Abstract
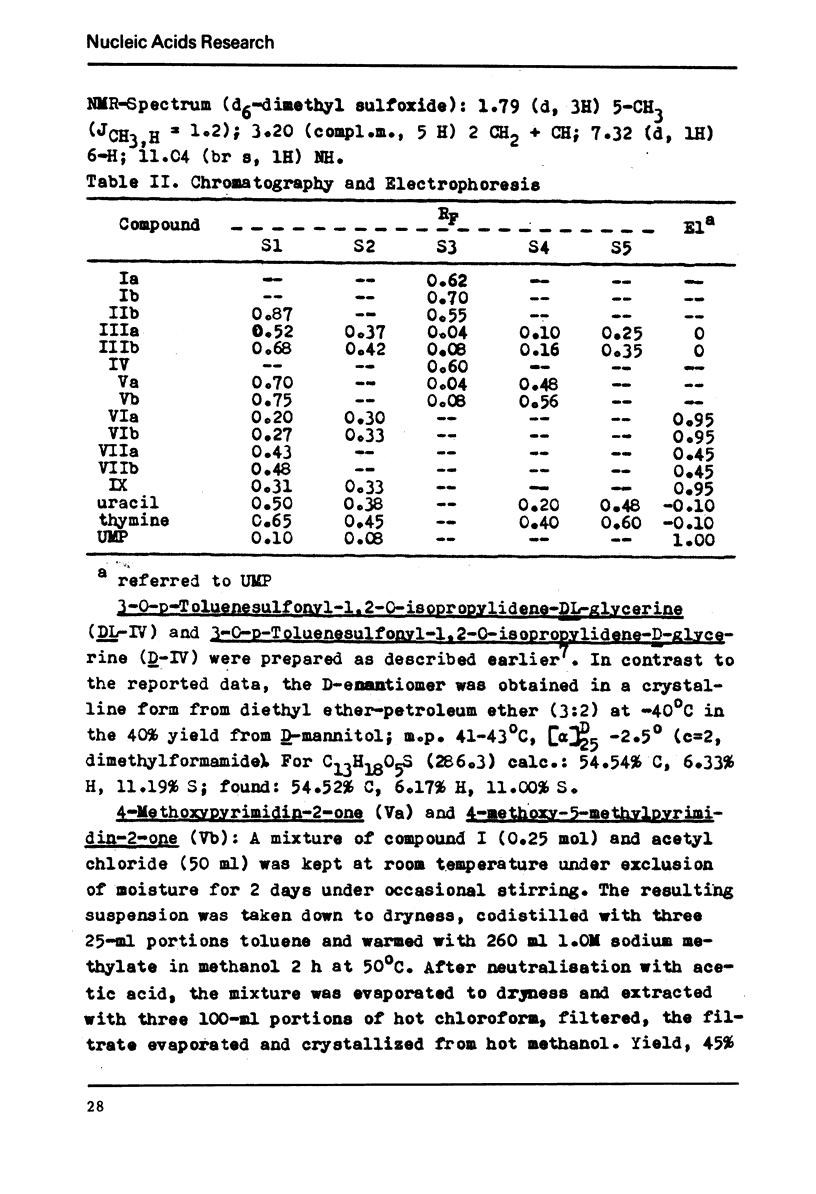
DL-1-(2,3-Dihydroxypropyl)thymine was prepared by Hilbert-Johnson reaction of 2,4-dinethoxy-5-methylpyrimidine with allyl bromide followed by the osmium tetroxide catalyzed hydroxylation of the l-allyl-4-methoxy-5-methylpyrimidin-2-one obtained as an intermediate. The D-glycero enantiomer, R-1-(2,3-dihydroxypropyl)thymine and the corresponding 1-substituted uracil derivative were prepared from 3-O-p-toluenesulfonyl-1, 2-O-isopropylidene-D-glycerine and sodium salt of 4-methoxy-5-methylpyrimidin-2-one or 4-methoxypyrimidin-2-one followed by treatment with hydrogen chloride in ethanol. The phosphorylation of the above 2,3-dihydroxypropyl derivatives with phosphoryl chloride in triethyl phosphate afforded the corresponding 3-phosphates which were transformed into the 2′,3′-cyclic phosphates by the condensation with N,N′-dicyclohexylcarbodiimide. The latter compounds of the D-glycero configuration are split by some microbial RNases to the 3-phosphates.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezborodova S. I., Bagdasarian Z. N. Issledovanie vnekletochnykh PNKaz Penicillium claviforme. Mikrobiologiia. 1972 Sep-Oct;41(5):773–781. [PubMed] [Google Scholar]
- Ghangas G. S., Fondy T. P. Stereospecific synthesis of D-1-fluorodeoxyglycerol 3-phosphate and its effects on glycerol 3-phosphate dehydrogenase. Biochemistry. 1971 Aug 17;10(17):3204–3210. doi: 10.1021/bi00793a007. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]


