Chandipura virus glycoprotein ectodomain (Gth) was purified and crystallized at pH 7.5. X-ray diffraction data set was collected to a resolution of 3.1 Å.
Keywords: viral fusion proteins, glycoproteins, intermediates, rhabdoviruses
Abstract
Fusion in members of the Rhabdoviridae virus family is mediated by the G glycoprotein. At low pH, the G glycoprotein catalyzes fusion between viral and endosomal membranes by undergoing a major conformational change from a pre-fusion trimer to a post-fusion trimer. The structure of the G glycoprotein from vesicular stomatitis virus (VSV G), the prototype of Vesiculovirus, has recently been solved in its trimeric pre-fusion and post-fusion conformations; however, little is known about the structural details of the transition. In this work, a soluble form of the ectodomain of Chandipura virus G glycoprotein (CHAV Gth) was purified using limited proteolysis of purified virus; this soluble ectodomain was also crystallized. This protein shares 41% amino-acid identity with VSV G and thus its structure could provide further clues about the structural transition of rhabdoviral glycoproteins induced by low pH. Crystals of CHAV Gth obtained at pH 7.5 diffracted X-rays to 3.1 Å resolution. These crystals belonged to the orthorhombic space group P21212, with unit-cell parameters a = 150.3, b = 228.2, c = 78.8 Å. Preliminary analysis of the data based on the space group and the self-rotation function indicated that there was no trimeric association of the protomers. This unusual oligomeric status could result from the presence of fusion intermediates in the crystal.
1. Introduction
The entry of enveloped viruses into host cells requires the binding of viral particles to cellular receptors followed by fusion of the viral envelope with the cellular membrane (Smith & Helenius, 2004 ▶). In rhabdoviruses, as in many other enveloped viruses, this process is mediated by glycoproteins embedded on the virion surface (Albertini, Baquero et al., 2012 ▶; Roche et al., 2008 ▶).
The complete mature G glycoprotein (G) is about 500 amino acids in length and is composed of an ectodomain, which forms the bulk mass of G located outside the viral membrane, a transmembrane domain which anchors the protein to the viral surface and a cytoplasmic tail. Similar to many other viral fusion proteins, G undergoes a structural rearrangement from a pre-fusion to a post-fusion conformation that is associated with a decrease in the internal pH of the endosome. This transition is reversible and there is a pH-dependent equilibrium between the different conformations of G (Roche & Gaudin, 2002 ▶; Albertini, Merigoux et al., 2012 ▶). The crystal structures of pre-fusion and post-fusion trimers of the vesicular stomatitis virus glycoprotein (VSV G; Vesiculovirus genus) revealed that during this transition the fusion domain and the C-terminal segment flip relative to a rigid block (Roche et al., 2006 ▶, 2007 ▶). Our group has recently demonstrated using several biophysical techniques that the rearrangement of VSV G proceeds through monomerization and that the threefold symmetry is recovered in the post-fusion form (Albertini, Merigoux et al., 2012 ▶). Intermediate conformations of VSV G were also visualized by electron microscopy of the surface of viral particles incubated at pH 6.7 (Albertini, Merigoux et al., 2012 ▶); however, the structural details of this process remain elusive owing to difficulty in trapping these transient fusion intermediates.
Site-directed mutagenesis has demonstrated the importance of many residues in the stabilization of secondary structure and, in the case of influenza virus haemagglutinin (HA), a class I viral glycoprotein, has allowed the characterization of the crystal structure of an early fusion intermediate (Xu & Wilson, 2011 ▶). Intermediate conformations of HA and paramyxovirus PIV5 F have also been detected using electron microscopy (Kim et al., 2011 ▶).
Comparison of crystal structures of other rhabdoviral glycoproteins that are expected to have a similar structure to VSV G may provide clues about the structural transition of G and it is possible that some of their physicochemical properties could lead to the production of fusion intermediates that are sufficiently stable to be trapped in a crystalline form. Chandipura virus (CHAV; Vesiculovirus genus) is associated with deadly encephalopathy outbreaks, mainly in India (Basak et al., 2007 ▶; Rao et al., 2004 ▶). To date, no structural information is available on CHAV glycoprotein; since it shares 41% amino-acid identity with VSV G, it is likely that its structure resembles the pre-fusion and post-fusion conformations of VSV G, thus constituting a good model for studying the relationship between structure and fusogenic activity.
In this study, we took advantage of a recombinant VSV in which the gene encoding G had been replaced by that encoding CHAV glycoprotein (Rose et al., 2000 ▶). This recombinant virus grows quite well and we found conditions that allowed cleavage of the CHAV G ectodomain by thermolysin at the surface of the viral particles. We were able to crystallize this thermolysin-generated G ectodomain (CHAV Gth) at pH 7.5. Analysis of the data set from CHAV Gth crystals suggested an oligomerization status that differed from those of the previously reported pre-fusion and post-fusion trimers of VSV Gth (Roche et al., 2006 ▶, 2007 ▶).
2. Experimental
2.1. Protein production and purification
A VSV recombinant expressing Chandipura virus G glycoprotein (CHAV G; GenBank AAA42916) instead of VSV G was recovered using the plasmid pVSV(GCh)XN-1 described in Rose et al. (2000 ▶). BSR cells (a clone of BHK cells) were infected with this recombinant at a multiplicity of infection of 0.3. Highly concentrated viral stocks were purified from 1 d post-infected BSR cultures as described by Libersou et al. (2010 ▶) with slight modifications (Fig. 1 ▶). The preparation of CHAV Gth was adapted from the protocol used for VSV Gth (Albertini, Merigoux et al., 2012 ▶). The purified viral particles were incubated with phosphate–citrate buffer pH 6.0 to induce the structural transition of G towards the post-fusion conformation at the surface of the virus. At this pH, the C-terminal segment of G is accessible to the enzymatic activity of proteases; thermolysin makes a single cut between Ser419 and Ile420 (as determined by mass spectrometry), allowing the soluble G ectodomain (Gth; residues 1–419) to be obtained. Digestion of G with thermolysin was carried out at 310 K for 1.5 h using a 1:0.8(w:w) ratio of virus:protease. The reaction was stopped using a protease-inhibitor cocktail (Complete, Roche) as recommended by the manufacturer.
Figure 1.
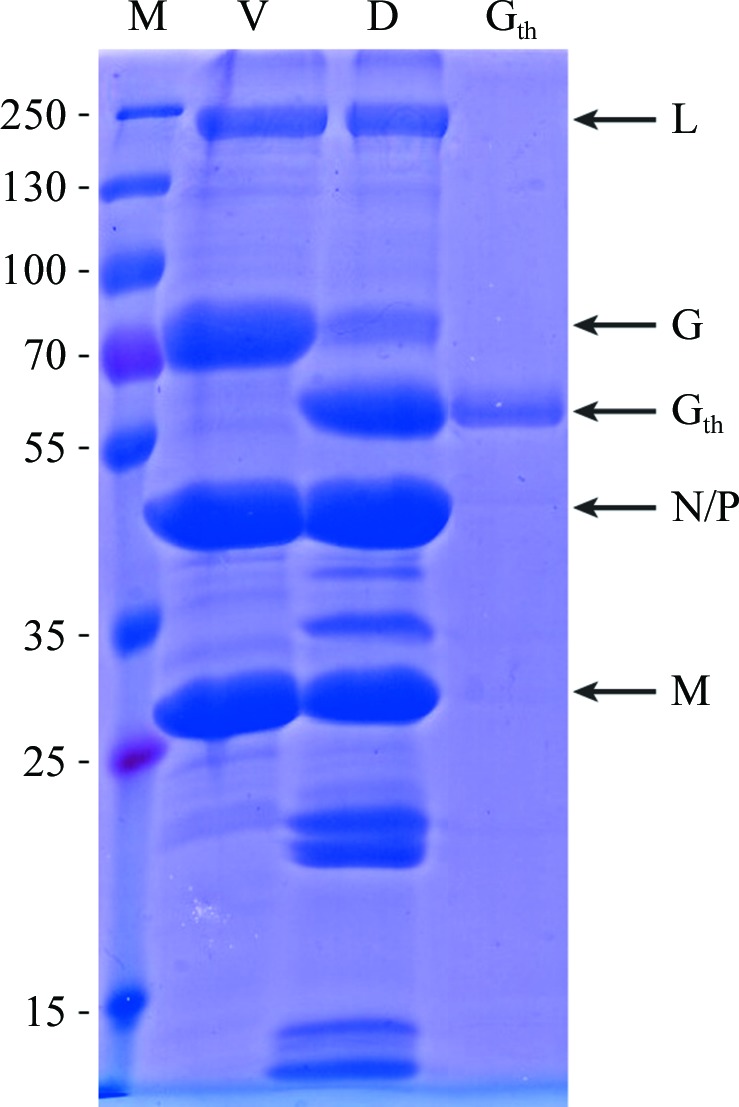
SDS–PAGE analysis of CHAV Gth purification. Lane M, molecular-mass marker (labelled in kDa); lane V, recombinant VSV encoding CHAV G; lane D, virus digested with thermolysin; lane Gth, CHAV Gth after purification. Viral proteins are also indicated (L, large protein, G, glycoprotein; N, nucleoprotein; P, phosphoprotein; M, matrix protein).
Gth was solubilized in a buffer consisting of 50 mM HEPES pH 7.5 and 2% glycerol and this solution was clarified by centrifugation at 125 000g (SW 32 Ti rotor; Beckman Coulter) on a 20% sucrose cushion.
Gth was then purified using two consecutive chromatographic procedures. Firstly, the protein was loaded onto a DEAE Trisacryl column (Sigma) equilibrated with washing buffer (20 mM Tris–HCl pH 8.8, 0.5 mM PMSF, 1 mM EDTA) and eluted with an increasing concentration of NaCl. Fractions containing Gth were pooled and injected onto a Superdex 200 gel-filtration column (GE Healthcare) equilibrated with washing buffer. The eluted pure Gth fractions were pooled and concentrated to 8 mg ml−1 by ultrafiltration (Amicon Ultracel, 30 kDa molecular-weight cutoff, Millipore). The concentrated protein was kept at 193 K until use (Fig. 1 ▶).
2.2. Crystallization
Purified Gth at 8 mg ml−1 was screened for initial crystallization conditions using commercially available kits (The Classics, PEGS, PEGS II and MBClass Suites from Qiagen). Experiments were set up with a Cartesian robot in which 576 conditions were screened by the sitting-drop vapour-diffusion method. Drops were set up by mixing 100 nl protein solution with 100 nl reservoir solution and were equilibrated against 100 µl reservoir solution. In this initial screening only two conditions, which contained polyethylene glycol (PEG) and had a pH of above 8.0, yielded needle-like crystals after one week of incubation. These conditions were optimized by hand using the hanging-drop vapour-diffusion method, varying the buffer, pH and the salt, precipitant and protein concentrations. All crystallization experiments were performed at 293 K. Good-quality crystals were obtained by mixing 1 µl Gth solution (4 mg ml−1) with 1 µl reservoir solution (14% PEG 3350, 0.3 M Na2SO4, 0.1 M HEPES pH 7.6; final pH 7.5) and equilibrating against 500 µl reservoir solution. Using these conditions, cube-shaped crystals appeared after two weeks and continued to grow for up to four weeks to reach final dimensions of between 60 and 100 µm (Fig. 2 ▶).
Figure 2.
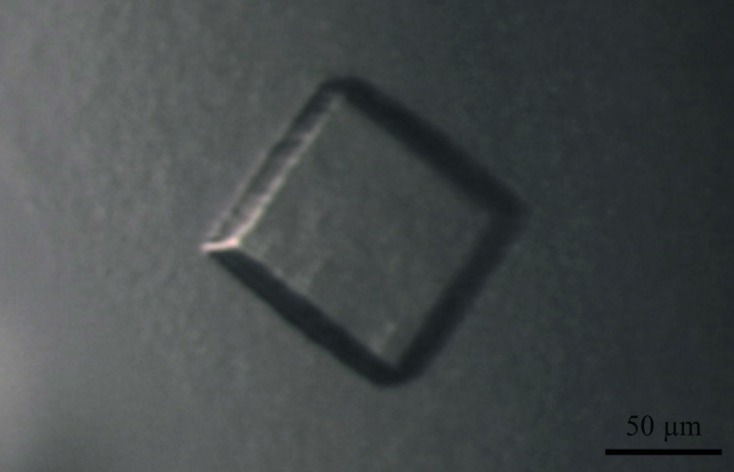
Crystal of Gth obtained using 14% PEG 3350, 0.3 M Na2SO4, 0.1 M HEPES pH 7.6.
2.3. X-ray data collection and analysis
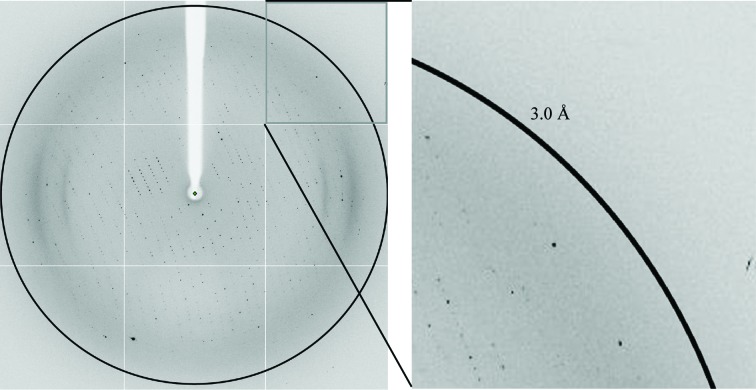
Crystals were flash-cooled in liquid N2 after a short soak in a cryoprotectant solution consisting of reservoir solution supplemented with 30% glycerol. The crystals diffracted to 3.1 Å resolution (Fig. 3 ▶). Diffraction data were collected on the PROXIMA 1 and ID14-4 beamlines at the SOLEIL and ESRF synchrotrons in France equipped with PILATUS and ADSC Quantum Q315r detectors, respectively. All data were collected at 100 K. Diffraction data sets were processed with iMOSFLM (Battye et al., 2011 ▶) or XDS (Kabsch, 2010 ▶). The self-rotation function was calculated using MOLREP (Vagin & Teplyakov, 2010 ▶). In order to detect noncrystallographic twofold and threefold symmetry axes, the κ angle was set to 180° and 120°, respectively. The calculations were performed with an integration radius of 40 Å in the resolution range 3.3–49 Å (Fig. 4 ▶).
Figure 3.
X-ray diffraction image from Gth crystals obtained at intermediate pH. The black circle indicates the diffraction limit at 3.0 Å resolution.
Figure 4.
Self-rotation functions of a Gth data set obtained from crystals grown at pH 7.5: (a) χ = 180.0°, (b) χ = 120.0°.
3. Results and discussion
The production of recombinant glycoproteins is often difficult and often affords poor yields of protein when conventional recombinant systems are employed. In this study, the use of a recombinant VSV/mammalian cell system to express CHAV G allowed the efficient production of G for crystallization experiments. The advantage of this method is that viral glycoproteins can be expressed and processed by higher eukaryote post-translational systems. VSV is an ideal vector for expression since it has a short multiplication cycle and can be produced in high amounts. To date, several surface proteins have been recombinantly expressed in VSV vectors, including various vesiculovirus glycoproteins, HIV glycoprotein (Rose et al., 2000 ▶), Ebola virus glycoprotein (Takada et al., 1997 ▶) and even proteins involved in cell–cell fusion (Avinoam et al., 2011 ▶). Additionally, the purification process from recombinant VSV particles allows the easy removal of impurities from cell cultures. Employing a recombinant VSV could be considered in order to produce other (rhabdo)viral glycoproteins for structural studies.
Initial crystallization screening of purified Gth resulted in only two hits, both with a pH above 8. Optimization of these conditions allowed the growth of crystals in the orthorhombic space group P21212 (Table 1 ▶) at a pH known to favour intermediate states of VSV Gth (Albertini, Merigoux et al., 2012 ▶). The unit-cell parameters and space group indicate that each molecule of CHAV Gth occupies about 8% (or ∼10% accounting for carbohydrates) of the volume of the asymmetric unit. This is consistent with the presence of 3–6 molecules per asymmetric unit, with a solvent content in the range of 40–70% and a Matthews coefficient of 2.05–4.09 Å3 Da−1 (Matthews, 1968 ▶). The self-rotation function showed no trace of a threefold noncrystallographic symmetry axis, but revealed the presence of extra twofold noncrystallographic symmetry within the asymmetric unit (Fig. 2 ▶). Thus, the association of Gth in the crystal seems to differ from the typical trimeric conformations observed in the previously determined pre-fusion and post-fusion crystalline forms of VSV G (Roche et al., 2006 ▶, 2007 ▶).
Table 1. Crystal parameters and data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Beamline | ID14-4, ESRF |
| Wavelength (Å) | 0.976 |
| Detector | ADSC Q315r |
| Space group | P21212 [No. 18] |
| Unit-cell parameters (Å) | a = 150.3, b = 228.2, c = 78.8 |
| Resolution range (Å) | 50–3.1 (3.2–3.1) |
| No. of observations | 332651 (30800) |
| Unique reflections | 50106 (4504) |
| Completeness (%) | 99.8 (100) |
| 〈I/σ(I)〉 | 18.27 (2.1) |
| R merge † (%) | 11.2 (79.5) |
R
merge = 
 , where Ii(hkl) is the intensity of reflection hkl,
, where Ii(hkl) is the intensity of reflection hkl,  is the sum over all reflections,
is the sum over all reflections,  is the sum over i measurements of reflection hkl and 〈I(hkl)〉 is the weighted average intensity of all observations i of reflection hkl.
is the sum over i measurements of reflection hkl and 〈I(hkl)〉 is the weighted average intensity of all observations i of reflection hkl.
In class II viral fusion glycoproteins a pH-induced conformational change drives moleculur assembly from a metastable dimer to a stable post-fusion trimer. In both class I and III viral fusion glycoproteins the transition is from a pre-fusion trimer to a more stable post-fusion trimer (Bullough et al., 1994 ▶; Roche et al., 2008 ▶). The protomers in the pre-fusion and post-fusion trimers are attached to the membrane by a linker segment that connects the ectodomains to the transmembrane domains. Both are located outside the volume formed by the trimer. The topological issue in the structural transition resides in the fact that the protomers must change their orientation in order to expose their fusion loops towards the target membrane and undergo refolding and relative movement of domains to acquire a more extended conformation. Since it is difficult for a trimer to undergo these changes as a unique entity, the most plausible solution would be that this transition occurs in monomers and the trimeric symmetry is finally recovered in the post-fusion form (Albertini, Baquero et al., 2012 ▶; Albertini, Merigoux et al., 2012 ▶). Preliminary molecular replacement with the three individual domains confirms the presence of four copies of Gth with no threefold symmetry and at least two distinct conformations in the asymmetric unit of the pH 7.5 crystals (not shown). This should provide molecular details of the refolding of rhabdoviral glycoproteins.
The crystal structure of CHAV Gth at pH 7.5 and its comparison with the previously determined structures of VSV G pre-fusion and post-fusion trimers should enable us to obtain a more complete view of the structural transition that occurs in viral fusion proteins during the fusion process.
Acknowledgments
This work was supported by the EU through the Marie Curie ITN VIRUS ENTRY (project reference 235649) to YG (with predoctoral funding to EB) and a grant from ANR (ANR-08-BLAN-0256) to YG and SB (with postdoctoral funding to AAA). We acknowledge the European Synchrotron Radiation Facility (Grenoble, France) and synchrotron SOLEIL (Saint-Aubin, France) for the provision of radiation facilities. We acknowledge the Structural Biology and Proteomics Pole of the IMAGIF integrated platform (https://www.imagif.cnrs.fr/?nlang=en) for access to crystallization and mass-spectrometric (Manuela Argentini and David Cornu) services.
References
- Albertini, A. A., Baquero, E., Ferlin, A. & Gaudin, Y. (2012). Viruses, 4, 117–139. [DOI] [PMC free article] [PubMed]
- Albertini, A. A., Mérigoux, C., Libersou, S., Madiona, K., Bressanelli, S., Roche, S., Lepault, J., Melki, R., Vachette, P. & Gaudin, Y. (2012). PLoS Pathog. 8, e1002556. [DOI] [PMC free article] [PubMed]
- Avinoam, O., Fridman, K., Valansi, C., Abutbul, I., Zeev-Ben-Mordehai, T., Maurer, U. E., Sapir, A., Danino, D., Grünewald, K., White, J. M. & Podbilewicz, B. (2011). Science, 332, 589–592. [DOI] [PMC free article] [PubMed]
- Basak, S., Mondal, A., Polley, S., Mukhopadhyay, S. & Chattopadhyay, D. (2007). Biosci. Rep. 27, 275–298. [DOI] [PMC free article] [PubMed]
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Bullough, P. A., Hughson, F. M., Skehel, J. J. & Wiley, D. C. (1994). Nature (London), 371, 37–43. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 133–144. [DOI] [PMC free article] [PubMed]
- Kim, Y. H., Donald, J. E., Grigoryan, G., Leser, G. P., Fadeev, A. Y., Lamb, R. A. & DeGrado, W. F. (2011). Proc. Natl Acad. Sci. USA, 108, 20992–20997. [DOI] [PMC free article] [PubMed]
- Libersou, S., Albertini, A. A., Ouldali, M., Maury, V., Maheu, C., Raux, H., de Haas, F., Roche, S., Gaudin, Y. & Lepault, J. (2010). J. Cell Biol. 191, 199–210. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Rao, B. L., Basu, A., Wairagkar, N. S., Gore, M. M., Arankalle, V. A., Thakare, J. P., Jadi, R. S., Rao, K. A. & Mishra, A. C. (2004). Lancet, 364, 869–874. [DOI] [PMC free article] [PubMed]
- Roche, S., Albertini, A. A., Lepault, J., Bressanelli, S. & Gaudin, Y. (2008). Cell. Mol. Life Sci. 65, 1716–1728. [DOI] [PMC free article] [PubMed]
- Roche, S., Bressanelli, S., Rey, F. A. & Gaudin, Y. (2006). Science, 313, 187–191. [DOI] [PubMed]
- Roche, S. & Gaudin, Y. (2002). Virology, 297, 128–135. [DOI] [PubMed]
- Roche, S., Rey, F. A., Gaudin, Y. & Bressanelli, S. (2007). Science, 315, 843–848. [DOI] [PubMed]
- Rose, N. F., Roberts, A., Buonocore, L. & Rose, J. K. (2000). J. Virol. 74, 10903–10910. [DOI] [PMC free article] [PubMed]
- Smith, A. E. & Helenius, A. (2004). Science, 304, 237–242. [DOI] [PubMed]
- Takada, A., Robison, C., Goto, H., Sanchez, A., Murti, K. G., Whitt, M. A. & Kawaoka, Y. (1997). Proc. Natl Acad. Sci. USA, 94, 14764–14769. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Xu, R. & Wilson, I. A. (2011). J. Virol. 85, 5172–5182. [DOI] [PMC free article] [PubMed]