Abstract
Purpose of the review
The purpose of this review is to highlight the advances in magnetic resonance (MR) imaging of the small intestine in patients with Crohn's disease. MR imaging of the gut has become more feasible with improved spatial resolution and speed of the MR sequences allowing parallel evaluation for both disease activity and extra-enteric complications.
Recent findings
Recent literature highlights excellent diagnostic accuracy of MR enterography (MRE) that is comparable to CT enterography (CTE). Compared to CTE the image quality is not quite as good, and there is slightly more inter-observer variability in interpretation. Despite these performance characteristics, the overall diagnostic yield of MRE is comparable to CTE. The lack of radiation exposure related to MRE is a significant strength, especially in the Crohn's population that by virtue of their younger age, body habitus and potential need for repeated imaging, is at highest risk of cancer from radiation exposure due to diagnostic imaging. MRE should not be viewed as a “safer” version of a CTE. The physics of MRI allows the application of unique sequences that add novel insights not possible with other imaging modalities.
Summary
MRE is a highly effective technique for assessing Crohn's disease. We are only starting to explore new MRI sequences and the future of this technology is extremely exciting.
Keywords: magnetic resonance imaging, Crohn's disease, natural history
Introduction
Mucosal healing is becoming the standard for assessing therapeutic benefit in Crohn's disease replacing composite clinical measures such as the Crohn's disease activity index (CDAI). Endoscopic evaluation and radiographic imaging are the two techniques currently at our disposal for assessing the mucosa of the gut. The various endoscopic and radiographic techniques are complementary and are best applied with a clear understanding of the goals of the evaluation with consideration of the strengths and weaknesses of each technique. Due to its broad availability and high special resolution, Computerized tomography (CT)-based imaging, especially CT enterography (CTE), has become the most widely used cross-sectional imaging technology for Crohn's disease and has nearly completely replaced small bowel follow through at many centers.1,2 CTE has become the “gold standard” to which other radiographic imaging techniques are compared. Growing concern and increased awareness about the potential risks associated with the cumulative radiation exposure, particularly in young patients, have lead to growing interest in alternative imaging modalities3. At the same time, improved spatial resolution of MR-based techniques, along with more effective methods to deal with bowel motion and improved technique availability, have driven a rapid increase in the use of MR enterography (MRE) for Crohn's imaging. Some consider MRI-based imaging, specifically MR enterography (MRE) and pelvic MRI, the primary modalities for cross sectional imaging in Crohn's disease. However, technical limitations and practical considerations such as patients’ acceptance, availability, and cost have caused others to choose CT over MRI for all but specific circumstances. In the past year the advances in MRI technology for assessing Crohn's disease have centered around two areas: 1) comparing MRE to other diagnostic imaging techniques to assess test characteristics including inter-observer and inter-modality agreement, sensitivity and specificity and 2) assessing the usefulness of specific sequences such as diffusion and magnetization transfer that are unique to MRI and may offer novel insight into Crohn's not available with other technologies. These studies have added to the mounting academic research validating the appropriateness of using the radiation-free MR technique to answer important clinical questions in the management of the patients with Crohn's disease.
Cross Sectional Imaging: CT enterography
Cross sectional radiographic imaging techniques provide exquisite details of the entire length of the bowel and the relationship between loops of bowel and nearby structures. Imaging techniques have greatly facilitated our ability to determine Crohn's disease extent and severity, and identify complications such as strictures, abscesses and fistulae. They have provided insights into patients’ symptoms and have helped us more appropriately direct therapy or intervene with surgery. They are complementary to colonoscopy and capsule endoscopy, adding significant information about the bowel wall and involvement of adjacent tissues. Traditionally, small bowel follow through has been the imaging most widely used to assess the small intestine. In recent years, the increased availability of cross sectioning imaging techniques, especially those using a combination of “neutral” oral contrast (low Hounsfield density) and intravenous contrast, have increased our ability to assess the superficial layers of the bowel and the extra-luminal structures.
CTE provides exquisite bowel images that lend amazing insight into disease pathology. The introduction of multi-detector technology has allowed faster examinations leading to higher temporal and spatial resolution of mucosal/bowel wall details. The introduction of neutral “low density” enteric oral contrast allows for the evaluation of the mucosal details by achieving the needed bowel distension and creating the visual contrast needed for the evaluation of the mucosal details and enhancement patterns4. Cross sectional diagnostic imaging can evaluate the extent of disease throughout the small bowel and the large bowel in the same setting; detects the presence of strictures with or without proximal dilatation, as well as detects signs of penetrating disease such as fistula and extraluminal abscess formation. CTE is not as sensitive as endoscopic techniques for early changes of Crohn's disease that may primarily only include mucosal aphtous ulceration and therefore, the cross sectional studies may be more suitable for evaluation of patients with moderate to severe disease or with stricturing/penetrating disease5. Supporting its usefulness in clinical practice, Higgins et al showed that CTE can add unique and unsuspected information to the clinician assessment, especially in detecting strictures, and that this additional information can change the clinicians’ assessment of the likelihood of successful medical therapy6.
Effects of cumulative radiation dosage from diagnostic imaging have gained attention in the medical community and in the lay press3. Measurement of effective radiation doses in CT is dependent on several factors including scanning technique and patient body habitus. One study by Jaffe et al7 found that the mean effective dose for abdominopelvic multidetector CT was 16.1 mSv which was up to five times higher than SBFT examination. They emphasized that the long term biologic impact of this type of radiation exposure is not known, although other studies estimated the lifetime attributable cancer mortality risk at around 0.08% for full-body multidetector CT, while an effective dose of 10 mSv may lead to an excess risk of fatal cancer of 1 in 2,000. In addition, studies suggest that cumulative exposure of lower-dose radiation may have a similar effect as a single acute dose. More recently several changes were introduced to CT scanning techniques that would allow the acquisition of “low dose CT” leading to decrease in the overall dose of radiation delivered to the patient undergoing CT examination while trying to maintain image quality. These changes include lowering the tube current (mA) and voltage (kVp) settings used in the CT scanner along with introduction of more effective algorithms for image reconstruction that aim at reducing the increased image noise typically associated with these techniques8.
MR enterography
MRI techniques are based on the energy released by unpaired protons when they are released from alignment after application of radio frequency pulses that are applied while they are placed in a high magnetic field. The complex physics of MRI has been applied to imaging human tissue. Scanners are “tuned” to evaluate T1 and T2 relaxation times generating weighted images that highlight different bodily substances. Radiologists rely on Gadolinium (Gd)-enhanced T1 weighted images to assess the bowel wall enhancement and better visualize the surrounding vasculature and tissues. The diagnostic efficacy of T1 weighted Gd-enhanced imaging is comparable to contrast enhanced CT. T2 weighted imaging highlights the tissue fluid content like no other imaging technique.9
The MRI scanning protocols consist of various combinations of sequences that highlight different aspects of the tissue. The exact sequences depend on the desired scanning time and center experience. Specific sequences: 1. assist in localization of findings (e.g. T2 weighted single-shot fast spin-echo SSFSE (or HASTE)), 2. highlight the bowel wall and mesenteric vessels (e.g. axial & coronal steady-state free precession FIESTA (or true FISP, Balanced-FFE)), 3. highlight the bowel wall and mesenteric edema (T2 weighted fast spin-echo FSE (or TSE) with fat-saturation), 4. highlight pathologic edema (Diffusion-weighted echoplanar imaging (EPI)), 5. assess mucosal and mural enhancement as well as the mesenteric vessels (pre and post Gadolinium contrast 3D SPGR-LAVA (or VIBE, eTHRIVE) with fat-saturation) , and 6. evaluate for the extra-luminal abnormalities (delayed post contrast 2D T1 weighted SPGR with fat-saturation; Figure 1).
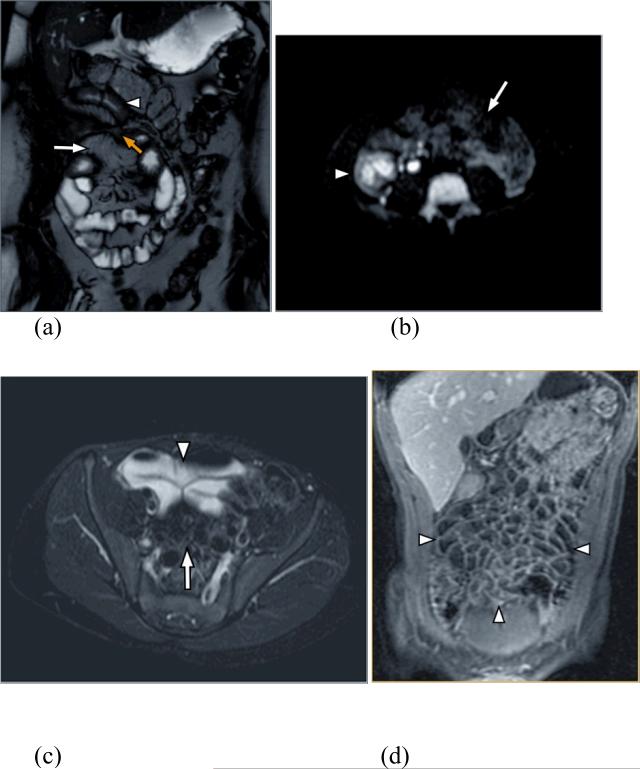
Figure 1.
(a) Coronal FIESTA allows depiction of bowel wall thickening (arrowhead) and the mesenteric fat and vessels (log arrow). Yellow arrow depicts enteroenteric fistula. (b) Diffusion weighted images showing restricted diffusion due to edema in the right lower quadrant (arrowhead) in contrast to normal bowel with no restricted diffusion in the left side of the abdomen (log arrow). (c) Axial T2 weighted image shows high signal of the intraluminal fluid within the lumen (arrowhead) with good depiction of the mesenteric fat (log arrow). (d) Coronal T1 weighted 3D SPGR after contrast administration shows the contrast between the hypointense lumen and the enhancing bowel wall improving the ability to assess abnormal enhancement
The small intestine provides unique challenges for MR imaging including uniform bowel distention, mucosal and bowel wall contrast, and intestinal motility that have been addressed by specific protocols and sequences. Biphasic oral contrast agents enable evaluation of the bowel wall demonstrating low T1 and high T2 characteristics reproducing water intensity. Methylcellulose, VoLumen (EZ-E-M, Westbury, NY), and polyethylene glycol are examples of biphasic oral contrast agents used in MRE. These agents allow assessment of mucosal enhancement on T1 weighted images, and on T2 weighted images create high contrast between the wall (low T2 signal) and the lumen (high T2 signal) allowing sensitive assessment of fold and wall thickness (Figure 2).10 Some centers use MR enteroclysis with oral contrast administered through a naso-jejunal enteric tube. While providing more uniform bowel distention, enteroclysis is time consuming, less well tolerated by patients, and does not greatly improve the overall diagnostic yield. Therefore, most centers rely on patients’ ability to ingest oral contrast. The deleterious effect of random bowel motility on MRI resolution, effects that are not improved by breath holding or gaiting as is done for respiratory or cardiac motion compensation, is partially controlled by the use of anti-peristaltic agents such as iv glucagon or similar agents administered during the sequence acquisition. Finally, iv gadolinium and application of fat suppression highlight tissue edema on the T2 weighted images. When optimally applied in a patient with optimal body weight, who is able to cooperate with breath holding, the quality of the images obtained by MRE vs. CT are indistinguishable to most gastroenterologists (Figure 3). However, studies demonstrate that the overall image quality is less for MRE than it is for CTE.11
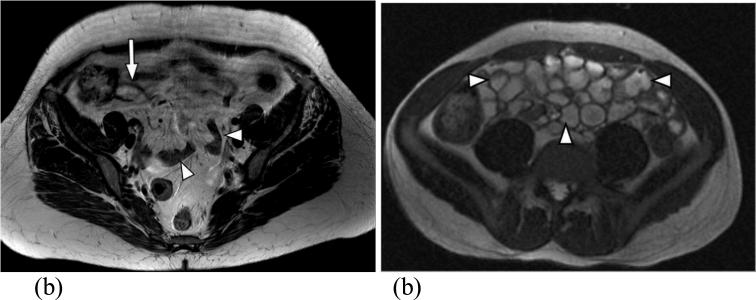
Figure 2.
(a) Axial T2 SSFSE from a routine MRI examination demonstrates lack of bowel distension (arrowheads) significantly limiting the evaluation of the bowel wall. The terminal ileum is partially distended (long arrow) and is poorly defined. (b) Axial T2 weighted SSFSE after the administration of biphasic oral contrast demonstrates excellent bowel distension (arrowheads) improving significantly the bowel wall assessment.
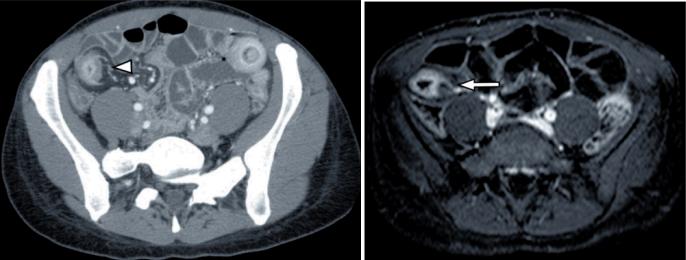
Figure 3.
(a) Axial CT image shows the abnormal terminal ileum with increased mucosal enahcnement, stranding of the surroundiung fat and engorged vessels (arrowhead). (b) Axial T1 weighted MRI examination in the same patient shows practically the same findings (arrow).
In most studies comparing histology to MRE findings, mural thickness and signal intensity on T2-weighted, fat-saturated, images and a pattern of mural enhancement correlate with histologic inflammation.12,13,14 It is possible, however, that mural enhancement is not indentifying inflammation in general but rather signaling a phase of inflammation with increased blood flow. One careful study of gadolinium enhancement determined that early enhancement did not correlate with histologic inflammation or indicators of clinical inflammation such as CRP, but rather correlated with blood flow and angiogenesis. Tissue inflammation was present even in the absence of enhancement.15
Recent studies have explored the most informative application of gadolinium to best evaluate Crohn's disease. Several studies have addressed the timing of the scan in relation to the injection of gadolinium iv contrast.16,17,18 One study applied a pharmacokinetic model to quantitatively analyze dynamic contrast enhanced MRI for the ability to detect bowel inflammation. In certain cancer types, kinetic monitoring is used clinically to monitor effectiveness of anti-angiogenic cancer drugs.19,20 The optimal timing of iv contrast is a major issue in CT were each phase of injection, arterial and venous, requires separate passes adding additional radiation exposure to the patient. As with CTE, mural enhancement is greatest within 1 minute of iv contrast administration. The optimal timing for MR imaging balancing the additional information to be derived from multiple phases vs. the additional scan time for the patient is being evaluated. To complicate things further, the finding of bowel wall enhancement is particulary prone to inter-observer variability.21
The ability of MRE to detect fibrosis or distinguish between inflammation and fibrosis is less well established. Theoretically, MRI should be able to differentiate inflammatory strictures from fixed fibrostenotic lesions due to differential water content of the two tissue types.22 This is an area of enormous clinical importance because the presence of a fibrotic stricture without significant inflammation would direct a patient to surgical therapy rather than continued medical therapy. Authors have equated mural thickening without enhancement to fibrostenotic disease though based on correlative studies with histology, this likely represents a phase of inflammation with less vascular engorgement. The idea that the absence of MRE signs of inflammation indicate fibrosis is supported by one study where 5 of 6 patients with wall thickening without enhancement of MRI had no histologic inflammation on surgical specimen.23 However, caution should be used in equating lack of mural enhancement with fibrosis. Studies specifically addressing fibrosis are few and conclusions are based on only a few surgical samples. Our experience is that very few if any surgical samples have only fibrosis; the best predictor of fibrosis is the presence of inflammation.24 Inflammation and fibrosis seems so closely linked pathologically that we agree with Zappa et al who found that fibrosis correlated well with inflammation and that the two are inseparable and that “it may not be relevant to make an exclusive distinction, as is usually done, between inflammatory patients and fibrotic patients”. 14
New sequences have been applied to specifically detect fibrosis. Magnetization transfer (MT) technique takes advantage of a different set of molecular properties than standard T1 and T2 imaging. MT MRI reflects the energy transferred from protons in free mobile water molecules to protons in water molecules associated with large molecules such as collagen. Therefore, stiff body substances such as muscle or fibrotic tissue have a high MT effect, whereas MT is relatively insensitive to inflammation and tissue edema. Our group has demonstrated that MT MRI can semi-quantitatively detect collagen in an animal model of Crohn's disease. Further, the technique is sensitive to the development of fibrosis over time and with treatment.25 Early human experience with MT sequence added to traditional MRE protocols suggests that MT can predict clinical phenotype in patients with Crohn's disease.26
Other novel sequences have been recently applied to MRI imaging in Crohn's disease. Diffusion weighted imaging (DWI) reflects the changes in the water mobility caused by interactions with cell membranes, macromolecules and alterations of the tissue environment. This technique has been widely used for intracranial disease, and has shown promise in the abdomen for evaluation of various hepatic, renal and pancreatic diseases. DWI is being explored for the evaluation of Crohn's disease where it may provide additional structural information.27
MRE Performance Characteristics
Several recent manuscripts add important information to our understanding of MRE as a tool to assess disease severity and identify complications of Crohn's disease. Foriano et al showed that CTE and MRE similarly identify disease localization, presence of wall thickening, bowel wall enhancement (with MRE being slightly more sensitivity for ileal wall enhancement than CTE), presence of fistula and mesenteric adenopathy. In this study, stricture identification was significantly more sensitive with MRE than CTE. Sensitivities and specificities of MRE for small intestinal findings in CD were similar to other reported studies with 0.88 (0.78-0.99, CI 95%) sensitivity and 0.88 (0.68-1.0, CI 95%) specificity for localization of disease, bowel wall thickening, bowel wall enhancement. Identification of enteroenteric fistulas was broadly similar between CTE and MRE (0.04 vs 0.02; p=0.08), respectively. The study concluded that both CTE and MRE are highly effective techniques in assessing ileocolonic Crohn's disease with broadly similar accuracy.28
Jensen et al compared CTE and MRE with respect to image quality in addition to disease evaluation, with assessment of both inter-observer and inter-modality agreement for four different reviewers. As noted on earlier studies, the image quality was superior for CTE which is not degraded by motion artifact due to the fast acquisition time, especially with the use of multi-detector CT technology. For disease evaluation, the inter-observer agreement was high for CTE and moderate for MRE. On the other hand the inter-modality agreement was fair to substantial depending on the reader.11 This suggests that the evaluation of small bowel Crohn's disease is both observer and modality dependent. However, despite these differences, both techniques had comparable diagnostic yields. Therefore, given an experienced radiologist, MRE offers an acceptable alternative to CTE despite the difference in image quality.
Perianal Crohn's disease
Perianal Crohn's disease is the one clinical setting where the superiority of MRI vs. other imaging techniques is less hotly debated. An MRI based grading system has been proposed by the St. James University group and has been validated by surgically proved cases. (10 in 14).29 MRI has sensitivity for detecting perianal fistulas of over 80% with an accuracy of over 90%.30,31 Diffusion weighted MRI has recently been applied to perianal fistulae and may be an adjunct to T2 weighted imaging especially in patients with risk factors for contrast agents.32 Endoscopic ultrasound (EUS) is also a useful technique for assessing perianal fistulae and, when performed by experienced personnel, has similar test characteristics. CT-based scans perform less well in the evaluation of perianal Crohn's disease due to the decreased contrast resolution and poor definition of the anal sphincter complex as compared to MRI. MRI is superior in detecting the fistula tract and also in assessing the relationship of the fistula to the internal sphincter which is of paramount importance for surgical fistula management. The advantage of EUS is in expense and ease, particularly with an experienced operator. MRI has the advantage over EUS of giving more of an overview of the disease process and probable superiority for high or complex fistulas. Imaging of perianal fistulas has been well reviewed recently by Ziech, Felt-Bersma and Stoker.33
Specific Clinical Situations
MRI imaging has been studied in several relevant and practical clinical situations. In the postoperative setting endoscopic findings have been shown to predict clinical outcomes.34 MR enteroclysis and colonoscopy were shown to have similar value to predict disease recurrence in postoperative patients suggesting that it may be possible to substitute a non-invasive MRE for a more invasive colonoscopy for evaluation of Crohn's patients for post operative disease recurrence.35 Another common clinical setting exists when a Crohn's disease patient visits the emergency department for evaluation of abdominal pain. Typically, a standard positive oral contrast abdominal CT scan is performed. An follow up negative oral contrast MRE is often ordered in the following days or weeks in an attempt to determine more subtle details of the small bowel that were not seen due to the positive oral contrast used in the standard scan. Schreyer et at determined that the additional MRE added little to the diagnostic yield and was not necessary.36
Conclusion
MRI technology has continued to improve to the point where it is a useful modality for imaging in Crohn's disease. The utility in perianal disease is well demonstrated and pelvic MRI has become the mainstay for fistula evaluation. Recent studies have shown that despite somewhat lower resolution and image quality, in experienced hands, the diagnostic yield of MRE is equivalent to CTE for evaluation of the small bowel. Incompletely addressed to date are the issues related to cost, availability, experience of radiologists, delay in MRI examination due to scheduling, longer time of examinations, more patient restrictions related to the presence of metallic hardware, claustrophopia, allergies etc. So how does a clinician make the decision about the optimal technique on an individual patient? Like many decisions we make in caring for these challenging patients, we balance risks and benefits, incorporating the literature, local expertise, and patient factors, and then make the best decision possible. In many cases this will mean choosing MRE over CTE in young patients, saving CTE for difficult cases where defining pathology using the technique with the optimal special resolution is essential. One strategy that is frequently employed at our institution and advocated by others is to use CTE for the initial exam, especially if an abscess or entero-enteric fistulae is suspected, but use MRE for monitoring progress if follow up scans are required. Looking to the future, we are only scratching the surface of information to be derived from novel and unique sequences many of which are used clinically in other diseases. Studies are refining existing sequences and adding novel sequences such as diffusion and magnetization transfer that are possible due to the physics of MRI and not available with CT or other modalities. This novel information will likely make MRI the technology of the future for imaging the small bowel and pelvis in patients with Crohn's disease.
Acknowledgements
Grant for Ellen Zimmermann RO1 DK073992
Funding: Ellen M. Zimmermann, NIH RO1 DK073992
Contributor Information
Ellen M. Zimmermann, Department of Internal Medicine Division of Gastroenterology 6520 MSRB I, 0682 University of Michigan Medical Center Ann Arbor, MI 4910 734-647-2964 ezimmer@umich.edu.
Mahmoud M. Al-Hawary, Department of Radiology Abdominal Division University of Michigan Medical Center alhawary@umich.edu.
References
- 1.Kroeker KI, Lam S, Birchall I, et al. Patients with IBD are Exposed to High Levels of Ionizing Radiation Through CT Scan Diagnostic Imaging: A Five-year Study. J Clin Gastroenterol. doi: 10.1097/MCG.0b013e3181e5d1c5. (In press.) [DOI] [PubMed] [Google Scholar]
- 2.Palmer L, Herfarth H, Porter CQ, et al. Diagnostic ionizing radiation exposure in a population-based sample of children with inflammatory bowel diseases. Am J Gastroenterol. 2009;104(11):2816–23. doi: 10.1038/ajg.2009.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 4.Huprich JE, Fletcher JG. CT Enterography: Principles, technique and utility in Crohn's disease. Eur J Radiol. 2009;69(3):393–7. doi: 10.1016/j.ejrad.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Dionisio PM, Gurudu SR, Leighton JA, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn's disease: A meta-analysis. Am J Gastroenterol. 2010;105(6):1240–8. doi: 10.1038/ajg.2009.713. quiz 1249. [DOI] [PubMed] [Google Scholar]
- 6.Higgins PD, Caoili E, Zimmermann EM, et al. Computed tomographic enterography adds information to clinical management in small bowel Crohn's disease. Inflam Bowel Dis. 2007;13(3):262–8. doi: 10.1002/ibd.20013. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe TA, Gaca AM, Delaney S, et al. Radiation Doses from Small-Bowel Follow-Through and Abdominopelvic MDCT in Crohn's Disease. Am J Radiol. 2007;189:1015–1022. doi: 10.2214/AJR.07.2427. [DOI] [PubMed] [Google Scholar]
- 8.Summers RM. Dose reduction in CT: The time is now. Acad Radiol. 2010;17(10):1201–2. doi: 10.1016/j.acra.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maccioni F. Double-contrast magnetic resonance imaging of the small and large bowel: Effectiveness in the evaluation of inflammatory bowel disease. Abdom Imaging. 2010;35(1):31–40. doi: 10.1007/s00261-008-9482-7. [DOI] [PubMed] [Google Scholar]
- 10.Tolan DJ, Greenhalgh R, Zealley IA, et al. MR enterographic manifestations of small bowel Crohn's disease. Radiographics. 2010;30(2):367–84. doi: 10.1148/rg.302095028. [DOI] [PubMed] [Google Scholar]
- 11.Jensen M, Ormstrup T, Vagn-Hasen C, et al. Inter-observer and inter-modality agreement for detection of small bowel Crohn's disease with MRI and CT enterography. Inflam Bowel Dis. doi: 10.1002/ibd.21534. (In press.) [DOI] [PubMed] [Google Scholar]
- 12.Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, et al. Mural inflammation in Crohn's disease: Location-matched histologic validation of MR imaging features. Radiology. 2009;252(3):712–20. doi: 10.1148/radiol.2523082167. [DOI] [PubMed] [Google Scholar]
- 13.Parisinos CA, McIntyre VE, Heron T. Magnetic resonance follow-through imaging for evaluation of disease activity in ileal Crohn's disease: An observational retrospective cohort study. Inflam Bowel Dis. 2010;16(7):1219–26. doi: 10.1002/ibd.21168. [DOI] [PubMed] [Google Scholar]
- 14*.Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn's disease? A retrospective comparison with surgical pathologic analysis. Inflam Bowel Dis. 2010 Aug 18; doi: 10.1002/ibd.21414. [There is a paucity of studies correlating histologic with radiographic findings. This paper exposes the dogma that fibrotic disease and inflammatory disease are separable by either means.] [DOI] [PubMed] [Google Scholar]
- 15.Taylor SA, Punwani S, Rodriguez-Justo M, et al. Mural Crohn's disease: Correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination--pilot study. Radiology. 2009;251(2):369–79. doi: 10.1148/radiol.2512081292. [DOI] [PubMed] [Google Scholar]
- 16.Knuesel PR, Kubik RA, Crook DW, et al. Assessment of dynamic contrast enhancement of the small bowel in active Crohn's disease using 3D MR enterography. Eur J Radiol. 2010;73(3):607–13. doi: 10.1016/j.ejrad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Horsthuis K, Nederveen AJ, de Feiter MW, et al. Mapping of T1-values and Gadolinium-concentrations in MRI as indicator of disease activity in luminal Crohn's disease: A feasibility study. J Magn Reson Imaging. 2009;29(2):488–93. doi: 10.1002/jmri.21535. [DOI] [PubMed] [Google Scholar]
- 18.Punwani S, Rodriguez-Justo M, Bainbridge A, et al. Mural inflammation in Crohn's disease: Location-matched histologic validation of MR imaging features. Radiology. 2009;252(3):712–20. doi: 10.1148/radiol.2523082167. [DOI] [PubMed] [Google Scholar]
- 19.Padhani AR, Hayes C, Landau S, et al. Reproducibility of quantitative dynamic MRI of normal human tissues. NMR Niomed. 2002;15:143–153. doi: 10.1002/nbm.732. [DOI] [PubMed] [Google Scholar]
- 20.Oto A, Fan X, Mustafi D, et al. Quantitative analysis of dynamic contrast enhanced MRI for assessment of bowel inflammation in Crohn's disease pilot study. Acad Radiol. 2009;16(10):1223–30. doi: 10.1016/j.acra.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Sharman A, Zealley IA, Greenhalgh R, et al. MRI of small bowel Crohn's disease: Determining the reproducibility of bowel wall gadolinium enhancement measurements. Eur Radiol. 2009;19(8):1960–7. doi: 10.1007/s00330-009-1371-0. [DOI] [PubMed] [Google Scholar]
- 22.Baidoo L, Regueiro M. Radiologic testing in Crohn's disease. Inflam Bowel Dis. 2008;14(Suppl):2S181–2. doi: 10.1002/ibd.20614. [DOI] [PubMed] [Google Scholar]
- 23.Lawrance IC, Welman CJ, Shipman P, et al. Correlation of MRI-determined small bowel Crohn's disease categories with medical response and surgical pathology. World J Gastrol. 2009;15(27):3367–75. doi: 10.3748/wjg.15.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeremy Adler, Darashana Punglia, Jonathan R. Dillman, et al. CT Enterography Findings Correlate with Tissue Inflammation But Not Fibrosis in Resected Small Bowel Crohn's Disease. Gastroenterol. 2009;134:S1178. [Google Scholar]
- 25*.Adler J, Swanson S, Schmiedlin-Ren P, et al. Magnetization transfer MRI: A non-invasive method to detect intestinal fibrosis in Crohn's disease. Radiology. doi: 10.1148/radiol.10091648. (in press) [This study applies a MRI sequence that semi-quantitatively detects fibrosis. Once validated in humans, this could become a means to monitor the natural history of Crohn's disease in the setting of clinical care or clinical trials.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeremy Adler, Mahmoud M. Al-Hawary, Jonathan R. Dillmann, et al. Magnetization Transfer MRI for In Vivo Imaging Bowel Fibrosis in Patients With Crohn's Disease. 2010 Abst. M1230. DDW. [Google Scholar]
- 27.Taylor SA, Punwani S, Rodriguez-Justo M, et al. Mural Crohn's disease: Correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination--pilot study. Radiology. 2009;251(2):369–79. doi: 10.1148/radiol.2512081292. [DOI] [PubMed] [Google Scholar]
- 28**.Fiorino G, Bonifacio C, Peyrin-Biroulet L, et al. Prospective comparison of Computed tomography and Magnetic resonance enterography for assessment of disease activity and complications in ileo-colonic Crohn's disease. Inflam Bowel Dis. doi: 10.1002/ibd.21533. In press. [This article prospectively compares MRE with the ‘gold standard’ CTE highlighting the strengths and weaknesses of both techniques.] [DOI] [PubMed] [Google Scholar]
- 29.Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20:623–635. doi: 10.1148/radiographics.20.3.g00mc15623. [DOI] [PubMed] [Google Scholar]
- 30.Chapple KS, Spencer JA, Windsor AC, et al. Prognostic value of magnetic resonance imaging in the management of fistula-in-ano. Dis Colon Rectum. 2000;43:511–516. doi: 10.1007/BF02237196. [DOI] [PubMed] [Google Scholar]
- 31.Buchanan GN, Halligan S, Bartram CL, et al. Clinical examination, endosonography and MR imaging in preoperative assessment of fistula in ano: Comparison and outcome-based reference standard. Radiology. 2004;233:674–681. doi: 10.1148/radiol.2333031724. [DOI] [PubMed] [Google Scholar]
- 32.Hori M, Oto A, Orrin S, et al. Diffusion-weighted MRI: A new tool for the diagnosis of fistula in ano. J Magn Reson Imaging. 2009;30(5):1021–6. doi: 10.1002/jmri.21934. [DOI] [PubMed] [Google Scholar]
- 33.Ziech M, Felt-Bersma R, Stoker J. Imaging of perianal fistulas. Clin Gastroenterol & Hep. 2009;7(10):1037–45. doi: 10.1016/j.cgh.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 34.Rutgeerts P, Van Assche G. What is the role of endoscopy in the postoperative management of Crohn's disease? Inflam Bowel Dis. 2008;14(Suppl 2):S179–80. doi: 10.1002/ibd.20655. [DOI] [PubMed] [Google Scholar]
- 35**.Koilakou S, Sailer J, Peloschek P, et al. Endoscopy and MR enteroclysis: Equivalent tools in predicting clinical recurrence in patients with Crohn's disease after ileocolic resection. Inflam Bowel Dis. 2010;16(2):198–203. doi: 10.1002/ibd.21003. [This paper addresses a common clinical scenario where non-invasive imaging would be clinical useful, that is, to identify disease recurrence in the post operative setting.] [DOI] [PubMed] [Google Scholar]
- 36**.Schreyer AG, Hoffstetter P, Daneschnejad M, et al. Comparison of conventional abdominal CT with MR-enterography in patients with active Crohn's disease and acute abdominal pain. Acad Radiol. 2010;17(3):352–7. doi: 10.1016/j.acra.2009.10.023. [Patients with Crohn's disease who visit the emergency department for abdominal pain almost invariably receive a standard abdominal CT scan to screen for abscesses etc. This scan protocol is not optimal for visualizing Crohn's, disease but in most case, an additional more disease-specific scan, such as MRE, adds little clinically useful information.] [DOI] [PubMed] [Google Scholar]