YgjG is a putrescine:2-oxoglutarate aminotransferase that belongs to the class III aminotransferases. In this study, YgjG from E. coli was overexpressed, purified and crystallized using the hanging-drop vapour-diffusion method.
Keywords: YgjG, putrescine:2-oxoglutarate aminotransferases, polyamine degradation
Abstract
Putrescine, one of the polyamines that are found in virtually all living organisms, has been implicated as an important biological material. The protein YgjG is involved in the putrescine-degradation pathway in Escherichia coli. The enzyme is a putrescine:2-oxoglutarate aminotransferase that belongs to the class III aminotransferases. In this study, YgjG from E. coli was overexpressed, purified and crystallized using the hanging-drop vapour-diffusion method. Diffraction data were collected to 2.1 Å resolution using synchrotron radiation. The crystal belonged to the primitive orthorhombic space group P212121, with unit-cell parameters a = 121.1, b = 129.5, c = 131.3 Å, and is estimated to contain four molecules of YgjG per asymmetric unit.
1. Introduction
Polyamines, including putrescine, spermidine and spermine, are essential for cell growth and development in all organisms (Tabor & Tabor, 1984 ▶). The cationic character of polyamines allows them to bind to polyanionic macromolecules such as DNA, RNA, phospholipids and several enzymes (Igarashi et al., 1982 ▶). The major physiological function of polyamines is related to interaction with RNA; they thus stimulate protein synthesis at the level of transcription and translation (Watanabe et al., 1991 ▶; Miyamoto et al., 1993 ▶). Although polyamines are clearly necessary for the normal growth of cells, the accumulation of polyamines can lead to inhibition of cellular growth and protein synthesis (He et al., 1993 ▶; Pegg, 1988 ▶). Therefore, the cellular level of polyamines needs to be adjusted by means of synthesis, degradation, uptake and excretion systems (Wallace et al., 2003 ▶).
Putrescine, the major polyamine in Escherichia coli, is degraded by two metabolic pathways: the Puu pathway involving γ-glutamylation of the metabolic intermediates (Kurihara et al., 2008 ▶) and a pathway in which γ-aminobutyraldehyde is produced by YgjG, which exhibits putrescine:2-oxoglutarate (2-OG) aminotransferase (PATase) activity (Samsonova et al., 2003 ▶). Recombinant YgjG protein shows preferential substrate specificity towards putrescine as an amino-group donor and α-ketobutyrate as an amino-group acceptor. The observed K m values for putrescine and 2-OG are in the millimolar range, which is relatively high compared with other aminotranferases. However, the high K m values show good agreement with the cellular concentration of putrescine in E. coli, which has been measured at about 32 mM (Igarashi & Kashiwagi, 2000 ▶). In addition, the identified enzymatic activity of YgjG is further supported by the fact that the ygjG gene is under the control of the σ54 promoter, which is induced under nitrogen-starvation conditions (Zimmer et al., 2000 ▶).
YgjG belongs to subclass III of the pyridoxal phosphate (PLP) dependent aminotransferase family, which includes GABA aminotransferase and acetylornithine δ-aminotransferase based on amino-acid sequence alignment (Mehta et al., 1993 ▶). Therefore, the catalytic mechanism of YgjG should be similar to those of other aminotransferases in which PLP and l-glutamate form pyridoxamine phosphate (PMP) and α-ketoglutarate in one half of the reaction (Toney, 2011 ▶). Putrescine is converted to γ-aminobutyaldehyde in the other half of the reaction, together with the conversion of PMP to PLP. The unique substrate specificity of the enzyme might be confined to the part that interacts with putrescine or γ-aminobutyraldehyde. The other part of the reaction is common to most aminotransferases, as expected.
Although many crystal structures of aminotransferases have been reported to date, no structural information is available for PATase. In the present study, we overexpressed, purified and crystallized YgjG as a first step towards elucidating the molecular structure and the enzymatic mechanism of YgjG. Details of the atomic structure of YgjG should help us to understand the regulation mechanism of putrescine in cells and the structural basis of substrate recognition.
2. Materials and methods
2.1. Cloning, expression and purification of YgjG
The gene encoding YgjG (UniProt accession No. P42588) was amplified by polymerase chain reaction (PCR) with Pfu DNA polymerase from chromosomal DNA of E. coli K-12. The primers were 5′-GATATACATATGATACGCGAGCCTCCGGAGC-3′ for the forward primer and 5′-TGGTGCTCGAGCGCTTCTTCGACACTTACTCGC-3′ for the reverse primer, and contained NdeI and XhoI sites, respectively (bold). The PCR product was subcloned into the pET30a vector (Merck, Germany) to generate an expression plasmid encoding residues 1–459 of YgjG with a C-terminal His6 tag. The YgjG gene insertion was confirmed by DNA sequencing. The resulting pET30a expression vector was transformed into E. coli strain B834 (DE3) and the cells were grown in LB medium containing kanamycin at 310 K. After induction with 1.0 mM IPTG for a further 16 h at 295 K, the cells were harvested by centrifugation at 5000g at 277 K. The cell pellet was resuspended in ice-cold buffer A (30 mM Tris–HCl pH 8.0, 0.3 M NaCl) and lysed by sonication. The lysate was centrifuged at 15 000g for 30 min and the supernatant was loaded onto Ni–NTA resin (Quagen, USA) equilibrated with buffer A. After washing with buffer A containing 20 mM imidazole, the bound protein was eluted using a solution consisting of 30 mM Tris–HCl pH 8.0, 100 mM NaCl, 200 mM imidazole. The target protein was bound to a 5 ml HiTrap Q column (GE Healthcare, Sweden) and eluted with a 20-column-volume linear gradient from 50 to 500 mM NaCl in a buffer consisting of 30 mM Tris–HCl pH 8.0. Finally, the protein sample was further purified using a Superdex 200 column (GE Healthcare, Sweden) equilibrated with 10 mM Tris–HCl pH 8.0, 100 mM NaCl. Fractions containing YgjG were pooled and concentrated to 23 mg ml−1. Aliquots were flash-cooled in liquid nitrogen and stored 193 K for subsequent use in crystallization.
2.2. Crystallization
Initial crystallization conditions were screened at 295 K by the sitting-drop vapour-diffusion method in 96-well Intelli-Plates (Hampton Research, USA) using commercial kits from Hampton Research (Crystal Screen, Crystal Screen 2, SaltRX, Index HT and PEG/Ion HT) and Emerald BioStructures (Wizard I and II). Initial crystals of YgjG were grown on plates by equilibrating a mixture consisting of 0.4 µl protein solution (23 mg ml−1 protein in 10 mM Tris pH 8.0, 100 mM NaCl) and 0.4 µl reservoir solution No. 75 from PEG/Ion HT (0.2 M sodium formate, 12% PEG 3350 pH 7.0) against 70 µl reservoir solution. A hit from an initial crystallization condition was further optimized by varying the concentration of protein and PEG 3350 precipitant and the pH and by using detergent screening. Crystals appeared within 1 d and grew to maximum dimensions of 0.1 × 0.1 × 0.6 mm in the presence of 0.1 mM n-dodecyl-N,N-dimethylglycine, 0.2 M sodium formate, 15% PEG 3350, 0.1 M HEPES pH 7.5 using a protein concentration of 15 mg ml−1.
2.3. X-ray data collection
For X-ray data collection, a single crystal was briefly immersed into reservoir solution containing 15% glycerol as a cryoprotectant and immediately flash-cooled in a 100 K nitrogen stream. Native X-ray diffraction data were collected with a 2 × 2 mosaic CCD detector on beamline 26B1 at SPring-8, Japan using 1° oscillations at a crystal-to-detector distance of 200 mm. The crystal was exposed for 3 s per image. A data set was collected to 2.1 Å resolution from a single crystal. The data were indexed and scaled with the HKL-2000 software package (Otwinowski & Minor, 1997 ▶). The data-collection statistics are summarized in Table 1 ▶.
Table 1. Diffraction statistics.
Values in parentheses are for the highest resolution shell. During the scaling process, a 0σ cutoff filter was applied.
| X-ray source | Beamline 26B1, SPring-8 |
| Wavelength (Å) | 1.000 |
| Resolution (Å) | 50.0–2.1 (2.13–2.10) |
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 121.1, b = 129.5, c = 131.3 |
| Completeness (%) | 89.0 (81.9) |
| R merge † (%) | 6.6 (39.6) |
| Multiplicity | 5.2 (3.4) |
| Average I/σ(I) | 12.6 (2.4) |
R
merge = 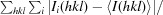
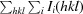 , where Ii(hkl) and 〈I(hkl)〉 are the observed intensity and the mean intensity of related reflections, respectively.
, where Ii(hkl) and 〈I(hkl)〉 are the observed intensity and the mean intensity of related reflections, respectively.
3. Results and discussion
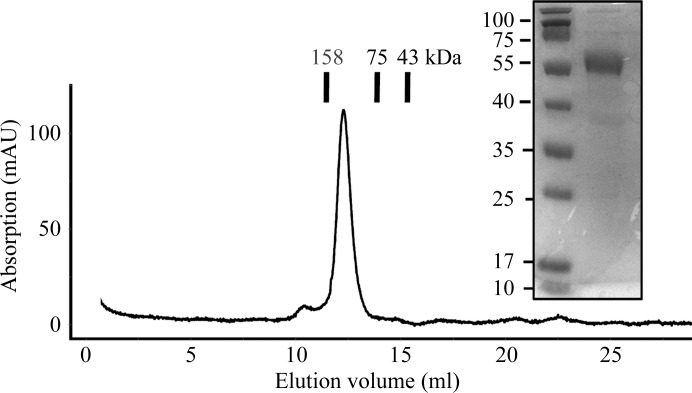
Recombinant YgjG protein was successfully overexpressed and purified to homogeneity using sequential chromatographic steps. A three-step purification method consisting of affinity chromatography and anion-exchange chromatography followed by gel-filtration chromatography produced 98% pure YgjG protein as analyzed by SDS–PAGE (Fig. 1 ▶). The yield of purified protein was ∼20 mg per litre of E. coli culture. The calculated monomeric molecular mass of YgjG including the C-terminal His tag was 50 700 Da and it eluted at approximately 100 kDa in gel-filtration chromatography, suggesting that it exists as a homodimer in solution, as observed for other aminotransferases (Jortzik et al., 2010 ▶).
Figure 1.
Gel-filtration chromatography profile and SDS–PAGE of purified E. coli YgjG.
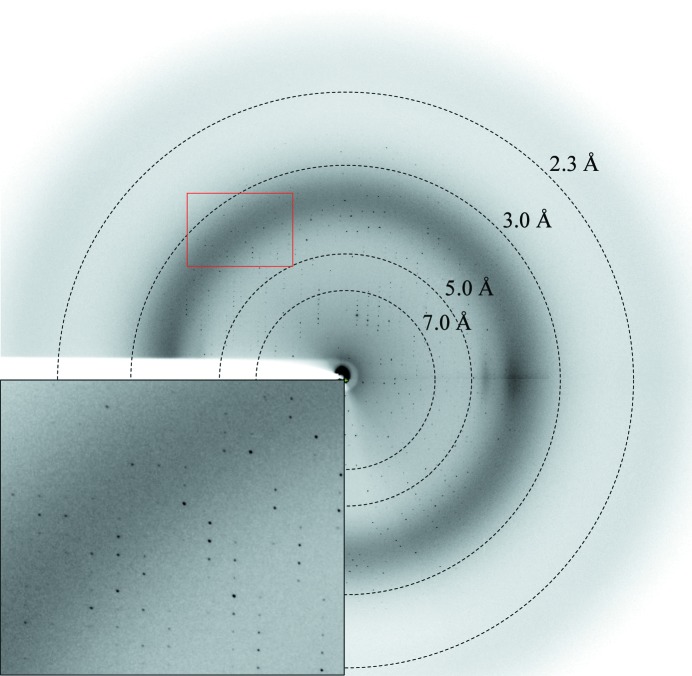
An initial clustered crystal that diffracted poorly was obtained from PEG/Ion HT condition No. 75 (Hampton Research). The quality of the crystals was improved by lowering the protein concentration and was further optimized by using n-dodecyl-N,N-dimethylglycine as an additive from a detergent-screening kit (Hampton Research). After optimizing the crystallization conditions, shiny single crystals were obtained by hanging-drop vapour diffusion by mixing equal volumes (2 µl) of protein solution (15 mg ml−1) and reservoir solution composed of 15%(w/v) PEG 3350, 0.2 M sodium formate, 0.1 M HEPES pH 7.5, 0.1 mM n-dodecyl-N,N-dimethylglycine. The best crystals grew within 7 d to final dimensions of approximately 0.1 × 0.1 × 0.6 mm (Fig. 2 ▶). The YgjG crystal diffracted to a resolution of 2.1 Å (Fig. 3 ▶) and belonged to space group P212121, with unit-cell parameters a = 121.1, b = 129.5, c = 131.2 Å. The diffraction data set was 96.7% complete, with an R merge of 5.8% (Table 1 ▶). The asymmetric unit contained four YgjG molecules of 50.7 kDa, resulting in a crystal volume per protein weight of 2.15 Å3 Da−1 and a solvent content of 43% (Matthews, 1968 ▶). A preliminary structure solution of the YgjG protein was obtained using the molecular-replacement (MR) method (MOLREP program; Vagin & Teplyakov, 2010 ▶) with the structure of acetylornithine aminotransferase from Thermus thermophilus (PDB entry 1vef; RIKEN Structural Genomics/Proteomics Initiative, unpublished work), which is the most closely related structure to YgjG (34% amino-acid sequence identity), as a search model. The best MR model gave a correlation coefficient of 52.4% and an R factor of 50.2% in the resolution range 30–3.0 Å. Analysis of the MR model showed good crystal packing and no clashes were found between symmetry-related molecules, indicating that the solution is correct and sufficient to refine to the full model.
Figure 2.

Crystals of E. coli YgjG. The crystals grew within one week at 295 K to maximum dimensions of approximately 0.1 × 0.1 × 0.6 mm.
Figure 3.
Representative X-ray diffraction image of the YgjG crystal. The crystal was exposed for 3 s over a 1° oscillation range.
Acknowledgments
This work was supported by the Marine Extreme Genome Research Center Program of the Ministry of Maritime of Land, Transportation and Maritime Affairs, Republic of Korea.
References
- He, Y., Kashiwagi, K., Fukuchi, J., Terao, K., Shirahata, A. & Igarashi, K. (1993). Eur. J. Biochem. 217, 89–96. [DOI] [PubMed]
- Igarashi, K. & Kashiwagi, K. (2000). Biochem. Biophys. Res. Commun. 271, 559–564. [DOI] [PubMed]
- Igarashi, K., Sakamoto, I., Goto, N., Kashiwagi, K., Honma, R. & Hirose, S. (1982). Arch. Biochem. Biophys. 219, 438–443. [DOI] [PubMed]
- Jortzik, E., Fritz-Wolf, K., Sturm, N., Hipp, M., Rahlfs, S. & Becker, K. (2010). J. Mol. Biol. 402, 445–459. [DOI] [PubMed]
- Kurihara, S., Oda, S., Tsuboi, Y., Kim, H. G., Oshida, M., Kumagai, H. & Suzuki, H. (2008). J. Biol. Chem. 283, 19981–19990. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Mehta, P. K., Hale, T. I. & Christen, P. (1993). Eur. J. Biochem. 214, 549–561. [DOI] [PubMed]
- Miyamoto, S., Kashiwagi, K., Ito, K., Watanabe, S. & Igarashi, K. (1993). Arch. Biochem. Biophys. 300, 63–68. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Pegg, A. E. (1988). Cancer Res. 48, 759–774. [PubMed]
- Samsonova, N. N., Smirnov, S. V., Altman, I. B. & Ptitsyn, L. R. (2003). BMC Microbiol. 3, 2. [DOI] [PMC free article] [PubMed]
- Tabor, C. W. & Tabor, H. (1984). Annu. Rev. Biochem. 53, 749–790. [DOI] [PubMed]
- Toney, M. D. (2011). Biochim. Biophys. Acta, 1814, 1407–1418. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Wallace, H. M., Fraser, A. V. & Hughes, A. (2003). Biochem. J. 376, 1–14. [DOI] [PMC free article] [PubMed]
- Watanabe, S., Kusama-Eguchi, K., Kobayashi, H. & Igarashi, K. (1991). J. Biol. Chem. 266, 20803–20809. [PubMed]
- Zimmer, D. P., Soupene, E., Lee, H. L., Wendisch, V. F., Khodursky, A. B., Peter, B. J., Bender, R. A. & Kustu, S. (2000). Proc. Natl Acad. Sci. USA, 97, 14674–14679. [DOI] [PMC free article] [PubMed]