The bacterial selenocysteine synthase SelA from Aquifex aeolicus was crystallized and the diffraction resolution was improved by lysine-residue methylation, truncation of N-terminal region (ΔN), and Lys-to-Ala point mutations. Phases were determined by using a selenomethionine-substituted crystal of the ΔN mutant.
Keywords: SelA, Aquifex aeolicus, selenocysteine synthase
Abstract
Selenocysteine (Sec), the 21st amino acid, is synthesized on its specific tRNA (tRNASec) via a multi-step process. In bacteria, tRNASec is ligated first with serine by seryl-tRNA synthetase, which is followed by Ser-to-Sec conversion by Sec synthase (SelA). To elucidate its structure and catalytic mechanism, Aquifex aeolicus SelA was crystallized. Although wild-type SelA crystals diffracted X-rays poorly (to up to 8 Å resolution), the resolution was improved by introducing a quadruple point mutation targeting the loop regions and by methylating the lysine residues, which yielded 3.9 Å resolution diffraction data from a full-length SelA crystal. Truncation of the N-terminal region (ΔN) also improved the resolution. A 3.3 Å resolution data set for phase determination was obtained from a crystal of selenomethionine-substituted Lys-methylated SelA-ΔN.
1. Introduction
Selenocysteine (Sec) is a selenium-containing amino acid known as the 21st amino acid and is translationally incorporated into proteins (Böck et al., 1991 ▶). Sec is widely distributed in all three domains of life: Bacteria, Archaea and Eukarya. In contrast to the canonical amino acids, Sec is synthesized on Sec-specific tRNA (tRNASec) via a multi-step process. tRNASec is ligated first with serine by seryl-tRNA synthetase (SerRS) to form seryl-tRNASec (Ser-tRNASec; Leinfelder et al., 1988 ▶). In Bacteria, the hydroxyl group of the seryl moiety is replaced with a selenol group by Sec synthase (SelA or SecS) to form selenocysteinyl-tRNASec (Sec-tRNASec; Forchhammer & Böck, 1991 ▶). In contrast, the eukaryotic/archaeal Sec synthesis characteristically employs an intermediate, O-phosphoseryl-tRNASec (Sep-tRNASec). The hydroxyl group of Ser-tRNASec is phosphorylated by O-phosphoseryl-tRNA kinase (PSTK) to yield Sep-tRNASec (Carlson et al., 2004 ▶), which is then converted to Sec-tRNASec by the homotetrameric enzyme Sep-tRNA:Sec-tRNA synthase (SepSecS; Yuan et al., 2006 ▶; Xu et al., 2007 ▶). SelA and SepSecS are both members of the fold-type-I superfamily of pyridoxal phosphate (PLP) dependent enzymes (Tormay et al., 1998 ▶; Ganichkin et al., 2008 ▶; Araiso et al., 2008 ▶), but their sequence similarity is limited to the PLP-binding motif. The selenophosphate generated by selenophosphate synthetase is utilized as the reactive selenium donor for Sec synthesis in both Bacteria and Eukarya/Archaea (Ehrenreich et al., 1992 ▶; Low et al., 1995 ▶).
A previous study of SelA, including electron-microscopic analyses, revealed that SelA exists as an ∼500 kDa homodecamer (Engelhardt et al., 1992 ▶). However, because of the low resolution, the catalytic mechanism and substrate specificity of SelA, as well as the biological significance of the homodecameric quaternary structure, have not been clarified. In this communication, we report the crystallization of SelA and a strategy for improving the diffraction resolution.
2. Materials and methods
2.1. Overexpression and purification of SelA
The Aquifex aeolicus SelA gene (NCBI Gene ID 1193774; locus tag aq_1031) was cloned into the NdeI and SalI restriction sites of the vector pET25b (Novagen). The mutant SelA gene corresponding to SelA-ΔN (amino-acid residues 62–452) was cloned into the NdeI and XhoI restriction sites of the vector pET22b (Novagen). The wild-type SelA encoded in the vector does not have any artificial sequences at its N- or C-termini, while SelA-ΔN only has an artificially introduced initiating Met residue followed by residues 62–452. Since the cloned genes retained their natural stop codons, the C-terminal His tags that were encoded in the vectors were not expressed.
The point mutations were introduced using the QuikChange site-directed mutagenesis kit (Stratagene). Escherichia coli strain Rosetta 2 (DE3) (Stratagene) was transformed with the expression plasmids and the proteins were overexpressed in LB medium at 310 K. Selenomethionine-substituted SelA-ΔN was overexpressed in E. coli Rosetta 2 (DE3) transformed by the same vector as that encoding the native protein. The cells were grown in M9 minimal medium at 310 K to an OD600 of 0.4, and 60 mg l−1 l-selenomethionine, 100 mg l−1 each of l-threonine, l-lysine and l-phenylalanine and 50 mg l−1 each of l-leucine, l-isoleucine and l-valine were then added to the medium. After a 15 min pre-incubation, gene expression was induced by adding 0.5 mM isopropyl β-d-1-thiogalactopyranoside. The cells were further cultured at 310 K overnight prior to harvesting.
The harvested cells expressing wild-type SelA were resuspended in 20 mM Tris–HCl buffer pH 8.1 containing 500 mM NaCl, 500 mM MgCl2 and 10 mM β-mercaptoethanol (β-ME) and disrupted using an ultrasonic homogenizer. The supernatant of the lysate was incubated at 358 K for 30 min to denature and remove most of the E. coli proteins. After centrifugation, ammonium sulfate was added to the supernatant (to a final concentration of 2 M), which was then applied onto a Toyopearl Phenyl-650 column (Tosoh). The column was washed with Tris–HCl buffer pH 7.5 containing 2.0 M ammonium sulfate and 10 mM β-ME and the protein was eluted with 20 mM Tris–HCl buffer pH 7.5 containing 10 mM β-ME. The protein was subsequently loaded onto an SP-Sepharose Fast Flow column (GE Healthcare) and eluted with a linear gradient of 0–1.2 M NaCl. It was dialyzed against 20 mM Tris–HCl buffer pH 7.5 containing 200 mM NaCl and 10 mM β-ME and concentrated to 5.2 mg ml−1. The mutant SelAs were purified in a similar manner to the wild type.
2.2. Gel-filtration analysis
The total molecular mass of SelA was analyzed by gel-filtration analysis. Wild-type SelA (2 mg) was loaded onto a HiLoad 26/60 Superdex 200 gel-filtration column (GE Healthcare) and chromatographed using Tris–HCl buffer pH 7.5 containing 300 mM NaCl and 10 mM β-ME. An elution peak was detected by monitoring the absorbance at 280 nm (protein) and 412 nm (protein-bound PLP). The column was calibrated using a standard protein marker kit (Sigma) and the molecular mass of SelA was calculated from its elution volume.
2.3. Lysine methylation
Lysine-residue methylation of the wild-type and mutant SelAs was performed using formaldehyde and dimethylamine–borane complex as described by Walter et al. (2006 ▶). Prior to methylation, the proteins were diluted with 20 mM HEPES–NaOH buffer pH 7.5 containing 0.4–0.8 M NaCl and 10 mM β-ME. The methylation reagents were removed by repeated dialyses against 20 mM Tris–HCl buffer pH 7.5 containing 0.2–0.3 M NaCl and 10 mM β-ME.
2.4. Crystallization and diffraction data collection
Crystallization was performed at 293 K by the sitting-drop vapour-diffusion method by mixing 0.75 µl protein solution with 0.75 µl reservoir solution. Seven screening kits, Crystal Screen, Crystal Screen 2, Natrix, SaltRx, Index (Hampton Research) and Wizard I and II (Emerald BioSystems), were used for initial screening of the crystallization conditions. These conditions were refined by optimizing the reservoir-solution composition, including the precipitant concentration, the buffer pH and the use of additives.
Prior to the X-ray diffraction experiment, crystals were transferred into a cryoprotective solution and flash-cooled in a cryocooled N2 stream (90 K). Diffraction data were collected on BL41XU at SPring-8, Hyogo, Japan and on BL5A, BL17A and NW12A at the Photon Factory, Tsukuba, Japan. The data were processed with HKL-2000 (Otwinowski & Minor, 1997 ▶) and the solvent content was estimated using MATTHEWS_COEF (Winn et al., 2011 ▶).
2.5. Soaking of the heavy-atom reagent
The crystals obtained were washed with reservoir solution and transferred into a solution of the heavy-atom reagent in reservoir solution. After a 10–15 h incubation, the crystals were transferred into a cryoprotective solution and flash-cooled. The X-ray absorption fine structure (XAFS) was measured to monitor the heavy-atom labelling and to estimate the peak and edge wavelengths of the anomalous scattering.
3. Results
3.1. Crystallization and diffraction analysis of wild-type SelA
We performed a gel-filtration analysis to determine whether the recombinant A. aeolicus SelA is indeed a homodecameric protein. The elution peak monitored by the absorbance of aromatic protein residues (280 nm) and protein-bound PLP (412 nm) indicated a molecular mass as large as 517 kDa (Fig. 1 ▶), which was in agreement with the theoretical value (10 × 50.8 kDa).
Figure 1.
Gel-filtration analysis of wild-type SelA. The elution volume was measured by monitoring the absorbance at 280 nm (protein) and 412 nm (protein-bound PLP). The calculated molecular mass was 517 kDa, which is close to the theoretical value (508 kDa) for the SelA decamer.
Initial crystallization screening of wild-type SelA performed by mixing 5.2 mg ml−1 protein solution and the reservoir solutions identified many crystallization conditions. Seven screening kits, Crystal Screen, Crystal Screen 2, Natrix, SaltRX, Index and Wizard I and II, were used and crystals were obtained under 72 conditions within two months. The main precipitants in each condition were polyethylene glycol (PEG), 2-methyl-2-propanol, (±)-2-methyl-2,4-pentanediol (MPD), Jeffamine M-600, ammonium sulfate, NaNO3, NaCl, trisodium citrate, sodium formate, Na2HPO4/KH2PO4, sodium malonate, KSCN, (NH4)2HPO4, diammonium tartrate and potassium sodium tartrate. However, most of them yielded needle-shaped or thin plate-shaped crystals. Three-dimensional crystals were only obtained using crystallization reagents that contained MPD as the precipitant, such as Wizard II condition No. 2 (100 mM MES–NaOH buffer pH 6.0 containing 35% MPD and 200 mM Li2SO4). The optimized reservoir solution (100 mM Na MES–HCl buffer pH 6.5 containing 35% MPD and 200 mM Li2SO4) generated hexagonal column crystals (Fig. 2 ▶ a). The crystals only diffracted to 8 Å resolution using snapshot X-ray diffraction experiments. The unit-cell parameters were a = 150, b = 165, c = 295 Å (primitive orthorhombic).
Figure 2.
Crystals of wild-type SelA and mutant SelAs. Typical crystals of wild-type SelA (a), Lys-methylated wild-type SelA (b), SelA-ΔN (c), SeMet-substituted SelA-ΔN with Lys methylation (d) and Lys-methylated SelA4KA (e) are shown. The yellow colour of the crystals arises from the PLP cofactor. The scale bars indicate 200 µm.
3.2. Effect of Lys methylation
In order to improve the diffraction resolution, methylation of the lysine residues of SelA was examined. Lys-methylated wild-type SelA (14 mg ml−1) required initial screening of crystallization conditions. Four screening kits, Crystal Screen 2, Natrix and Wizard I and II, were used and crystals were obtained under 12 conditions within two weeks. The main precipitant reagent in each condition was PEG 2000–8000. The initially obtained crystals were all needle-shaped or thin rod-shaped and the best reservoir solution was Wizard II condition No. 43 [100 mM Tris–HCl buffer pH 7.0 containing 10%(w/v) PEG 8000 and 200 mM MgCl2]. During reservoir-solution optimization, we noticed that octahedral crystals were obtained at a high NiCl2 concentration. Finally, the following reservoir solution was employed: 100 mM Tris–HCl buffer pH 7.5 containing 10–12%(w/v) PEG 4000, 200 mM MgCl2, 60–120 mM NiCl2, 50 mM Na2S2O3 and 50 mM l-serine (Fig. 2 ▶ b). The crystals diffracted to 6 Å resolution using an optimized cryoprotective solution (50 mM Tris–HCl buffer pH 7.5 containing 25% ethylene glycol, 12% PEG 1000, 12% PEG 8000 and 20 mM MgCl2). The unit-cell parameters were a = b = 172, c = 250 Å (primitive tetragonal). Diffraction improvement (from 8 to 6 Å resolution) and alteration of the crystal system were observed upon Lys methylation. However, the diffraction resolution was not sufficient to determine the crystal structure.
3.3. Improvement by the truncation of the N-terminal region
In addition to the core and C-terminal regions that are conserved among PLP-dependent enzymes, SelA possesses a unique N-terminal region. To improve the diffraction resolution, the truncated mutant SelA-ΔN lacking the N-terminal region (residues 1–61) was examined. Iinitial crystallization screening of SelA-ΔN (2.4 mg ml−1) was also performed. Six screening kits, Crystal Screen, Crystal Screen 2, Natrix, SaltRX and Wizard I and II, were used and crystals were obtained under 47 conditions within two months. The main precipitants in the conditions were PEG, ethanol, 2-propanol, 2-methyl-2-propanol, 1,6-hexanediol, 1,4-butanediol, MPD, Jeffamine M-600, ammonium sulfate, Li2SO4, NaCl, trisodium citrate, sodium formate, sodium acetate, ammonium acetate, Na2HPO4/KH2PO4, (NH4)2HPO4 and potassium sodium tartrate. Like the wild-type SelA crystals, most of the mutant protein crystals were needle-shaped or thin plate-shaped. The best reservoir solution was Crystal Screen 2 condition No. 36 (Na HEPES–HCl buffer pH 7.5 containing 4.3 M NaCl) and rod-shaped crystals were obtained by using a further concentrated protein solution (20 mg ml−1) and the optimized reservoir solution 100 mM Na MES–HCl buffer pH 6.5 containing 2.5 M NaCl (Fig. 2 ▶ c). The crystals diffracted to 4.5 Å resolution using the cryoprotective solution 100 mM Na MES–HCl buffer pH 6.5 containing 4.0 M LiCl, 1.0 M NaCl, 100 mM l-serine and 200 mM Na2S2O3. The crystals belonged to space group P21, with unit-cell parameters a = 93, b = 347, c = 141 Å, β = 108°.
Lys methylation of SelA-ΔN was also performed. Two screening kits, Crystal Screen 2 and Natrix, were used for initial crystallization screening of Lys-methylated SelA-ΔN (6.9 mg ml−1) and crystals were obtained under eight conditions within two weeks. The main precipitant in each condition was PEG 4000–20 000 or ammonium sulfate. Most conditions yielded three-dimensional crystals, although they were smaller than 30 µm in size. The best reservoir solution was Natrix condition No. 44 [50 mM Tris–HCl buffer pH 7.5 containing 10%(w/v) PEG 4000, 200 mM KCl and 50 mM MgCl2], which was optimized to the following reservoir: 100 mM Tris–HCl buffer pH 7.5 containing 12% PEG 4000, 800 mM NaNO3, 160 mM KCl, 50 mM l-serine and 50 mM Na2S2O3. The crystals diffracted to 3.25 Å resolution using a cryoprotective solution (100 mM Tris–HCl buffer pH 7.5 containing 35% PEG 4000, 800 mM NaBr and 160 mM KCl; Table 1 ▶). The unit-cell parameters were a = 92, b = 116, c = 125 Å, α = 102, β = 93, γ = 106° (P1). However, this cryoprotective solution was not sufficiently optimized, as most of the crystals only diffracted to ∼4.0 Å resolution.
Table 1. Data-collection and phasing statistics.
Values in parentheses are for the highest resolution shell.
| SelA-ΔN, SeMet, Lys methylation | |||||
|---|---|---|---|---|---|
| SelA-ΔN, Lys methylation | Peak | Edge | SelA-ΔN, SeMet, Lys methylation, K2PtCl4 derivative | SelA4KA, Lys methylation | |
| Data collection | |||||
| Beamline | BL5A, Photon Factory | BL41XU, SPring-8 | BL17A, Photon Factory | BL41XU, SPring-8 | |
| Wavelength (Å) | 1.00000 | 0.97917 | 0.97947 | 1.07117 | 1.00000 |
| Space group | P1 | P21 | P21 | P41212 or P43212 | |
| Unit-cell parameters (Å, °) | a = 92.4, b = 116.4, c = 124.8, α = 102.1, β = 93.4, γ = 106.1 | a = 92.9, b = 280.9, c = 98.1, β = 113.5 | a = 92.8, b = 280.9, c = 97.6, β = 113.4 | a = b = 167.0, c = 211.1 | |
| Resolution (Å) | 50.0–3.25 (3.37–3.25) | 50.0–3.30 (3.42–3.30) | 50.0–3.40 (3.52–3.40) | 50.0–3.20 (3.31–3.20) | 50.0–3.90 (4.04–3.90) |
| Unique reflections | 75475 (7499) | 69327 (6903) | 63781 (6362) | 75145 (7212) | 26396 (2358) |
| Completeness (%) | 99.3 (98.6) | 99.8 (99.9) | 99.9 (99.8) | 99.5 (96.0) | 94.1 (86.8) |
| Multiplicity | 3.9 (3.8) | 7.5 (7.0) | 7.5 (7.0) | 7.4 (6.2) | 9.1 (8.1) |
| R merge † | 0.129 (0.538) | 0.114 (0.859) | 0.107 (0.891) | 0.090 (0.674) | 0.103 (0.736) |
| 〈I/σ(I)〉 | 12.7 (2.05) | 18.1 (2.47) | 19.2 (2.17) | 22.4 (2.50) | 15.3 (2.02) |
| Phasing | |||||
| No. of identified Se sites | 60 | ||||
| Phasing power | |||||
| Isomorphous (acentric/centric) | — | 0.254/0.213 | |||
| Anomalous | 0.729 | 0.364 | |||
| FOM (before DM/after DM)‡ | 0.250/0.598 | ||||
R
merge = 
 , where Ii(hkl) is the intensity of the ith measurement of hkl and 〈I(hkl)〉 is the average value of Ii(hkl) for all ith measurements.
, where Ii(hkl) is the intensity of the ith measurement of hkl and 〈I(hkl)〉 is the average value of Ii(hkl) for all ith measurements.
FOM, figure of merit; DM, density modification (solvent flattening and NCS averaging).
To obtain phase information, SeMet-substituted SelA-ΔN with Lys methylation (6.0 mg ml−1) was first crystallized in the same reservoir solution as used for the native protein. Further optimization yielded the following reservoir solution: 100 mM Tris–HCl buffer pH 7.5 containing 11% PEG 4000, 400 mM CaCl2, 160 mM KCl, 5.0 mM NiCl2, 50 mM Na2S2O3 and 50 mM l-serine (Fig. 2 ▶ d). Optimization of the cryoprotective solution was also required. Finally, the following cryoprotective solution was employed: 50 mM Tris–HCl buffer pH 7.5 containing 38% PEG 4000, 100 mM NaNO3 and 20 mM MgCl2, which also contributed to the improvement of the resolution. A multiwavelength anomalous dispersion (MAD) data set for phasing was collected to a resolution of 3.3 Å (Fig. 3 ▶ a and Table 1 ▶). On the other hand, the highest resolution data (3.20 Å) were obtained from a platinum-labelled crystal of SeMet-substituted Lys-methylated SelA-ΔN prepared by soaking in 0.2 mM K2PtCl4 (Table 1 ▶). The unit-cell parameters were a = 93, b = 281, c = 98 Å, β = 113° (P21). One SelA decamer is expected to be present in the asymmetric unit, with a solvent content of ∼55%.
Figure 3.
X-ray diffraction images. Typical X-ray diffraction images from crystals of SeMet-substituted SelA-ΔN with Lys methylation (a) and Lys-methylated SelA4KA (b) are shown.
3.4. Improvement by point mutations
To improve the diffraction resolution, point mutations targeted to the basic residues in the loop regions were introduced into full-length SelA. There are two sets of three consecutive Lys/Arg residues: Lys19-Lys20-Lys21 and Lys46-Arg47-Lys48. Both of them are found in the N-terminal region and thus the quadruple mutation K19A-K21A-K46A-K48A (4KA) was introduced. The structure of SelA4KA is expected to be identical to that of the wild type, since these mutated residues are not conserved among SelAs. A 3.9 Å resolution diffraction data set was obtained from Lys-methylated SelA4KA using 11.3 mg ml−1 protein solution and the same reservoir and cryoprotective solutions as used for Lys-methylated wild-type SelA (Figs. 2 ▶ e and 3 ▶ b). The crystal belonged to space group P41212 or P43212, with unit-cell parameters a = b = 167, c = 211 Å (Table 1 ▶). One half of the SelA decamer (five subunits of SelA) is expected to be present in the asymmetric unit, with a solvent content of ∼58%. The point mutations drastically improved the diffraction resolution (from 6 to 3.9 Å).
3.5. Phase determination
The two-wavelength data set for SeMet-substituted SelA-ΔN with Lys methylation was analyzed with SHELXC and SHELXD (Sheldrick, 2008 ▶) and 26 putative Se sites were detected. Subsequently, three of the 26 Se sites were deleted and 39 new sites were picked during several automated cycles of Se-site-based phasing using autoSHARP (Vonrhein et al., 2007 ▶). Finally, a total of 62 sites were obtained.
Each SelA-ΔN subunit contains eight Met residues; two of them are located in the N-terminal region and are presumably disordered. Therefore, we postulated that the 62 Se sites corresponded to the ordered 6 × 10 SeMet residues in decameric SelA-ΔN. The rearrangement of the 62 Se sites by the crystallographic symmetry operations revealed one fivefold and five twofold rotation axes of noncrystallographic symmetry (NCS). Five Se sites which did not agree with the NCS were deleted manually and three new sites were picked based on the NCS and the anomalous difference Fourier map (Fig. 4 ▶ a). The resulting 60 Se sites were superposed on each other and nine NCS operators (72, 144, −72 and −144° rotations around the fivefold axis and 180° rotations around the five twofold axes) were calculated using LSQKAB (Winn et al., 2011 ▶).
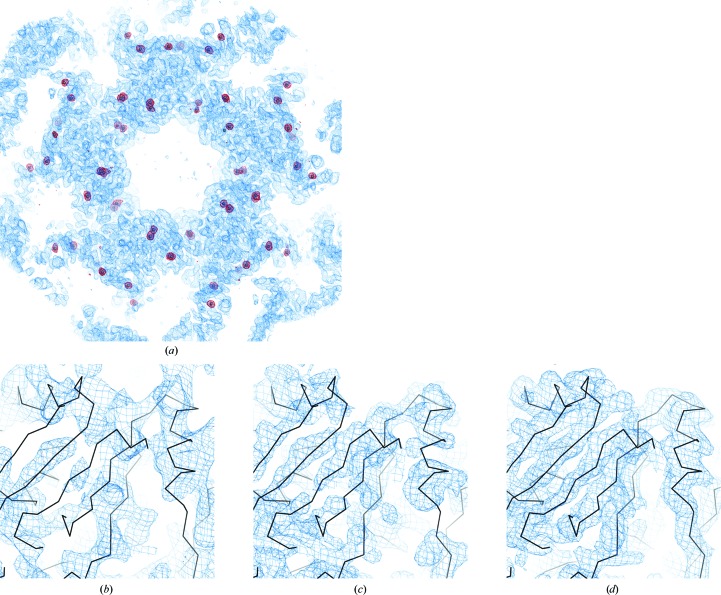
Figure 4.
Electron-density maps of SeMet-substituted SelA-ΔN with Lys methylation. (a) The overall F o map (1.0σ level) calculated from the phase after density modification (solvent flattening and NCS averaging) is shown as a blue mesh. The 60 identified Se sites, represented as spheres, revealed the NCS rotation axes (one fivefold and five twofold axes). The anomalous difference Fourier map (3.5σ level), indicating the ordered SeMet Se sites, is shown as a red mesh. (b–d) Typical close-up F o maps calculated from the phases without density modification (b), after density modification with solvent flattening (c) and after density modification with solvent flattening and NCS averaging (d). The putative Cα traces are shown.
The phase was recalculated based on the 60 updated Se sites using SHARP (Vonrhein et al., 2007 ▶). Solvent flattening and NCS averaging using the calculated operators were performed with DM (Winn et al., 2011 ▶). The phase was drastically improved by NCS averaging (Figs. 4 ▶ b, 4 ▶ c and 4 ▶ d). The phasing statistics are shown in Table 1 ▶. The overall F o map clearly indicated that the asymmetric unit contains a pentamer of dimers (Fig. 4 ▶ a).
4. Discussion
The wild-type A. aeolicus SelA contains 49 lysine residues (10.84%), while SelA-ΔN contains 38 lysine residues (9.69%). The theoretical isoelectric points of wild-type SelA and SelA-ΔN are 9.4 and 9.2, respectively. In both full-length SelA and SelA-ΔN, Lys methylation changed the crystallization conditions and the crystal forms. The high lysine content is probably one of the reasons for these changes. Lys methylation also facilitated crystal formation and growth. Furthermore, a significant improvement in the diffraction resolution was observed.
Truncation of the N-terminal region caused drastic improvements (from 6 to 3.25 Å resolution with Lys methylation and from 8 to 4.5 Å resolution without Lys methylation). Accordingly, SelA-ΔN crystallized under different conditions and in different space groups from the full-length SelAs (wild type and the 4KA mutant). These results suggest that the N-terminal domain has some flexibility that negatively affects the crystallographic protein–protein contacts.
The point mutations introduced into the loop regions had no effect on either the crystallization conditions or the appearance of the crystals. However, a drastic improvement in the diffraction resolution was observed (from 6 to 3.9 Å). The unit-cell volume was reduced from 7.40 × 106 to 5.88 × 106 Å3 (20.4% shrinkage). Therefore, the improvement in resolution may have resulted from a slippage that reinforces the intermolecular interactions at the crystal-packing interface.
Acknowledgments
We thank the beamline staff of BL41XU at SPring-8, Harima, Japan and the Photon Factory, Tsukuba, Japan for assistance with data collection. We also thank A. Ishii and T. Nakayama for assistance in preparation of the manuscript. This work was supported in part by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (A) to SY and (C) to SS, the JSPS Global Centers of Excellence Program (Integrative Life Science Based on the Study of Biosignalling Mechanisms) and the Targeted Proteins Research Program of the Ministry of Education, Culture, Sports, Science and Technology. YI was supported by Research Fellowships from JSPS.
References
- Araiso, Y., Palioura, S., Ishitani, R., Sherrer, R. L., O’Donoghue, P., Yuan, J., Oshikane, H., Domae, N., Defranco, J., Söll, D. & Nureki, O. (2008). Nucleic Acids Res. 36, 1187–1199. [DOI] [PMC free article] [PubMed]
- Böck, A., Forchhammer, K., Heider, J., Leinfelder, W., Sawers, G., Veprek, B. & Zinoni, F. (1991). Mol. Microbiol. 5, 515–520. [DOI] [PubMed]
- Carlson, B. A., Xu, X.-M., Kryukov, G. V., Rao, M., Berry, M. J., Gladyshev, V. N. & Hatfield, D. L. (2004). Proc. Natl Acad. Sci. USA, 101, 12848–12853. [DOI] [PMC free article] [PubMed]
- Ehrenreich, A., Forchhammer, K., Tormay, P., Veprek, B. & Böck, A. (1992). Eur. J. Biochem. 206, 767–773. [DOI] [PubMed]
- Engelhardt, H., Forchhammer, K., Müller, S., Goldie, K. N. & Böck, A. (1992). Mol. Microbiol. 6, 3461–3467. [DOI] [PubMed]
- Forchhammer, K. & Böck, A. (1991). J. Biol. Chem. 266, 6324–6328. [PubMed]
- Ganichkin, O. M., Xu, X.-M., Carlson, B. A., Mix, H., Hatfield, D. L., Gladyshev, V. N. & Wahl, M. C. (2008). J. Biol. Chem. 283, 5849–5865. [DOI] [PubMed]
- Leinfelder, W., Zehelein, E., Mandrand-Berthelot, M. A. & Böck, A. (1988). Nature (London), 331, 723–725. [DOI] [PubMed]
- Low, S. C., Harney, J. W. & Berry, M. J. (1995). J. Biol. Chem. 270, 21659–21664. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tormay, P., Wilting, R., Lottspeich, F., Mehta, P. K., Christen, P. & Böck, A. (1998). Eur. J. Biochem. 254, 655–661. [DOI] [PubMed]
- Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. (2007). Methods Mol. Biol. 364, 215–230. [DOI] [PubMed]
- Walter, T. S., Meier, C., Assenberg, R., Au, K. F., Ren, J., Verma, A., Nettleship, J. E., Owens, R. J., Stuart, D. I. & Grimes, J. M. (2006). Structure, 14, 1617–1622. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Xu, X.-M., Carlson, B. A., Mix, H., Zhang, Y., Saira, K., Glass, R. S., Berry, M. J., Gladyshev, V. N. & Hatfield, D. L. (2007). PLoS Biol. 5, e4. [DOI] [PMC free article] [PubMed]
- Yuan, J., Palioura, S., Salazar, J. C., Su, D., O’Donoghue, P., Hohn, M. J., Cardoso, A. M., Whitman, W. B. & Söll, D. (2006). Proc. Natl Acad. Sci. USA, 103, 18923–18927. [DOI] [PMC free article] [PubMed]