H2O dissolved in a liter of artificial seawater and harvested by a centrifuge at 3000 rpm for 10 min [9]. The samples were freeze-dried and stored at 4°C before use.
2.2. Lipid Extractions under Various Extraction Conditions
As shown in Figure 1 (a flow diagram of algal lipid extraction), to select the best extraction solvent, we used five of the most common lipid extraction solvents such as, acetone, 95% hexane, hexane : ether (1 : 1, v/v), 70% ethanol, and chloroform : methanol (2 : 1, v/v). Detailed extraction procedures were as follows: at first, 1 g of the freeze-dried sample was extracted with 50 mL of each solvent in a 1 L batch type extractor at room temperature (400 rpm, 24 hrs). Then, the extracts were filtered through a filter paper (Watman, no. 5), and the filtrates and residual algal lipid droplets were stained with 10% Nile red. The sample was observed under a fluorescence microscope (Eclipe 50i, Nikon, Japan) and a confocal laser microscope (FV300, Olympus, Japan) [9]. As a control, the dried biomass without any solvent treatment was stained with Nile red to show the intact cellular lipids.
Figure 1.
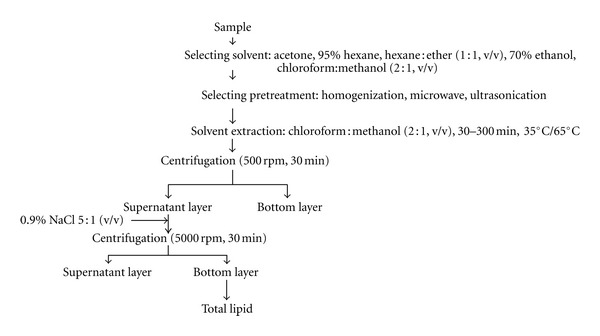
A flow diagram of lipid extraction from Scenedesmus sp.
After selecting chloroform : methanol (2 : 1 v/v) as an optimal extraction solvent, 1 g of the sample was treated with 30 ml of solvent (500 rpm, 12 hrs at 65°C). Then, the extracts were centrifuged at 5000 rpm for 30 min, and the supernatant was added to five times volume of 0.9% NaCl solution. The bottom layer of the extracts was dried and the weight of total lipids was measured for estimating extraction yields.
To evaluate the performance of microwave, ultrasonification, and homogenization methods, each pretreatment process was applied before being extracted with chloroform : methanol (2 : 1, v/v): For the microwave pretreatment, the sample was put in a microwave oven (Mr-216 mr, 1200 W, Korea) at a frequency of 2450 MHz for 5 min; for the ultrasonification pretreatment, an ultrasonicator (Bransonic 5210, USA) was used on the solvent at 47 kHz for 10 min; for the homogenization pretreatment,ahigh-pressure homogenizer (Micronox Inc. Korea)was used as shown in Figure 2, where 70 μm diameter Y-type diamond nozzle was installed in a 1 m long stainless steel pipe at room temperature. The sample was passed through a nozzle at 1200 psi pressure. The average size of the sample was reduced to 1–4 μm by the shear stress when it was released externally by cavitation [10]. Samples obtained after each pretreatment processes were extracted independently by following the method described in the previous paragraph except that the temperature was reduced to 35°C. As a control, nonpretreated sample was directly used for lipid extraction, exactly as given in the previous paragraph.
Figure 2.
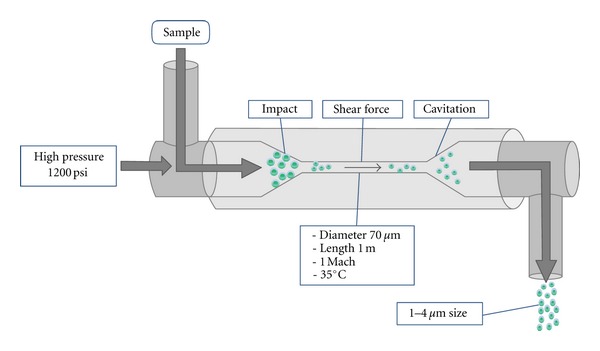
A schematic diagram of high-pressure homogenizer employed for pretreatment of algal biomass.
Before and after extracting the pretreated samples with the solvent, the surface textures were observed by field emission scanning electron microscope (FE-SEM: Hitachi S-4300, Japan) at 200~5000x magnified mode.
2.3. Kinetic Analysis of Lipid Extraction
To fit the kinetic models to lipid extraction process, the following first-order or second-order rate equations were employed since these models were widely applied for the analysis of dissolution and leaching within inhomogeneous liquid-solid systems [11].
First-order equation:
| (1) |
second-order equation:
| (2) |
Here, Cs and Ct represent the concentrations of extracted lipids and total cellular lipids, respectively, at an arbitrary time t; k1 and k2 represent first-order and second-order rate constants, respectively.
3. Results and Discussion
3.1. Comparative Analysis of Solvent Condition on the Lipid Extraction
Figure 3 illustrates the Nile red stained cellular lipid droplets after being extracted by five different extraction solvents including the control; no treatment (a), acetone (b), 95% hexane (c), hexane : ether (1 : 1, v/v) (d), 70% ethanol (e), and chloroform : methanol (2 : 1, v/v) (f). When compared to the control (a) with many yellow triglycerides (neutral lipids), a large proportion of red colored polar lipids were observed in the residue after being extracted by solvent (f), which suggested that solvent (f), unlike the other solvents, extracted most of neutral lipids from the biomass and left only polar lipids in the residue. By using this solvent, approximately 15% (w/w) of extraction yield was obtained, as compared to 1.3%, 2.1%, 4.5%, and 11.1% (w/w) lipid obtained by using acetone, 95% hexane, hexane : ether (1 : 1, v/v), and 70% ethanol, respectively. It implies that the most proper extraction solvent for this alga would be chloroform : methanol (2 : 1, v/v), which could also be supported by the results that show mixing two or three solvents would be better for lipid extraction [5, 12].
Figure 3.
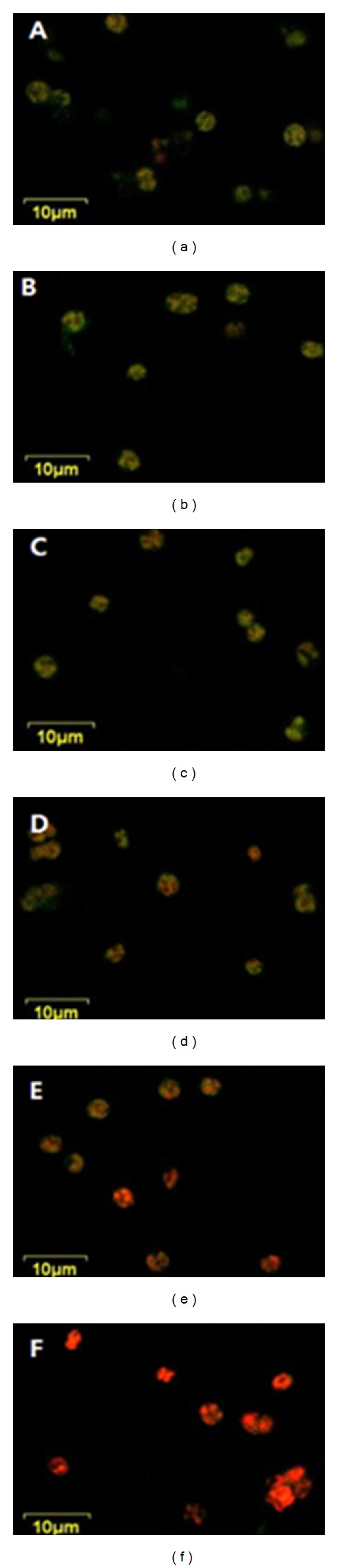
Comparison of the confocal laser microscopic images of Scenedesmus sp. illustrates the Nile red stained residual cellular lipid droplets after being extracted by five different extraction solvents including the control; (a) control (not extracted with any solvent), (b) acetone, (c) 95% hexane, (d) hexane : ether (1 : 1, v/v), (e) 70% ethanol, and (f) chloroform : methanol (2 : 1, v/v).
3.2. Effect of High-Pressure Homogenization on Cellular Lipid Extraction
Figure 4 compares the lipid extraction patterns from two different processes: conventional Folch extraction at 65°C versus combined extraction at 35°C. For combined extraction process, the algal biomass was passed through a high-pressure homogenizer at 1200 psi, then extracted with chloroform : methanol (2 : 1, v/v) at 35°C. Because, in general, it was known that high-pressure homogenization could effectively disrupt the cell membrane at room temperature due to high shear stress application inside the orifice and large pressure drop at the outlet [13]. It was found that the conventional extraction process showed a first-order reaction release pattern while the combined process followed second-order kinetics possibly because of easy disruption of relatively rigid algal cell membranes [14]. It was observed that during the conventional extraction process the external mass transfer resistance was important since generally the dissolution of lipids by the solvent and the external mass transfer occur in the opposite direction in the extraction processes, including the penetration of outer cell walls by the solvent [15]. It was also interesting that two steps were observed in extracting the lipids during the conventional extraction process: the initial slow stage for the first 1 hr and the later faster stage after 2–5 hrs. This implies that the initial step worked as the rate-limiting stage for this process. Compared with the case of no pretreatment process, the combined extraction method showed the typical second-order reaction kinetics which consists of two consecutive processes. The first process is a fast elution process for the first 30 min followed by a slower process in which after the elution, the internal diffusion, and the external diffusion from the liquid of the solute occur. Such phenomena occurred because when the biomass was exposed to fresh solvent, the lipid on its surface was solubilized and extracted rapidly. While at the initial stage of extraction, as the concentration of lipid was low in the solvent layer, the diffusion from the solid to the liquid occurred due to the mass transfer effect. As shown in the Figure 4, when a reaction time of 30 min was elapsed, a balanced state reaction was observed for the homogenized sample with a lipid extraction yield of 24.90% (w/w), which was slightly higher than the yield (19.80%, w/w) obtained for nonhomogenized sample after extended treatment for about 5 hrs. It was found that this combined extraction process followed second-order reaction kinetics, which means that most of the cellular lipids were extracted for the first periods of extraction, probably within 30 min. In contrast, during the conventional extraction process, the cellular lipids were slowly and continuously extracted for <5 hrs by following first-order kinetics. The results clearly showed that the destruction of cells through high-pressure homogenization and the combined solvent extraction method could help to increase the lipid extraction during the same time. In Figure 4, the extraction pattern was determined to follow second-order reaction kinetics. To confirm the reaction kinetics, the homogenization pretreatment data given in Figure 4 were fitted to (1) and (2) given in materials and methods. Based on Figure 5(b), the data fit well to the second-order kinetic (2) with a 0.9997 linear regression coefficient while the result did not linearly fit to the first-order (1) in Figure 5(a). In Figure 5(b), the rate constant of the second-order rate equations based on lipid extraction kinetics for the homogenization treatment was estimated to be 0.0053 min−1, which was much higher than 0.0000527 min−1 of extracting lipid from macroalga, Ulva lactuca which shows first-order kinetics [16]. This result clearly implies that the destruction of cells by applying the high-pressure could efficiently extract the lipids with a significant minimization in the extraction time (~9 times) as compared to nonhomogenized sample subjected to conventional extraction process.
Figure 4.
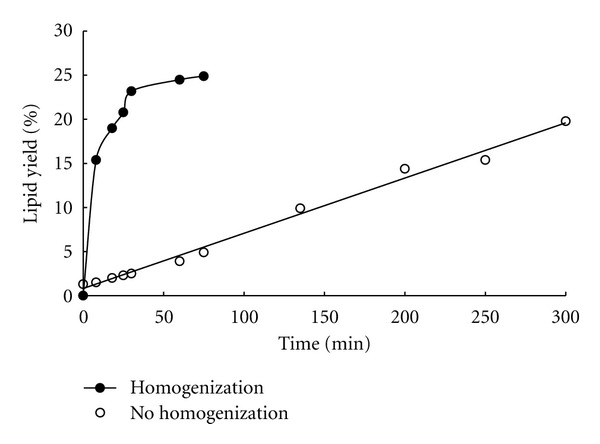
Comparison of the lipid extraction profile with (●) and without (○) homogenization as a pretreatment extracted with chloroform : methanol ( 2 : 1, v/v) solvent. Extraction yield was calculated by the weight ratio of the extracted lipids to the total dried biomass.
Figure 5.
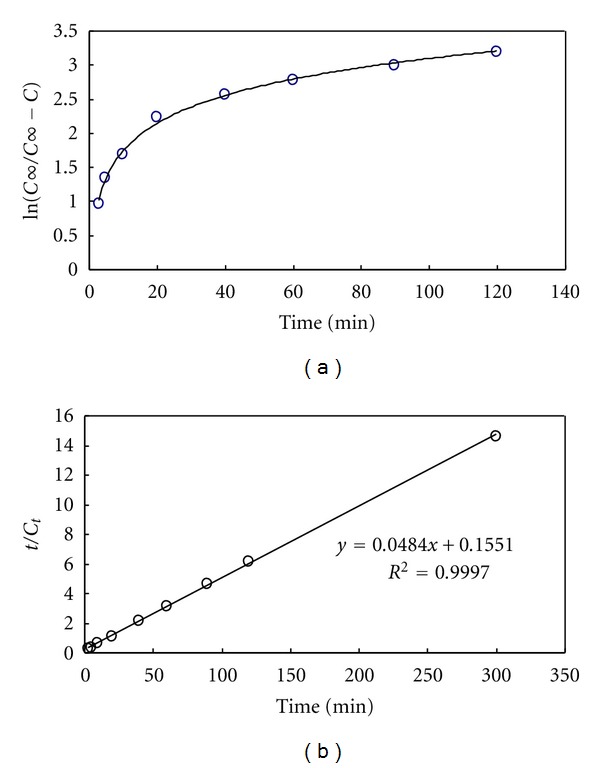
Result of first-order (a) and second-order (b) plots of lipid extraction from Scenedesmus sp. with homogenization pretreatment.
3.3. Comparative Analysis of Lipid Extraction Patterns According to Pretreatment Processes
From the above results, it was found that the extraction yield could be significantly increased by a high-pressure pretreatment process because the external mass transfer acted as the primary resistance due to the rigid structure of the algal cell walls [17]. Figure 6 shows characteristics of the residual cellular-lipid droplets in several pretreatment conditions. In control (a), most of the lipids in the residue remained as the yellow-colored droplets with the least extractions of neutral lipids. On the contrary, for others, after treating with any pretreatment, more neutral lipids were extracted even though there was an observable difference among the treatment conditions. As expected, the homogenization pretreatment process (b) maintained the best performance on extracting neutral lipids from the biomass compared to the processes such as microwave (c) and ultrasonic (d) pretreatments, showing that only polar lipids were left behind in the homogenized residue. After completion of 30 min. extraction time, a lipid yield of about 15.6% and 11.5% was obtained for the sample pretreated with microwave and ultrasonication, respectively, which was relatively lesser as compared to the yield (~25%), obtained for the sample pretreated with high-pressure homogenization. These results indicate that the extraction of neutral lipid can be significantly increased through the efficient destruction of cell walls by properly preconditioning the samples. To confirm this hypothesis, the surface textures of the samples were investigated by using SEM in Figure 7. For the case of homogenization pretreatment (b), many structures were spherically formed with about a 5 μm diameter, which was seldom observed in other samples such as microwave pretreatment (c) and ultrasonification pretreatment (d) whose shapes were similar to control (a). Based on this result, it was understood that due to high-pressure homogenization preconditioning, the large sizes of spherical masses (a) of the nonpreconditioned biomass were broken down into very small individual shapes, which resulted in high extraction of neutral lipids with less damage than other processes. This may also have occurred since at the initial stage of solvent extraction, the individual samples were disintegrated due to the highly increased surface area leading to the improved extraction yield and also considering that the extraction yield increases dramatically during the solvent extraction.
Figure 6.
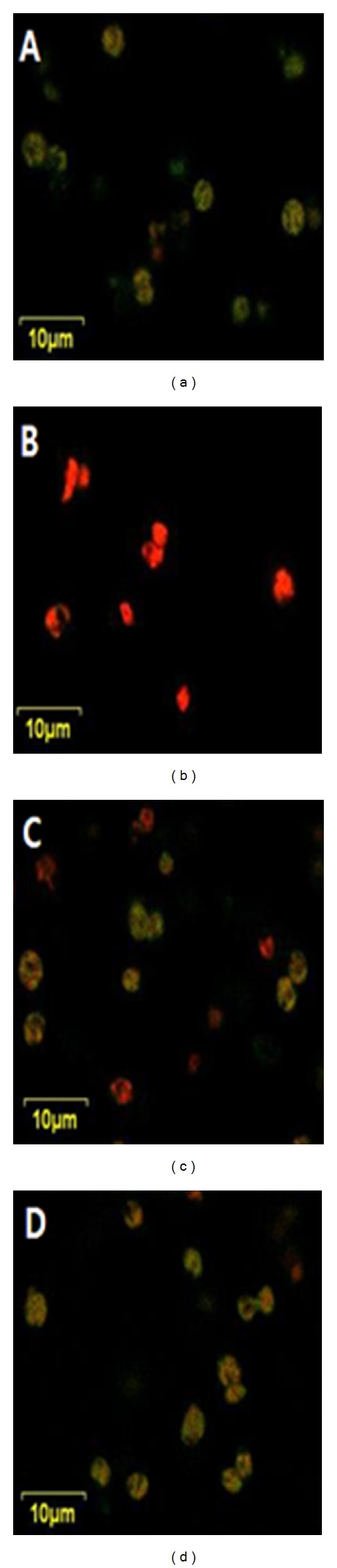
Comparison of confocal laser micrograph of Scenedesmus sp. stained with Nile red showing residual cellular lipid droplets; (a) control (only solvent extraction without any pretreatment), (b) solvent extraction with homogenization pretreatment, (c) solvent extraction with microwave pretreatment, and (d) solvent extraction with ultrasonification pretreatment.
Figure 7.
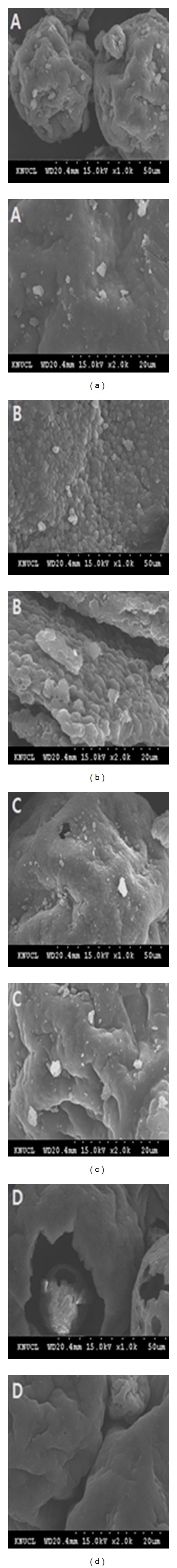
Scanning electron micrograph (SEM) of Scenedesmus sp. after several extraction processes (top: 1000x, bottom: 2000x); (a) control (only solvent extraction without any pretreatment), (b) solvent extraction with homogenization pretreatment, (c) solvent extraction with microwave pretreatment, and (d) solvent extraction with ultrasonification pretreatment.
4. Conclusion
The high-pressure homogenization pretreatment-coupled Folch method described in this study is found efficient than conventional lipid extraction method. This process works greatly at moderate temperature. The efficiency of this process was confirmed by analyzing residual algal cellular lipids using Nile red dye. By kinetic analysis, it was found that this combined process followed second-order extraction kinetics showing that most of the lipids were extracted during the first period of extraction. While most solvent extraction methods follow first-order reaction kinetics where the lipids are continuously extracted as the extraction time is increased, which results in a longer processing time. From confocal laser images and scanning electron microscope observation, it can be seen that this could be due to the effective destruction of the rigid cell walls at high-pressure homogenization. These results imply that this simple and efficient process could be employed to economically obtain mass amounts of good quality lipids from marine resources.
Acknowledgments
This work was supported by Human Resources Development (2008-N-BL-HM-E-06) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea Government Ministry of Knowledge Economy. This study was carried out with the support of Korea Institute of Ocean Science & Technology (Project No. PE98592), Korea.
References
- 1.Amin S. Review on biofuel oil and gas production processes from microalgae. Energy Conversion and Management. 2009;50(7):1834–1840. [Google Scholar]
- 2.Hossain ABMS, Salleh A, Boyce AN, Chowdhury P, Naqiuddin M. Biodiesel fuel production from algae as renewable energy. American Journal of Biochemistry and Biotechnology. 2008;4(3):250–254. [Google Scholar]
- 3.Mandal S, Mallick N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Applied Microbiology and Biotechnology. 2009;84(2):281–291. doi: 10.1007/s00253-009-1935-6. [DOI] [PubMed] [Google Scholar]
- 4.Sayyar S, Abidin ZZ, Yunus R, Muhammad A. Extraction of oil from Jatropha seeds-optimization and kinetics. American Journal of Applied Sciences. 2009;6(7):1390–1395. [Google Scholar]
- 5.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 6.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 7.Wiltshire KH, Boersma M, Möller A, Buhtz H. Extraction of pigments and fatty acids from the green alga Scenedesmus obliquus (Chlorophyceae) Aquatic Ecology. 2000;34(2):119–126. [Google Scholar]
- 8.Walker TH, Cochran HD, Hulbert GJ. Supercritical carbon dioxide extraction of lipids from Pythium irregulare. Journal of the American Oil Chemists’ Society. 1999;76(5):595–602. [Google Scholar]
- 9.Matsunaga T, Matsumoto M, Maeda Y, Sugiyama H, Sato R, Tanaka T. Characterization of marine microalga, Scenedesmus sp. strain JPCC GA0024 toward biofuel production. Biotechnology Letters. 2009;31(9):1367–1372. doi: 10.1007/s10529-009-0029-y. [DOI] [PubMed] [Google Scholar]
- 10.Choi WY, Lee HY, Ahn JH, et al. Enhancement of sacchaarification yield of Ulva pertusa Kjellman by high-pressure homogenization process for bioethanol production. Korean Society for Biotechnology and Bioengineering Journal. 2011;26(1):400–406. [Google Scholar]
- 11.Rakotondramasy RL, Havet JL, Porte C, Fauduet H. Solid-liquid extraction of protopine from Fumaria officinalis L.-analysis determination, kinetic reaction and model building. Separation and Purification Technology. 2007;54(2):253–261. [Google Scholar]
- 12.Grimal O, Masson DP, Bertrand L, Yelon A. Evidence of weak phonon coupling to the Si-H stretching modes in a-Si:H. Physical Review B. 1994;49(15):10242–10247. doi: 10.1103/physrevb.49.10242. [DOI] [PubMed] [Google Scholar]
- 13.Chisti Y, Moo-Young M. Review: disruption of microbial cells for intracellular products. Enzyme and Microbial Technology. 1986;8(4):194–204. [Google Scholar]
- 14.Ru’an C, Jun T, Hong G, et al. Kinetics of leaching flavonoids from pueraria lobata with ethanol. Chinese Journal of Chemical Engineering. 2006;14(3):402–406. [Google Scholar]
- 15.Ly M, Margaritis A, Jajuee B. Effect of solvent concentration on the extraction kinetics and diffusivity of Cyclosporin A in the fungus Tolypocladium inflatum. Biotechnology and Bioengineering. 2007;96(1):67–79. doi: 10.1002/bit.21180. [DOI] [PubMed] [Google Scholar]
- 16.Suganya T, Renganathan S. Optimization and kinetic studies on algal oil extraction from marine microalgae Ulva lactuca. Bioresource Technology. 2012;107:319–326. doi: 10.1016/j.biortech.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Oh SH, Han JG, Ha JH, et al. Enhancement of immune activity of Spirulina maxima by low temperature ultrasonification extraction. Korean Journal of Food Science and Technology. 2009;41(3):313–319. [Google Scholar]


