The mature protein of the potent anti-HIV lectin actinohivin has been crystallized with high reproducibility in a new form without packing disorder.
Keywords: anti-HIV lectin, actinohivin, high-mannose-type glycans
Abstract
Actinohivin (AH) is a new potent anti-HIV lectin of microbial origin. In order to modify it to produce a more efficient drug, its three-dimensional structure has previously been determined with and without the target α(1–2)mannobiose moiety of the high-mannose-type glycan (HMTG) attached to HIV-1 gp120. However, ambiguity remained in the structures owing to packing disorder that was possibly associated with peptide fragments attached at the N-terminus. To resolve these problems, the duration of cultivation of the AH-producing strain was examined and it was found that in a sample obtained from a 20 d culture the heterogeneous fragments were completely removed to produce mature AH with high homogeneity. In addition, the purification procedures were simplified in order to increase the yield of AH and the addition of solvents was also examined in order to increase the solubility of AH. AH thus obtained was successfully crystallized with high reproducibility in a different form to the previously obtained crystals. The crystal diffracted well to beyond 1.90 Å resolution and the crystallographic data suggested that it contained no packing disorder.
1. Introduction
HIV/AIDS is a major health concern; it is a global pandemic which remains a relatively uncontrolled infectious disease. Over 20 kinds of inhibitors targeting HIV enzymes (e.g. reverse transcriptase, integrase and protease) are currently in use as medicines to disturb the life cycle of HIV after its entry into cells (Jegede et al., 2008 ▶). These antiretroviral drugs have recently been further evaluated for their dual effects (Cohen, 2011 ▶) as treatments for and in the prevention of HIV infection (Sigal et al., 2011 ▶; Cohen et al., 2011 ▶). In addition, some proteins which are able to bind to the surface glycoprotein of HIV are now expected to prevent the entry of HIV into cells (Balzarini, 2007 ▶).
This entry-inhibition effect is also applicable to help suppress the spread of infection. Structurally, trimeric gp120 protruding from the HIV surface binds to human CD4+ cells to initiate entry (Berger et al., 1999 ▶). Each gp120 is highly glycosylated to cover the surface with high-mannose-type glycans (HMTGs; Leonard et al., 1990 ▶). As candidates for suppressing gp120 binding to susceptible cells, several carbohydrate-binding proteins (lectins) have been isolated and characterized. Among them, cyanovirin-N (CV-N) has been intensively investigated in order to determine its structural properties, carbohydrate-binding potential and antiviral activity (Bewley & Otero-Quintero, 2001 ▶; Bewley et al., 2002 ▶; Fromme et al., 2008 ▶; Tsai et al., 2004 ▶). Another lectin, griffithsin (GRFT), isolated from a red alga (Griffithsia sp.) also exhibits a high binding affinity for the HMTG of gp120 (Moulaei et al., 2010 ▶; Ziółkowska et al., 2007 ▶). These lectins are strong candidates for development as microbicides to prevent HIV transmission.
Similarly, we independently succeeded in discovering a new lectin, actinohivin (hereafter designated AH), from an actinomycete, Longispora albida K97-0003T (Matsumoto et al., 2003 ▶). This lectin possesses potent specific anti-HIV activity that inhibits the entry of various HIV-1 and HIV-2 strains into susceptible cells as well as T-cell-tropic and macrophage-tropic syncytium formation (Chiba et al., 2001 ▶). Initially, we used a sample purified according to the published method (Chiba et al., 2001 ▶) in order to determine the three-dimensional structure of wild-type AH (Tanaka et al., 2009 ▶; Takahashi et al., 2010 ▶, 2011 ▶). Its crystal structure was successfully solved, revealing that AH is composed of three repeated modules that are closely associated with each other (PDB entry 3a07; Tanaka et al., 2009 ▶). An N-terminal extension of a fragment which is part of the linker connecting the signal peptide to AH (Inokoshi et al., 2001 ▶) was also found, but only two residues were visible in the crystal. However, further detailed structural knowledge of the binding of AH to the target HMTG was required in order to develop AH as a useful drug. We attempted to prepare crystals of AH in complex with α(1–2)mannobiose (hereafter referred to as MB), which is the target moiety in the three branched ends of HMTG. A crystal obtained by chance in the presence of MB was analyzed by X-ray diffraction to reveal the structure of the complex, in which AH was disordered by a ±120° rotation around the crystallographic threefold axis1 (PDB entry 4den; structural information will be published elsewhere). Therefore, ambiguity remained in the structures owing to packing disorder, which was possibly associated with the peptide fragments attached at the N-terminus. Subsequently, all attempts to obtain crystals with and without MB resulted in failure, despite using AH prepared by the same method. To resolve the low reproducibility, the preparation method was examined by changing the duration of cultivation of the AH-producing strain; the purification protocol was also revised.
This paper presents a revised method which yields homogeneous AH (114 amino-acid residues2) that completely lacks the linker fragments, in addition to conditions under which AH can be crystallized with high reproducibility. Finally, this method was successful in obtaining a crystal of the AH–MB complex without packing disorder.
2. Materials and methods
2.1. Time-course analyses for maturation of AH
The AH-producing strain L. albida K97-0003T was cultivated as described previously (Chiba et al., 2001 ▶) except that the glucose concentration was reduced from 2.0 to 1.0%. Aliquots of the culture were centrifuged daily to obtain a supernatant and were subjected to SDS–PAGE analysis.
SDS–PAGE analyses were performed using a 15–20%(w/v) acrylamide gel (Bio Craft, Japan). A protein solution consisting of 10% glycerol, 2% SDS, 0.01% bromophenol blue and 5% β-mercaptoethanol in 63 mM Tris buffer pH 6.8 was heated at 373 K for 5 min and then loaded at 20 mA and 300 V (Bio-Rad, California, USA). A broad-range marker (TEFCO, Japan) was used to calibrate the molecular weights. Protein bands were stained with Coomassie Brilliant Blue R-250. Throughout the experiments, pH values and absorption spectra were measured using an HM-30G pH meter (Toa, Japan) and a BioSpec-mini spectrophotometer (Shimadzu, Japan), respectively.
Mass-spectrometric analyses were carried out using a Voyager-DE STR reflecting time-of-flight mass spectrometer (Applied Biosystems, California, USA) equipped with a 337 nm nitrogen laser operating in the linear positive-ion mode with an accelerating voltage of +25 kV and an extraction delay of 800 ns. A timed ion selector was used to deflect ions of low m/z (<500) from the detector. The spectra were acquired by averaging data from 200 laser shots in order to improve the data quality and ion statistics. Mass spectra were calibrated using myoglobin as an external mass standard. The spectra were processed using the Data Explorer software.
2.2. Purification of matured AH
Culture supernatant obtained as described above was subjected to 45% saturated ammonium sulfate fractionation. The resultant precipitate was dissolved in 50% methanol and loaded onto a hydroxyapatite column (Type I, 40 µm; Bio-Rad, California, USA). The absorbed materials were eluted with 20% methanol. The AH-containing fractions, as confirmed by SDS–PAGE, were merged and lyophilized.
2.3. Crystallization
AH for crystallization was prepared several times by the abovementioned method from cells cultivated for 20 d. To examine the solubility of AH, trifluoroacetate, propanol, 2-methyl-2,4-pentanediol (MPD) or acetonitrile was added to aqueous AH solutions at different concentrations and their effects were compared. Prior to crystallization, aqueous AH solutions were washed with 30% acetonitrile to increase the solubility of the AH and lyophilized again to remove unnecessary acetonitrile. The lyophilized AH was dissolved in water and its concentration was adjusted to 20 mg ml−1. MB was purchased from Sigma (St Louis, Missouri, USA). An aqueous MB solution was adjusted to 20 mg ml−1. The two solutions were mixed in equal volumes to prepare a protein solution for crystallization. The MB:AH molar ratio was calculated to be 12:1, taking into account that three MBs bind to one AH. Crystallization screening of AH in complex with MB was carried out by the hanging-drop vapour-diffusion method at 298 K. In each well, a droplet of 2.0 µl protein solution mixed with the same volume of reservoir solution was equilibrated against 700 µl reservoir solution. Commercially available crystallization kits (from Hampton Research, California, USA and Emerald BioSystems, Washington, USA) were used in the initial trials. From several conditions under which crystalline precipitates appeared, a suitable condition was further optimized.
2.4. X-ray diffraction experiment
As the crystals were obtained from a solution that contained 50%(v/v) MPD, they were regarded to already be cryoprotected. A crystal suitable for X-ray analysis was picked up and mounted in a CryoLoop (Hampton Research). X-ray experiments were performed at 100 K using synchrotron radiation of wavelength 1.00 Å on the BL-5A beamline at Photon Factory (PF), Tsukuba, Ibaraki, Japan. Diffraction images were recorded on an ADSC Quantum 210r CCD detector (Area Detector Systems). A total of 180 frames were collected in 1° oscillation steps with 1 s exposure per frame. Raw data images were indexed and intensities around Bragg spots were integrated to a resolution of 1.90 Å and then scaled and merged using iMOSFLM (Battye et al., 2011 ▶) from the CCP4 suite (Winn et al., 2011 ▶). Intensities were converted to structure-factor amplitudes using TRUNCATE from the CCP4 suite. Crystallographic data and intensity measurement statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics and crystallographic data.
Values in parentheses are for the highest resolution shell.
| Data collection | |
| X-ray source | BL-5A, PF |
| Wavelength | 1.00 |
| Resolution | 12.45–1.90 (2.00–1.90) |
| Crystal system | Orthorhombic |
| Observed reflections | 137072 (20500) |
| Unique reflections | 19664 (2855) |
| Completeness (%) | 98.6 (100.0) |
| R merge † | 0.053 (0.064) |
| 〈I/σ(I)〉 | 25.0 (22.1) |
| Crystal data | |
| Space group | P22121 |
| Unit-cell parameters (Å) | |
| a | 54.8 |
| b | 66.9 |
| c | 66.9 |
| Z ‡ | 2 |
| V M (Å3 Da−1) | 2.49 |
R
merge = 100 × 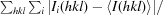
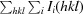 , where Ii(hkl) is the ith measurement of the intensity of reflection hkl and 〈I(hkl)〉 is its mean value.
, where Ii(hkl) is the ith measurement of the intensity of reflection hkl and 〈I(hkl)〉 is its mean value.
Number of protein molecules in the asymmetric unit.
3. Results and discussion
3.1. Effect of the duration of cultivation
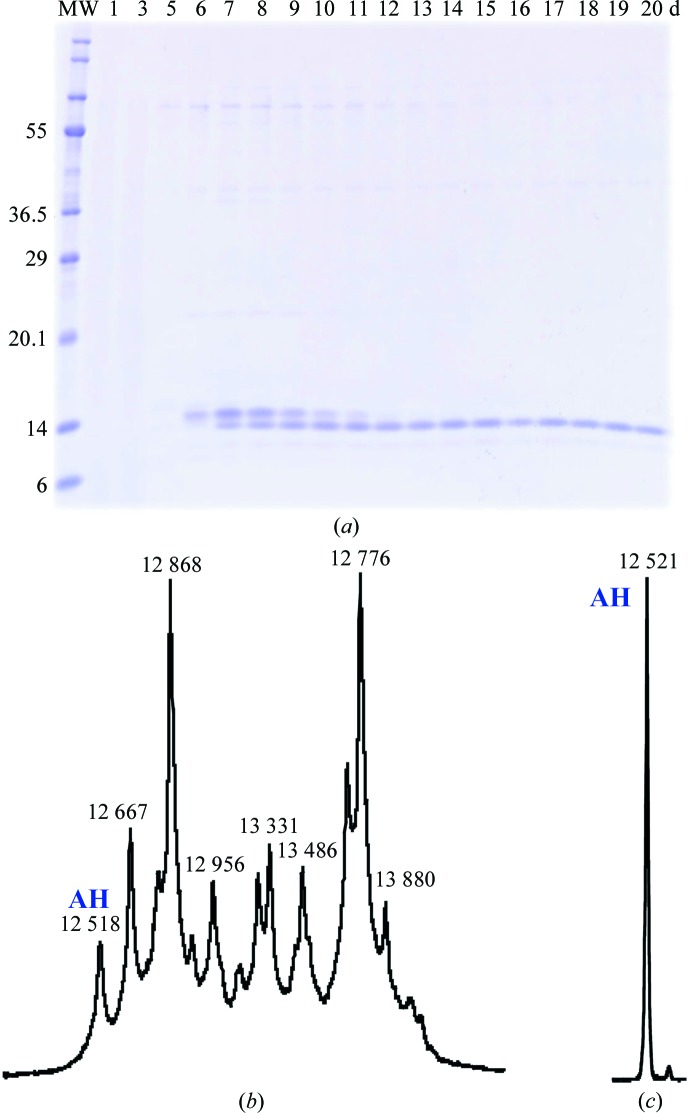
An SDS–PAGE of 15 samples of the supernatants obtained from various cultivations (Fig. 1 ▶ a) shows the behaviour of two main bands appearing at molecular weights close to that corresponding to AH. The first band appears at 6 d and disappears at 12 d, while the second band starts to appear at 7 d and remains until the end (20 d). A mass spectrum at 7 d (Fig. 1 ▶ b) indicates that several fragments appear around the two main peaks corresponding to the two bands in SDS–PAGE. The estimated molecular weight of the peak appearing at low m/z corresponds to that of mature AH, indicating that the fragments of the linker which connects the signal peptide to AH have been completely removed. The estimated molecular weights of the other peaks are consistent with the sequence of the linker region (Inokoshi et al., 2001 ▶). It might be difficult to isolate the mature AH from such a heterogeneous mixture. The AH sample used in previous X-ray analyses was derived from several samples obtained using 6–7 d cultivations. This heterogeneous situation (Figs. 1 ▶ a and 1 ▶b) is consistent with the two additional residues found in the X-ray structure of apo-form AH. In contrast, the sample obtained after 20 d cultivation showed a single peak in the mass spectrum (Fig. 1 ▶ c) which corresponds to mature AH.
Figure 1.
Contents and purity of the samples. (a) SDS–PAGE of samples fractionated with ammonium sulfate after x days of cultivation; x = 1, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 and 20. Lane MW contains size markers (molecular weights are labelled in kDa). (b, c) The mass spectra of (b) a sample obtained after 7 d cultivation and (c) a sample purified by hydroxyapatite column chromatography after 20 d cultivation.
3.2. Purification of mature AH
Another purpose of the present study is to establish a purification protocol that gives a high yield of homogeneous AH with high purity. The distinct insolubility of AH in ionic solutions suggested the application of the ammonium sulfate fractionation method. In practice, this technique gave a good result, as observed in Fig. 1 ▶(a). A subsequent hydroxyapatite column removes unnecessary substances to leave high-purity AH, as observed in the mass spectrum in Fig. 1 ▶(c). Thus, the present purification protocol reduced the number of steps to one compared with the previous method involving three steps of column chromatography.
3.3. Crystallization conditions
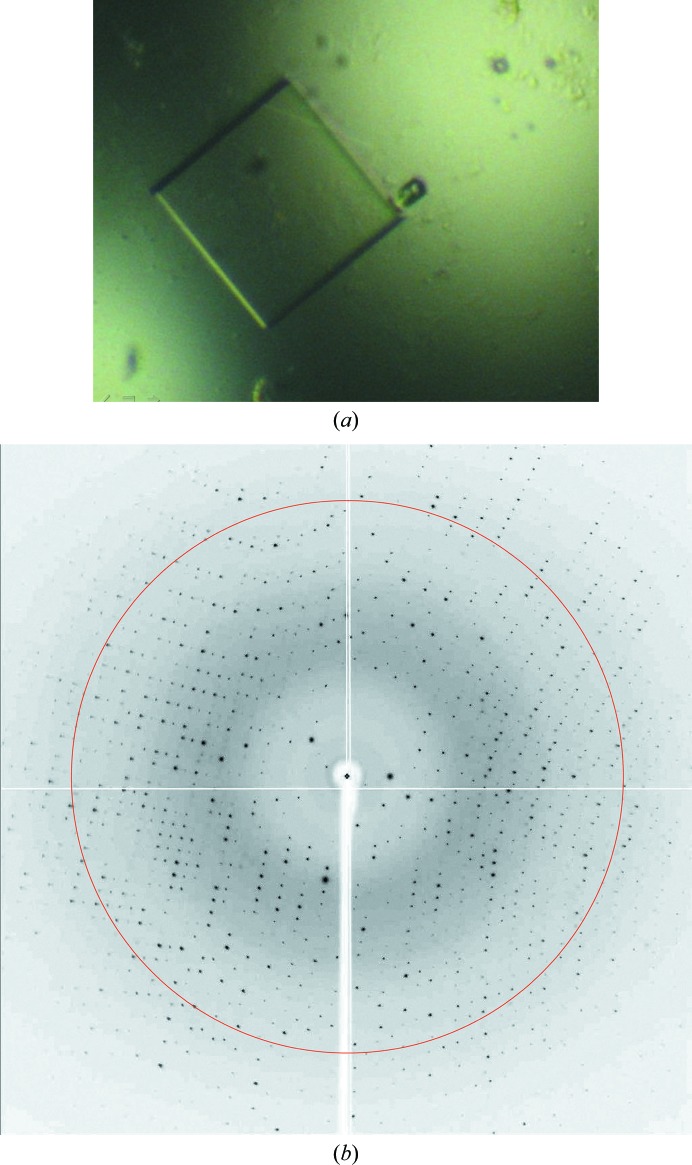
AH exhibits low solubility in neutral buffer solutions, but is soluble under acidic conditions below pH 4 or in solutions containing organic compounds such as TFA, alcohol, acetonitrile etc. and is stable under such conditions. This property limits the possibilities for the crystallization of AH. In practice, the addition of MB (which is a type of alcohol) also increased the solubility of AH. The solubility tests showed that acetonitrile was more effective than other solvents. In the final treatment, therefore, acetonitrile was added to AH and the excess was removed by vacuum freeze-drying. As the acetonitrile treatment increased the solubility of AH, crystallization was possible at a higher AH concentration (20 mg ml−1) and this reduced the duration of crystal growth from many months to a few months. A photograph of a crystal is shown in Fig. 2 ▶. This crystal, which was about 200 × 200 × 200 µm in size, was obtained at 298 K from a drop consisting of 0.2 M ammonium dihydrogen phosphate and 50%(v/v) MPD in 0.1 M Tris–HCl buffer solution pH 8.5. This condition differs from the previous condition [20%(w/v) polyethylene glycol 1000 and 0.2 M sodium chloride in 0.1 M sodium/potassium phosphate buffer pH 6.2].
Figure 2.
(a) A crystal obtained under the condition described in the text from a 20 d cultivation and (b) its X-ray diffraction pattern. The circle indicates 1.9 Å resolution. Many diffraction spots can be observed beyond this limit.
3.4. X-ray diffraction analysis
As given in Table 1 ▶, the number of observed reflections was 137 072 in the resolution range 12.45–1.90 Å and the R merge value was 5.3% for 19 664 unique reflections with a completeness of 98.6%. The space group was P22121, with unit-cell parameters a = 54.8, b = 66.9, c = 66.9 Å. These statistics were quite different from those of the previous crystal (space group P213, a = 56.2 Å). The previous crystal had a threefold crystallographic symmetry axis, around which AH was disordered by a ±120° rotation. The disorder in the P213 crystal could be ascribed to the noncrystallographic molecular threefold symmetry between the three structural modules (see Tanaka et al., 2009 ▶). However, the present crystal has no such threefold crystallographic symmetry. Therefore, it could be expected there would be no such disorder of the AH molecule. The calculated Matthews coefficient (V M) and solvent content (2.49 Å3 Da−1 and 50.6%, respectively) suggested that the asymmetric unit contained two AH molecules. This crystal may reveal not only the detailed interaction geometry, but also other effects on the structures of both AH and the bound MB.
Acknowledgments
We thank Y. Yamada, N. Matsugaki, N. Igarashi and S. Wakatsuki (Photon Factory, Tsukuba, Japan) for their assistance in data collection and with the synchrotron facility. This work was supported in parts by grants from the Grants-in-Aid for Scientific Research (No. 24790049), the Ministry of Education, Culture, Sports, Science and Technology of Japan (KS), the Tokyo Biochemical Research Foundation (AT), the JAXA-GCF project (MT), the Science Research Promotion Fund, the Promotion and Mutual Aid Corporation for Private Schools of Japan (HT), the Japan Ministry of Health, Labour and Welfare Research and the Japan Health Science Foundation on Drug Innovation (HT).
Footnotes
The asymmetric unit of the cubic crystal contains one third of an AH molecule.
The precursor AH is composed of 160 residues, the N-terminal 46 residues of which are removed in mature AH (NCBI accession No. Q9KWN0.1).
References
- Balzarini, J. (2007). Nature Rev. Microbiol. 5, 583–597. [DOI] [PMC free article] [PubMed]
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Berger, E. A., Murphy, P. M. & Farber, J. M. (1999). Annu. Rev. Immunol. 17, 657–700. [DOI] [PubMed]
- Bewley, C. A., Kiyonaka, S. & Hamachi, I. (2002). J. Mol. Biol. 322, 881–889. [DOI] [PubMed]
- Bewley, C. A. & Otero-Quintero, S. (2001). J. Am. Chem. Soc. 123, 3892–3902. [DOI] [PubMed]
- Chiba, H., Inokoshi, J., Okamoto, M., Asanuma, S., Matsuzaki, K., Iwama, M., Mizumoto, K., Tanaka, H., Oheda, M., Fujita, K., Nakashima, H., Shinose, M., Takahashi, Y. & Omura, S. (2001). Biochem. Biophys. Res. Commun. 282, 595–601. [DOI] [PubMed]
- Cohen, J. (2011). Science, 334, 1628. [DOI] [PubMed]
- Cohen, M. S. et al. (2011). N. Engl. J. Med. 365, 493–505.
- Fromme, R., Katiliene, Z., Fromme, P. & Ghirlanda, G. (2008). Protein Sci. 17, 939–944. [DOI] [PMC free article] [PubMed]
- Inokoshi, J., Chiba, H., Asanuma, S., Takahashi, A., Omura, S. & Tanaka, H. (2001). Biochem. Biophys. Res. Commun. 281, 1261–1265. [DOI] [PubMed]
- Jegede, O., Babu, J., Di Santo, R., McColl, D. J., Weber, J. & Quiñones-Mateu, M. (2008). AIDS Rev. 10, 172–189. [PubMed]
- Leonard, C. K., Spellman, M. W., Riddle, L., Harris, R. J., Thomas, J. N. & Gregory, T. J. (1990). J. Biol. Chem. 265, 10373–10382. [PubMed]
- Matsumoto, A., Takahashi, Y., Shinose, M., Seino, A., Iwai, Y. & Omura, S. (2003). Int. J. Syst. Evol. Microbiol. 53, 1553–1559. [DOI] [PubMed]
- Moulaei, T., Shenoy, S. R., Giomarelli, B., Thomas, C., McMahon, J. B., Dauter, Z., O’Keefe, B. R. & Wlodawer, A. (2010). Structure, 18, 1104–1115. [DOI] [PMC free article] [PubMed]
- Sigal, A., Kim, J. T., Balazs, A. B., Dekel, E., Mayo, A., Milo, R. & Baltimore, D. (2011). Nature (London), 477, 95–98. [DOI] [PubMed]
- Takahashi, A., Inokoshi, J., Hachiya, A., Oka, S., Omura, S. & Tanaka, H. (2011). J. Antibiot. 64, 551–557. [DOI] [PubMed]
- Takahashi, A., Inokoshi, J., Tsunoda, M., Suzuki, K., Takenaka, A., Sekiguchi, T., Omura, S. & Tanaka, H. (2010). J. Antibiot. 63, 661–665. [DOI] [PubMed]
- Tanaka, H., Chiba, H., Inokoshi, J., Kuno, A., Sugai, T., Takahashi, A., Ito, Y., Tsunoda, M., Suzuki, K., Takénaka, A., Sekiguchi, T., Umeyama, H., Hirabayashi, J. & Omura, S. (2009). Proc. Natl Acad. Sci. USA, 106, 15633–15638. [DOI] [PMC free article] [PubMed]
- Tsai, C.-C., Emau, P., Jiang, Y., Agy, M. B., Shattock, R. J., Schmidt, A., Morton, W. R., Gustafson, K. R. & Boyd, M. R. (2004). AIDS Res. Hum. Retroviruses, 20, 11–18. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Ziółkowska, N. E., Shenoy, S. R., O’Keefe, B. R., McMahon, J. B., Palmer, K. E., Dwek, R. A., Wormald, M. R. & Wlodawer, A. (2007). Proteins, 67, 661–670. [DOI] [PMC free article] [PubMed]