Abstract
Current artificial lungs and respiratory assist devices designed for carbon dioxide removal (CO2R) are limited in their efficiency due to the relatively small partial pressure difference across gas exchange membranes. To offset this underlying diffusional challenge, bioactive hollow fiber membranes (HFMs) increase the carbon dioxide diffusional gradient through the immobilized enzyme carbonic anhydrase (CA), which converts bicarbonate to CO2 directly at the HFM surface. In this study, we tested the impact of CA-immobilization on HFM CO2 removal efficiency and thromboresistance in blood. Fiber surface modification with radio frequency glow discharge (RFGD) introduced hydroxyl groups, which were activated by 1M CNBr while 1.5M TEA was added drop wise over the activation time course, then incubation with a CA solution covalently linked the enzyme to the surface. The bioactive HFMs were then potted in a model gas exchange device (0.0084 m2) and tested in a recirculation loop with a CO2 inlet of 50mmHg under steady blood flow. Using an esterase activity assay, CNBr chemistry with TEA resulted in 0.99U of enzyme activity, a 3.3 fold increase in immobilized CA activity compared to our previous method. These bioactive HFMs demonstrated 108 ml/min/m2 CO2 removal rate, marking a 36% increase compared to unmodified HFMs (p < 0.001). Thromboresistance of CA-modified HFMs was assessed in terms of adherent platelets on surfaces by using lactate dehydrogenase (LDH) assay as well as scanning electron microscopy (SEM) analysis. Results indicated HFMs with CA modification had 95% less platelet deposition compared to unmodified HFM (p < 0.01). Overall these findings revealed increased CO2 removal can be realized through bioactive HFMs, enabling a next generation of more efficient CO2 removal intravascular and paracorporeal respiratory assist devices.
Keywords: Artificial lung, Carbonic anhydrase, CO2 removal, hollow fiber membrane
1. Introduction
Over the last 10 years carbon dioxide (CO2) removal has been emphasized to improve survival outcomes for patients suffering from acute lung failure. Due to significant limitations in its clinical application, the lifesaving efforts of traditional therapy such as mechanical ventilation (MV) are often marred by unfavorable effects which initiate and more often exacerbate lung injury, substantially contributing to patient morbidity and mortality(1). Consequently, breathing support independent of the lungs through low blood flow CO2 removal devices are needed as a tool for the management of acute and possibly chronic respiratory insufficiency. Considering current respiratory assist devices designed for extracorporeal carbon dioxide removal can require blood flow rates of 1 L/min for to extract a therapeutic level of carbon dioxide, advances in CO2 removal membranes with sufficient clinical impact has yet to be realized. Development of a bioactive hollow fiber membrane (HFMs) which facilitates CO2 removal may provide the next generation of highly efficient CO2 removal membranes for respiratory assist devices.
Medical treatment for acute and chronic respiratory failure remains a significant healthcare problem, with over several hundred thousand adult patients in the US(2,3). High tidal volume ventilation known to be a major contributor to ventilator induced lung injury could be avoided by decreasing ventilatory needs of the patient through extracorporeal carbon dioxide removal. The seminal acute respiratory distress syndrome (ARDS) Network trial demonstrated that delivery of low tidal volume MV at 6mL/kg instead of 12 mL/kg resulted in reduced lung injury and improved survival(4). Evidence suggests however, that even more protective MV settings are needed as alveolar over-distention is still seen at 6mL/kg exposing the ARDS lung to 25- to 30-times the mechanical stress of a normal lung(5,6). These patients are susceptible to micro vascular occlusion and alveolar dead space which lead to under perfused respiratory units limited in gas exchange(7,8). Control of PCO2 independent of alveolar ventilation is possible through extracorporeal carbon dioxide removal, decreasing MV requirements and facilitating lung rest. For patients suffering from acute exacerbation of chronic obstructive pulmonary disease (COPD), MV could be avoided as CO2 removal itself maybe adequate. Zwischenberger et al. have shown improved survival with arterio-venous CO2 removal (AVCO2R) compared to conventional mechanical ventilation, high frequency percussive ventilation and low tidal volume ventilation, observing recovered lung cell proliferation and tissue architecture along with reduced apoptosis through AVCO2R treatments in model sheep ARDS(9,10). More recently, the CESAR study demonstrated enhanced survival benefit for lung-rest ventilation in tandem with ECMO as compared to protective ventilation alone(11). These studies demonstrate CO2 removal is a powerful alternative to MV in patients suffering from acute lung failure.
A highly efficient, low flow CO2 removal device offers an adjunct therapy for acute and possibly chronic respiratory insufficiency. Several novel respiratory assist devices have been developed and/or proposed which mechanically enhance gas exchange by actively mixing the blood flow over the HFM surface. These devices could decrease patient ventilator needs by more than 50% relieving dyspnea and distress while requiring blood flow rates as small as 300–500 mL/min(6). Novalung (Baden-Württemberg, Germany) and Hemolung (Alung Technologies, Pittsburgh PA) are a realization of low flow partial CO2 removal devices, capable of eliminating 20–30% of resting metabolic CO2 production. Pumpless, percutaneous and arteriovenous, the Novalung device has been recognized for its CO2 removal capacity in facilitating lung protective MV settings(12). Despite the promising de-escalation of invasive ventilatory variables, arterial canulation systems have shown complication rates as high as 24% leading to limb ischemia, compartment syndrome, and intracranial hemorrhage, in part due to their demand for approximately 25% of cardiac output(13). To avoid such difficulties, the Hemolung employs a venovenous system incorporating the pump and hollow fiber membranes (HFMs) as one cartridge. While offering the first steps in CO2 removal these devices are limited in their efficiency, and therefore limited in their audience for therapeutic potential. An ideal low flow CO2 removal device would eliminate up to 200 ml/min carbon dioxide to meet the metabolic needs for an adult patient on cardiopulmonary bypass(14). To achieve this goal the devices must overcome the small CO2 partial pressures (50mmHg) across HFMs limiting CO2 removal. Since more than 90% of blood CO2 is carried as HCO3− (bicarbonate), a chemical means of increasing gas exchange efficiency is needed.
We propose a bioactive HFM to facilitate CO2 removal by increasing the diffusional gradient at the HFM surface through immobilization of carbonic anhydrase (CA), an enzyme which dehydrates bicarbonate to CO2. Our tissues face the same diffusional challenges as HFM devices; however they employ CA within red blood cells and on the endothelial surfaces of lung capillaries to accelerate diffusion. In a biomimetic approach, we reported the development of a carbonic anhydrase (CA) immobilized bioactive HFM coating(15). Through radio frequency flow discharge (RFGD) treatment hydroxyl groups were deposited upon the fiber membrane. Scanning electron microscopy (SEM) and gas permeance tests demonstrated an exposure time of 30 sec and 25W did not affect the integrity and gas permeability of the HFMs. After CNBr activation and CA coupling, further gas permeance data indicated CA does not impede gas diffusion across the membrane. These CA HFMs demonstrated the ability to improve CO2 removal rates by 75% from PBS in small gas exchange devices (0.0074 m2) compared to unmodified devices(15).
In respiratory assist devices where HFM surface area can be greater than 1 m2, hemocompatibility is a major concern. A wide variety of surface modification techniques currently exist and have been evaluated to reduce the thrombogenicity of blood-material interaction(16–18). However, to date there are few reports specifically focused on HFMs utilized in artificial lung applications, especially those employing plasma polymerization and bioconjugation techniques. Platelet adhesion and subsequent protein-based coagulation cascades are well known mechanisms of thrombus formation on the surfaces of polymeric materials(19). As a result, large blood-contacting surface presents significant challenges in terms of hemocompatibility for HFMs, where clots can reduce performance of respiratory assist devices and even prevent blood flow(20). Next generation respiratory assist devices will need improved hemocompatibility of HFMs for the successful continued operation. While heparin coated materials are well known for enhanced hemocompatibility(21), a single coating capable of significantly enhancing gas exchange while simultaneously decreasing thrombogenicity could afford the next generation of low flow CO2 removal device with minimal complications.
This present study sought to further improve CA immobilization upon HFMs through optimized CNBr chemistry, quantify HFM CO2 removal performance in blood and assess hemocompatibility of a CA coating. HFMs were plasma RFGD treated to create surface functional groups, to which CNBr activation chemistry was optimized with TEA and finally incubated with CA solution for covalent conjugation to the HFM surface. The improved CA modified HFMs were fabricated into model gas exchange devices to assess CO2 removal performance from blood under a mass transfer environment similar to those of commercially available oxygenators. Platelet deposition was quantified through lactate dehydrogenase assay and SEM. Our study demonstrates CA immobilized HFMs are a viable means of augmenting CO2 removal and hemocompatibility for artificial lung devices.
2. Materials and methods
2.1. Materials
Carbonic anhydrase (CA) from bovine erythrocytes, cyanogen bromide (CNBr), triethylamine (TEA), paranitrophenol acetate (pNPA), N,N′-dimethylbarbituric acid, Triton X-100, glutaraldehyde and acetone were purchased from Sigma-Aldrich (St. Louis, MO). A commercial polymethyl-pentene (PMP) fiber (Oxyplus™, OD: 380 μm, ID: 200 μm) was obtained from Membrana GmbH (Wuppertal, Germany). Bovine blood for the gas exchange experiments was purchased from Lampire Biological Laboratories (Pipersville, PA). All other reagents were purchased from Sigma-Aldrich and were of analytical grade or purer.
2.2. CA Immobilization on HFMs
HFMs were modified with water plasma using a RFGD instrument (March Plasma Systems, Concord, CA) under conditions of 25 W and 30 s treatment time. After plasma modification, the HFMs were immersed in 60% acetone solution equlibrated to −15C° through an ice bath, to which a solution of cyanogen bromide (CNBr) in acetone was added to a yield a final 1M CNBr concentration. While vigorously mixed, 1.5 M TEA was added drop wise over 3 minutes to yield a final ratio of 1:1.5 CNBr:TEA. The HFMs were subsequently rinsed 3 times for 3 minutes each with deionized water (DIW). The reaction time was optimized through the cyanate ester assay to maximize cyanate ester production, while the rinse time was optimized to remove any residual CNBr. The activated HFMs were then incubated with 1 mg/mL of CA in 50 mM sodium phosphate buffer (pH 7.5) for 3 h at 25C°. Any loosely bound CA was removed through three 15 minute phosphate buffer wash sessions.
HFMs for hemocompatibility assessment (section 2.6) were modified in similar fashion but without the addition of TEA catalyst. The modifications listed below are the only changes. After plasma treatment, the HFMs were immersed in 2 M of sodium carbonate to which a solution of CNBr in acetonitrile was added to yield a final concentration of 1M. The solution was incubated for 10 min. At the completion of the activation reaction, the HFMs were washed with deionized water. The CNBr activated HFMs were then incubated with 1 mg/mL of CA in 100 mM sodium carbonate buffer (pH 8.0) for 3 h.
2.3. Assay for Cyanate Ester Active Groups
A quantitative assessment of CNBr/TEA activation was performed through colormetric quantification of cyanate ester active groups(22). The assay buffer was created by mixing 0.75 g of N,N′-dimethylbarbituric acid in 45mL analytical grade pyridine. The modified HFMs were cut into 1–2 mm segments, placed in assay buffer (4 mL) and vigorously mixed using a magnetic stirrer for 10 minutes. Reaction of cyanate ester with the assay buffer yields a strong purple color. Activation quality was measured spectrophotometrically using a Genesys 5 UV spectrophotometer (Thermo Spectronic, Somerset, NJ) by monitoring absorbance change at 588nm.
2.4. Esterase Assay of CA Activity
The enzyme activity on HFMs was assayed using the substrate p-nitrophenyl acetate (p-NPA) as described previously(15). Enzyme activity was measured spectrophotometrically by monitoring the hydrolysis of p-nitrophenyl acetate (p-NPA) to p-nitrophenol (p-NP) at 412 nm. The modified HFMs were cut into 1–2 mm segments and placed in assay buffer (4 mL; 50 mM phosphate buffer, pH 7.5) to measure the esterase activity. The reaction was initiated by the addition of p-NPA (40 μL) and vigorously mixed using a magnetic stirrer. Absorbance measurements were recorded every 3 min over a 9 min period, and plotted as a function of time. One activity unit was defined as the amount of enzyme required to generate 1 μmol pNP per minute.
2.5 In Vitro CO2 Exchange in a Model Oxygenator
A scaled-down model gas exchange module was fabricated by inserting HFMs (70 fibers, 18 cm) into a 1/4 in. ID polycarbonate-tubing (McMaster Carr, Elmhurst, IL) to which single luer locks were UV-glued 1.25 in. from each end in opposing directions (Figure 1A). Both ends of the HFMs were secured to the tubing using an epoxy adhesive (Devcon, Danvers, MA) and then trimmed to the length of the tubing to expose the HFM lumens, yielding 10 cm of HFM uncovered within the module for a total active surface area of 0.0074 m2. An in vitro recirculating test loop was used to assess CO2 exchange rates using CA-immobilized and unmodified HFMs (Figure 1B).
Figure 1.
A. Diagram of a model respiratory device employed for measuring CO2 removal rates of unmodified and modified HFMs. Bovine blood or PBS was perfused over the outside of the fibers while oxygen sweep gas was passed through the fiber lumens in the opposite direction. B. Experimental setup for the in vitro CO2 gas exchange assessment. Both the blood reservoir and de-oxygenator employed the use of a heat exchanger to maintain blood temperature at 37C°.
2.5.1. PBS CO2 Removal
The loop consisted of a fluid reservoir, peristaltic pump, oxygenator, vacuum pump and the model gas exchange device. The testing fluid (180 mL of PBS), flowed from a MasterFlex L/S peristaltic pump (Vernon Hills, IL) to a Terumo CAPIOX RX05 Baby RX Oxygenator (Ann Arbor, Michigan), then to the model gas exchange testing module and finally back to the reservoir. The inlet partial pressure of CO2 (PCO2) was adjusted to 50 mmHg and measured with a RAPIDLAB 248 Blood-Gas analyzer (Siemens, Deerfield, IL). Pure oxygen sweep gas was pulled by vacuum through a GR Series Gas Regulator (Fathom, Round Rock, TX), through the model gas exchange testing module HFM lumens, moisture trap condenser immersed in ice, KNF Lab UN811KV.45P Vacuum Pump (USA) and finally a WMA-4 CO2 Analyzer (PP Systems, Amesbury, MA). The fluid flow rate through the module was set at 10 mL/min and the sweep gas through the HFM lumens at 50 mL/min. The fluid temperature was maintained at 37C° by heat bath.
The rate of CO2 removal (VCO2) for each model oxygenator device was calculated using the sweep gas flow rate ( ) and CO2 fraction (FCO2) exiting the model respiratory assist device and then normalized to 50mmHg to correct for small deviations in the inlet PCO2:
| (Eq. 1) |
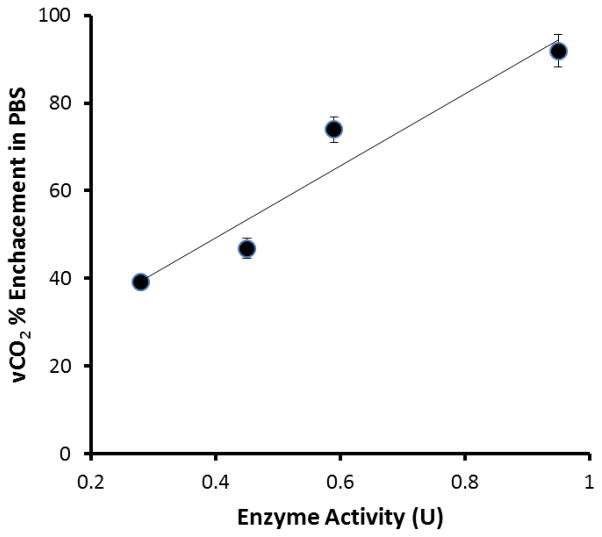
For each device the fluid inlet PCO2 and resulting FCO2 were measured in triplicate. The VCO2 for each model gas exchange device is reported as an average of these measurements. The percent enhancement in CO2 removal from PBS was determined for various levels of immobilized enzyme activity ranging from 0.30 to 0.99 U (Figure 3). By modifying the time of enzyme immobilization between 0.5 and 3 hours this spectrum of enzyme activity could be created.
Figure 3.
Increasing immobilized CA activity results in a proportional increase in CO2 removal from PBS. (N=2)
2.5.2. Blood CO2 Removal
To match CO2 mass transfer environment of commercially available oxygenator devices, the gas exchange module surface area was increased to 0.0084 m2, the recirculating loop liquid flow rate was set to 60 mL/min and a sweep gas flow rate to 300 mL/min. Under these conditions the HFM CO2 removal efficiency (ml/min/m2) matches commercially available devices. Prior to testing, blood glucose was equilibrated to 200 mg/dL and allowed to incubate for 1 hour. A solution of PBS was circulated through the testing loop for 15 minutes to remove bubbles. Blood was introduced to the loop through a wet connection (no bubbles between the PBS/blood interface) to limit hemolysis. The PBS/blood mixture was allowed to flow through the loop and drain into a waste container until no signs of blood dilution were present, upon which the loop was reconnected to the blood reservoir filled to 180mL. For each device the fluid inlet PCO2 and resulting FCO2 were measured in triplicate and averaged. The VCO2 for modified devices is reported as an average of four separate devices and trials.
2.6. Blood collection and assessment of acute thrombotic deposition
The HFM samples (0.6 cm2) were placed into Vacutainer® blood collection tubes (BD Biosciences, Franklin Lakes, NJ) filled with 5 mL of heparinized ovine blood and incubated for 2 h at 37°C on a hematology mixer (Fisher Scientific, Pittsburgh, PA). The number of platelets deposited on the samples was determined by a lactate dehydrogenase (LDH)(23) assay with an LDH cytotoxicity detection kit (Takara Bio, Otsu, Shiga, Japan). The samples were rinsed thoroughly with 50 mL of PBS and then immersed in 1 mL of 2% Triton X-100 solution for 20 min to lyse surface deposited platelets. Surface imaging studies were performed after continuous rocking of blood for 2 h, as mentioned above. The HFM sample surfaces were then rinsed with 50 mL PBS and immersed in 2.5% glutaraldehyde solution for 2 h at 4°C to fix the surface deposited platelets in order to perform scanning electron microscopic (SEM) analysis(16).
2.7. Statistical Analyses
All data are presented as a mean with standard deviation. A Student’s t-test assuming equal sample variance was used to determine p-values and assess any statistically significant differences between unmodified and CA-modified HFMs. Differences were considered statistically significant for p<0.05.
3. Results
CNBr activation of surface hydroxyl groups was optimized to yield the highest level of enzyme immobilization on HFMs. In our previous study, a maximal activity of 0.30 U was reported, producing a 75% enhancement in CO2 removal from PBS(15). Using the optimized method described here, an immobilized enzyme activity of .99 U was obtained (Figure 2), marking a 3.3 fold increase in maximal immobilized enzyme activity compared to the method in our previous study. It is important to note the in-vitro testing loop employed in these studies differs from the loop of our previous study(15), which limits direct comparison. The percent increase in CO2 removal from PBS was determined for various levels of immobilized enzyme activity ranging from 0.30 to 0.99 U (Figure 3). Within this invitro test loop, immobilized enzyme activity (0.30 U) produced a 40% enhancement in vCO2. Increasing immobilized enzyme activity resulted in a proportional increase in CO2 removal where maximal enzyme activity (0.99 U) increased vCO2 by 95%.
Figure 2.
Esterase activity units of CA-modified HFMs. Traditional CNBr (Kaar et al.) activation reflects the activity level resulting from our previous work. CNBr/TEA activation demonstrates the enhanced activity of the optimized method. *p < 0.005
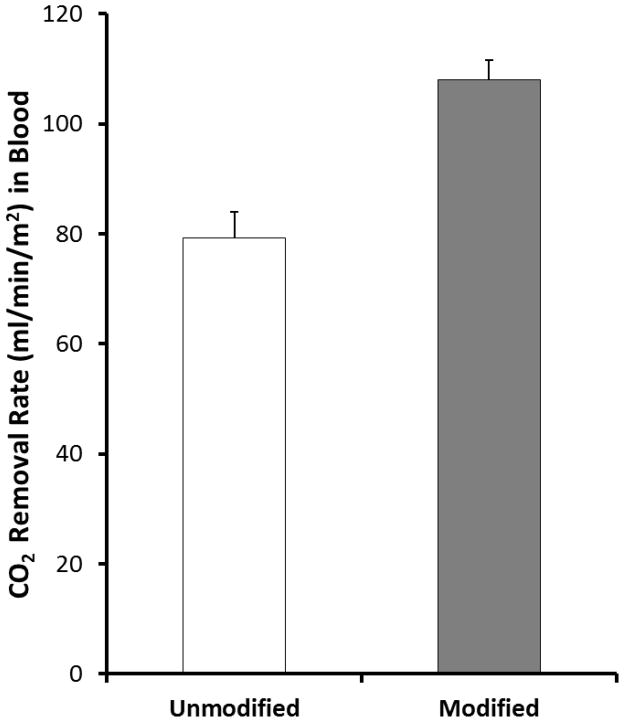
The CO2 removal capacity of the CA-immobilized HFMs was also assessed in bovine blood. To mimic the mass transfer environment of commercially available oxygenators, a few modifications were made to the model oxygenator device used in PBS. As described in section 2.5.2, the surface area was increased to 0.0084 cm2 to match the void fraction of current commercial oxygenators. Furthermore the liquid flow rate was increased to 60 mL/min and sweep gas flow rate to 300 L/min match the mass transfer coefficient of commercial oxygenators. Figure 4 illustrates the impact of CA- immobilized HFMs on CO2 removal in blood. The unmodified testing module removed 79 ± 4.7 mL/min/m2 of CO2 while the bioactive (0.99 U) testing module removed 108 ± 3.7 mL/min/m2, marking a 36% increase compared to unmodified HFMs (p< 0.001).
Figure 4.
CO2 removal by unmodified (control) and modified (CA immobilized) HFMs in bovine blood in a model respiratory assist device (N=4). The modified fibers demonstrated a 36% increase in CO2 removal capacity. *p <0.005
Thromboresistance of CA-modified HFMs was assessed in terms of adherent platelets on surfaces by using LDH assay as well as SEM analysis. Figure 5 illustrates the impact of surface modification on thromboresistance by measuring platelet adhesion to the HFMs. Quantification through LDH assay indicated the CA fiber surface reduced platelet deposition by 95% relative to unmodified fiber. The SEM micrographs support the results from the indirect platelet deposition assay, with CA-modified HFM surfaces showing less platelet deposition compared to unmodified HFM (Figure 6).
Figure 5.
Platelet deposition onto surfaces after contact with ovine blood for 2 h as determined by a lactate dehydrogenase (LDH) assay (N=3).
Figure 6.
SEM images of testing hollow fiber membrane surfaces after contact with heparinized ovine blood for 2 h at 37°C. (A) Unmodified PMP; (B) CA-modified PMP. Images were recorded using an accelerating voltage of 5 kV at a magnification of 1,000× (scale bar = 10 μm).
4. Discussion
The present study sought to demonstrate the increased CO2 removal capacity of CA modified HFMs could be further developed as a viable technology for HFM respiratory assist devices. By activating the hydroxyl groups introduced to the HFM surface using CNBr/TEA, free amine groups on the enzyme surface are able react with the HFM, covalently linking the enzyme to the surface. Improvements in CNBr activation chemistry were made to enhance enzyme loading and consequently increase CO2 removal efficiency. A proportional increase between immobilized enzyme activity and percent enhancement of CO2 removal was demonstrated in PBS. Once fully optimized, the CO2 removal performance of the CA-HFMs was quantified in bovine blood under a CO2 mass transfer environment similar to those of commercially available oxygenators. Furthermore hemocompatibility of the CA-modified fibers was explored. To our knowledge, this is the first report assessing the potential enhancement of CO2 removal from blood using CA-immobilized HFMs.
We hypothesized through immobilization of CA on HFMs, HCO3− in plasma could be converted directly to gaseous dissolved CO2 at the membrane surface (Eq. 2), creating a gradient in which HCO3− diffuses to the fiber surface.
| (Eq. 2) |
Consequently, a “facilitated diffusion” of CO2 is created in the form of HCO3− diffusion, increasing the rate of CO2 removal. Our results here demonstrate the viability of CA immobilized HFMs as a means of enhancing the CO2 removal capacity of commercially available oxygenators. The linear increase in vCO2 with immobilized enzyme activity suggests even greater improvements in CO2 removal performance could be realized through enhanced immobilization techniques.
The CNBr activation of hydroxyl groups employed here was first described in 1967 by means of a basic pH where highly amine reactive cyanate ester and less reactive imidocarbonate functional groups were generated with limited efficiency(24). While at the time popular, this technique also formed inert carbamates which only contaminated the surface. TEA has since emerged as a reaction catalyst to enhance the efficiency of CNBr activation chemistry at a neutral pH(25,26). This works by the formation of N-cyanotriethylammonium (CTEA) boride, which by the von Braun reaction is unstable and will decay at temperatures above −10°C(27). We employed the CNBr/TEA method to increase the level of cyanate esters generated in our activation, as a means of increasing immobilized enzyme activity on the HFM surface. To avoid the decay of reactive groups, an ice bath of propanol and dry ice was used to maintain a reaction temperature of −15C. Subsequent reaction with CA under mild conditions yielded primarily N-substituted imidocarbonate linkages between lysines and the fiber membrane(28,29). In our previous study, an average 0.30 U enzyme activity, as determined by the esterase assay was observed. After employing the CNBr/TEA activation technique, an average of 0.99 U was observed, marking a 330% increase in overall immobilized enzyme activity. When comparing the 0.99 U activity level to previous work(15), we cannot accurately predict the percent surface coverage based upon monolayer CA immobilization through the esterase activity assay without first determining the Km for the immobilized enzyme state. Since enzyme attachment to cyanogen bromide-activated substrates is only stable in solution over a period of days to weeks(30), the immobilization chemistry is a suitable model for proof of principle of CA-facilitated diffusion. However, alternate methods of attaching CA may need to be explored to extend the operational stability of CA-modified HFMs in respiratory assist devices. This would also include study of enzyme loading versus enzyme activity, as a means to understand the efficiency of the various bioconjugation chemistries.
This work demonstrated bioactive CA-HFMs are capable of accelerating CO2 removal efficiency by 36% from blood. While this level of CA enhancement does not afford low flow CO2 removal devices capable of fully supporting adult resting metabolic CO2 production, it can potentiate more efficient and even smaller CO2 removal devices. Comparison of the mass transfer environment for the scaled-down control testing module with surface area (0.0084 m2) and CO2 removal efficiency of 79 ml/min/m2, to the Novalung with surface area (1.3 m2) and a mean CO2 removal rate of 119.3 ml/min, would yield a comparable 102.7 ml/min CO2 removal rate for the scaled-down module(31). The equivalent CO2 removal rates between the Novalung and the scaled-down control testing module demonstrate the clinical relevance of the mass transfer environment for the scaled-down module in this work. By increasing the immobilized CA activity upon HFMs a proportional enhancement in CO2 removal efficiency was observed (Figure 3). This behavior suggests continued optimization in CA immobilized activity could offer even greater increases in HFM CO2 removal efficiency. Based upon our previous study, immobilizing CA on the HFM has negligible effect on HFM permeance and therefore does not increase diffusional resistance of carbon dioxide(15). Further work will assess strategies to improve CA immobilization activity with more efficient bioconjugation chemistries, spacer molecules(32), and site specific immobilization(33,34).
The percent enhancement in CO2 removal rate by 0.99U HFMs diverged from PBS to blood as a result of differences in mass transfer environment. Since CA-modified HFMs demonstrated improved thromboresistance, platelet deposition is not likely the cause. Instead, competition with native CA in blood may lower the impact of CA-modified HFMs. Our current gas exchange modules have relatively low CO2 mass transfer and removal rates (~100 mL/min/m2) which may diminish the impact of CA on CO2 removal. We believe the increase in CO2 removal with CA-immobilized HFMs can be further improved by employing a new active mixing gas exchange module which can increase the mass transfer rate through decreasing boundary layer thickness. Considering carbonic anhydrase catalyzes 1×106 reactions per second, working nearly at the rate of diffusion, active mixing of bicarbonate contacting fluid over HFMs can enhance the effect of CA coatings by delivering more substrate to the enzyme.
Several types of biomolecules have been immobilized onto HFMs in an effort to improve their surface hemocompatibility, including chitosan, heparin and albumin. Liu et al.(35) and Sperling et al.(36) demonstrated that albumin-coated surfaces exhibited significant inhibition of platelet adhesion. The albumin mechanism acts similar to an inactive protein, lacking cell adhesion, but also occupying potential sites of protein adsorption. A passivating protein layer might serve as an ideal non-thrombogenic surface if adhesive plasma proteins, such as fibrinogen, are minimally adsorbed. Several studies observed that reused hemodialyzers have better thromboresistance compared to new ones, due to such a bland protein layer acting to discourage thrombogenic activity(37). In the present study, commercial PMP HFMs (Oxyplus) modified with CA reduced platelet adhesion by 95% (Figures 5 & 6). As platelets may become activated without adhering to the HFMs, previous work has demonstrated a reduction in activated platelets within the bulk phase of blood with CA-HFMs(31). Our findings indicate that surface attached CA is associated with reduced acute platelet deposition, which may be caused by a similar effect related to albumin attachment. CA occupies adsorption sites that otherwise might be bound with adhesive plasma proteins. However new work suggests CA may play a role in nitric oxide (NO) generation(39), which is a well-known inhibitor of platelet adhesion and activation on normal blood vessel walls(40).
5. Conclusions
In conclusion, we have assessed the impact of CA-modified HFMs on both hemocompatibility and CO2 removal from blood for improved respiratory assist devices. Surface modification of HFM with CA has significantly less platelet deposition compared to unmodified PMP HFMs. A statistically significant 36% increase in the blood CO2 removal rate was achieved using CA-immobilized HFMs. These findings suggest that bioactive HFMs may result in the design of smaller, more hemocompatible HFM-based artificial lungs. However, further study is required to increase the CA loading on HFMs and to develop a new active mixing gas exchange device to improve the impact of CA on CO2 removal. If successful, the bioactive HFMs could be used in artificial lungs or respiratory support devices to improve their ability to treat patients with severe respiratory failure. Highly efficient CO2 removal will enable the next generation of compact, intravascular and paracorporeal respiratory assist devices.
Highlights.
CA-immobilization on hollow fiber membranes (HFMs)
CA-HFMs increased CO2 removal efficiency from blood by 36%
CA-HFMs had 95% less platelet deposition
Acknowledgments
This publication was made possible by Grant Number R01 HL70051 from the National Institutes of Health, National Heart, Lung and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. This work was also funded by research grants from the Pennsylvania Department of Health (SAP #4100030667, #4100035341, and #4100041556). We would like to recognize the University of Pittsburgh’s McGowan Institute for Regenerative Medicine for support of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tremblay LN, Slutsky AS. Applied Physiology in Intensive Care Medicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. Ventilator-induced lung injury: from the bench to the bedside; pp. 357–66. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342(18):1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Brower R, Matthay MA. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2000 May 4;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal Hyperinflation During Low Tidal Volume Ventilation in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2006 Oct 12;:200607-915OC. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 6.Pesenti A, Patroniti N, Fumagalli R. Carbon dioxide dialysis will save the lung. Critical Care Medicine. 2010 Oct;38:S549–54. doi: 10.1097/CCM.0b013e3181f1fe0c. [DOI] [PubMed] [Google Scholar]
- 7.Greene R. Pulmonary vascular obstruction in the adult respiratory distress syndrome. Journal of Thoracic Imaging. 1986;1(3) doi: 10.1097/00005382-198607000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Tomashefski JF, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983 Jul;112(1):112–26. [PMC free article] [PubMed] [Google Scholar]
- 9.Vertrees RA, Nason R, Hold MD, Leeth AM, Schmalstieg FC, Boor PJ, et al. Smoke/Burn Injury-Induced Respiratory Failure Elicits Apoptosis in Ovine Lungs and Cultured Lung Cells, Ameliorated With Arteriovenous CO2 Removal*. Chest. 2004 Apr 1;125(4):1472–82. doi: 10.1378/chest.125.4.1472. [DOI] [PubMed] [Google Scholar]
- 10.Schmalstieg FC, Keeney SE, Rudloff HE, Palkowetz KH, Cevallos M, Zhou X, et al. Arteriovenous CO2 Removal Improves Survival Compared to High Frequency Percussive and Low Tidal Volume Ventilation in a Smoke/Burn Sheep Acute Respiratory Distress Syndrome Model. Ann Surg. 2007 Sep;246(3):512–23. doi: 10.1097/SLA.0b013e318148c6e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009 Oct 17;374(9698):1351–63. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann M, Bein T, Arlt M, Philipp A, Rupprecht L, Mueller T, et al. Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: a prospective pilot study. Critical Care. 2009;13(1):R10. doi: 10.1186/cc7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bein T, Weber F, Philipp A, Prasser C, Pfeifer M, Schmid F-X, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia*. Critical Care Medicine. 2006 May;34(5):1372–7. doi: 10.1097/01.CCM.0000215111.85483.BD. [DOI] [PubMed] [Google Scholar]
- 14.Galletti P, Colton C. Artificial Lungs and Blood–Gas Exchange Devices. In: Bronzino JD, editor. The Biomedical Engineering Handbook. CRC Press LLC; Boca Raton: 2000. pp. 1–19. [Google Scholar]
- 15.Kaar JL, Oh H-I, Russell AJ, Federspiel WJ. Towards improved artificial lungs through biocatalysis. Biomaterials. 2007 Jul;28(20):3131–9. doi: 10.1016/j.biomaterials.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye S, Johnson CA, Jr, Woolley JR, Snyder TA, Gamble LJ, Wagner WR. Covalent surface modification of a titanium alloy with a phosphorylcholine-containing copolymer for reduced thrombogenicity in cardiovascular devices. Journal of Biomedical Materials Research Part A. 2009 Oct 1;91A(1):18–28. doi: 10.1002/jbm.a.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye S-H, Johnson CA, Jr, Woolley JR, Murata H, Gamble LJ, Ishihara K, et al. Simple surface modification of a titanium alloy with silanated zwitterionic phosphorylcholine or sulfobetaine modifiers to reduce thrombogenicity. Colloids and Surfaces B: Biointerfaces. 2010 Sep 1;79(2):357–64. doi: 10.1016/j.colsurfb.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Progress in Polymer Science. 2007 Jul;32(7):698–725. [Google Scholar]
- 19.Mao C, Qiu Y, Sang H, Mei H, Zhu A, Shen J, et al. Various approaches to modify biomaterial surfaces for improving hemocompatibility. Advances in Colloid and Interface Science. 2004 Jun 30;110(1–2):5–17. doi: 10.1016/j.cis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Florchinger B, Philipp A, Klose A, Hilker M, Kobuch R, Rupprecht L, et al. Pumpless Extracorporeal Lung Assist: A 10-Year Institutional Experience. Ann Thorac Surg. 2008 Aug 1;86(2):410–7. doi: 10.1016/j.athoracsur.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Michanetzis GPA, Katsala N, Missirlis YF. Comparison of haemocompatibility improvement of four polymeric biomaterials by two heparinization techniques. Biomaterials. 2003 Feb;24(4):677–88. doi: 10.1016/s0142-9612(02)00382-4. [DOI] [PubMed] [Google Scholar]
- 22.Kohn J, Wilchek M. A colorimetric method for monitoring activation of sepharose by cyanogen bromide. Biochemical and Biophysical Research Communications. 1978 Sep 14;84(1):7–14. doi: 10.1016/0006-291x(78)90255-3. [DOI] [PubMed] [Google Scholar]
- 23.Tamada Y, Kulik EA, Ikada Y. Simple method for platelet counting. Biomaterials. 1995;16(3):259–61. doi: 10.1016/0142-9612(95)92126-q. [DOI] [PubMed] [Google Scholar]
- 24.Grigat E, Pütter R. Chemie der Cyansäureester, I. Cyansäureester aus Hydroxylverbindungen und Halogencyan. Chemische Berichte. 1964 Nov 1;97(11):3012–7. [Google Scholar]
- 25.Kohn J, Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochemical and Biophysical Research Communications. 1982 Aug 16;107(3):878–84. doi: 10.1016/0006-291x(82)90604-0. [DOI] [PubMed] [Google Scholar]
- 26.Jurado LA, Mosley J, Jarrett HW. Cyanogen bromide activation and coupling of ligands to diol-containing silica for high-performance affinity chromatography: Optimization of conditions. Journal of Chromatography A. 2002 Sep 20;971(1–2):95–104. doi: 10.1016/s0021-9673(02)00964-0. [DOI] [PubMed] [Google Scholar]
- 27.Kohn J, Wilchek M. The use of cyanogen bromide and other novel cyanylating agents for the activation of polysaccharide resins. Appl Biochem Biotechnol. 1984 Jun;9(3):285–305. [Google Scholar]
- 28.Axéan R, Ernback S. Chemical Fixation of Enzymes to Cyanogen Halide Activated Polysaccharide Carriers. European Journal of Biochemistry. 1971 Feb 1;18(3):351–60. doi: 10.1111/j.1432-1033.1971.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 29.Duerksen PJ, Wilkinson KD. Immobilization of proteins via arginine residues. Analytical Biochemistry. 1987 Feb 1;160(2):444–54. doi: 10.1016/0003-2697(87)90074-1. [DOI] [PubMed] [Google Scholar]
- 30.Schall CA, Wiencek JM. Stability of nicotinamide adenine dinucleotide immobilized to cyanogen bromide activated agarose. Biotechnology and Bioengineering. 1997 Jan 5;53(1):41–8. doi: 10.1002/(SICI)1097-0290(19970105)53:1<41::AID-BIT7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X, Loran DB, Wang D, Hyde BR, Lick SD, Zwischenberger JB. Seventy-two hour gas exchange performance and hemodynamic properties of NOVALUNG®iLA as a gas exchanger for arteriovenous carbon dioxide removal. Perfusion. 2005 Dec 1;20(6):303–8. doi: 10.1191/0267659105pf838oa. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z-G, Wan L-S, Liu Z-M, Huang X-J, Xu Z-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. Journal of Molecular Catalysis B: Enzymatic. 2009 Apr;56(4):189–95. [Google Scholar]
- 33.Algar WR, Prasuhn DE, Stewart MH, Jennings TL, Blanco-Canosa JB, Dawson PE, et al. The Controlled Display of Biomolecules on Nanoparticles: A Challenge Suited to Bioorthogonal Chemistry. Bioconjugate Chem. 2011;22(5):825–58. doi: 10.1021/bc200065z. [DOI] [PubMed] [Google Scholar]
- 34.Wong LS, Khan F, Micklefield J. Selective Covalent Protein Immobilization: Strategies and Applications. Chem Rev. 2009;109(9):4025–53. doi: 10.1021/cr8004668. [DOI] [PubMed] [Google Scholar]
- 35.Liu T-Y, Lin W-C, Huang L-Y, Chen S-Y, Yang M-C. Hemocompatibility and anaphylatoxin formation of protein-immobilizing polyacrylonitrile hemodialysis membrane. Biomaterials. 2005 Apr;26(12):1437–44. doi: 10.1016/j.biomaterials.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 36.Sperling C, Houska M, Brynda E, Streller U, Werner C. In vitro hemocompatibility of albumin–heparin multilayer coatings on polyethersulfone prepared by the layer-by-layer technique. Journal of Biomedical Materials Research Part A. 2006 Mar 15;76A(4):681–9. doi: 10.1002/jbm.a.30519. [DOI] [PubMed] [Google Scholar]
- 37.Lin W-C, Liu T-Y, Yang M-C. Hemocompatibility of polyacrylonitrile dialysis membrane immobilized with chitosan and heparin conjugate. Biomaterials. 2004 May;25(10):1947–57. doi: 10.1016/j.biomaterials.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 38.Oh H, Ye S, Johnson CA, Jr, Woolley JR, Federspiel WJ, Wagner WR. Hemocompatibility Assessment of Carbonic Anhydrase Modified Hollow Fiber Membranes for Artificial Lungs. Artificial Organs. 2010 May 1;34(5):439–42. doi: 10.1111/j.1525-1594.2009.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. American Journal of Physiology - Heart and Circulatory Physiology. 2009 Dec 1;297(6):H2068–74. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 40.Frost MC, Reynolds MM, Meyerhoff ME. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005 May;26(14):1685–93. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]