Abstract
Urinary concentrations of metabolites of the anti-androgenic xenobiotic di-(2-ethylhexyl) phthalate (DEHP) were previously shown to be weakly associated with serum levels of several hormones in two disparate US populations; partners of pregnant women participating in the Study for Future Families, and partners in an infertile couple from Massachusetts General Hospital infertility clinic. The observed associations between phthalate metabolites and reproductive hormones were robust and insensitive to the characteristics of the subpopulation or the laboratory in which the hormones were measured, despite the fact that these two populations span a range of fertility, urinary phthalate metabolites and reproductive hormone levels. We therefore examined associations between urinary metabolites of DEHP and reproductive hormones (follicle stimulating hormone, luteinizing hormone, testosterone (T), inhibin B and estradiol (E2), and sex hormone-binding globulin (SHGB) in the pooled population. The magnitude of the associations seen were similar to those reported for each population separately, but effect estimates were more precise due to the increased sample size, and the greater range of phthalate metabolite concentrations and hormone levels. Urinary concentrations of three metabolites of DEHP [mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP)] were inversely associated with the free androgen index (FAI = T/SHBG) and calculated free testosterone (FT). Urinary concentrations of MEHHP and MEOHP were positively associated with SHBG, and MEHP was inversely associated with E2. No other phthalate metabolites were associated with serum hormones, consistent with results in each population. Our results in this diverse population suggest that DEHP exposure is robustly associated with some male sex steroid hormones.
Keywords: anti-androgens, DEHP metabolites, endocrine disruptor, male hormones
INTRODUCTION
Recent studies have reported secular shifts in male reproductive hormone levels (Andersson et al, 2007; Travison et al, 2007) which might be associated with decreases in semen quality (Carlsen et al, 1992; Swan et al, 2007). While exposure data are limited, it has been hypothesized that these changes may, at least in part, reflect the widespread use, and human exposure to, environmental endocrine-disrupting compounds (EDCs) (Jørgensen et al, 2010; Sharpe and Skakkebæk, 2008).
Phthalates, man-made chemicals extensively used in industry and commerce, are among the most widely studied EDCs, and several, including di(2-ethylhexyl) phthalate (DEHP) and di-n butyl phthalate (DBP) have been shown to have anti-androgenic activity (ATSDR, 2002; CDC, 2011). A growing body of literature has shown relationships between several of these phthalates and adverse reproduction and development (Hauser and Calafat, 2005; NRC, 1999; Talsness et al, 2009; Thompson et al, 2009). Laboratory studies have shown that DEHP and/or its metabolites are associated with the induction of testicular toxicity in neonatal, pubertal and adult rodents (Heindel et al, 1989; Li et al, 1998; 2000; Parmar et al, 1986; Srivastava et al, 1990). However, adult animals are usually less sensitive than young pubertal animals or animals exposed in utero (Dostal et al, 1988; Higuchi et al, 2003). For example, several toxicological studies have demonstrated that DEHP, DBP, benzylbutyl phthalate (BzBP), and di-isononyl phthalate (DiNP) disrupt reproductive tract development (e.g. hypospadias, reduced fetal testosterone synthesis) in male rodents due to anti-androgenic action (Gray et al, 2000; Parks et al, 2000). Nevertheless, only a small number of human studies have investigated the relationship between male reproductive hormones and phthalate exposures. In those studies relationships have been shown between human prenatal and peri-natal exposure to some phthalate metabolites and alterations in reproductive hormones [sex hormone-binding globulin (SHBG), luteinizing hormone (LH) and free testosterone (FT)] (Main et al, 2006), and markers of male reproductive development (Swan et al, 2005; Swan, 2008). In a population of young men, Jönsson et al. (2005) reported an inverse association between urinary monoethyl phthalate (MEP) concentrations and circulating LH, though no associations were found between other phthalate metabolites and reproductive hormones. Pan et al. (2006) studied adult men occupationally exposed to some phthalates (DEHP and DBP), and reported that phthalate exposure was inversely associated with serum FT levels. Meeker and collaborators (2009) investigated this issue and extended their previous work (Duty et al, 2005) by including a larger sample size and expanding the number of hormones and phthalate metabolites measured. In a male population attending a fertility clinic, the authors reported an association between increased urinary concentration of mono(2-ethylhexyl) phthalate (MEHP) with decreased testosterone (T), estradiol (E2) and free androgen index (FAI) levels, showing that exposure to DEHP might be associated with altered steroid hormones in these men. Recently, Mendiola et al. (2010) investigated these associations in a population of fertile men. Both Meeker et al. (2009) and Mendiola et al. (2010) showed significant inverse association between FAI levels and urinary concentrations of several DEHP metabolites. In both studies SHBG was positively associated with urinary concentrations of MEHP, but not with other DEHP metabolites. Neither study found notable associations between metabolites of any other phthalate and hormones under investigation. There were, however, some discrepancies between these studies. For instance, Duty et al. (2005) reported a dose-response relationship between monobenzyl phthalate (MBzP) and follicle stimulating hormone (FSH) and mono-n-butyl phthalate (MBP) and inhibin B but no strong evidence of an association between MEHP and T. Meeker et al. (2009) reported a significant relationship between MEHP and T, and mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) and FAI (p<.05) but for FAI and MEHP the adjusted p-value was <0.1. Mendiola et al. (2010) reported a significant association between several DEHP metabolites and FAI but no relationship between DEHP metabolites and T.
The aim of the current study was to use a pooled analysis of a large heterogeneous population of both fertile (Mendiola et al, 2010) and infertile men (Meeker et al, 2009) to more precisely examine the relationships of urinary phthalate metabolite concentrations with serum reproductive hormone levels. Although data from both populations were previously published, this new pooled analysis adds to our understanding of the human health effects of phthalates by allowing us to systematically investigate whether associations differed by populations based on fertility status.
MATERIALS AND METHODS
Study populations
The present study includes men from two large ongoing studies of environmental influences on reproductive health. One of these, the Study for Future Families (SFF) (n=425), is a multicenter study of pregnant women and their male partners, conducted at prenatal clinics affiliated with university hospitals in five US cities (Harbor-UCLA and Cedars-Sinai Medical Center in Los Angeles, CA; University of Minnesota Medical Center in Minneapolis, MN; University Physicians in Columbia, MO; Mt. Sinai School of Medicine, New York City, NY and University of Iowa, Iowa City, IA) between 1999 and 2005. In this study couples were eligible only if the pregnancy was conceived without assisted reproduction (Swan et al, 2003). The second study included men who were male partners of infertile couples seeking evaluation at the Vincent Memorial Obstetrics and Gynecology Service, Andrology Laboratory and In Vitro Fertilization Unit, Massachusetts General Hospital (MGH) (n=425) in Boston between January 2000 and May 2004 (Meeker et al, 2009). That infertility clinic population includes men with male factor infertility as well as men who are partners of women with female factor infertility. Methods for clinical examination, data collection, and semen analysis have been described previously for each study (Meeker et al, 2009; Swan et al, 2003). Briefly, in both studies the men completed a questionnaire and gave urine, blood and semen specimens. Information was collected on demographics, medical history, and lifestyle factors. Human subject approvals were obtained from Institutional Review Boards at all participating institutions. The involvement of Centers for Disease Control and Prevention (CDC) laboratory in SFF was limited and determined not to constitute engagement in human subjects research.
Serum hormone analysis
In both populations venous blood samples were drawn, and the serum was separated and frozen at − 80°C, on the same day the urinary sample was collected. Samples were analyzed for hormones in two different laboratories, SFF samples at the Rigshospitalet Andrology Laboratory (Copenhagen, Denmark) and MGH samples at the REU Laboratory at MGH, Boston, MA. Each methodology has been described previously elsewhere (Asklund et al, 2007; Bang et al, 2005; Meeker et al, 2009; Mendiola et al, 2010). The MGH lab is a Clinical Laboratory Improvement Amendments (CLIA)-certified (Centers for Medicare and Medicaid Services, Department of Health and Human Services, Baltimore, MD, USA) and the Rigshospitalet Andrology Laboratory participates in Bio-Rad Laboratories external quality Immunoassay program (Bio-Rad Laboratories, Copenhagen, Denmark). Table 1 summarizes the serum hormone analysis methods that were employed at the two laboratories. FAI was calculated as total testosterone ×100/SHBG, and FT concentration was calculated using the equation of Vermeulen et al. (1999).
Table 1.
Methods for serum hormone analyses at the two laboratories (MGH and SFF).
| MGH assay details | |||||
|---|---|---|---|---|---|
| Hormone | Method | Manufacturer/System | Sensitivity | CVs | |
| Intra-assay | Inter-assay | ||||
| FSH | Microparticle Enzyme Immunoassay (MEIA) | Abbott AxSYM | 1.1 IU/L | 3–7% | 2–5% |
| LH | Microparticle Enzyme Immunoassay (MEIA) | Abbott AxSYM | 1.2 IU/L | 4–7% | 2–7% |
| Testosterone | Radioimmunoassay (RIA) | Coat-A-Count kit, Diagnostic Products Corp. | 0.14 nmol/L | 10% | 12% |
| SHBG | Solid-phase two-site enzyme chemiluminescent immunometric assay | Immulite, Diagnostic Products Corp. | 1 nmol/L | 2–5% | 4–8% |
| Inhibin B | Double antibody enzyme-linked immunosorbent assay (Double antibody ELISA) | Oxford Bioinnovation | 50 pg/mL | 8% | 20% |
| Estradiol | Microparticle Enzyme Immunoassay (MEIA) | Abbott AxSYM | 73 pmol/L | 3–11% | 5–15% |
| SFF assay details | |||||
|---|---|---|---|---|---|
| Hormone | Method | Manufacturer/System | Sensitivity | CVs | |
| Intra-assay | Inter-assay | ||||
| FSH | Time-resolved immunofluorometric assay (TR-IFMA) | DELFIA, Perkin Elmer | 0.05 IU/L | 1.3–2.1 % | 2.8–4.6 % |
| LH | Time-resolved immunofluorometric assay (TR-IFMA) | DELFIA, Perkin Elmer | 0.05 IU/L | 1.5–3.0 % | 4.0–4.5 % |
| Testosterone | Time-resolved fluoroimmunoassay (TR-FIA) | DELFIA, Perkin Elmer | 0.23 nmol/L | 1.4–2 % | 6–8 % |
| SHBG | Time-resolved immunofluorometric assay (TR-IFMA) | DELFIA, Perkin Elmer | 0.23 nmol/L | 3–5 % | 4–5 % |
| Inhibin B | Specific two-sided enzyme immunometric assay | (Oxford Bioinnovation, in-house standard) | 20 pg/ml | 15 % | 18 % |
| Estradiol | Radioimmunoassay (RIA) | Pantex, USA | 18 pmol/L | 3–8 % | 11–13 % |
CVs: coefficients of variation
Urinary phthalate metabolites measures
In both populations the concentrations of urinary phthalate metabolites were determined at the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) (Atlanta, GA, USA), which had no access to participant data. SFF samples were analyzed in 2006 and MGH samples were analyzed throughout a 3-year period (2003–2006). Urinary samples were frozen and stored at −80 °C, and then shipped to CDC on dry ice. Phthalate metabolites were measured in urine to avoid potential sample contamination from the parent diester and because the metabolites (not the parent diesters) are the active toxicants (Li et al, 1998). The analytical approach for the analysis of urinary phthalate metabolites in the MGH men population has been previously described (Meeker et al, 2009; Silva et al, 2007). A modification of that approach was used in the SFF population and has been described and published elsewhere (Swan et al, 2005). Limits of detection (LOD) are in the low nanogram per milliliter range (see Table 4). Isotopically labeled internal standards were used along with conjugated internal standards to increase precision and accuracy of the measurements. The method is accurate (spiked recoveries are near 100%), and precise with between-day relative standard deviations of < 10%. Quality control samples and laboratory blanks were analyzed along with unknown samples to monitor performance of the method (Swan et al, 2005). Concentrations are reported in ng/mL. While different metabolites were assessed in our separate studies, we report here only the six urinary phthalate metabolites that were measured in both populations: MEHP, MEHHP, MEOHP, MEP, MBzP and MBP (as sum of MBP and mono-iso-butyl phthalate concentrations). We also calculated the percent of these DEHP metabolites excreted as MEHP (MEHP%). To calculate MEHP%, we converted MEHP, MEHHP and MEOHP concentrations to nanomoles per milliliter, divided MEHP concentrations by the sum of concentrations of MEOHP, MEHHP and MEHP, and multiplied by 100 (Hauser et al, 2006).
Table 4.
Summary statistics for the urinary concentrations (in ng/mL) of DEHP metabolites (non creatinine-adjusted) in men from both studies separately and combined (N=850)
| Study | Geometric Mean | Selected Percentiles | P valuef | |||||
|---|---|---|---|---|---|---|---|---|
| LODa | % > LODb | 10th | 50th | 90th | ||||
| Variables | ||||||||
| MEHP (ng/mL) | ||||||||
| SFF | 3.7 | 1.2 | 77 | 0.85 | 3.2 | 17.8 | ||
| MGH | 8.2 | 1.0 | 83 | 1.0 | 7.9 | 64.3 | ||
| TOTAL | 4.9 | 80 | 0.9 | 4.4 | 39.2 | < 0.001 | ||
| MEHHP (ng/mL) | ||||||||
| SFF | 23.3 | 0.7 | 99 | 4.6 | 23.7 | 104 | ||
| MGHc | 55.6 | 1.3 | 100 | 13.2 | 47.0 | 272 | ||
| TOTALd | 27.6 | 99.5 | 5.4 | 25.3 | 170 | < 0.001 | ||
| MEOHP (ng/mL) | ||||||||
| SFF | 12.9 | 0.7 | 97 | 2.7 | 12.9 | 57.4 | ||
| MGHc | 36.2 | 1.1 | 99 | 8.4 | 32.2 | 193 | ||
| TOTALd | 16.1 | 98 | 3.2 | 15.4 | 110 | < 0.001 | ||
| MEP (ng/mL) | ||||||||
| SFF | 206 | 0.8 | 100 | 31.8 | 205 | 1358 | ||
| MGH | 179 | 1.1 | 100 | 30.2 | 153 | 1376 | ||
| TOTAL | 173 | 100 | 23.6 | 170 | 1259 | < 0.001 | ||
| MBP (ng/mL) | ||||||||
| SFF | 19.2 | 0.6 | 98 | 4.0 | 24.5 | 65.3 | ||
| MGH | 17.1 | 0.8 | 97 | 5.1 | 17.7 | 50.8 | ||
| TOTAL | 16.3 | 97.5 | 3.4 | 18.8 | 58.2 | < 0.001 | ||
| MBzP (ng/mL) | ||||||||
| SFF | 11.2 | 0.3 | 98 | 2.1 | 12.5 | 49.8 | ||
| MGH | 7.7 | 0.7 | 97 | 2.3 | 8.2 | 24.9 | ||
| TOTAL | 8.4 | 97.5 | 1.6 | 9.8 | 41.2 | < 0.001 | ||
| MEHP%e | ||||||||
| SFF | 9.4 | 3.9 | 10.1 | 18.8 | ||||
| MGHc | 9.4 | 3.5 | 10.3 | 24.3 | ||||
| TOTALd | 9.4 | 3.7 | 10.1 | 21.6 | 0.59 | |||
Limit of detection (LOD) in ng/mL for each phthalate metabolite.
Percentage of samples above the LOD for each phthalate metabolite
N= 221
N= 646
To calculate MEHP%, we transformed MEHP, MEHHP and MEOHP concentrations to nanomoles per milliliter, divided MEHP levels by the sum of concentrations of MEHP, MEHHP and MEOHP, and then multiplied by 100
Mann-Whitney U Test
Statistical analyses
Data from Meeker et al. (2009) and Mendiola et al. (2010) were pooled for statistical analysis. Serum hormones (except E2) and urinary phthalate metabolite concentrations were log transformed (log10) to normalize their asymmetric distributions. In preliminary analyses, we used Mann-Whitney U test and Pearson correlation coefficients to explore the relationship between each hormone concentration and each phthalate metabolite concentration. We then used multiple linear regression analysis to control for appropriate covariates, including age, age square, body mass index (BMI), smoking status (current smoker vs. never smoked), ethnicity (African American vs. others), time of sample collection (hours after 7:00 am), and time of sample collection squared. Urinary dilution was measured differently in the two populations; SFF models were adjusted by urinary creatinine concentrations and MGH models by specific gravity (SG). Although these methods of adjusting for urinary concentration are different, the rank of urinary concentrations assigned by each method should be comparable (Box and Tidwell 1962). Therefore, the measure of urinary dilution used in the combined analysis was the rank of creatinine or SG in the respective data sets. We also included a term for study center (SFF vs. MGH), which reflects between-center differences, including those due to differing methods of hormone analysis and measurement for urinary dilution. Age, BMI and time of collection were modeled as continuous variables, all others as dichotomous indicator variables. Most metabolite concentrations were above the LOD; those below the LOD were assigned the value LOD divided by the square root of 2, which has been recommended when the data are not highly skewed (i.e. geometric standard deviation <3) (Hornung and Reed 1990), as was the case in the present analysis. Two analysts (J.D.M. and J.M.) conducted all analyses independently using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
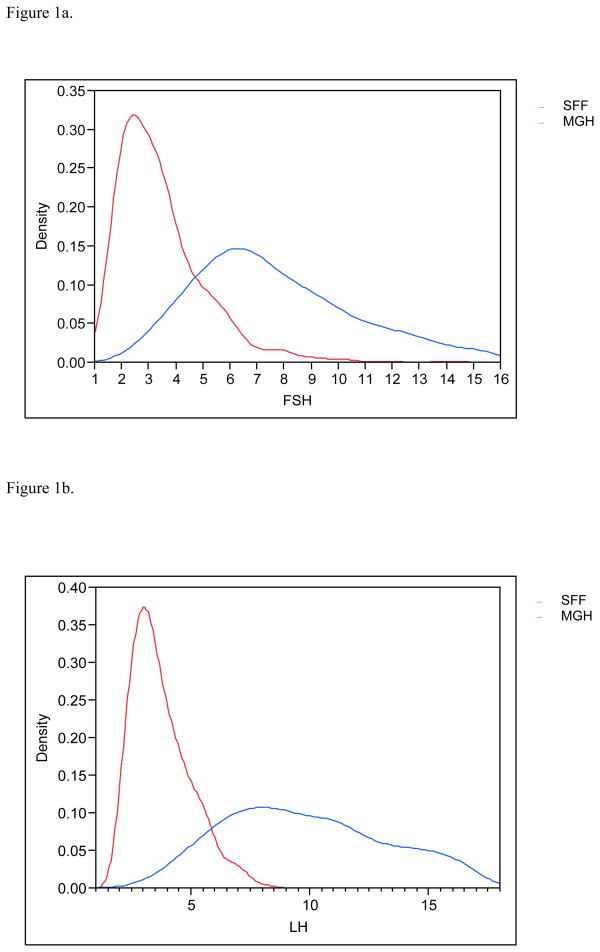
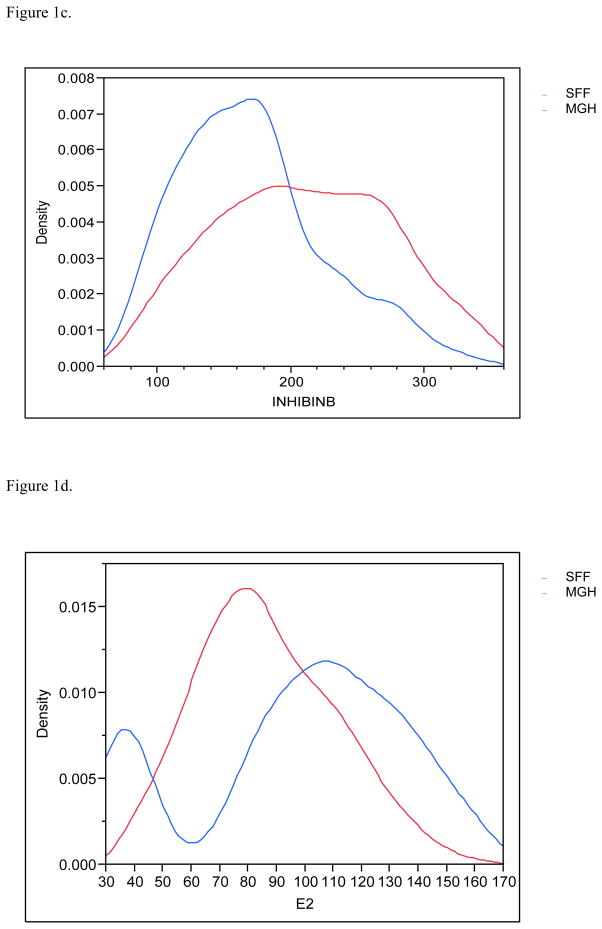
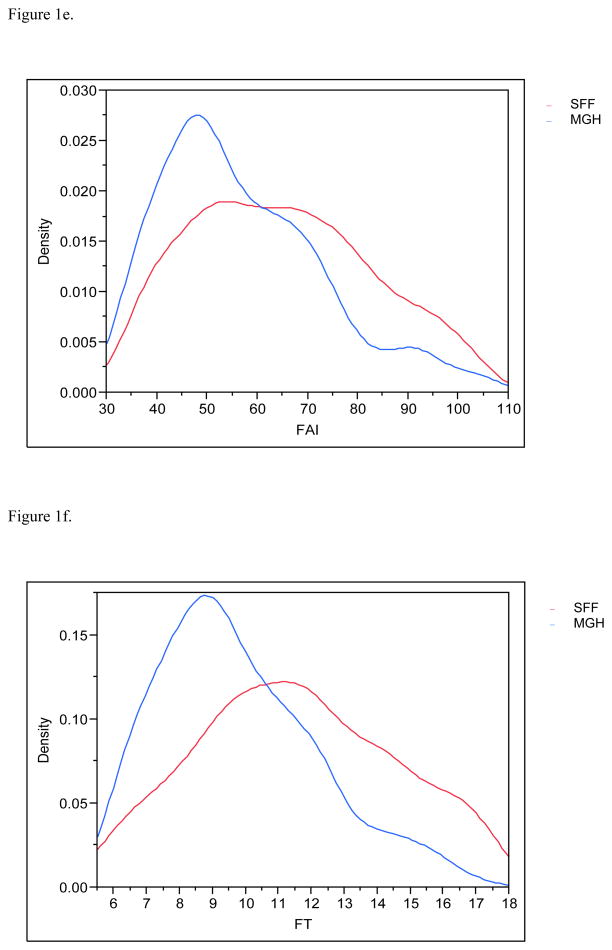
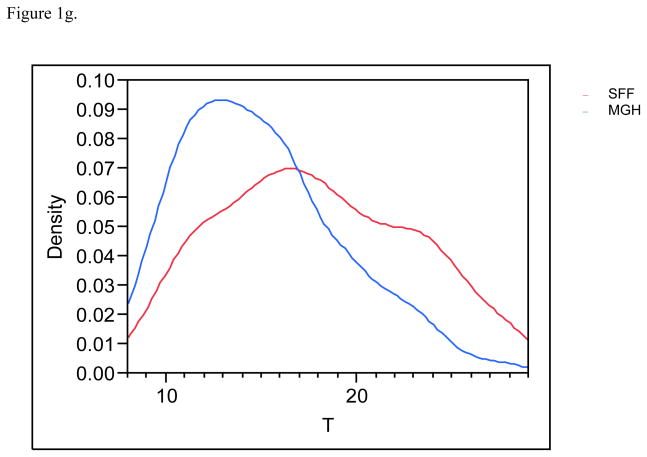
Serendipitously, 425 men in each population provided urine and blood. Estradiol and inhibin B serum levels were available for 830 and 849 males respectively and 783 had complete information on all covariates and were included in the final multivariate analyses. MEHHP and MEOHP urinary concentrations were measured in 646 men, as these metabolites were not incorporated into the MGH study until after the study had already begun. Basic demographic data are presented in Table 2, including information about reproductive parameters in the separate and joint populations; Figures 1a–1g present the frequency distribution of the reproductive hormones measured in the two populations. Summary statistics for the serum concentrations of men’s reproductive hormones are presented in Table 3. All hormone levels differed significantly between the two populations. Both FSH and LH were about three-fold higher in MGH men compared to SFF men, and inhibin B levels were lower in MGH men.
Table 2.
Characteristics of the SFF and MGH study populations
| SFF N=425 |
MGH N=425 |
Total N=850 |
|
|---|---|---|---|
| Mean (SD) | |||
| Age (years) | 32.2 (6.2) | 36 (5.3) | 34.3 (6.1) |
| BMI (kg/m2) | 28.2 (5.4) | 28 (4.5) | 28.1 (4.9) |
| Percent of men | |||
| Current smoker | 21 | 9 | 15 |
| White, non Hispanic | 72.3 | 85 | 79 |
| Sperm concentration < 20 ×106/mL | 7.8 | 15.3 | 12 |
| Sperm motility (A+B) < 50% | 37.4 | 45.9 | 42 |
| Made a partner pregnanta | 100 | 41.6 | 71 |
| Had trouble fathering a childb | 4.3 | 100 | 52 |
SFF: Study for Future Families
MGH: Massachusetts General Hospital
SD: Standard deviation
In SFF, all men were partners of pregnant women. In MGH, this is the percent of men who self-reported that they had ‘ever made a partner pregnant’
In MGH, all men were in a couple seeking evaluation or treatment for infertility. In SFF this is the percent of men who responded positively to the question: ‘Have you ever seen a doctor because you thought you might be having trouble fathering a child?’
Figure 1.
Figure 1a. Distribution (density) of the serum follicle stimulating hormone (FSH) profiles for SFF and MHG. All data have been truncated to fall between the 5th and 95th percentiles.
Figure 1b. Distribution (density) of the serum luteinizing hormone (LH) profiles for SFF and MHG. All data have been truncated to fall between the 5th and 95th percentiles.
Figure 1c. Distribution (density) of the serum inhibin B profiles for SFF and MHG. All data have been truncated to fall between the 5th and 95th percentiles.
Figure 1d. Distribution (density) of the serum estradiol (E2) profiles for SFF and MHG. All data have been truncated to fall between the 5th and 95th percentiles.
Figure 1e. Distribution (density) of the free androgen index (FAI) profiles for SFF and MHG. All data have been truncated to fall between the 5th and 95th percentiles.
Figure 1f. Distribution (density) of the free testosterone (FT) profiles for SFF and MHG. All data have been truncated to fall between the 5th and 95th percentiles.
Figure 1g. Distribution (density) of the serum testosterone (T) profiles for SFF and MHG. All data have been truncated to fall between the 5th and 95th percentiles.
Table 3.
Summary statistics for serum reproductive hormone levels in men from both studies separately and combined (N=850)
| Study | Geometric Mean | Selected Percentiles | P valuee | |||
|---|---|---|---|---|---|---|
| 10th | 50th | 90th | ||||
| Variables | ||||||
| FSH (IU/L) | ||||||
| SFF | 2.9 | 1.5 | 2.9 | 5.5 | ||
| MGH | 8.0 | 4.3 | 7.5 | 15.7 | ||
| TOTAL | 4.8 | 1.9 | 4.8 | 11.8 | < 0.001 | |
| LH (IU/L) | ||||||
| SFF | 3.3 | 1.9 | 3.3 | 5.4 | ||
| MGH | 10.1 | 5.8 | 9.9 | 17.2 | ||
| TOTAL | 5.7 | 2.4 | 5.5 | 14.5 | < 0.001 | |
| T (nmol/L) | ||||||
| SFF | 17.7 | 10.9 | 18.1 | 28.7 | ||
| MGH | 13.8 | 8.8 | 14.1 | 21.1 | ||
| TOTAL | 15.6 | 9.7 | 15.9 | 25 | < 0.001 | |
| Inhibin B (pg/mL) | ||||||
| SFFa | 207 | 120 | 218 | 333 | ||
| MGH | 147 | 81.6 | 160 | 262 | ||
| TOTALc | 174 | 101 | 182 | 299 | < 0.001 | |
| E2 (pmol/L) | ||||||
| SFFb | 80.1 | 53 | 83 | 121 | ||
| MGH | 96.2 | 36.7 | 110 | 165 | ||
| TOTALd | 88 | 36.8 | 94.5 | 143 | < 0.001 | |
| SHBG (nmol/L) | ||||||
| SFF | 27.6 | 15 | 28 | 50.4 | ||
| MGH | 25.8 | 15.3 | 26 | 44 | ||
| TOTAL | 26.7 | 15 | 27 | 47 | 0.01 | |
| FAI | ||||||
| SFF | 64.2 | 39.7 | 65 | 100 | ||
| MGH | 53.4 | 34.9 | 52.1 | 83.6 | ||
| TOTAL | 58.6 | 37.1 | 58.4 | 93.8 | < 0.001 | |
| FT | ||||||
| SFF | 11.5 | 7.4 | 11.8 | 17.5 | ||
| MGH | 9.0 | 6.1 | 9.1 | 12.9 | ||
| TOTAL | 10.2 | 6.5 | 10.3 | 15.9 | < 0.001 | |
| T/E2 ratio | ||||||
| SFFb | 0.22 | 0.11 | 0.23 | 0.40 | ||
| MGH | 0.14 | 0.08 | 0.13 | 0.32 | ||
| TOTALd | 0.18 | 0.09 | 0.18 | 0.37 | < 0.001 | |
N= 424 for Inhibin B
N= 405 for E2 and T/E2 ratio
N= 849 for Inhibin B
N= 830 for E2 and T/E2 ratio,
Mann-Whitney U Test
The urinary concentrations (in ng/mL) of DEHP metabolites (before urine dilution adjustment) are shown in Table 4, together with the LOD and percent of samples above the LOD. Urinary concentrations of DEHP metabolites were notably higher in MGH men than men in SFF, while MEP, MBP and MBzP were higher in SFF men. MEHP% was similar in the two populations.
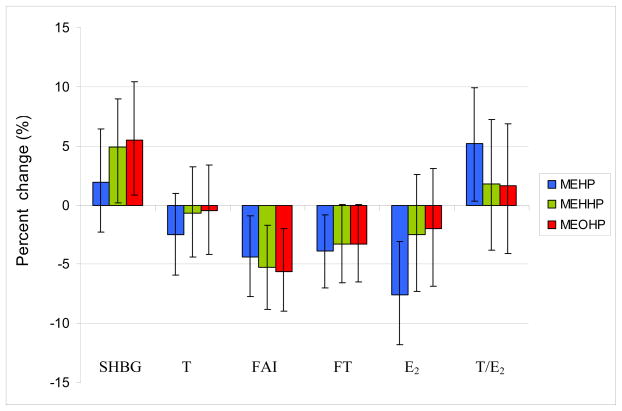
Table 5 shows correlation coefficients for reproductive hormones and unadjusted urinary DEHP metabolite concentrations from initial bivariate analyses. We observed no associations between any hormone levels and any urinary metabolites of phthalates other than DEHP (data available on request). Therefore, here we report only the associations involving the three measured metabolites of DEHP (MEHP, MEHHP and MEOHP). Table 6 shows the results of the multivariate analysis for reproductive hormones and urinary DEHP metabolite concentrations in both populations separately and combined. After adjustment for covariates many of the relationships (as described by the β coefficients) were consistent with previously published results (Meeker et al, 2009; Mendiola et al, 2010), though the effect estimate for E2 strengthened in the pooled analysis. Overall, an increase in statistical power due to increased sample size resulted in increased precision in the effect estimates compared to the individual studies. There were no significant associations between T and any urinary DEHP metabolites. FAI and FT were both inversely associated with the urinary concentrations of all three urinary DEHP metabolites measured in the study (MEHP, MEHHP and MEOHP). Serum gonadotropin levels (FSH and LH) were not associated with DEHP metabolite concentrations in the separate or combined populations. There was a significant inverse association between E2 levels and urinary MEHP concentrations, but not with the other DEHP metabolites. T/E2 ratio was positively associated with urinary MEHP metabolite concentrations. SHBG levels were positively related to urinary MEHHP and MEOHP concentrations but not MEHP concentration. Figure 2 shows the percent change in men’s reproductive hormones expected with an inter-quartile increase in urinary DEHP metabolite concentrations for a 34-year-old non-smoker with BMI of 28 kg/m2. For this typical subject, an increase in urinary concentrations of MEHP and the oxidative metabolites (MEHHP and MEOHP) from the 25th to the 75th percentile would be predicted to decrease steroid hormone levels the amount ranging from 3.5% and 7%, for T and E2 respectively.
Table 5.
Correlation coefficients for reproductive hormones and DEHP metabolites1 concentrations in men (bivariate analysis) (n=850)
| Study | MEHP | MEHHP | MEOHP | ||||
|---|---|---|---|---|---|---|---|
| R | 95% CI | R | 95% CI | R | 95% CI | ||
| FSH | |||||||
| SFF | .003 | (−.09, .10) | −.01 | (−.11, .09) | −.01 | (−.11, .09) | |
| MGH | .04 | (−.05, .14) | −.03 | (−.16, .10) | −.04 | (−.17, .09) | |
| TOTAL | .16 | (.09, .23)d | .10 | (.03, .18)d | .14 | (.06, .22)d | |
| LH | |||||||
| SFF | −.01 | (−.11, .09) | −.02 | (−.12, .08) | −.03 | (−.13, .07) | |
| MGH | −.04 | (−.14, .05) | −.01 | (−.15, .12) | −.01 | (−.14, .13) | |
| TOTAL | .14 | (.07, .21)d | .12 | (.04, .20)d | .16 | (.08, .24)d | |
| T | |||||||
| SFF | −.07 | (−.17, .03) | −.09 | (−.19, .01) | −.10 | (−.20, −.001)c | |
| MGH | −.15 | (−.24, −.05)c | −.13 | (−.25, .001) | −.12 | (−.25, .01) | |
| TOTAL | −.16 | (−.23,−.10)d | −.15 | (−.23, −.08)d | −.17 | (−.25, −.09)d | |
| E2 | |||||||
| SFFa | −.06 | (−.16, .04) | −.02 | (−.12, .08) | −.02 | (−.12, .08) | |
| MGH | −.12 | (−.22, −.03)c | −.07 | (−.20, .06) | −.06 | (−.19, .08) | |
| TOTALb | −.04 | (−.10, .03) | −.02 | (−.10, .06) | −.01 | (−.09, .07) | |
| SHBG | |||||||
| SFF | .06 | (−.04, .16) | −.03 | (−.13, .07) | −.03 | (−.13, .07) | |
| MGH | −.05 | (−.15, .05) | .03 | (−.10, .16) | .04 | (−.09, .17) | |
| TOTAL | −.01 | (−.08, .05) | −.02 | (−.10, .06) | −.02 | (−.10, .06) | |
| FAI | |||||||
| SFF | −.15 | (−.25, −.06)d | −.06 | (−.16, .04) | −.07 | (−.17, .03) | |
| MGH | −.08 | (−.18, .01) | −.17 | (−.29, −.04)d | −.17 | (−.29, −.04)d | |
| TOTAL | −.15 | (−.22,−.09)d | −.14 | (−.21, −.06)d | −.16 | (−.23, −.08)d | |
| FT | |||||||
| SFF | −.12 | (−.22, −.03)d | −.09 | (−.19, .01) | −.10 | (−.20, −.001)c | |
| MGH | −.16 | (−.25, −.06)d | −.19 | (−.31, −.06)d | −.19 | (−.31, −.05)d | |
| TOTAL | −.19 | (−.26,−.13)d | −.17 | (−.25, −.10)d | −.19 | (−.27, −.12)d | |
| T/E2 | |||||||
| SFFa | −.002 | (−.10, .10) | −.05 | (−.15, .05) | −.06 | (−.16, .04) | |
| MGH | .03 | (−.07, .13) | −.01 | (−.14, .12) | −.02 | (−.15, .11) | |
| TOTALb | −.07 | (−.14, .−.01)c | −.09 | (−.17, −.01)c | −.11 | (−.19, −.03)d | |
non-creatinine-adjusted/non-SG-adjusted
N= 405 for E2
N= 830 for E2
P value≤.05
P value≤.01
R= correlation coefficient
CI= confidence interval
Log-transformations of phthalate metabolites and men sex hormones, except for E2 were used
Table 6.
Multivariate analysis for reproductive hormones and DEHP metabolites concentrations in men (n=783)1
| Study | MEHP | MEHHP | MEOHP | ||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | ||
| FSH | |||||||
| SFF | .01 | (−.03, .06) | .01 | (−.04, .06) | .01 | (−.04, .05) | |
| MGH | .02 | (−.02, .05) | −.02 | (−.07, .04) | −.02 | (−.07, .03) | |
| TOTAL | .01 | (−.01, .04) | −.01 | (−.04, .03) | −.01 | (−.05, .02) | |
| LH | |||||||
| SFF | .01 | (−.03, .05) | −.01 | (−.05, .03) | −.01 | (−.05, .03) | |
| MGH | −.01 | (−.04, .02) | −.002 | (−.05, .04) | .001 | (−.04, .05) | |
| TOTAL | −.01 | (−.03, .01) | −.02 | (−.05, .01) | −.02 | (−.05, .01) | |
| T | |||||||
| SFF | .01 | (−.03, .04) | −.01 | (−.04, .03) | −.01 | (−.04, .03) | |
| MGH | −.02 | (−.04, −.003)c | −.02 | (−.05, .01) | −.02 | (−.05, .02) | |
| TOTAL | −.01 | (−.03, .005) | −.01 | (−.03, .02) | −.01 | (−.03, .02) | |
| E2 | |||||||
| SFFa | −4.6 | (−10.4, 1.1) | −2.6 | (−8.4, 3.2) | −2.9 | (−8.8, 3.0) | |
| MGH | −3.1 | (−5.7, −.46)c | −1.9 | (−5.9, 2.1) | −1.5 | (−5.3, 2.4) | |
| TOTALb | −7.9 | (−12.4, −3.5)d | −3.4 | (−8.8, 1.9) | −3.0 | (−8.4, 2.3) | |
| SHBG | |||||||
| SFF | .05 | (.02, .09)d | .03 | (−.01, .07) | .03 | (−.01, .07) | |
| MGH | −.01 | (−.03, .02) | .02 | (−.02, .06) | .03 | (−.01, .07) | |
| TOTAL | .01 | (−.01, .03) | .03 | (.002, .06)c | .03 | (.005, .06)c | |
| FAI | |||||||
| SFF | −.05 | (−.08, −.02)d | −.04 | (−.07, −.004)d | −.04 | (−.07, −.01)d | |
| MGH | −.01 | (−.03, .01) | −.03 | (−.06, −.001)c | −.03 | (−.06, −.002)c | |
| TOTAL | −.02 | (−.04, .−.01)c | −.04 | (−.06, −.01)d | −.04 | (−.06, −.01)d | |
| FT | |||||||
| SFF | −.02 | (−.05, .01) | −.02 | (−.05, .01) | −.02 | (−.05, .01) | |
| MGH | −.02 | (−.04, −.004)c | −.03 | (−.06, .001) | −.03 | (−.06, .001) | |
| TOTAL | −.02 | (−.04, .−.004)c | −.02 | (−.04, −.001)c | −.02 | (−.04, −.001)c | |
| T/E2 | |||||||
| SFFa | .03 | (−.02, .07) | .004 | (−.04, .05) | .003 | (−.04, .05) | |
| MGH | .03 | (−.008, .06) | .02 | (−.04, .08) | .02 | (−.04, .08) | |
| TOTALb | .03 | (.001, .05)c | .01 | (−.02, .05) | .01 | (−.03, .05) | |
Controlling for age, age square, BMI, smoking status (current smoker vs. never smoked), ethnicity (African-American vs. others), study center (SFF vs. MGH), time of sample collection and time of sample collection squared. In addition, to take into account urinary dilution, SFF model also was adjusted by urinary creatinine values, MGH by specific gravity and the joint analysis (TOTAL) by ranking both variables.
N= 346 for E2
N= 766 for E2
P value≤.05,
P value≤.01
β= regression coefficient, CI= confidence interval
Log-transformations of phthalate metabolites and men sex hormones, except for E2 were used
Figure 2.
Percent change in men’s reproductive hormones expected with an increase from the 25th to the 75th percentile in DEHP metabolite concentrations for a standard subject (34 years old, non-smoker with BMI of 28 kg/m2). Error bars indicate the 95% confidence intervals.
DISCUSSION
This is the first study to examine the associations between urinary concentrations of phthalate metabolites and reproductive hormone serum levels in a large cohort including both fertile men and male partners of infertile couples. Our results suggest that exposure to DEHP at environmental concentrations is associated with statistically significant declines in free testosterone (both FAI and FT) and serum estradiol (E2). The other phthalate monoester metabolites we examined (MEP, MBP and MBzP) were not associated with any reproductive hormones. These associations are not substantially different from those reported in the separate analyses, which in turn do not differ appreciably between the two populations (Meeker et al, 2009; Mendiola et al, 2010). However, each of the individual studies provides information only about a limited subset of the total population. When the two populations are combined, the effect estimates are more precise and more generalizable to men of reproductive age.
In this combined population of fertile and subfertile men, we saw no significant associations with total T levels and any phthalate metabolites. However, both FT and FAI were both inversely associated with urinary DEHP metabolite concentrations. This may be accounted for by a positive association between serum SHBG levels and urinary MEHP concentrations in the SFF cohort and with MEHHP and MEOHP in the combined analysis. Significant positive associations were seen between SHBG and MEHHP and MEOHP in the combined analysis. However, associations between SHBG and MEHP differed in these two cohorts, with a significant positive association in SFF men, but a non-significant negative association in the MGH cohort. This resulted in a non-significant positive association between SHBG and MEHP in the combined analyses. It should be noted that the serum SHBG concentration in all the subjects are within the physiological range of adult men. Thus, the small increases in serum SHBG levels associated with greater DEHP may result in a small reduction of FT without affecting the total serum T levels.
We did not see an association between DEHP metabolite concentrations and LH in this combined population of fertile and infertile men. In this mixed population the small changes in FT and FAI associated with DEHP may not be sufficient to elicit the negative feedback that would be expected to produce a positive association between LH and DEHP metabolites.
Although all men had serum steroid hormones within the laboratory reference ranges, our findings suggest a somewhat anti-androgenic effect of DEHP. This is consistent with data showing that phthalates may inhibit expression of genes involved in steroidogenesis (cholesterol transport and the biosynthesis of testosterone) in rat fetal testis after in-utero exposure to large doses of DEHP (Borch et al, 2006).
Estradiol plays a role in male germ cell survival in vitro (Pentikainen et al, 2000). In our study urinary MEHP concentrations were inversely associated with serum E2 levels and positively associated with T/E2 ratio. In vitro and animal studies have shown that aromatase activity, and E2 production, can be lowered by DEHP and/or MEHP (Andrade et al, 2006; Davis et al, 1994; Lovekamp and Davis, 2001; Noda et al, 2007). Our results suggest that, as in rodent models, DEHP may be associated with a reduced aromatase activity.
We compared unadjusted urinary concentrations of DEHP metabolites in our subjects to those from men participants in the 2007–2008 National Health and Nutrition Examination Survey (NHANES) (CDC, 2011). Median MEHP concentration was almost twice as high in our combined population (4.4 ng/mL compared to 2.3 ng/mL), while the other DEHP metabolites were similar (e.g., medians 20.9 and 23.2 ng/mL for MEHHP in NHANES and our population).
Our data were limited by the use of a single urine and blood sample to assess DEHP exposure and hormone function, respectively. However, several studies have reported that although phthalate metabolite concentrations are variable within an individual over time, the average concentration over the course of days, weeks or months can be satisfactorily predicted by a single sample (Hauser et al, 2004; Hoppin et al, 2002; Teitelbaum et al, 2008). Similarly, a single sample can be used to classify reproductive hormone levels in men (Bjornerem et al, 2006).
It is generally accepted that hormone levels obtained in different laboratories or/and with different methods are likely to differ. The variations among laboratories are more marked for steroid hormone levels at low levels (e.g. T and E2 levels in men) than for gonadotropins (Pitteloud et al, 2008; Rosner et al, 2007; Sikaris et al, 2005; Taieb et al, 2003; Wang et al, 2004). We included a center effect in our multivariate models to reflect between-laboratory differences. Adding this covariate did not alter associations between urinary DEHP metabolites and androgens (T, FT and FAI). However, it did slightly increase effect estimates for E2 and SHBG and decreased them for LH and FSH.
One limitation of all previously published studies on phthalate metabolites and reproductive parameters is that their study populations (fertile men or men in infertility clinics) are not representative of the general population. Our combined analysis includes a wider range of men, though still not a representative sample of adult men.
In conclusion, our results in this population, including both fertile and infertile men, suggest that DEHP exposure is associated with some changes in circulating levels of male sex steroid hormones, consistent with the known anti-androgenic effect of this chemical.
Acknowledgments
The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the CDC. The authors gratefully acknowledge Manori J. Silva, Ella Samandar, James Preau and Jack Reidy (CDC, Atlanta, GA) for measuring the urinary concentrations of the phthalate metabolites. This study is supported by the following grants: The Danish Agency for Science, Technology and Innovation, grant no. 271070678 to N.J.; University of Iowa Center for Health Effects of Environmental Contamination cooperative project grant to A.S.; the General Clinical Research Center at Harbor-UCLA Medical Center (MO1 RR00425); NIEHS grant ES009718 to R.H.
References
- Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. 2007;92:4696–4705. doi: 10.1210/jc.2006-2633. [DOI] [PubMed] [Google Scholar]
- Andrade A, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227:185–192. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Asklund C, Jørgensen N, Skakkebaek NE, Jensen TK. Increased frequency of reproductive health problems among fathers of boys with hypospadias. Hum Reprod. 2007;22:2639–2646. doi: 10.1093/humrep/dem217. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP) Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2002. [PubMed] [Google Scholar]
- Bang AK, Carlsen E, Holm M, Petersen JH, Skakkebaek NE, Jørgensen N. A study of finger lengths, semen quality and sex hormones in 360 young men from the general Danish population. Hum Reprod. 2007;20:3109–3113. doi: 10.1093/humrep/dei170. [DOI] [PubMed] [Google Scholar]
- Bjornerem A, Straume B, Oian P, Berntsen GK. Seasonal variation of estradiol, follicle stimulating hormone, and dehydroepiandrosterone sulfate in women and men. J Clin Endocrinol Metab. 2006;91:3798–3802. doi: 10.1210/jc.2006-0866. [DOI] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223:144–155. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Box GEP, Tidwell PW. Transformation of the independent variables. Technometrics. 1962;5:531–550. [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during the past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; National Center for Environmental Health; Atlanta, GA: 2011. http://www.cdc.gov/exposurereport/pdf/Updated_Tables.pdf. [Google Scholar]
- Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2-ethylhexyl) phthalate suppresses estradiol production independent of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994;128:224–228. doi: 10.1006/taap.1994.1201. [DOI] [PubMed] [Google Scholar]
- Dostal LA, Chapin RE, Stefanski SA, Harris MW, Schwetz BA. Testicular toxicity and reduced Sertoli cell numbers in neonatal rats by di(2-ethylhexyl)phthalate and the recovery of fertility as adults. Toxicol Appl Pharmacol. 1988;95:104–121. doi: 10.1016/s0041-008x(88)80012-7. [DOI] [PubMed] [Google Scholar]
- Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani DC, Hauser R. Phthalate exposure and human semen parameters. Epidemiology. 2003;14:269–277. [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. Phthalate exposure and reproductive hormones in adult men. Hum Reprod. 2005;20:604–610. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Gulati DK, Mounce RC, Russell SR, Lamb JC., 4th Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol. Fundam Appl Toxicol. 1989;12:508–518. doi: 10.1016/0272-0590(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Herr C, Zur Nieden A, Koch HM, Schuppe HC, Fieber C, Angerer J, Eikmann T, Stilianakis NI. Urinary di(2-ethylhexyl) phthalate (DEHP) Metabolites and male human markers of reproductive function. Int J Hyg Environ Health. 2009;212:648–653. doi: 10.1016/j.ijheh.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Higuchi TT, Palmer JS, Gray LE, Jr, Veeramachaneni DN. Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure. Toxicol Sci. 2003;72:301–313. doi: 10.1093/toxsci/kfg036. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:48–51. [Google Scholar]
- Jönsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16:487–493. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- Jørgensen N, Rajpert-De Meyts E, Main KM, Skakkebæk NE. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int J Androl. 2010;33:298–303. doi: 10.1111/j.1365-2605.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- Li LH, Jester WF, Jr, Orth JM. Effects of relatively low levels of mono-(2-ethylhexyl) phthalate on cocultured Sertoli cells and gonocytes from neonatal rats. Toxicol Appl Pharmacol. 1998;153:258–265. doi: 10.1006/taap.1998.8550. [DOI] [PubMed] [Google Scholar]
- Li LH, Jester WF, Jr, Laslett AL, Orth JM. A single dose of Di-(2-ethylhexyl) phthalate in neonatal rats alters gonocytes, reduces sertoli cell proliferation, and decreases cyclin D2 expression. Toxicol Appl Pharmacol. 2000;166:222–229. doi: 10.1006/taap.2000.8972. [DOI] [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172:217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson AM, Toppari J, Skakkebaek NE. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl. 2009;30:287–297. doi: 10.2164/jandrol.108.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Jørgensen N, Andersson AM, Calafat AM, Silva MJ, Redmon JB, Sparks A, Drobnis EZ, Wang C, Liu F, Swan SH. Associations between urinary metabolites of di(2-ethylhexyl) phthalate and reproductive hormones in fertile men. Int J Androl. 2010 doi: 10.1111/j.1365–2605.2010.01095.x. in press. Epub ahead of print 14 Jul 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Ohno S, Nakajin S. Mono-(2-ethylhexyl) phthalate (MEHP) induces nuclear receptor 4A subfamily in NCI-H295R cells: a possible mechanism of aromatase suppression by MEHP. Mol Cell Endocrinol. 2007;274:8–18. doi: 10.1016/j.mce.2007.05.004. [DOI] [PubMed] [Google Scholar]
- NRC. Hormonally active agents in the environment. National Research Council, National Academies Press; Washington, DC: 1999. [PubMed] [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of din-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006;114:1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE., Jr The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Parmar D, Srivastava SP, Seth PK. Effect of di(2-ethylhexyl) phthalate (DEHP) on spermatogenesis in adult rats. Toxicology. 1986;42:47–55. doi: 10.1016/0300-483x(86)90091-0. [DOI] [PubMed] [Google Scholar]
- Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab. 2000;85:2057–2067. doi: 10.1210/jcem.85.5.6600. [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, Crowley WF, Jr, Hayes FJ. The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab. 2008;93:2686–2692. doi: 10.1210/jc.2007-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Utility, limitations, and pitfalls in measuring Testosterone: An Endocrine Society Position Statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebæk NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89(2 Suppl):33–38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Sikaris K, McLachlan RI, Kazlauskas R, de KD, Holden CA, Handelsman DJ. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab. 2005;90:5928–5936. doi: 10.1210/jc.2005-0962. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Srivastava SP, Srivastava S, Saxena DK, Chandra SV, Seth PK. Testicular effects of di-n-butyl phthalate (DBP): biochemical and histopathological alterations. Arch Toxicol. 1990;64:148–152. doi: 10.1007/BF01974401. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997;105:1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, Redmon JB, Wang C, Overstreet JW. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1381–1395. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philos Trans R Soc Lond B Biol Sci. 2009;364:2079–2096. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Moore CJ, vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc Lond B Biol Sci. 2009;364:2153–2166. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, O’Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92:196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]