Abstract
Dendritic cells (DCs) are central to innate and adaptive immunity of early kidney ischemia–reperfusion injury (IRI), and strategies to alter DC function may provide new therapeutic opportunities. Sphingosine 1-phosphate (S1P) modulates immunity through binding to its receptors (S1P1–5), and protection from kidney IRI occurs in S1P3-deficient mice. Through a series of experiments we determined that this protective effect was owing in part to differences between S1P3-sufficient and -deficient DCs. Mice lacking S1P3 on bone marrow cells were protected from IRI, and S1P3-deficient DCs displayed an immature phenotype. Wild-type (WT) but not S1P3-deficient DCs injected into mice depleted of DCs prior to kidney IR reconstituted injury. Adoptive transfer (i.e., i.v. injection) of glycolipid (Ag)-loaded WT but not S1P3-deficient DCs into WT mice exacerbated IRI, suggesting that WT but not S1P3-deficient DCs activated NKT cells. Whereas WT DC transfers activated the Th1/IFN-γ pathway, S1P3-deficient DCs activated the Th2/IL-4 pathway, and an IL-4–blocking Ab reversed protection from IRI, supporting the concept that IL-4 mediates the protective effect of S1P3-deficient DCs. Administration of S1P3-deficient DCs 7 d prior to or 3 h after IRI protected mice from IRI and suggests their potential use in cell-based therapy. We conclude that absence of DC S1P3 prevents DC maturation and promotes a Th2/IL-4 response. These findings highlight the importance of DC S1P3 in modulating NKT cell function and IRI and support development of selective S1P3 antagonists for tolerizing DCs for cell-based therapy or for systemic administration for the prevention and treatment of IRI and autoimmune diseases.
Introduction
The pathogenesis of kidney injury following kidney ischemia–reperfustion (IR) involves a complex interaction between altered microcirculatory hemodynamics, endothelial and epithelial cells, and infiltration of immune cells (1, 2). Dendritic cells (DCs), the major subset of leukocytes in the kidney (3–5), contribute to innate and adaptive immunity of kidney IR injury (IRI) (6) through activation of NKT cells and T cells (7–9). NKT cells, a specialized innate T cell subpopulation, recognize glycolipid Ags presented by CD1d, a nonclassical MHC class I (MHCII) molecule (10). Additionally, IL-12 and IL-23 release from DCs (11, 12), interaction of CD40/CD40L, and CD1d/glycolipid presentation lead to NKT cell activation (11, 12) in the early innate immune response of IRI (3, 7). Infiltration and activation of IFN-γ–producing NKT cells leads to the downstream production of CXCL1, CXCL1-mediated neutrophil infiltration (7), and neutrophil-dependent IFN-γ and IL-17 production (12). In contrast, IL-18 mediates a pro-Th2–like response by enhancing NKT cell IL-4 production independently of IL-12 (13), indicating that different conditions skew NKT cell Th1–Th2 polarity. NKT cell activation has beneficial or detrimental effects depending on activation-induced polarization of Th1 or Th2 responses. Unlike IRI, activation of NKT cells in autoimmune diseases results in amelioration of disease by shifting the balance toward a Th2 response.
Sphingosine 1-phosphate (S1P), a sphingolipid that is produced by phosphorylation of sphingosine by sphingosine kinases, is the natural ligand for a family of five G protein-coupled receptors (S1P1–5) and evokes diverse cellular signaling responses (14–16). Most of the S1P effects are mediated through the S1P receptor family, which includes the ubiquitously expressed S1P1, S1P2, and S1P3 subtypes (17). T cells express S1P1 at a higher amount compared to S1P4 (18, 19), and human DCs express S1P1–4 (20).
Tissue injury and repair are modulated by subtype-specific S1P receptors. Whereas S1P1 expressed in proximal tubule cells attenuates kidney IRI (21), S1P3 activation initiates fibrosis in the heart (22), and S1P3 on bone marrow (BM) mesenchymal stem cells mediates fibrosis in the liver (23, 24). S1P3 regulates vascular permeability (25), arterial vasodilatation (26), and splenic endothelial sinus organization (27). In DCs, S1P3 signaling is coupled to protease activated receptor 1, leading to lethal outcomes in sepsis (28). In contrast, in some studies S1P3 is beneficial. Although activation of S1P3 protects hearts from IRI (29, 30), sphingosine 1-phosphate-3 receptor (S1pr3)−/− mice are protected from kidney IRI (31). Cell-specific expression of S1P3 likely contributes to the heterogeneity of outcomes. Given the importance of the DC/NKT pathway in kidney IRI (7) and that S1P3 is expressed on DCs (32) to regulate critical cytokines that participate in inflammation (28), we hypothesized that DC S1P3 activation of NKT cells plays an important role in kidney IRI.
Materials and Methods
All animals were handled and procedures were performed in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Virginia Institutional Animal Care and Use Committee.
Generation of BM chimeric mice
S1pr3−/− mice (a gift from Dr. Richard Proia, National Institutes of Health) and S1P3 wild-type (WT) littermate control male mice (C57BL/6 background) were used for BM transplantation as previously described (33). Briefly, lethally irradiated recipient mice were injected with 107 donor BM cells (i.v.), and the resulting chimeric mice were maintained for 6–8 wk before experimentation.
Diphtheria toxin-mediated DC ablation
CD11c-diphtheria toxin receptor (DTR) transgenic (Tg) mice (B6.FVB-Tg Itgax-DTR/GFP 57Lan/J) harboring a transgene encoding a simian DTR-GFP fusion protein under the transcriptional control of mouse CD11c promoter were purchased (The Jackson Laboratory). DCs were depleted in male CD11c-DTR Tg mice (8 wk old, 25–28 g) by a single injection of DT (Sigma-Aldrich; 4 ng/g body weight, i.v.), and ablation was confirmed by immunohistochemical staining for CD11c+MHCII+ DCs in kidney (6). DT-treated CD11c-DTR Tg littermates negative for the transgene (CD11c-DTR−) were used as controls. WT or S1pr3−/− BMDCs (0.5 × 106) were injected i.v. 20 h after DT injection into CD11c-DTR Tg and control mice, and IRI was performed 6 h after DC injection.
Renal IRI and blocking IL-4
Male mice (8–12 wk old, C57BL/6, from the National Cancer Institute, Frederick, MD) were subjected to bilateral IRI (24–28 min ischemia, then 24 h reperfusion) as previously described (3, 7, 12). Control, sham-operated mice underwent a similar procedure, but the renal pedicles were not clamped. Mice injected with BM-derived DCs (BMDCs) that were loaded with α-galactosylceramide (α-GalCer; Alexis Biochemicals; isolated and primed as described below) underwent mild ischemia of 24 min with 24 h reperfusion. An IL-4–blocking mAb (clone 11B11; eBioscience; 50, 75, or 100 μg, i.p.) or rat IgG1 isotype control Ab was administered to WT or S1pr3−/− mice 24 h before kidney IR.
Assessment of kidney function and histology
Plasma creatinine, as a measure of kidney function, was determined using a colorimetric assay according to the manufacturer’s protocol (Sigma-Aldrich). For histology, kidneys were fixed overnight in 0.2% sodium periodate/1.4% dl-lysine/4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and embedded in paraffin. Kidneys were prepared for H&E staining as previously described (3) and viewed by light microscopy (Zeiss Axioskop). Photographs were taken and brightness/contrast adjustment was made with a SPOT RT camera (software version 3.3; Diagnostic Instruments). The histological change in the outer medulla was expressed as acute tubular necrosis (ATN), scored as previously described (34).
Immunohistochemical analysis
Kidneys were fixed in 0.2% sodium periodate/1.4% dl-lysine/1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) overnight, incubated in 30% sucrose for 48 h at 4°C, and embedded and frozen in OCT (Ted Pella). Frozen sections (7 μm) were permeabilized with 0.3% Triton X-100, and nonspecific binding was blocked with 10% horse serum and anti-mouse CD16/32 (10 μg/ml; clone 2.4G2; StemCell Technologies). Sections were labeled with FITC-labeled anti-neutrophil mAb (7 μg/ml; clone 7/4; Cedarlane Laboratories) for 1 h. Kidney sections stained for DCs were permeabilized and blocked as described above and then treated with 3% H2O2 in PBS for 15 min followed by incubation for 1 h with biotinylated anti-CD11c (1:100, clone HL3; BD Pharmingen), HRP-conjugated streptavidin (Jackson ImmunoResearch Laboratories, 1:100 in TNB buffer [PerkinElmer]), biotin-tyramide amplification (PerkinElmer), and with streptavidin-Alexa 555 (1:500; Invitrogen). All specimens were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen) to label cell nuclei. Images were acquired using a Zeiss Axiovert 200 microscopy system with ApoTome imaging and AxioVision software (Carl Zeiss).
BMDC culture and NKT1.2 hybridomas
Eight-week-old WT and S1pr3−/− male mice were used for generating highly pure DCs from whole BM precursors (35). Briefly, freshly isolated BM was cultured with 2 ng/ml recombinant mouse GM-CSF (R&D Systems) for 10 d with media changed every 3 d. Ninety-eight percent of resulting cells were CD11c+ DCs as determined by flow cytometry with CD11c and MHCII Abs. BMDCs were primed with 100 ng/ml α-GalCer or vehicle (0.1% DMSO) for 2 d in culture medium. Mouse (Vα14) and human (Vα24) invariant NKT cells can recognize α-GalCer, a glycolipid originally extracted from marine sponges (36, 37). Cells were washed, and 0.5 × 106 cells per mouse were introduced i.v. to naive mice 1 d prior to kidney IR surgery with a moderate (24 min) ischemic injury. For in vitro studies, WT or S1pr3−/− DCs loaded with α-GalCer or vehicle were cultured overnight with NKT1.2 hybridoma cells (DN3A4-1.2; a gift from Dr. Kronenberg, La Jolla Institute for Allergy and Immunology) (38) at a ratio of 1:5, and changes in IL-4 cytokine levels in the cell supernatant were measured with ELISA (BD Pharmingen). As control, NKT1.2 cells were cultured in the absence of DCs and vice versa.
S1P3 knockdown with small interfering RNA
WT DCs were seeded in medium without FBS 1 h before transfection. Two ready-to-use validated double-stranded 21-nucleotide small interfering RNAs (siRNAs) for S1P3 (J-040957-07-0010) were transfected into DCs along with ON-TARGETplus nontargeting siRNA (D-001810-01; Dharmacon, Lafayette, CA) using GenePORTER (Genlanis, San Diego, CA) and following the manufacturers’ protocols.
Activation and detection of cytokine-producing leukocyte
WT and S1pr3−/− total spleen cells or T cells isolated by negative selection from spleen (CD3+) were cultured for 72 h in supplemented RPMI 1640 under Th17-inducing conditions (2 ng/ml TGF-β, 10 ng/ml rIL-23, 50 ng/ml IL-6 [all eBioscience], 2 μg/ml anti-CD3ε Ab [BD Biosciences], 1 μg/ml LPS [Sigma-Aldrich]). Cells were restimulated by 1 μl/ml leukocyte activation kit (BD Biosciences) for 4–5 h and cytokine-producing cells were analyzed by flow cytometry.
Quantitative real-time RT-PCR
Total RNA was extracted from kidneys with TRI Reagent (Invitrogen) according to the manufacturer’s protocol and single-stranded cDNA was synthesized as previously described (34). Gene sequences were obtained from the GenBank database. Primers were designed using PrimerQuest (Integrated DNA Technologies, http://www.idtdna.com). Primer sequences were as previously published (12, 21). RT-PCR was performed using the iScript one-step RT-PCR kit with SYBR Green (Bio-Rad); samples were normalized to GAPDH. Melting curves were inspected to ensure specificity of product detection. The following PCR protocol was used: initial denaturation (95°C for 3 min); denaturation, annealing, and elongation program repeated 35 times (95°C for 45 s, 52°C for 60 s, and 72°C for 60 s); final elongation (72°C for 7 min); and finally, a holding step at 4°C.
FACS analysis
Flow cytometry was used to analyze kidney leukocyte content. In brief, kidneys were extracted, minced, digested, and passed through a filter and a cotton column as described (7). After blocking nonspecific Fc binding with anti-mouse CD16/32 (2.4G2), fresh kidney suspensions were incubated with fluorophore-tagged anti-mouse CD45 (30-F11) to determine total leukocyte cell numbers. CD45-labeled samples were further used for labeling with different combinations of anti-mouse F4/80 (BM8), GR-1 (Ly6G), CD11b, CD11c, IA (MHCII), TCR-β, and CD1d tetramer loaded with PBS-57 (1:100), an analog of α-GalCer (National Institutes of Health Tetramer Facility, Emory University, Atlanta, GA) (39). Unloaded CD1d tetramer was used as control. 7-Aminoactinomycin D (7-AAD; BD Biosciences) was added 15 min before analyzing the sample to separate live from dead cells. Intracellular staining for IFN-γ was performed using the BD Biosciences (San Jose, CA) Fix/Perm buffer set according to the manufacturer’s protocol and as described previously (7, 12). Appropriate fluorochrome-conjugated, isotype-matched, irrelevant mAbs were used as negative controls. Flow cytometry data acquisition was performed on a FACSCalibur (Becton Dickinson). Data were analyzed by FlowJo software 9.0 (Tree Star). All Abs (except as noted) were from eBioscience and were used at a concentration of 5 μg/ml.
Statistical analysis
GraphPad Instat 3 (GraphPad Software), SigmaPlot 11.0 (Systat Software), and Canvas X (ACD Systems of America) were used to analyze and present the data. Data were analyzed, after transformation when needed to generate a normal distribution, by two-tailed t test or one- or two-way ANOVA with post hoc analysis as appropriate. A p value <0.05 was used to indicate significance.
Results
Mice deficient in BM S1P3 are protected from kidney IRI
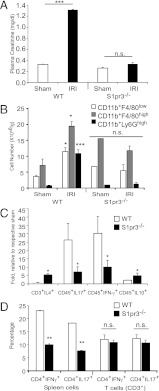
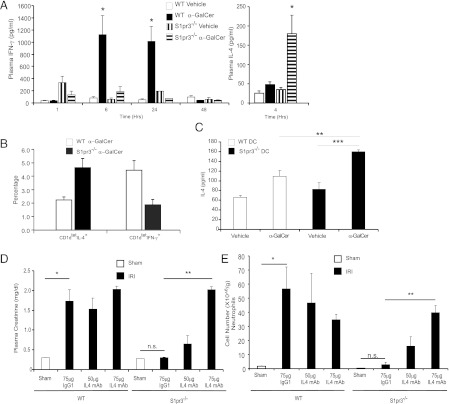
Compared to WT mice after IRI, which have increased plasma creatinine levels indicative of reduced kidney function, S1pr3−/− mice showed no increase in creatinine (Fig. 1A), no change in infiltration of neutrophils, DCs, and macrophages (Fig. 1B), and better kidney morphology after IRI (Supplemental Fig. 1A). Chemokines and proinflammatory cytokines contribute to leukocyte infiltration following kidney IRI. WT mouse kidneys expressed more CXCL1 after IRI compared with S1pr3−/− mice; CXCL1 mRNA levels were 15.2 ± 2.7- and 0.89 ± 0.4-fold higher compared with sham in WT and S1pr3−/− mice, respectively (p < 0.01). S1pr3−/− mouse kidneys had fewer CD45+ leukocytes expressing proinflammatory cytokines IL-17 and IFN-γ and more CD45+ leukocytes and CD3+ T cells expressing anti-inflammatory cytokines IL-10 and IL-4 after IRI compared with WT mice (Fig. 1C). Additionally, the percentage of CD4+ spleen cells that express IFN-γ and IL-17 was lower in S1pr3−/− mice compared with WT mice but was comparable in CD3+ T cells isolated from spleens of WT and S1pr3−/− mice (Fig. 1D); therefore, differentiation into Th1 and Th17 cells was lower in S1pr3−/− spleens compared with WT spleens. Additionally, these data suggest that S1pr3−/− T cells can respond normally but that other leukocytes involved in the regulation of T cells are less responsive to the stimulus.
FIGURE 1.
S1pr3−/− mice are protected from kidney IRI. Kidney IRI (26 min ischemia and 24 h reperfusion) was performed in WT and S1pr3−/− mice. (A) There was no difference in baseline plasma creatinine between sham-operated WT and S1pr3−/− mice. WT mice have a significant rise in plasma creatinine compared with S1pr3−/− mice after IRI (n = 4–6/group). ***p < 0.001. (B) FACS analysis of total live (7-AAD−) leukocytes (CD45+) gated for CD11b+F4/80low (macrophages), CD11b+F4/80high (DCs), and CD11b+Ly6Ghigh (neutrophils) in WT and S1pr3−/− mice after IRI (n = 4–6/group). ***p < 0.001 and *p < 0.05 compared with respective sham-operated mice. (C) FACS analysis of total live (7-AAD−) leukocytes (CD45+) and T cells (CD3+) expressing pro- (IL-17, IFN-γ) and anti-inflammatory (IL-4, IL-10) cytokines after IRI (n = 2–5/group). *p < 0.05 compared with WT mice. (D) Leukocyte expression of IL-17 and IFN-γ in WT and S1pr3−/− total spleen cells and T cell subset (CD3+) cultured under Th17-inducing conditions. Data are expressed as percentage of total CD4+ cells (n = 3–5/group). **p < 0.01 compared with WT mice. Data are expressed as means ± SEM.
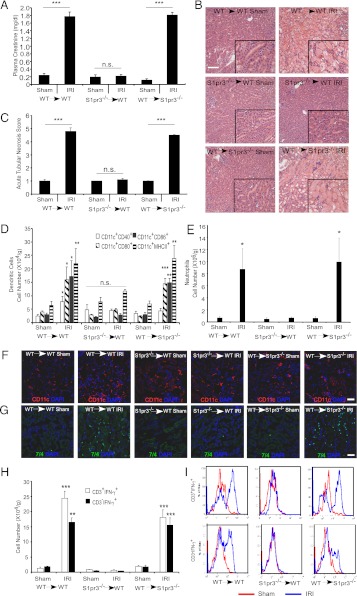
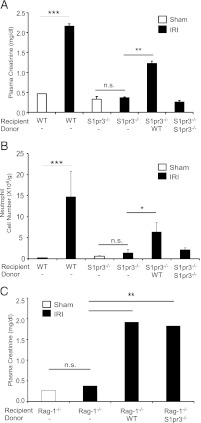
To determine the contribution of S1P3 on BM-derived cells to kidney IRI, we made BM chimeras wherein WT mice were lethally irradiated and BM was reconstituted with S1pr3−/− (S1pr3−/−→WT) or WT (WT→WT) BM cells. In a similar manner, we created a WT→S1pr3−/− chimera. After 8 wk, mice were subjected to IRI; there was a marked increase in plasma creatinine in WT→WT and WT→S1pr3−/− chimeras but not in S1pr3−/−→WT chimeras (Fig. 2A). Kidneys of S1pr3−/−→WT chimeras had less tubular necrosis (Fig. 2B) and lower ATN scores (Fig. 2C) after IR than did WT→WT and WT→S1pr3−/− chimeras. Kidney mRNA levels of proinflammatory cytokines and chemokines (TNF-α, IL-12p40, IL-1β, IL-6, CXCL1, CXCL2 and CXCL5) increased in WT→WT and WT→S1pr3−/− mice after IRI compared with sham but not in kidneys of S1pr3−/−→WT mice (Table I). These results indicate that S1P3 on BM-derived cells is necessary for kidney injury induced by IR, and in its absence, the kidney is protected from IRI and there is no increase in kidney proinflammatory cytokine and chemokine expression.
FIGURE 2.
Kidneys of mice with S1P3-deficient BM-derived cells (S1pr3−/−→WT) are protected from IRI. Kidney IRI (28 min ischemia, 24 h reperfusion) was performed in BM chimeric mice: WT→WT, S1pr3−/−→WT and WT→ S1pr3−/−. (A) Plasma creatinine levels (n = 8–10/group). ***p < 0.001. (B) H&E staining of kidney sections after IRI; insets show a ×2.5 magnified image. Scale bar, 100 μm. (C) Semiquantitative measure of tubular injury in H&E-stained kidney sections using a scale of 0–5 as described in Materials and Methods (n = 8–10/group). ***p < 0.001. (D) FACS analysis of DC subsets of total live (7-AAD−) leukocytes (CD45+) (n = 6–8/group). *p < 0.05, **p < 0.01, and ***p < 0.001 versus respective sham-operated mice. (E) FACS analysis of total live neutrophils (CD11b+GR-1high) (n = 6–8/group). *p < 0.05 versus respective sham-operated mice. (F and G) Immunofluorescence labeling of DCs (CD11c; red) (F) in the kidney outer medulla or neutrophils (7/4, green) or (G) in kidney sections of chimeric mice after sham or IRI; nuclei are labeled with DAPI (blue). Scale bars, 40 μm. (H) FACS analysis of T cell (CD3+) and non-T cell (CD3−) subsets of total live IFN-γ–producing leukocytes in kidney after IRI (n = 6–8/group). **p < 0.01 and ***p < 0.001 versus respective sham-operated mice. (I) Representative histograms (total live leukocytes gated on CD3+ and CD3− cells that produce IFN-γ) show a rightward shift after IR (red) compared with sham (blue), indicative of increased IFN-γ–producing cells in WT→WT and WT→S1pr3−/− mice but not in S1pr3−/−→WT mice. Data are expressed as means ± SEM.
Table I. Number of NKT cells, macrophages, and DCs and mRNA levels of cytokines and chemokines in kidneys of WT→WT, S1pr3−/−→WT, and WT→S1pr3−/− BM chimeras after ischemia and 24 h reperfusion.
| WT→WT | S1pr3−/−→WT | WT→S1pr3−/− | |
|---|---|---|---|
| Immune cellsa | |||
| NKT cells (×104) | 4.0 ± 0.84* | 4.1 ± 1.53 | 3.8 ± 1.37 |
| Macrophages (×105) | 2.6 ± 0.49* | 0.9 ± 0.31 | 3.1 ± 0.51* |
| CD11c cells (×105) | 1.1 ± 0.27 | 1.7 ± 0.52 | 0.8 ± 0.24 |
| mRNA levelsb | |||
| TNF-α | 3.82 ± 0.92** | 1.56 ± 0.56 | 4.22 ± 0.91* |
| IL-12p40 | 2.21 ± 0.06* | 1.51 ± 0.16 | 2.79 ± 1.06 |
| IL-1β | 10.75 ± 3.35* | 1.94 ± 0.58 | 12.46 ± 3.90** |
| IL-6 | 23.63 ± 6.46*** | 1.31 ± 0.35 | 12.47 ± 2.83* |
| CXCL1 | 31.26 ± 9.47** | 1.23 ± 0.24 | 12.76 ± 2.23** |
| CXCL2 | 34.67 ± 12.15* | 1.77 ± 0.66 | 30.68 ± 10.96** |
| CXCL5 | 21.92 ± 6.69** | 4.69 ± 1.23 | 11.62 ± 3.61** |
For immune cells, the total numbers of NKT (CD45+7-AAD−CD19−TCRβlowCd1dtet) cells, macrophages (CD45+7-AAD−CD11b+F4/80low), and CD11c (CD45+7-AAD−CD11c+) cells, expressed as fold ratios relative to respective sham-operated mice and as determined by flow cytometry, are shown (n = 3–6/group). Data are means ± SEM. *p < 0.05 relative to sham-operated mice.
mRNA expression levels shown are relative to GAPDH and are expressed as fold ratios relative to respective sham-operated mice (n = 4–9/group). Data are means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 relative to sham-operated mice.
Mice deficient in BM S1P3 have immature DCs in kidney after IRI and reduced infiltration of NKT cells and neutrophils compared with mice with WT BM cells
The following observations prompted further investigation of the role of BM cell S1P3 in kidney IRI: 1) key DC cytokines (TNF-α, IL-6, and IL-12p40) are regulated by S1P3 in kidney IRI (Table I) and systemic inflammation (28), 2) DCs interact with NKT cells to promote neutrophil infiltration in kidney IRI (7), and 3) S1pr3−/−→WT chimeras were protected from kidney IRI. We examined by flow cytometry the number of NKT cells (CD1dtetTCRβlow), macrophages (CD11b+F4/80low), and neutrophils (CD11b+Ly6Ghigh), and the number and phenotype of DCs (CD11c+) in kidney obtained from WT→WT, S1pr3−/−→WT, and WT→S1pr3−/− mice after IRI. The total number of DCs (fold relative to sham) did not change after IRI (Table I). The total number of DCs expressing activation markers (CD11c+MHCII+), including positive costimulatory molecules (CD11c+CD40+, CD11c+CD80+, and CD11c+CD86+), increased in kidneys of WT→WT and WT→S1pr3−/− mice compared with the respective sham-operated mice, and this increase was not observed in S1pr3−/−→WT mice (Fig. 2D). Total number of CD11c+CD40+ cells did not increase in WT→S1pr3−/− mice. Activated DCs in WT→WT and WT→S1pr3−/− (but not S1pr3−/−→WT) mice after IRI also represented a larger percentage of total CD11c+ DCs (data not shown). Immunofluorescent localization revealed no obvious differences in distribution of CD11c+ cells in kidney outer medulla from any of the chimeric mice after sham or IRI (Fig. 2F). These results demonstrate that S1P3 expressed on BM cells is important in mediating the increase in mature DCs observed in the kidney following IRI, and not simply for increased infiltration of activated DCs.
Using FACS analysis, we found an increase in the total number of infiltrating macrophages (Table I), NKT cells (Table I), and neutrophils (Fig. 2E) in kidney after IRI (consistent with our prior results in WT mice; Ref. 3) in WT→WT mice and WT → S1pr3−/− mice compared with sham, but there was no change in S1pr3−/−→WT kidneys. Confirming the flow cytometry data, immunofluorescence labeling of infiltrating neutrophils (7/4+ cells) increased in kidneys of WT→WT and WT → S1pr3−/− mice after IRI compared with sham but not in S1pr3−/−→WT chimeras (Fig. 2G). Thus, BM-derived S1P3 is necessary for neutrophil infiltration following kidney IRI. Following kidney IRI, the increase in kidney content of IFN-γ–producing leukocytes contributes to tissue inflammation (7). We next sought to determine whether S1P3 on BM-derived cells regulates IFN-γ production after kidney IRI. There was a significant increase in the total number of IFN-γ–producing leukocytes (CD3+ T cells and CD3− non-T cells) (Fig. 2H) in kidney in WT→WT and WT→S1pr3−/− mice after IR and in IFN-γ content in CD3+ and CD3– cell populations (Fig. 2I), but this increase was not observed in S1pr3−/−→WT mice.
S1P3-deficient DCs attenuate kidney IRI
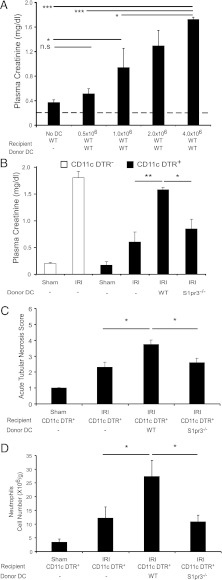
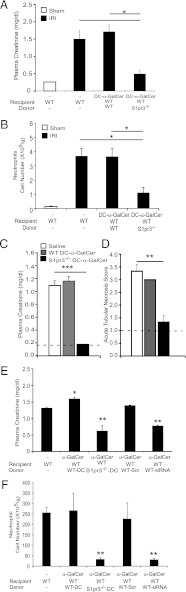
Following IRI, key DC cytokines (TNF-α, IL-12p40, and IL-1β) from S1pr3−/−→WT mouse kidneys were attenuated compared with control chimeras, suggesting that the lack of immune response in mice with S1P3-deficient BM could be due to S1P3-deficient DCs. To demonstrate the functional role of DCs expressing S1P3 in kidney IRI, we performed adoptive transfer studies in mice depleted of DCs. First, the optimal number of DCs for these reconstitution studies was determined. Mild ischemia alone (no DCs) caused only a minimal rise in plasma creatinine compared with sham-operated mice (dashed line), but injection of WT DCs exacerbated injury in a dose-dependent manner as indicated by increased plasma creatinine (Fig. 3A), kidney histology (Supplemental Fig. 1B), and tubular injury (data not shown). A dose of 0.5 × 106 DCs, which did not exacerbate injury in intact WT mice, was selected for adoptive transfer studies. DCs were depleted in DT-sensitive recipient mice that express the simian DTR under the control of the CD11c promoter (CD11c-DTR+); littermates lacking DTR transgene expression (CD11c-DTR−) were used as controls. Depletion of DCs by DT administration to CD11c-DTR+ mice 24 h prior to kidney IR reduced kidney DC content (6) and in the present study led to a marked attenuation of kidney injury (as measured by plasma creatinine) (Fig. 3B) and kidney histology (Supplemental Fig. 1C). Kidney mRNA levels of proinflammatory cytokines and chemokines increased markedly in WT mice after IRI, but TNF-α (1.08 ± 0.39), IL-12p40 (0.19 ± 0.49), IL-1β (0.47 ± 0.68), IL-6 (1.64 ± 0.76), CXCL1 (6.48 ± 0.45), CXCL2 (5.63 ± 0.32), and CXCL5 (4.68 ± 0.67) (fold expression relative to sham) did not increase significantly after IR in DC-depleted mice, demonstrating that DCs are necessary to initiate the inflammatory immune response after kidney IRI. The reduction in injury in DC-depleted mice was reversed by adoptive transfer of WT but not S1pr3−/− BMDCs (Fig. 3B–D). Kidney neutrophil infiltration, a hallmark of IRI, which increased only modestly after IRI in DC-depleted CD11c-DTR+ mice relative to sham, was higher after adoptive transfer of WT but not S1pr3−/− DCs (Fig. 3D). These results indicate that DC S1P3 is necessary in mediating injury following IR. Additionally, protection from injury is not due to compensatory changes in expression of the other S1P receptor subtypes in DCs of S1pr3−/− mice, as expression was not different from WT DCs (mRNA by RT-PCR, data not shown).
FIGURE 3.
Kidney injury after IR is blunted in DC-depleted mice reconstituted with S1pr3−/− BMDCs. (A) Injection (i.v.) of increasing numbers of WT BMDCs to WT mice 18 h prior to IRI (24 min ischemia) induces kidney injury (n = 4–6/group). *p < 0.05, ***p < 0.001. (B–D) DCs were depleted in DT-sensitive, human DTR-expressing mice (CD11c-DTR+) but not in CD11c-DTR− mice by treatment with DT; 20 h later mice were injected with either 1× PBS (−), DCs from WT mice, or DCs from S1pr3−/− mice, and mice were subjected to IR (26 min ischemia) 6 h after DC injection. (B) CD11c-DTR+ mice reconstituted with WT DCs had more injury after IR than did CD11c-DTR+ mice with no DCs or with S1pr3−/− DCs. (C) Semiquantitative measure of tubular injury in H&E-stained kidney sections. (D) Reconstitution of DC-depleted CD11c-DTR+ mice with WT but not S1pr3−/− DCs increased neutrophil infiltration in kidneys (n = 3–6/group). Data are expressed as means ± SEM. *p < 0.05, **p < 0.01.
DCs require S1P3 to initiate inflammation following IRI
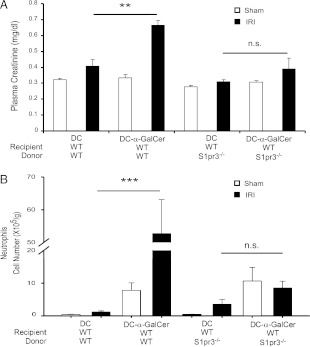
DC-mediated activation of NKT cells depends on stimulation of the invariant TCR through glycolipid presentation, costimulation, and cytokine production. To further explore the role of DC S1P3 in injury, we investigated whether S1P3 is necessary for DCs to activate NKT cells. CD1d expressed by DCs presents either self-glycolipid, such as isoglobotrihexosylceramide (40), or in experimental protocols, foreign glycolipid, such as the marine sponge-derived α-GalCer (41), to NKT cells. Following a widely used experimental paradigm to probe the role of NKT cell activation, BMDCs from WT and S1pr3−/− mice were loaded with vehicle (DMSO) or α-GalCer and were administered (i.v.) 24 h before a subthreshold “mild” kidney IRI in mice. We used a mild injury model to detect the anticipated increase in injury following activation of NKT cells by α-GalCer–loaded DCs. Consistent with prior findings that DC-α-GalCer–mediated NKT cell activation exacerbates kidney IRI (12), WT mice that received α-GalCer–loaded WT DCs showed significant functional (Fig. 4A) and morphological (Supplemental Fig. 1D) evidence of injury; injury was not elicited by α-GalCer–loaded S1pr3−/− DCs. Similarly, mRNA expression of proinflammatory cytokines and chemokines (TNF-α, IL-12p40, IL-1β, IL-6, CXCL1, CXCL2, and CXCL5) increased after IRI compared with sham in mice that received α-GalCer–loaded WT but not α-GalCer–loaded S1pr3−/− DCs (Table II). Mild ischemia did not increase infiltration of NKT cells and neutrophils; however, increased cell infiltration produced by α-GalCer–loaded WT DCs in sham-operated mice was further enhanced by IRI, particularly for neutrophil infiltration. In contrast, α-GalCer–loaded S1pr3−/− DCs did not stimulate neutrophil infiltration after IRI (Fig. 4B). These results indicate that DCs harboring S1P3 are necessary for activation of NKT cells and downstream infiltration of neutrophils in kidney IRI. Furthermore, maturation of DCs, which can be induced by α-GalCer–activated NKT cells (42), also required DC S1P3. The subthreshold injury did not increase the number of mature DCs (data not shown), which was produced by our standard 28 min ischemia (Fig. 2D), but administration of α-GalCer–loaded WT DCs increased substantially the total CD11c+MHCII+, CD11c+CD40+, and CD11c+CD1d+ cells observed following mild IRI (Table II). In contrast, there was no enhancement of these populations of activated DCs when α-GalCer–loaded S1pr3−/− DCs were injected into mice prior to IRI. Therefore, not only are DCs expressing S1P3 necessary for activating NKT cells in IRI, but the accompanying DC maturation may be mediated by the S1P3-dependent DC-α-GalCer–stimulated activation of NKT cells.
FIGURE 4.
S1pr3−/− BMDCs loaded with α-GalCer are unable to induce injury after IR. (A) Mild ischemia (24 min) did not increase plasma creatinine. Injection of α-GalCer–loaded WT but not S1pr3−/− DCs caused a significant increase in plasma creatinine (A) and neutrophil infiltration (B) after mild IR (n = 4–9/group). Data are expressed as means ± SEM. **p < 0.01, ***p < 0.001.
Table II. Numbers of NKT cells and activated DCs (CD11c+) and mRNA levels of cytokines and chemokines in kidney after 24 h reperfusion from sham and ischemic mice injected with WT or S1pr3−/− BMDCs loaded with vehicle or α-GalCer.
| WT DC |
WT DC-α-GalCer |
S1pr3−/− DC |
S1pr3−/− DC-α-GalCer |
|||||
|---|---|---|---|---|---|---|---|---|
| Sham | IRI | Sham | IRI | Sham | IRI | Sham | IRI | |
| Immune cellsa | ||||||||
| NKT (×104) | 1.9 ± 0.5 | 2.2 ± 0.4 | 9.7 ± 0.6 | 15.1 ± 2.2*** | 0.9 ± 0.2 | 4.0 ± 1.1 | 5.6 ± 1.3 | 6.1 ± 1.3†† |
| CD11c+MHCII+ (×103) | 9.7 ± 3.4 | 38.4 ± 6.4 | 230 ± 84.5 | 1049.5 ± 294** | 7.8 ± 1.7 | 40.7 ± 18.5 | 246.4 ± 86 | 150 ± 54.5† |
| CD11c+CD40+ (×104) | 1.1 ± 0.2 | 1.5 ± 0.5 | 19.2 ± 66.1 | 86.9 ± 23.8** | 0.2 ± 0.1 | 2.1 ± 0.8 | 17.6 ± 4.6 | 12.6 ± 6.3†† |
| CD11c+CD1d+ (×103) | 0.4 ± 0.1 | 1.7 ± 0.5 | 55.9 ± 29.3 | 515.5 ± 125.9** | 0.3 ± 0.1 | 4.6 ± 2.6 | 89.3 ± 46 | 93.4 ± 51.7†† |
| mRNA levelsb | ||||||||
| TNF-α | 4.16 ± 0.67 | 1.04 ± 0.21 | 4.13 ± 0.64 | 0.74 ± 0.15 | ||||
| IL-12p40 | 3.24 ± 1.46 | 0.52 ± 0.14 | 0.22 ± 0.10 | 0.93 ± 0.67 | ||||
| IL-1β | 0.89 ± 0.40 | 3.17 ± 0.71** | 2.75 ± 0.67 | 0.69 ± 0.32 | ||||
| IL-6 | 10.21 ± 3.99 | 52.2 ± 82.81*** | 18.1 ± 9.35 | 0.46 ± 0.24 | ||||
| CXCL1 | 11.42 ± 3.56 | 18.63 ± 0.59 | 12.66 ± 1.37 | 1.52 ± 0.46 | ||||
| CXCL2 | 10.94 ± 4.86 | 84.45 ± 6.48** | 2.82 ± 0.51 | 0.51 ± 0.21 | ||||
| CXCL5 | 35.07 ± 13.43* | 61.40 ± 9.04* | 37.69 ± 15.02* | 6.32 ± 2.59 | ||||
For immune cells, total numbers of NKT (CD45+7-AAD−CD19−TCRβlowCd1dtet) and CD11c (CD45+7-AAD−CD11c+) cells as determined by flow cytometry are shown (n = 3–6/group). Data are means ± SEM. **p < 0.01, ***p < 0.001 compared with WT DC IRI; †p < 0.01, ††p < 0.001 compared with WT DC-α-GalCer IRI.
mRNA expression levels are shown relative to GAPDH and are expressed as fold ratios relative to respective sham-operated mice (n = 4–6/group). Data are means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 relative to sham-operated mice.
DCs deficient of S1P3 induce a Th2-like response in NKT cells
To determine whether S1P3 is important for cytokine production following DC-mediated activation of NKT cells, WT and S1pr3−/− mice were administered α-GalCer (10 μg/mouse, i.p.), and plasma IFN-γ levels were measured over time. NKT cells activated by this process rapidly release large amounts of both IL-4 and IFN-γ (43, 44). Plasma IFN-γ levels were higher in WT mice 6 h after treatment with α-GalCer in WT mice (and were sustained for up to 24 h) but did not increase in S1pr3−/− mice (Fig. 5A). Conversely, IL-4 plasma levels were higher in S1pr3−/− mice than in WT mice 4 h after α-GalCer administration. Liver NKT cell-dependent production of IL-4 and IFN-γ was measured by FACS 2 h after α-GalCer (1 μg/mouse, i.v.) as previously described (38) without restimulation. More liver NKT cells (CD1dtet-positive cells) from WT mice made IFN-γ than did NKT cells from S1pr3−/− mice, but more S1pr3−/− NKT cells made IL-4 than did WT NKT cells (Fig. 5B). To directly test the interaction of WT and S1pr3−/− DCs with NKT cells we cultured vehicle- and α-GalCer–loaded DCs with NKT1.2 hybridoma cells, which produce IL-4 but not IFN-γ upon stimulation with α-GalCer–loaded DCs (38). Higher levels of IL-4 were found after coculture with α-GalCer–loaded S1pr3−/− DCs than with vehicle-loaded S1pr3−/− DCs or α-GalCer–loaded WT DCs (Fig. 5C). These data suggest that DCs deficient of S1P3 induce a Th2-like response in NKT cells to produce more IL-4, although it is possible in vivo that conventional CD4+ T cells could contribute to increased plasma IL-4 levels and hence to the Th2 response.
FIGURE 5.
Protection of S1pr3−/− mice from IRI is IL-4–dependent. (A) Plasma levels of IFN-γ 1–48 h and IL-4 4 h after treatment of WT and S1pr3−/− mice with vehicle or α-GalCer (10 μg/mouse, i.p.) (n = 3–4/group). *p < 0.05 compared with respective vehicle control for each time point. (B) WT and S1pr3−/− mice were injected with α-GalCer (1 μg/mouse, i.v.), and liver NKT cell (CD1dtet) IFN-γ and IL-4 levels were measured by FACS. Compared to WT mice, S1pr3−/− mice had a higher percentage of liver CD1dtet-positive cells that made IL-4, and WT mice had higher percentage of CD1dtet-positive cells that made IFN-γ compared with S1pr3−/− mice. (C) DCs from WT and S1pr3−/− mice were loaded with vehicle or α-GalCer prior to coculture with NKT1.2 hybridoma cells, and IL-4 was measured in the culture medium. Little or no measurable IL-2 or IL-4 was detected in culture medium of WT DCs or NKT1.2 cells that were cultured alone (n = 3/group). **p < 0.01, ***p < 0.001. (D and E) WT or S1pr3−/− mice were injected with neutralizing IL-4 mAb (50, 75, or 100 μg) or isotype control Ab (75 μg) 1 d prior to kidney ischemia (26 min), and plasma creatinine (D) and kidney neutrophil infiltration (by flow cytometry) (E) were measured after 20 h of reperfusion (n = 3/group). Data are expressed as means ± SEM. *p < 0.05, **p < 0.01.
Protection of S1pr3−/− mice from IRI is IL-4–dependent
Our in vitro studies demonstrated that S1P3 deficiency in DCs leads to Th2 polarization with production of IL-4. Therefore to determine in vivo whether the protection observed in S1P3-deficient mice was IL-4–dependent, we injected WT and S1pr3−/− mice with a mAb to IL-4 (IL-4 mAb) 1 d prior to IR to block the effects of IL-4. IL-4 mAb had no effect on injury in WT mice but reversed the protection from injury in S1pr3−/− mice in a dose-dependent manner compared with isotype control (IgG1)-injected S1pr3−/− mice as measured by plasma creatinine (Fig. 5D) or kidney neutrophil infiltration (Fig. 5E). WT or S1pr3−/− mice injected with 100 μg IL-4 mAb died several hours after surgery (data not shown). Taken together these data suggest that the protection observed in S1pr3−/− mice was IL-4–dependent.
WT DCs reconstitute injury in S1pr3−/− mice
We next sought to determine whether S1P3 deficiency on non-DCs conferred kidney protection following IRI. In this experiment WT DCs were adoptively transferred into S1pr3−/− mice (containing S1pr3−/− NKT cells). S1pr3−/− mice were protected from severe IRI (28 min ischemia). Injury was reconstituted in S1pr3−/− mice injected with 0.5 × 106 WT DCs but not with 0.5 × 106 S1pr3−/− DCs (Fig. 6A). After IRI, S1pr3−/− mice also had significantly more tubular injury (Supplemental Fig. 1E) and neutrophil infiltration (Fig. 6B) after injection of WT DCs than after S1pr3−/− DCs, thus demonstrating that DC S1P3 is required for kidney injury. Additionally, non-DCs, including NKT cells, deficient of S1P3 do not confer kidney protection from IRI.
FIGURE 6.
Injection of WT DCs in S1pr3−/− mice reconstitutes injury after IR. (A) Plasma creatinine increased after IRI (26 min ischemia) in WT but not S1pr3−/− mice treated with vehicle. Injury was partially restored in S1pr3−/− mice after injection of WT but not S1pr3−/− DCs (0.5 × 106 cells were injected 24 h prior to surgery) as demonstrated by plasma creatinine (A) and neutrophil infiltration (B) (n = 3–4/group). *p < 0.05, **p < 0.01, ***p < 0.001. (C) Reconstitution of Rag-1−/− mice with WT or S1pr3−/− T conventional (CD4+CD25−) cells results in significantly higher plasma creatinine after kidney IRI (n = 3–4/group). Data are expressed as means ± SEM. **p < 0.01.
To further demonstrate there was no defect in the T cell or NKT cell response in S1pr3−/− mice, we isolated conventional CD4+ T cells (CD4+CD25−) from WT and S1pr3−/− mice. Equal numbers of conventional T cells were injected into Rag-1−/− mice a week before kidney IRI. Reconstitution of T and B cell-deficient Rag1−/− mice, which are resistant to injury, with WT or S1pr3−/− conventional T cells resulted in a similar rise in plasma creatinine, suggesting that Tcon cells from S1pr3−/− mice had no defect in their ability to contribute to the inflammatory response to IRI (Fig. 6C). These results demonstrate that NKT and T cells recognize and respond to DC-presented α-GalCer and are activated in IRI in the absence of NKT cell S1P3.
Prevention of acute kidney injury and treatment of established acute kidney injury
Our data strongly suggested that S1P3 controls DC phenotype and its absence renders DCs immature and tolerized. Therefore, we investigated the potential clinical application and efficacy in prevention of IRI by administering S1P3-deficient DCs 7 d prior to IRI. As in the results with 2 d pretreatment, 7 d pretreatment with α-GalCer–loaded S1pr3−/− DCs resulted in significantly less injury (reduced plasma creatinine; Fig. 7A), neutrophil infiltration (Fig. 7B), and tubular injury (by H&E; Supplemental Fig. 1F) after kidney IRI compared with pretreatment with WT DCs or α-GalCer–loaded WT DCs. Next, we sought to determine the effectiveness of this approach in treating established injury following IR. Mice were significantly protected from kidney injury when administration of α-GalCer–loaded S1pr3−/− DCs was delayed by 3 h after IR (Fig. 7C) and tubular injury score (Fig. 7D, by H&E; Supplemental Fig. 1G) compared with saline or WT DC-α-GalCer.
FIGURE 7.
S1pr3−/− DCs are effective both in preventing kidney IRI and in treating injury after IR. (A and B) WT mice were injected (i.v.) with WT DCs or S1pr3−/− DCs (0.5 × 106; loaded with α-GalCer) 7 d prior to IRI (26 min). (A) Plasma creatinine; (B) neutrophil infiltration in kidneys (n = 3–4/group). *p < 0.05, **p < 0.01, ***p < 0.001. (C) WT mice were subjected to 26 min ischemia and 24 h reperfusion and injected (i.v.) with WT DCs or S1pr3−/− DCs (0.5 × 106; loaded with α-GalCer) 3 h after ischemia; plasma creatinine was measured at the 24 h reperfusion. Dashed line, creatinine levels in sham-operated mice (n = 3–4). p < 0.001. (D) Semiquantitative measure of tubular injury in H&E-stained kidney sections. Dashed line, ATN in sham-operated mice. (E) Knockdown of S1pr3 in WT DCs results in less kidney injury in WT mice as indicated by plasma creatinine and (F) neutrophil infiltration. WT DCs transfected with S1P3 siRNA (WT-siRNA) or scrambled oligonucleotides (WT-Scr) or untreated WT or S1pr3−/− DCs were loaded with α-GalCer and injected into WT mice (0.5 × 106 cells) 24 h prior to IRI (n = 3–4/group). Data are means ± SEM. *p < 0.05, **p < 0.01.
Confirmation of results with Tg mice on the role of DC S1P3 in IRI was obtained in siRNA experiments. Knockdown of S1P3 expression in WT DCs using siRNA (WT-siRNA) prior to loading with α-GalCer and injection into WT mice resulted in less injury (Fig. 7E) and less neutrophil infiltration (Fig. 7F) after kidney IRI compared with WT DCs transfected with scrambled oligonucleotides (WT-Scr). WT-siRNA DCs had ∼76% less S1P3 mRNA, as determined by RT-PCR, compared with WT-Scr DCs (data not shown). Taken together, these data suggest that DC S1P3 is crucial in DC activation and downstream inflammation and infiltration of neutrophils. Furthermore, targeting S1P3 receptors for systemic antagonist delivery or specific cell-based therapy with DCs whose receptors are antagonized pharmacologically may be considered in future clinical studies.
Discussion
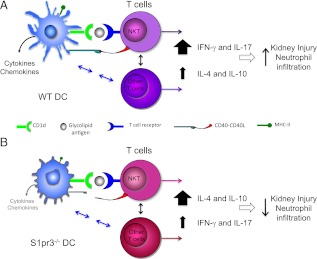
S1P is an important intracellular and extracellular signaling molecule that regulates cellular function and modulates the immune system. In this study, we defined a permissive role of S1P3 in the DC response to kidney IRI and in DC-mediated activation of NKT cells and neutrophil infiltration (Fig. 8). We found that DC S1P3 plays an important role in mediating kidney IRI, and its absence from DCs attenuated kidney injury. The absence of S1P3 blocked DC maturation, as evidenced by reduced expression of costimulatory molecules, MHCII, and proinflammatory cytokines and chemokines. Additionally, S1P3 on DCs is necessary for NKT cell activation, downstream neutrophil infiltration, and IFN-γ production in kidney IRI, but NKT cell S1P3 is likely not required for kidney injury mediated by IR-induced activation of the DC/NKT axis. Decreased kidney injury in S1pr3−/− mice is IL-4–dependent. These results indicate that during kidney IRI, the presence or absence of S1P3 in DCs polarizes NKT cells to Th1 or Th2 phenotype, respectively. Because DCs are the proximate immune cells in the inflammatory cascade of kidney IRI, antagonizing S1P3 may be a useful therapeutic strategy in treatment of acute kidney IRI.
FIGURE 8.
Absence of S1P3 in DCs polarizes T cells to a Th2 phenotype in kidney IRI. In kidney IRI, (A) WT DCs via their class I-like CD1d molecule present endogenous glycolipid or α-GalCer to NKT cells and along with CD40 costimulatory molecule–CD40L interaction cause– NKT cell activation. Additionally, DCs can interact with and activate conventional T cells and regulatory T cells through a variety of mechanisms. Activated NKT cells produce large amounts of IFN-γ (Th1 response), leading to neutrophil infiltration and kidney injury. (B) DCs lacking S1P3 (S1pr3−/− DCs) also present endogenous glycolipid or α-GalCer to NKT cells via their class I-like CD1d molecule but have reduced CD40 and cytokine/chemokine expression after kidney IRI. NKT cells stimulated by α-GalCer–loaded S1pr3−/− DCs produce large amounts of IL-4 (Th2 response) and IL-10 with low to minimal IFN-γ (Th1) and IL-17. Similarly, S1pr3−/− DCs in mice subject to kidney IRI may also fail to induce a Th1 response in conventional T cells and hence promote increased IL-4 production by conventional T cells. High levels of IL-4 result in less neutrophil infiltration and less kidney injury. Neutralization of IL-4 with blocking mAb reverses this protective effect of IL-4 in S1pr3−/− mice, leading to more kidney injury. The present studies focused on DC/NKT interactions in IRI, but other mechanisms, such as reduced IL-17 or increased IL-10, may also contribute to protection in S1pr3−/− mice and in WT mice treated with S1pr3−/− DCs prior to IRI. Mechanisms underlying the beneficial effects of S1pr3−/− DCs administered after established IRI have not yet been identified and could include enhanced repair processes.
DCs participate in innate and adaptive immunity, autoimmunity, allograft rejection, host defense, and IRI (1, 6, 45). Following reperfusion, intracellular events occur that lead to cellular dysfunction, apoptosis, and cell death (for reviews, see Ref. 1). The innate immune system, leading to the activation of BM-derived cells, endothelial cells, and epithelial cells, contributes to IRI and reduction in glomerular filtration rate (1, 6, 45). F4/80high resident DCs are the predominant leukocyte subset in kidney following IRI (3) and are a key population of cells for initiating inflammation in kidney IRI. Depletion of DCs by clodronate liposome (46) or by using transgenic CD11c-DTR mice (6), as confirmed in the present study, significantly protects kidneys from IRI. Using a different approach, we have now also found that injection of WT DCs exacerbates injury in a dose-dependent manner, therefore demonstrating by two independent means that DCs contribute to kidney injury following IR. Additionally, ablation of DCs attenuates inflammation associated with colitis (47). However, in some models DCs mediate anti-inflammatory and protective effects (48). By responding to the local microenvironment, which may be altered differentially by different types of injury processes and progressive stages of injury and repair, DCs may either adopt a proinflammatory phenotype or may become tolerized and provide protection. For example, hypoxia is known to elevate extracellular adenosine levels (49, 50), which may alter DC functions. Thus, targeting DCs offers an important therapeutic strategy for minimizing tissue injury.
Upon stimulation (inflammation), DCs transition to a mature cell type characterized by high levels of MHCII and costimulatory molecules and low phagocytic capacity. Mature DCs are specialized in Ag-specific T cell activation. However, DCs also participate in the innate immune response by releasing chemokines and cytokines (11, 51), interacting with NKT cells via CD40/CD40L, presenting glycolipids via the CD1d molecule and producing IL-12 cytokine to activate iNKT cells (52–56). Kidney DCs produce TNF-α in early time points following IRI (11). The present study demonstrates that the presence of S1P3 on DCs permits normal maturation and activation of DCs leading to tissue injury. In the absence of S1P3, despite the initial stimulus of IRI, DCs possess an immature phenotype, induce a Th2-like response from activated NKT cells, and do not induce immune cell infiltration; the marked increase in expression of macrophage- and DC-associated proinflammatory genes normally observed after IRI in kidneys was also absent. Furthermore, although S1P3 on DCs is necessary to activate the DC/NKT pathway that is important for initiating kidney injury, our data suggest that T cell S1P3 does not appear to be critical for this response, as T cells, and specifically NKT cells, from S1pr3−/− mice function normally in response to a stimulus or in contributing to IRI.
Mouse NKT cells express the invariant TCR, Vα14Jα18, and human NKT cells express an invariant Vα24 TCR (57). Both mouse and human NKT cells are dependent on CD1d for positive selection in the thymus and subsequent activation in the periphery (10, 58). DC-mediated presentation of endogenous self-glycolipid or α-GalCer via CD1d activates NKT cells, which results in IFN-γ production following reperfusion (7), but in the present study the number of CD45+ IFN-γ cells did not increase after IRI in S1P3-deficient mice. These findings provide further evidence for a role of DC S1P3 in DC-dependent glycolipid presentation and activation of NKT cells. They also support prior work from our laboratory and others showing that IFN-γ (59), and in particular type I NKT-dependent IFN-γ (7), mediates kidney (59) and liver (60) IRI and is an important target for A2A adenosine receptor agonists in mediating tissue protection (59). In a similar manner, compounds that block the more proximal DC S1P3 may attenuate kidney IRI. Therapeutic manipulations of Th1–Th2 functions of NKT cells to potentiate Th2 immune response have also been demonstrated in type 1 diabetes (61, 62) and other Th1-mediated autoimmune pathologies, such as autoimmune encephalomyelitis (63). Because mouse and human NKT cells recognize α-GalCer, our findings might be applicable to therapeutic interventions in human diseases (57).
Upon activation of the DC/NKT pathway, S1P3 deficiency skewed the NKT Th1–Th2 response to a Th2 response with higher plasma levels of IL-4 and lower IFN-γ. S1pr3−/− DCs produced a stronger IL-4 response in cocultures with NKT cells, suggesting that DCs lacking S1P3 polarize NKT cells toward a Th2 response, a mechanism that could reduce IRI (64, 65). S1P3-deficient DC-mediated Th2 polarization and prevention of increased CD40 expression after kidney IRI is consistent with other studies demonstrating that a lack of CD40 or CD28 costimulatory pathways polarizes NKT cells toward a Th2-like phenotype (66). It is possible that S1P3-deficient DC-mediated interactions with conventional T cells also contribute to the shift from Th1 to Th2 response, possibly with involvement of regulatory T cells. Future studies will determine whether the response of other leukocyte populations to IRI differs in S1pr3−/− mice. Recent studies have demonstrated that a hypoxia-induced increase in HIF-1α in T cells also induces a shift from Th1 to Th2 phenotype, which involves an increase in IL-10 and a decrease in IFN-γ (67). Furthermore, in kidney IRI, a functional role of hypoxia signaling has been demonstrated in renal proximal tubule cells. Hypoxia-dependent increase in HIF-1α upregulates sphingosine kinase 1-dependent S1P production, which binds to S1P1 resulting in improved cell survival (69); no role for S1P3 was shown in these studies. Additionally, blocking S1P2 in proximal tubule cells further enhances the cross-talk between HIF-1α and sphingosine kinase 1, resulting in improved cell survival (68). It is possible that similar mechanisms might exist in DCs that are deficient of S1P3.
Increased endocytosis in mature DCs by stimulation of S1P3 does not appear to alter Ag processing (69); however, S1P3-deficient DCs are unable to upregulate CD1d molecules and their ability to recognize foreign Ags in response to an inflammatory signal may be altered (S1pr3−/− DCs have a diminished ability to engage a T cell response in an MLR; A. Bajwa and M.D. Okusa, unpublished observations). Further studies are necessary to determine whether S1P3-deficient DCs have altered ability to recognize, process, and present α-GalCer to NKT cells. The immature phenotype of S1P3-deficient DCs may also be due to loss of a component of the stimulation of DC maturation by α-GalCer–activated NKT cells (42).
S1P regulates migration of immune cells and could play a role in migration of DCs in S1P3-dependent kidney injury (70). DCs deficient of S1P3 have reduced migration and endocytosis in response to S1P (69). Although it is possible that reduced DC migration contributes to the lack of injury in the absence of DC S1P3 in our kidney IR model, we did not observe any differences in DC migration in WT and S1pr3−/− mice using FITC skin painting and footpad injection of DCs treated with LPS (A. Bajwa and M.D. Okusa, unpublished observations). Furthermore, no significant differences in accumulation of i.v. injected DCs were observed between WT and S1pr3−/− DCs. Injection of unloaded or α-GalCer–loaded WT or S1pr3−/− DCs into CD45.1 mice resulted in accumulation of injected DCs in lymphoid and nonlymphoid organs, including spleen, liver, lung, and peripheral lymph nodes (data not shown); these results are similar to previously published findings (71). Thus, the effects of transferred DCs on IRI may be mediated at distant sites; identification of a mechanism will require further study.
A current strategy for reducing the incidence of rejection in transplantation is to produce immature DCs with a stable tolerogenic phenotype (72–79) using various pharmacological agents (80) or to deplete donor MHCII+ or CD45+ passenger leukocytes with mAbs prior to transplantation (81). Similarly, genetic or environmental factors that stimulate chronic maturation of tissue DCs can induce severe organ-specific autoimmune disease and systemic autoimmunity (82, 83). The proposed role of DC S1P3 in DC maturation could therefore be important in other diseases besides acute kidney injury, such as solid organ transplantation. The inability of S1pr3−/− DCs to mature in response to Ag stimulation (α-GalCer) or after kidney IRI results in less kidney injury and reduced infiltration of innate immune cells that further contribute to injury. BMDCs from S1pr3−/− mice (or WT DC-siRNA S1P3), owing to their inability to mature (decreased MHCII and CD40) and the presence of reduced cytokines (IL-12 and TNF-α), also have significantly lower translocation of NF-κB. WT DCs stimulated by α-GalCer increase phosphorylation of NF-κB compared with vehicle-treated cells; S1pr3−/− DCs do not increase phosphorylation of NF-κB after α-GalCer (data not shown). Taken together, these data suggest that S1pr3−/− DCs are unable to induce an inflammatory cytokine response and which also could explain the lower expression levels of costimulatory molecule CD40. Mechanistically, IRI leads to an aggravation of cell damage through production of reactive oxygen species derived from activated DCs, macrophages, and neutrophils and activation of inflammatory redox-sensitive transcription factors, such as NF-κB and AP-1, which contribute to IRI. NF-κB is a rapid response transcription factor that is critical to both the regulation of apoptosis and increased expression of many proinflammatory mediators (84, 85). DC maturation and immunostimulatory (CD40/80/86) ability depend on NF-κB–dependent gene transcription (86, 87). Inhibition of NF-κB by IκB (repressor gene) or by decoy NF-κB oligodeoxynucleotides ameliorates IRI in experimental lung (88) and heart (77) transplantation models and rat renal IRI (89). Therefore, NF-κB is a relevant therapeutic target that may be of clinical importance and may play a role in the S1P3 activation. The results of our present studies strongly support a rationale for development of a selective S1P3 antagonist, particularly in view of the absence of safe and effective Food and Drug Administration-approved drugs for the treatment of acute kidney injury. In contrast to our results, Park et al. (68) demonstrated that a commercially available S1P3 antagonist (CAY10444) did not provide protection from kidney IRI, but the dose and timing of administration may not have been optimal for blocking S1P3, and the specificity and efficacy of this compound at S1P3 has been questioned (90). Thus, the development of a potent and selective compound is necessary for future studies.
In summary, the present study demonstrates a role for sphingolipid S1P3 on DCs in kidney IRI. S1P3 is necessary for DCs to: 1) exhibit a mature phenotype, 2) regulate IFN-γ in the innate immune response to injury, and 3) regulate the innate immune response and NKT cell and neutrophil transmigration to the injured kidney following kidney IR. S1P3 controls NKT Th1–Th2 polarity: the absence of S1P3 leads to a Th2 phenotype and the presence of S1P3 leads to a Th1 phenotype. Our studies, however, do not exclude other potential mechanisms of DC S1P3 in the control of innate immune response to IRI, and further identification of the role of S1P3 on DCs will add to our understanding of the immunological pathways involved in kidney IRI. These studies should aid in development of new therapeutic strategies for patients with acute kidney injury.
Supplementary Material
Acknowledgments
We thank the University of Virginia Research Histology core for help with H&E staining and the Okusa Laboratory members for helpful suggestions.
This work was supported by National Institutes of Health Grants R01 DK085259 and R01 DK062324 (to M.D.O.), R01 DK083406 (to M.D.O. and P.I.L.), R01 GM067958 (to K.R.L.), and K01 DK09144 (to A.B.). The postdoctoral fellowship for A.B. was supported in part from a National Kidney Foundation Research Fellowship Award. A.B. was also the recipient of American Heart Association Scientist Development Grant 11SDG7000007.
The online version of this article contains supplemental material.
Abbreviations used in this article:
- 7-AAD
- 7-aminoactinomycin D
- ATN
- acute tubular necrosis
- BM
- bone marrow
- BMDC
- bone marrow-derived dendritic cell
- DC
- dendritic cell
- DT
- diphtheria toxin
- DTR
- diphtheria toxin receptor
- α-GalCer
- α-galactosylceramide
- IR
- ischemia–reperfusion
- IRI
- ischemia–reperfusion injury
- MHCII
- MHC class II
- siRNA
- small interfering RNA
- S1P
- sphingosine 1-phosphate
- S1pr3
- sphingosine 1-phosphate-3 receptor
- Tg
- transgenic
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bonventre J. V., Yang L. 2011. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 121: 4210–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grenz A., Bauerle J. D., Dalton J. H., Ridyard D., Badulak A., Tak E., McNamee E. N., Clambey E., Moldovan R., Reyes G., et al. 2012. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J. Clin. Invest. 122: 693–710 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Li L., Huang L., Sung S. S., Vergis A. L., Rosin D. L., Rose C. E., Jr., Lobo P. I., Okusa M. D. 2008. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 74: 1526–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson P. J. 2007. Renal ischemia-reperfusion injury: renal dendritic cells loudly sound the alarm. Kidney Int. 71: 604–605 [DOI] [PubMed] [Google Scholar]
- 5.Soos T. J., Sims T. N., Barisoni L., Lin K., Littman D. R., Dustin M. L., Nelson P. J. 2006. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70: 591–596 [DOI] [PubMed] [Google Scholar]
- 6.Li L., Okusa M. D. 2010. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin. Nephrol. 30: 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L., Huang L., Sung S. S., Lobo P. I., Brown M. G., Gregg R. K., Engelhard V. H., Okusa M. D. 2007. NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J. Immunol. 178: 5899–5911 [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Rabb H., Womer K. L. 2007. Ischemia-reperfusion and immediate T cell responses. Cell. Immunol. 248: 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burne M. J., Daniels F., El Ghandour A., Mauiyyedi S., Colvin R. B., O’Donnell M. P., Rabb H. 2001. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Invest. 108: 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey D. I., MacDonald H. R., Kronenberg M., Smyth M. J., Van Kaer L. 2004. NKT cells: what’s in a name? Nat. Rev. Immunol. 4: 231–237 [DOI] [PubMed] [Google Scholar]
- 11.Dong X., Swaminathan S., Bachman L. A., Croatt A. J., Nath K. A., Griffin M. D. 2007. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 71: 619–628 [DOI] [PubMed] [Google Scholar]
- 12.Li L., Huang L., Vergis A. L., Ye H., Bajwa A., Narayan V., Strieter R. M., Rosin D. L., Okusa M. D. 2010. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 120: 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leite-De-Moraes M. C., Hameg A., Pacilio M., Koezuka Y., Taniguchi M., Van Kaer L., Schneider E., Dy M., Herbelin A. 2001. IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: a pro-Th2 effect of IL-18 exerted through NKT cells. J. Immunol. 166: 945–951 [DOI] [PubMed] [Google Scholar]
- 14.Spiegel S., Milstien S. 2002. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277: 25851–25854 [DOI] [PubMed] [Google Scholar]
- 15.Spiegel S., Kolesnick R. 2002. Sphingosine 1-phosphate as a therapeutic agent. Leukemia 16: 1596–1602 [DOI] [PubMed] [Google Scholar]
- 16.Jo S. K., Bajwa A., Awad A. S., Lynch K. R., Okusa M. D. 2008. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 73: 1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takuwa Y., Takuwa N., Sugimoto N. 2002. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J. Biochem. 131: 767–771 [DOI] [PubMed] [Google Scholar]
- 18.Allende M. L., Dreier J. L., Mandala S., Proia R. L. 2004. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279: 15396–15401 [DOI] [PubMed] [Google Scholar]
- 19.Rosen H., Goetzl E. J. 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5: 560–570 [DOI] [PubMed] [Google Scholar]
- 20.Idzko M., Panther E., Corinti S., Morelli A., Ferrari D., Herouy Y., Dichmann S., Mockenhaupt M., Gebicke-Haerter P., Di Virgilio F., et al. 2002. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 16: 625–627 [DOI] [PubMed] [Google Scholar]
- 21.Bajwa A., Jo S. K., Ye H., Huang L., Dondeti K. R., Rosin D. L., Haase V. H., Macdonald T. L., Lynch K. R., Okusa M. D. 2010. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J. Am. Soc. Nephrol. 21: 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takuwa N., Ohkura S., Takashima S., Ohtani K., Okamoto Y., Tanaka T., Hirano K., Usui S., Wang F., Du W., et al. 2010. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc. Res. 85: 484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C., Jiang X., Yang L., Liu X., Yue S., Li L. 2009. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am. J. Pathol. 175: 1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C., Kong Y., Wang H., Wang S., Yu H., Liu X., Yang L., Jiang X., Li L., Li L. 2009. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 50: 1174–1183 [DOI] [PubMed] [Google Scholar]
- 25.Singleton P. A., Moreno-Vinasco L., Sammani S., Wanderling S. L., Moss J., Garcia J. G. 2007. Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. Am. J. Respir. Cell Mol. Biol. 37: 222–231 [DOI] [PubMed] [Google Scholar]
- 26.Tölle M., Levkau B., Keul P., Brinkmann V., Giebing G., Schönfelder G., Schäfers M., von Wnuck Lipinski K., Jankowski J., Jankowski V., et al. 2005. Immunomodulator FTY720 induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ. Res. 96: 913–920 [DOI] [PubMed] [Google Scholar]
- 27.Girkontaite I., Sakk V., Wagner M., Borggrefe T., Tedford K., Chun J., Fischer K. D. 2004. The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in splenic marginal sinus organization. J. Exp. Med. 200: 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niessen F., Schaffner F., Furlan-Freguia C., Pawlinski R., Bhattacharjee G., Chun J., Derian C. K., Andrade-Gordon P., Rosen H., Ruf W. 2008. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452: 654–658 [DOI] [PubMed] [Google Scholar]
- 29.Means C. K., Xiao C. Y., Li Z., Zhang T., Omens J. H., Ishii I., Chun J., Brown J. H. 2007. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 292: H2944–H2951 [DOI] [PubMed] [Google Scholar]
- 30.Theilmeier G., Schmidt C., Herrmann J., Keul P., Schäfers M., Herrgott I., Mersmann J., Larmann J., Hermann S., Stypmann J., et al. 2006. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 114: 1403–1409 [DOI] [PubMed] [Google Scholar]
- 31.Jo S. K., Bajwa A., Ye H., Vergis A. L., Awad A. S., Kharel Y., Lynch K. R., Okusa M. D. 2009. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int. 75: 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oz-Arslan D., Rüscher W., Myrtek D., Ziemer M., Jin Y., Damaj B. B., Sorichter S., Idzko M., Norgauer J., Maghazachi A. A. 2006. IL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipids. J. Leukoc. Biol. 80: 287–297 [DOI] [PubMed] [Google Scholar]
- 33.Day Y. J., Huang L., McDuffie M. J., Rosin D. L., Ye H., Chen J. F., Schwarzschild M. A., Fink J. S., Linden J., Okusa M. D. 2003. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J. Clin. Invest. 112: 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awad A. S., Ye H., Huang L., Li L., Foss F. W., Jr., Macdonald T. L., Lynch K. R., Okusa M. D. 2006. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am. J. Physiol. Renal Physiol. 290: F1516–F1524 [DOI] [PubMed] [Google Scholar]
- 35.Lutz M. B., Kukutsch N., Ogilvie A. L., Rössner S., Koch F., Romani N., Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223: 77–92 [DOI] [PubMed] [Google Scholar]
- 36.Brossay L., Chioda M., Burdin N., Koezuka Y., Casorati G., Dellabona P., Kronenberg M. 1998. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188: 1521–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieda M., Nicol A., Koezuka Y., Kikuchi A., Takahashi T., Nakamura H., Furukawa H., Yabe T., Ishikawa Y., Tadokoro K., Juji T. 1999. Activation of human Vα24NKT cells by α-glycosylceramide in a CD1d-restricted and Vα24TCR-mediated manner. Hum. Immunol. 60: 10–19 [DOI] [PubMed] [Google Scholar]
- 38.Sullivan B. A., Nagarajan N. A., Wingender G., Wang J., Scott I., Tsuji M., Franck R. W., Porcelli S. A., Zajonc D. M., Kronenberg M. 2010. Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J. Immunol. 184: 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Goff R. D., Zhou D., Mattner J., Sullivan B. A., Khurana A., Cantu C., III, Ravkov E. V., Ibegbu C. C., Altman J. D., et al. 2006. A modified α-galactosyl ceramide for staining and stimulating natural killer T cells. J. Immunol. Methods 312: 34–39 [DOI] [PubMed] [Google Scholar]
- 40.Zhou D., Mattner J., Cantu C., III, Schrantz N., Yin N., Gao Y., Sagiv Y., Hudspeth K., Wu Y. P., Yamashita T., et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science 306: 1786–1789 [DOI] [PubMed] [Google Scholar]
- 41.Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Sato H., Kondo E., Harada M., Koseki H., Nakayama T., et al. 1998. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc. Natl. Acad. Sci. USA 95: 5690–5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii S., Shimizu K., Smith C., Bonifaz L., Steinman R. M. 2003. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198: 267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sköld M., Behar S. M. 2003. Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 71: 5447–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronenberg M. 2005. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23: 877–900 [DOI] [PubMed] [Google Scholar]
- 45.Eltzschig H. K., Eckle T. 2011. Ischemia and reperfusion: from mechanism to translation. Nat. Med. 17: 1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Day Y. J., Huang L., Ye H., Linden J., Okusa M. D. 2005. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am. J. Physiol. Renal Physiol. 288: F722–F731 [DOI] [PubMed] [Google Scholar]
- 47.Berndt B. E., Zhang M., Chen G. H., Huffnagle G. B., Kao J. Y. 2007. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J. Immunol. 179: 6255–6262 [DOI] [PubMed] [Google Scholar]
- 48.Tadagavadi R. K., Reeves W. B. 2010. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J. Am. Soc. Nephrol. 21: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eltzschig H. K., Carmeliet P. 2011. Hypoxia and inflammation. N. Engl. J. Med. 364: 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morote-Garcia J. C., Rosenberger P., Nivillac N. M., Coe I. R., Eltzschig H. K. 2009. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136: 607–618 [DOI] [PubMed] [Google Scholar]
- 51.Ramesh G., Reeves W. B. 2002. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 110: 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomura M., Yu W. G., Ahn H. J., Yamashita M., Yang Y. F., Ono S., Hamaoka T., Kawano T., Taniguchi M., Koezuka Y., Fujiwara H. 1999. A novel function of Vα14+CD4+NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J. Immunol. 163: 93–101 [PubMed] [Google Scholar]
- 53.Kitamura H., Iwakabe K., Yahata T., Nishimura S., Ohta A., Ohmi Y., Sato M., Takeda K., Okumura K., Van Kaer L., et al. 1999. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189: 1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bendelac A., Savage P. B., Teyton L. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25: 297–336 [DOI] [PubMed] [Google Scholar]
- 55.Wu D., Xing G. W., Poles M. A., Horowitz A., Kinjo Y., Sullivan B., Bodmer-Narkevitch V., Plettenburg O., Kronenberg M., Tsuji M., et al. 2005. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc. Natl. Acad. Sci. USA 102: 1351–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinjo Y., Wu D., Kim G., Xing G. W., Poles M. A., Ho D. D., Tsuji M., Kawahara K., Wong C. H., Kronenberg M. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434: 520–525 [DOI] [PubMed] [Google Scholar]
- 57.Prussin C., Foster B. 1997. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J. Immunol. 159: 5862–5870 [PubMed] [Google Scholar]
- 58.Bendelac A., Rivera M. N., Park S. H., Roark J. H. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15: 535–562 [DOI] [PubMed] [Google Scholar]
- 59.Day Y. J., Huang L., Ye H., Li L., Linden J., Okusa M. D. 2006. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-γ. J. Immunol. 176: 3108–3114 [DOI] [PubMed] [Google Scholar]
- 60.Lappas C. M., Day Y. J., Marshall M. A., Engelhard V. H., Linden J. 2006. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J. Exp. Med. 203: 2639–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong S., Wilson M. T., Serizawa I., Wu L., Singh N., Naidenko O. V., Miura T., Haba T., Scherer D. C., Wei J., et al. 2001. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 7: 1052–1056 [DOI] [PubMed] [Google Scholar]
- 62.Laloux V., Beaudoin L., Jeske D., Carnaud C., Lehuen A. 2001. NK T cell-induced protection against diabetes in Vα14-Jα281 transgenic nonobese diabetic mice is associated with a Th2 shift circumscribed regionally to the islets and functionally to islet autoantigen. J. Immunol. 166: 3749–3756 [DOI] [PubMed] [Google Scholar]
- 63.Oh S. J., Chung D. H. 2011. Invariant NKT cells producing IL-4 or IL-10, but not IFN-γ, inhibit the Th1 response in experimental autoimmune encephalomyelitis, whereas none of these cells inhibits the Th17 response. J. Immunol. 186: 6815–6821 [DOI] [PubMed] [Google Scholar]
- 64.Yokota N., Burne-Taney M., Racusen L., Rabb H. 2003. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 285: F319–F325 [DOI] [PubMed] [Google Scholar]
- 65.Liu M., Agreda P., Crow M., Racusen L., Rabb H. 2009. Effects of delayed rapamycin treatment on renal fibrosis and inflammation in experimental ischemia reperfusion injury. Transplant. Proc. 41: 4065–4071 [DOI] [PubMed] [Google Scholar]
- 66.Hayakawa Y., Takeda K., Yagita H., Van Kaer L., Saiki I., Okumura K. 2001. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 166: 6012–6018 [DOI] [PubMed] [Google Scholar]
- 67.Ben-Shoshan J., Afek A., Maysel-Auslender S., Barzelay A., Rubinstein A., Keren G., George J. 2009. HIF-1α overexpression and experimental murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29: 665–670 [DOI] [PubMed] [Google Scholar]
- 68.Park S. W., Kim M., Brown K. M., D’Agati V. D., Lee H. T. 2012. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 23: 266–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeda Y., Matsuyuki H., Shimano K., Kataoka H., Sugahara K., Chiba K. 2007. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J. Immunol. 178: 3437–3446 [DOI] [PubMed] [Google Scholar]
- 70.Rivera J., Proia R. L., Olivera A. 2008. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8: 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lappin M. B., Weiss J. M., Delattre V., Mai B., Dittmar H., Maier C., Manke K., Grabbe S., Martin S., Simon J. C. 1999. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology 98: 181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ouyang J., Fan C., Wen D., Hou J., Du Y., Wang Y., Shi G. 2010. Donor antigen-loaded IKK2dn gene-modified dendritic cells prolong allograft survival. Scand. J. Immunol. 71: 336–344 [DOI] [PubMed] [Google Scholar]
- 73.Tiao M. M., Lu L., Tao R., Wang L., Fung J. J., Qian S. 2005. Prolongation of cardiac allograft survival by systemic administration of immature recipient dendritic cells deficient in NF-κB activity. Ann. Surg. 241: 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun W., Wang Q., Zhang L., Liu Y., Zhang M., Wang C., Wang J., Cao X. 2003. Blockade of CD40 pathway enhances the induction of immune tolerance by immature dendritic cells genetically modified to express cytotoxic T lymphocyte antigen 4 immunoglobulin. Transplantation 76: 1351–1359 [DOI] [PubMed] [Google Scholar]
- 75.Tiao M. M., Lu L., Tao R., Harnaha J., Fung J. J., Huang L. T., Qian S. 2004. Application of recipient-derived dendritic cells to induce donor-specific T-cell hyporesponsiveness. Transplant. Proc. 36: 1592–1594 [DOI] [PubMed] [Google Scholar]
- 76.Tao R., Wang L., Chen C. H., Wang S. H., Demarco R. A., Lotze M. T., Thai N. L., Fung J. J., Lu L., Qian S. 2006. Mechanistic insights into achievement of cardiac allograft long-term survival by treatment with immature dendritic cells and sub-dose sirolimus. J. Heart Lung Transplant. 25: 310–319 [DOI] [PubMed] [Google Scholar]
- 77.Bonham C. A., Peng L., Liang X., Chen Z., Wang L., Ma L., Hackstein H., Robbins P. D., Thomson A. W., Fung J. J., et al. 2002. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-κB oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J. Immunol. 169: 3382–3391 [DOI] [PubMed] [Google Scholar]
- 78.Morelli A. E. 2006. The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. Am. J. Transplant. 6: 254–261 [DOI] [PubMed] [Google Scholar]
- 79.Wang Z., Larregina A. T., Shufesky W. J., Perone M. J., Montecalvo A., Zahorchak A. F., Thomson A. W., Morelli A. E. 2006. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am. J. Transplant. 6: 1297–1311 [DOI] [PubMed] [Google Scholar]
- 80.Hackstein H., Thomson A. W. 2004. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 4: 24–34 [DOI] [PubMed] [Google Scholar]
- 81.Goldberg L. C., Bradley J. A., Connolly J., Friend P. J., Oliveira D. B., Parrott N. R., Rodger R. S., Taube D., Thick M. G. 1995. Anti-CD45 monoclonal antibody perfusion of human renal allografts prior to transplantation: a safety and immunohistological study. CD45 Study Group. Transplantation 59: 1285–1293 [PubMed] [Google Scholar]
- 82.Green E. A., Eynon E. E., Flavell R. A. 1998. Local expression of TNFα in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity 9: 733–743 [DOI] [PubMed] [Google Scholar]
- 83.Mehling A., Loser K., Varga G., Metze D., Luger T. A., Schwarz T., Grabbe S., Beissert S. 2001. Overexpression of CD40 ligand in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J. Exp. Med. 194: 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daemen M. A., de Vries B., Buurman W. A. 2002. Apoptosis and inflammation in renal reperfusion injury. Transplantation 73: 1693–1700 [DOI] [PubMed] [Google Scholar]
- 85.Barkett M., Gilmore T. D. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18: 6910–6924 [DOI] [PubMed] [Google Scholar]
- 86.Li Q., Verma I. M. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2: 725–734 [DOI] [PubMed] [Google Scholar]
- 87.Rescigno M., Martino M., Sutherland C. L., Gold M. R., Ricciardi-Castagnoli P. 1998. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 188: 2175–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishiyama T., Dharmarajan S., Hayama M., Moriya H., Grapperhaus K., Patterson G. A. 2005. Inhibition of nuclear factor κB by IκB superrepressor gene transfer ameliorates ischemia-reperfusion injury after experimental lung transplantation. J. Thorac. Cardiovasc. Surg. 130: 194–201 [DOI] [PubMed] [Google Scholar]
- 89.Cao C. C., Ding X. Q., Ou Z. L., Liu C. F., Li P., Wang L., Zhu C. F. 2004. In vivo transfection of NF-κB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int. 65: 834–845 [DOI] [PubMed] [Google Scholar]
- 90.Salomone S., Waeber C. 2011. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.