Abstract
Purpose
To characterize the phenotype and investigate the associations of intraretinal crystalline deposits in a large cohort of Type 2 Idiopathic Macular Telangiectasia (MacTel)
Design
Case-control study
Participants
Patients with and without retinal crystals from the Macular Telangiectasia Project, an international multi-centre prospective study of Type 2 MacTel.
Methods
Grading of stereoscopic 30° colour fundus (CF), confocal blue light reflectance (CBR), red-free (RF) and infrared (IR) images was performed according to the MacTel Natural History Study protocol and staged using the classification system devised by Gass & Blodi. SD-OCT and adaptive optics imaging were used for a finer analysis of the phenotype. Associations between crystals and other characteristics of the disease as well as potential risk factors were investigated.
Main outcome measures
Presence of crystals, fundus signs of MacTel, clinical characteristics, presence of potential risk factors of MacTel.
Results
Out of 443 probands enrolled in the MacTel study, 203 (46%) had crystalline deposits present; 60% of the cases were bilateral at baseline. Eyes with crystals had a mean letter score of 70.7 (SD=15.9) while those without crystals had a mean of 66.5 letters (SD=15.5, p<0.001). Crystals were present at all stages of the disease and showed high reflectivity within a wide wavelength range. They were located at the anterior surface of the nerve fibre layer, arranged along the nerve fibres, within an annular area centred on the fovea. Significant associations of crystalline deposits were found with a loss of retinal transparency, MPOD loss, fluorescein leakage, retinal thickness and a break in the IS/OS junction line. Associations with environmental risk factors were not found.
Conclusions
Intraretinal crystals are a frequent phenomenon associated with type 2 MacTel, they may appear at all stages and may aid in the early diagnosis of the disease. Their morphology further implicates Müller cells in the pathogenesis of the disease. Insight into their physical and chemical properties may provide clues to the metabolic pathways involved in the pathogenesis of the disease.
Idiopathic Macular Telangiectasia (MacTel) is a vascular anomaly affecting retinal capillaries in the juxtafoveal region.1 The aetiology and pathogenesis of the disease are little known.
According to a system originally devised by Gass and Blodi2 and subsequently simplified by Yanuzzi et al.3, MacTel is classified into two main categories, based on biomicroscopic and fluorescein angiographic features. Type 1 (Aneurysmal Telangiectasia) is a predominantly unilateral disease affecting mostly males, associated clinically with retinal thickening, prominent extensive telangiectasis, lipid exudates and cystoid changes, mainly in the temporal part of the macula. Type 2 (Perifoveal Telangiectasia) is a bilateral disease that affects both sexes, starting in the fifth to seventh decades. Early clinical features are more subtle and include a loss of retinal transparency, retinal crystals, intraretinal cystoid spaces at the fovea and a diffuse leakage in the fluorescein angiogram. The retina is thinner than normal. Subsequent vascular remodelling and fibrosis accompanied by pigment plaques and eventual subretinal neovascularisation, scarring and atrophy limit the prognosis for central vision. To date, no known therapeutic modality has been proven effective.
The presence of yellow-white refractile crystals in the superficial layers of the parafoveal retina is a characteristic and frequent feature of the disease phenotype.1, 4, 5 Their origin, composition or significance are unknown. Our aim was to characterize the phenotype and associations of these intraretinal crystalline deposits using multiple imaging modalities in a large cohort of type 2 MacTel.
METHODS
Patients
Patients with a diagnosis of Type 2 MacTel with and without retinal crystals from the MacTel project, an international multi-centre prospective study of Type 2 MacTel, currently involving 28 research centres worldwide were included in this analysis. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the respective institutional review board or local ethics committee at each participating centre. Before inclusion, written, informed consent was obtained from each participant after explanation of the nature of the study. Maximum retinal irradiance of lasers used was well below the limits established by the American National Standards Institute (ANSI Z136.1; 1993) and other international standards.
Imaging
Standard 30° stereo field 2 colour (CF) and red-free (RF) images were either recorded digitally or digitized at high resolution from colour transparencies. Confocal scanning laser ophthalmoscopes, cSLO (Heidelberg Retina Angiograph 2 or Spectralis HRA+OCT, Heidelberg Engineering GmbH, Dossenheim, Germany) were used for fundus autofluorescence (FAF), confocal blue-light reflectance (CBR), infrared (IR) imaging and Indocyanine Green (ICG) angiography. For FAF and CBR imaging the fundus was illuminated using an excitation light with a wavelength of 488nm. Fundus autofluorescence was recorded through a filter with a short-wavelength cut-off at 520nm. To reduce random noise, a master image was produced for each eye in the study, by averaging individually recorded images. For ICG angiography, a standard excitation wavelength of 790nm was used while for IR imaging a diode laser was used to illuminate the fundus at 820nm. Fluorescein Angiography (FA) was performed using a variety of imaging equipment available at respective centre participating in the MacTel study, including cSLOs and fundus cameras. Optical Coherence Tomography (OCT) of the macula was performed routinely using Stratus OCT-3s (Carl Zeiss Meditec, Inc.), complemented by high-resolution SD-OCT imaging where available (Spectralis HRA-OCT, Heidelberg Engineering GmbH, Dossenheim, Germany). Macular pigment optical density was assessed using a cSLO (Heidelberg Retina Angiograph, Heidelberg Engineering GmbH, Dossenheim, Germany) through a dual-wavelength (488/514nm) autofluorescence technique described previously.6 Additional high-resolution retinal images were obtained with the University of Rochester adaptive optics scanning laser ophthalmoscope (AOSLO).7, 8 Through-focus images of the nerve fibre layer and the retinal photoreceptors in the same retinal locations were acquired using near-infrared reflectance with <2 µm transverse resolution and 19-Hz video-rate. Confocal pinholes of 0.5 to 1.5 Airy disks improved contrast through optical sectioning. The Rochester AOSLO has an 847nm laser beacon, a Shack-Hartmann wavefront sensor (WFS), and Alpao hi-speed 97 actuator deformable mirror (DM). The scanning system has an adjustable 0.75 – 2.5° field of view. Infrared reflectance imaging of the retina was achieved with a 680 nm superluminescent diode source and a PMT for light detection. The raw data was registered and frame averaged using custom Matlab software (MathWorks, Inc., Natick, MA), and individual frames were montaged using Photoshop (Adobe Systems Inc., San Jose, CA).
Phenotyping
Detailed phenotyping based on the Gass and Blodi classification was performed by grading of field 2 CF, RF and FA images, according to the system defined in the MacTel Study Protocol.9 Briefly, a grid (adapted from the standard grid for ARM classification10, 11, consisting of three concentric circles with 1000, 3000 and 6000 micron diameters and four radial quadrant spokes dividing the central macula into nine subfields) was superimposed over the colour fundus image, centred on the fovea, in Adobe Photoshop CS4 (Adobe Systems Inc. San Jose, CA, USA). Images were graded for image quality and for fundus features of type 2 MacTel listed in Table 1, as well as for OCT and FAF characteristics as described previously.9 Grading was performed independently by three certified graders masked to the identity and clinical data of the patients. Gradings were compared for inter- and intra-observer reliability and final copy was created by adjudication. Staging of disease severity was based on the criteria devised by Gass and Blodi2 using the categories shown in Table 2.
Table 1.
Distribution of probands with retinal crystals by gender and race.
| No Crystals (n=232) |
Crystals (n=203) |
p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Male Gender | 87 | 37 | 83 | 41 | 0.47 |
| White Race | 193 | 83 | 159 | 78 | 0.2 |
Race was compared to non-Caucasian race given the small numbers in each individual race group. Non-Caucasian races included American Indian, Asian, African American, Native Hawaiian or Pacific Islander and Australian Aboriginal or Torres Strait Islander. Test: Chi-square test.
Table 2.
Associations with FAF and OCT characteristics
| No Crystals | Crystals | p-value | |
|---|---|---|---|
| % | % | ||
| Abnormal FAZ | 89 | 85 | 0.4 |
| Inner empty spaces | 46 | 50 | 0.29 |
| Outer empty spaces | 18 | 24 | 0.09 |
| Inner empty spaces | |||
| Centre not involved | 25 | 25 | 0.45 |
| Centre involved | 20 | 24 | |
| Outer empty spaces | 0.13 | ||
| Centre not involved | 10 | 17 | |
| Centre involved | 7 | 7 | |
| IS/OS PR Break | 50 | 68 | <0.001 |
| Increased AF within 1 DD of the fovea | 80 | 83 | 0.47 |
| Fovea abnormal | 85 | 83 | 0.72 |
| Large area of decreased AF | 7 | 8 | 0.65 |
Test: Chi-square test.
Registration of retinal crystals
In cases where grading detected the presence of crystals, composite images containing overlays of aligned CF, CBR, RF and IR images were created (in Adobe Photoshop CS4) and analyzed for the presence and location of crystals in images of respective modality. The number of crystals present per subfield within the grading grid was assessed in each imaging modality and graded using the categories 0, 1–5, 6–10 and >10.
Clinical data collection
Clinical information including age, gender, self-reported race/ethnicity, medical and ocular history was collected through standardized questionnaires.9 Monocular best-corrected visual acuities (BCVA) were determined according to a standardized protocol, using the Early Treatment Diabetic Retinopathy Study (ETDRS) LogMAR visual acuity charts at a distance of 4 meters, scoring of the test was based on the number of letters read correctly. Possible scores ranged from 0 (Snellen equivalent <20/800) to 100 (Snellen equivalent 20/12).12, 13 Participants also underwent a comprehensive ophthalmologic examination annually.9 Evaluation of vision-targeted health-related quality of life (HR-QoL) was measured using the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25).14
Statistical Methods
Comparisons of various characteristics of interest between participants/eyes with and without crystalline deposits were done using a Chi-square test. Generalized linear models were used to compare continuous image grading characteristics across the crystalline deposit groups. Kappa statistics were used to assess the agreement of the crystalline deposit grading across the various image modalities. A p-value of <0.05 was accepted as statistically significant. All analyses were conducted using commercially available statistical software (SAS version 9.1; SAS Institute, Cary, NC).
RESULTS
Demographics
Out of 443 patients enrolled in the MacTel study, 203 (46%) showed crystalline deposits. At enrolment, mean age of patients with crystals was 60.0 years (SD=8.8), of patients without 60.4 years (SD=9.8). Follow-up periods ranged from none to four years with a mean of 1.6 years (median=2, SD=1.2 years). The distributions by gender and race are shown in Table 1.
Phenotype
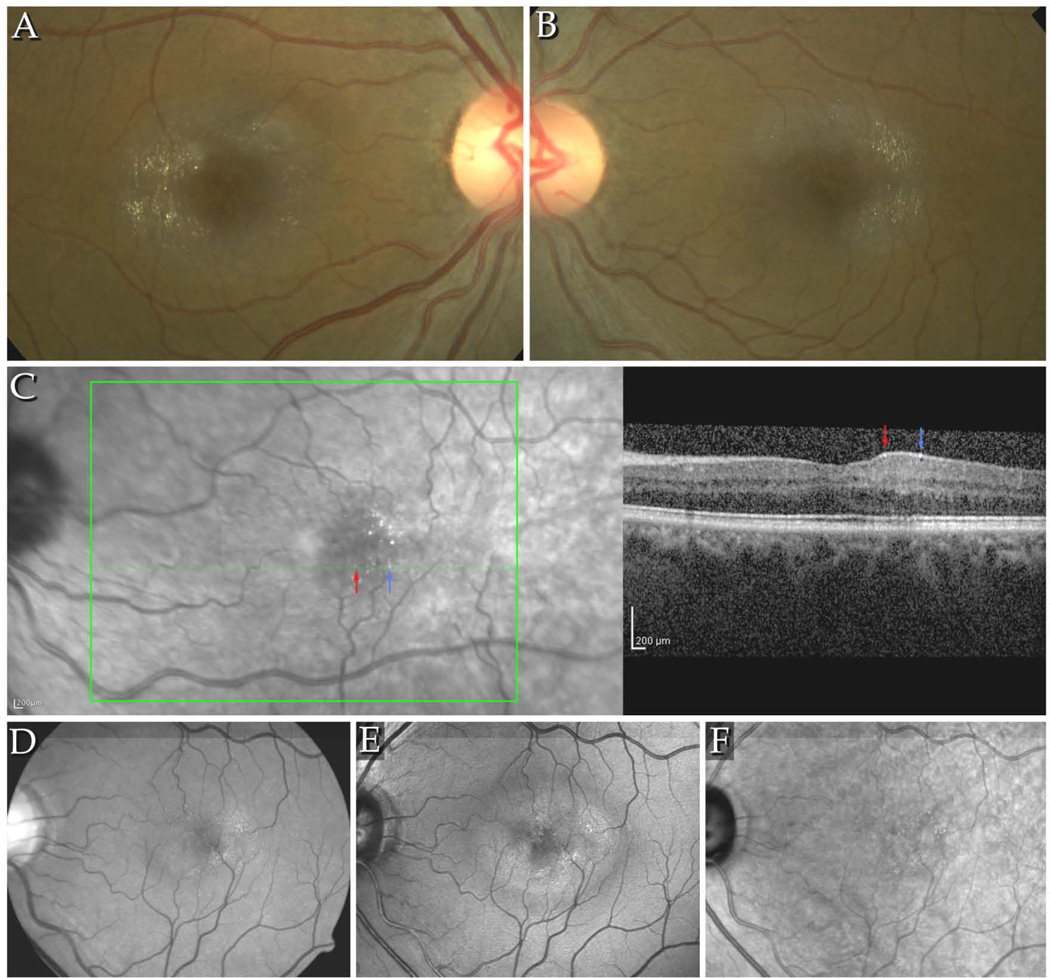
At baseline, of 203 participants showing retinal crystals, 121 cases (59.6%) were bilateral, 81 (39.9%) unilateral. Retinal crystals were located within a band approximately 4000µm in diameter centred on the fovea, with a sparing of the central approximately 700µm around the fovea. In 34 cases (16.8%) a pattern in the alignment of the crystals corresponding to that of the nerve fibre layer (NFL) was clearly distinguishable in fundus images (Figure 1A–B, Figure 2B–C). In 78% of cases with an NFL pattern, a gap in the pattern temporal of the fovea, corresponding to the raphe was apparent (Figure 1A–B). High-resolution imaging using AOSLO confirmed this pattern as well as the presence of the crystals within the same optical section as the nerve fibre bundles (Figure 2). Individual crystals measured at least 5µm or larger. Crystals appeared to be angular with sharply demarcated edges and were distributed in a distinct to subconfluent pattern. Based on the analysis of stereoscopic fundus images, retinal crystals appeared to be located at the inner surface of the retina. SD-OCT imaging also confirmed this observation, crystals appeared within the nerve fibre layer, NFL (Figure 1, C).
Figure 1.
A–B Colour fundus images of the right and left eyes of a patient with signs of stage 3 MacTel, including retinal crystals. The crystals are arranged in a pattern along the fibres of the NFL, within a round area with an approximate diameter of up to 4000µm, centred on the fovea. Typically, few crystals are seen at and immediately around the fovea. The area temporal of the fovea, corresponding to the raphe, contains also usually less crystals. C. IR+OCT image combination from the Heidelberg Spectralis. Red and blue arrows indicate identical retinal crystals in the IR image and the B-scan. The crystals appear to be located to the NFL. D–F. Detectability of crystals in different imaging modalities: D shows a red-free image taken with a fundus camera, E and F: confocal blue light reflectance (CBR) and infrared images (Heidelberg Spectralis HRA+OCT). Best detectability of crystals is provided by the CBR images. Crystals were located within the area of increased reflectivity in CBR.
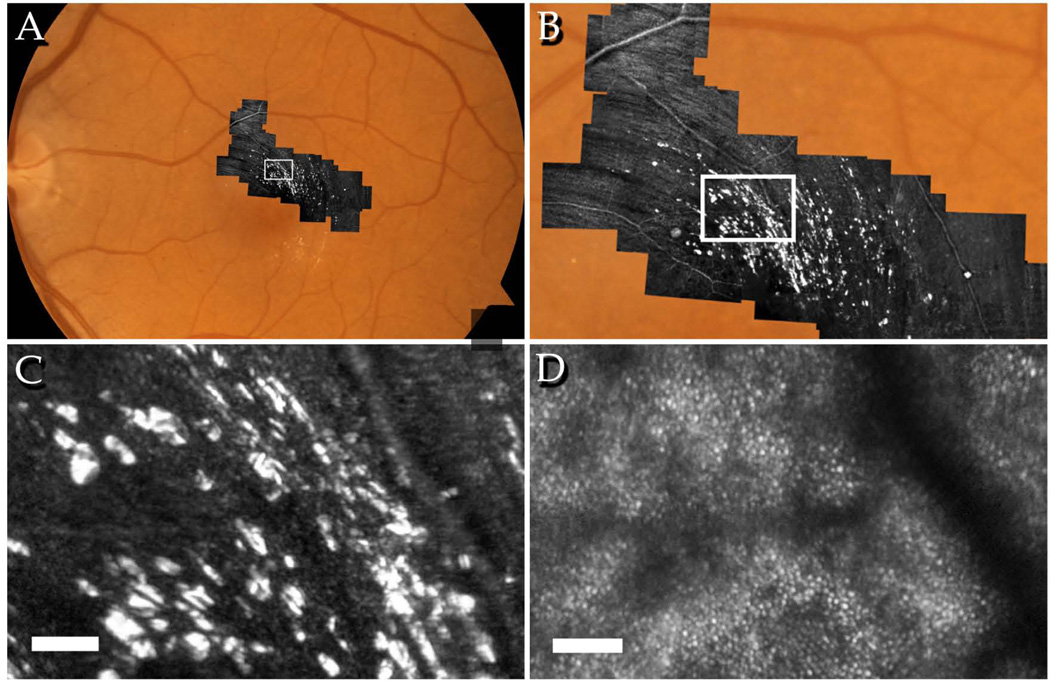
Figure 2.
AOSLO images of the left eye of a 60-year-old patient with MacTel, visual acuity 20/25. A: Montage of AOSLO images on standard 30-degree fundus photograph, B: distribution of crystals appears to be parallel to the ganglion cell axon bundles within the NFL, C: individual crystals appear with high contrast in the same focal plane as the NFL, D: the cone mosaic at the same retinal location appears regular with no evidence of crystals at this focal plane. Scale bars = 50µm.
In CF images the crystals appeared highly reflective, white or golden yellow. Crystals were also detectable in RF, CBR and IR images. Agreement of crystal counts grading based on CF images (in the temporal Zone 2 subfield) was substantial with red-free images κ=0.80 (0.77–0.83) and with CBR κ=0.69 (0.63–0.75), and moderate with that based on infrared images κ=0.47 (0.41–0.54). Values shown are weighted kappa values, 95% confidence intervals in parentheses. Red-free and CBR imaging provided the highest detection rates, while in IR images typically only a subset of crystals was seen. Crystals were not detectable in FA, FAF or ICG images.
Crystals were seen at all stages of the disease, most frequently in stages 3 and 4. The association of the amount of crystals with Gass&Blodi stage reached significance in zone 1 (p=0.003) and in the temporal subfield of zone 2 (P=0.035, Fisher's exact test).
Associations with features of the phenotype
The presence of crystals showed a strong association with the presence of a break in the IS/OS junction line but not with other FAF/OCT characteristics of the disease (Table 2).
The amount of crystals showed an association with loss of retinal transparency and fluorescein leakage in all subfields; and also with retinal thickness except in zone 1 and temporal zone 2 (Tables 3 and 4). The association with visible telangiectatic vessels, dilated retinal vessels and right-angled veins only reached significance in the nasal subfield of zone 2 (p=0.002, p=0.04 and p=0.03 respectively). No association was found with blunted veins. Associations with MPOD loss were significant in all subfields except the inferior subfield of zone 2 (zone 1 p= 0.04, zone 2: superior p=0.009, nasal p=0.02, inferior p=0.08 and temporal p=0.001, n=52 eyes).
Table 3.
The association of retinal thickness and loss of retinal transparency with the amount of crystals by subfield.
| Retinal thickness |
Loss of retinal transparency |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Zone 2 | Zone | Zone 2 | |||||||
| 1 | sup | nas | inf | temp | 1 | sup | nas | inf | temp | |
| Amount of crystals |
Mean (SE) |
Mean (SE) |
Mean (SE) |
Mean (SE) |
Mean (SE) |
N(%) | N(%) | N(%) | N(%) | N(%) |
| 0 | 190.4 (2.2) |
245.9 (1.6) |
245.9 (1.6) |
240.3 (1.6) |
239.0 (2.7) |
24 (6) |
84 (25) |
88 (24) |
55 (15) |
98 (55) |
| 1 | 193.5 (4.5) |
245.0 (3.4) |
254.7 (4.5) |
245.4 (4.4) |
233.8 (2.9) |
8 (9) | 39 (52) |
26 (55) |
14 (29) |
123 (80) |
| 2 | 187.1 (12.3) |
250.8 (6.0) |
260.2 (6.1) |
259.8 (6.1) |
239.4 (5.3) |
2 (18) |
16 (62) |
11 (48) |
12 (41) |
36 (80) |
| 3 | 200.5 (28.8) |
265.6 (4.3) |
273.0 (5.5) |
259.1 (4.7) |
246.1 (3.8) |
1 (50) |
31 (67) |
19 (56) |
24 (58) |
79 (79) |
| p-value | 0.9 | <0.001 | <0.001 | <0.001 | 0.09 | 0.02 | <0.001 | <0.001 | <0.001 | <0.001 |
Test: Generalized linear model.
Table 4.
The association of fluorescein leakage with the amount of crystals by subfield.
| RPE-level |
level of the outer capillary network |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zone | Zone 2 | Zone | Zone 2 | |||||||
| 1 | sup | nas | inf | temp | 1 | sup | nas | inf | temp | |
| Amount of crystals |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N(%) | N(%) | N (%) |
N (%) |
| 0 | 60 (17) |
45 (15) |
52 (15) |
33 (10) |
62 (36) |
64 (18) |
41 (13) |
63 (18) |
35 (10) |
89 (52) |
| 1 | 18 (24) |
14 (20) |
11 (26) |
7 (16) | 63 (44) |
23 (31) |
19 (27) |
16 (37) |
10 (23) |
95 (66) |
| 2 | 4 (44) | 6 (25) | 8 (35) | 2 (7) | 17 (41) |
1 (11) | 8 (33) | 11 (48) |
7 (26) | 31 (76) |
| 3 | 1 (50) | 10 (24) |
8 (27) | 6 (16) | 37 (42) |
0 | 16 (38) |
15 (50) |
6 (16) | 60 (67) |
| p-value | 0.01 | 0.05 | 0.01 | 0.29 | 0.41 | 0.22 | <0.001 | <0.001 | 0.02 | 0.01 |
Test: Generalized linear model.
Associations With Clinical Features
Eyes with crystals (n=520) had a mean letter score of 70.7 (SD=15.9), those without 66.5 letters (SD=15.5, n=343), differences were significant (p<0.001). Associations with diet or other clinical characteristics were not detectable (Table 5).
Table 5.
Associations with clinical characteristics
| No Crystals N = 232 |
Crystals N = 200 |
p-value | |
|---|---|---|---|
| Diabetes | 68 (29) | 74 (37) | 0.09 |
| Cerebral vascular incident | 6 (3) | 3 (2) | 0.43 |
| Coronary artery disease | 23 (10) | 29 (14) | 0.14 |
| Hypertension | 127 (55) | 103 (51) | 0.5 |
| Smoking* | |||
| Never | 110 (48) | 102 (51) | 0.39 |
| Former | 94 (41) | 71 (36) | |
| Current | 23 (10) | 26 (13) | |
| Medication exposure** | |||
| Cholesterol lowering agents | 88 (39) | 80 (40) | 0.73 |
| Tamoxifen | 6 (3) | 6 (3) | 0.81 |
| Vitamins | 89 (39) | 82 (41) | 0.64 |
| Dietary supplements | 65 (29) | 59 (30) | 0.79 |
| Multi-vitamin | 56 (25) | 40 (20) | 0.27 |
| Preservision | 13 (6) | 19 (10) | 0.13 |
| Vitamin A | 3 (1) | 6 (3) | 0.22 |
| Beta-carotene | 3 (1) | 3 (2) | 0.87 |
| Zinc | 4 (2) | 7 (4) | 0.25 |
| Lutein/zeaxanthin | 13 (6) | 13 (7) | 0.72 |
| NEI-VFQ | |||
| Overall NEI-VFQ score | 77.9 (13.7) | 78.1 (13.6) | 0.17 |
| General vision | 65.6 (15.7) | 64.6 (62.4) | 0.52 |
| Colour vision | 96.7 (10.8) | 96.8 (11.0) | 0.9 |
| Near activities | 70.9 (20.2) | 67.4 (20.6) | 0.08 |
| Distance activities | 76.9 (19.2) | 75.3 (21.3) | 0.41 |
Values are n (probands) with % shown in parentheses, or mean ± SD for the NEI-VFQ data. Note 3 participants did not have this data available.
Smoking data not available for 9 participants (5 - no crystals; 4 crystals).
10 participants did not have this data available, 5 with, 5 without crystals) Chi-square test was used for comparing percentages, t-test for comparing means.
DISCUSSION
Retinal crystals may be present in a wide range of disorders from hereditary to drug-induced, from systemic to local retinal.15, 16 In type 2 MacTel, retinal crystals have some distinguishing characteristics.1, 2, 4, 5
In our study, retinal crystals were present in 46% of 443 MacTel patients at baseline, and nearly two thirds of these cases were bilateral. Crystals appeared highly reflective at wavelengths ranging from 488nm (blue) to 820nm (IR). In infrared images, typically only a subset of crystals seen in BLR or RF images were detectable. Considering the confocal characteristics of the SLO, this may simply be due to a tilt of the retina relative to the focal plane. The crystals also exhibited specular reflection such that multiple frames of the same retinal area taken at slightly different angles showed a variation in the number and location of the crystals. As a rule, more crystals are likely to be present in the retina than those detectable in any single fundus image.
Retinal crystals in MacTel are typically distributed in an annular pattern within an approximately 4000µm diameter circular area centred on the fovea, with a sparing of the central approximately 700µm. A gap in this annular pattern was seen temporal of the fovea, corresponding to the horizontal raphe. This is remarkable as many other signs of the disease appear first or occur predominantly in this region.
Within this annular area, the crystals were arranged along the ganglion cell axons in the NFL. To our knowledge, this pattern of crystals is only seen in MacTel and in Sjögren-Larsson Syndrome (SLS).17, 18 At late stages of MacTel, the distortion of the retina due to vascular remodelling, fibrosis and pigment plaques may break up this pattern. In some late-stage eyes, only a few crystals were apparent surrounding the pigment plaques or fibrotic post-neovascular scars. This pattern has been reported also in other neovascular conditions and is thus probably not specific for MacTel.19
The retinal area affected by crystals coincides closely with that affected by other features of the disease. A circumfoveal band of increased reflectivity seen in CBR images, the loss of retinal transparency in CF and RF images, the loss of macular pigment and fluorescein leakage all seem to affect this region. Also, in our study, the amount of crystals showed statistically significant associations with the loss of retinal transparency, a loss of MPOD and with fluorescein leakage.
The implications of these observations are not known, however, they may suggest further Müller cell involvement in the pathogenesis of type 2 MacTel. Müller cells are radially oriented support cells traversing the retina from the inner vitreal border (ILM) to the distal end of the outer nuclear layer. They have an extended funnel shape, a higher refractive index than their surrounding tissue, and are oriented along the direction of light propagation. Müller cells have been shown to act as optical fibres within the retina.20 A disruption of their structure may explain a loss of retinal transparency even without significant oedema. In our study, retinal crystals did however also show a statistically significant association with a minimal level of retinal thickening in all subfields.
The cell processes of Müller cells enclose regions of the perikarya and fascicles of axons of the ganglion cells, within the NFL, processes from their footplates surround the nerve fibres bundles.21 In our study, high-resolution retinal images obtained using adaptive optics confirmed our findings based on stereoscopic fundus images and SD-OCT imaging, crystals seem to be located superficially within the NFL, in close proximity of the nerve fibres. Individual ganglion cell axon diameters vary in the range of 0.6–2.0µm depending on the type of ganglion cell of their origin. Midget ganglion cells with thinner axons are found more frequently within the macular area.22 We estimate the size of individual crystals to approximately 5µm or larger, this together with their often confluent distribution suggests that crystals are more likely to be located around the nerve fibre bundles, possibly within Müller cell foot-plates, rather than within individual axons. A similar conclusion was drawn in the case of tamoxifen crystals, and other crystals such as canthaxanthine and beta carotene also form in the innermost retina.
Müller cells play a role in retinoid metabolism including cone pigment regeneration and they show avid phagocytic activity.21 While the origin and chemical composition of the crystals are not known, based on their distribution and reflective properties, we hypothesize that they may be composed of retinoids originating in the visual cycle and be located within the Müller cell footplates and processes surrounding the nerve fibres. We also found a significant association between the amount of crystals and the presence of a break in the IS/OS junction line which may further support this hypothesis. Significant associations with increased retinoid intake via the diet or by supplementation were however not demonstrable in our study.
We found an association between the amount of crystals and fluorescein leakage. Macular pigment loss was also associated with the amount of retinal crystals in our study. Müller cells extend branches that interdigitate with retinal neurons and glial cells and their processes ensheath the retinal capillaries and may contribute to the blood-retina barrier.21 The role of Müller cells in the metabolism of macular pigment is not clear, however, in a histological study of a MacTel eye, Powner et al.23 found a depletion of Müller cells as well as vascular abnormalities within an area of the same extent as the loss of macular pigment. The distribution and associations of retinal crystals found in our study thus further implicate Müller cells in the pathogenesis of MacTel.
The reflective properties of the crystals are compatible with those of retinoids but also with those of lipids. Retinal crystals arranged along the nerve fibres, central cystoid spaces and a loss of luteal pigment seen in MacTel are also the ocular manifestations of SLS, which raises the possibility of similarities in the metabolic pathways responsible for the pathogenesis of the two diseases. SLS is an autosomal dominant hereditary systemic metabolic disorder characterized by deficient microsomal fatty aldehyde dehydrogenase (FALDH) activity, resulting in an accumulation of fatty aldehydes and alcohols in body tissues. SLS is however also associated with severe extraocular manifestations including cognitive deficiencies, spastic diplegia, and congenital ichtyosis.17, 18
Further insight into the physical and chemical properties of retinal crystals in MacTel may provide clues to the metabolic pathways involved in the pathogenesis of the disease.
ACKNOWLEDGMENTS
The authors wish to thank the Lowy Medical Foundation and the NIHR for providing support for funding this study, Dr Austin Roorda for valuable discussions concerning the reflective properties of the crystals and Dr Marcus Fruttiger for valuable discussions concerning microanatomic, histologic and cell biological aspects of the crystals.
Footnotes
COMMERCIAL DISCLOSURE
FB Sallo: none, I Leung: none, M Chung: none, UEK Wolf-Schnurrbusch: none, T Clemons: none, A Dubra: none, DR Williams: none, AC Bird: none, T Peto: none
REFERENCES
- 1.Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100(5):769–780. doi: 10.1001/archopht.1982.01030030773010. [DOI] [PubMed] [Google Scholar]
- 2.Gass JD, Blodi BA. Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology. 1993;100(10):1536–1546. [PubMed] [Google Scholar]
- 3.Yannuzzi LA, Bardal AM, Freund KB, et al. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124(4):450–460. doi: 10.1001/archopht.124.4.450. [DOI] [PubMed] [Google Scholar]
- 4.Moisseiev J, Lewis H, Bartov E, et al. Superficial retinal refractile deposits in juxtafoveal telangiectasis. Am J Ophthalmol. 1990;109(5):604–605. doi: 10.1016/s0002-9394(14)70699-3. [DOI] [PubMed] [Google Scholar]
- 5.Abujamra S, Bonanomi MT, Cresta FB, et al. Idiopathic juxtafoveolar retinal telangiectasis: clinical pattern in 19 cases. Ophthalmologica. 2000;214(6):406–41l. doi: 10.1159/000027534. [DOI] [PubMed] [Google Scholar]
- 6.Helb HM, Charbel Issa P, RL VDV, et al. Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina. 2008;28(6):808–816. doi: 10.1097/IAE.0b013e31816d81aa. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Vieyra A, Dubra A, Malacara-Hernandez D, Williams DR. First-order design of off-axis reflective ophthalmic adaptive optics systems using afocal telescopes. Opt Express. 2009;17(21):18906–18919. doi: 10.1364/OE.17.018906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scoles D, Gray DC, Hunter JJ, et al. In-vivo imaging of retinal nerve fiber layer vasculature: imaging histology comparison. BMC Ophthalmol. 2009;9:9. doi: 10.1186/1471-2415-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons TE, Gillies MC, Chew EY, et al. Baseline characteristics of participants in the natural history study of macular telangiectasia (MacTel) MacTel Project Report No. 2. Ophthalmic Epidemiol. 17(l):66–73. doi: 10.3109/09286580903450361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39(5):367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 12.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(l):91–96. [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98(5 Suppl):741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 14.Clemons TE, Gillies MC, Chew EY, et al. The National Eye Institute Visual Function Questionnaire in the Macular Telangiectasia (MacTel) Project. Invest Ophthalmol Vis Sci. 2008;49(10):4340–4346. doi: 10.1167/iovs.08-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drenser K, Sarraf D, Jain A, Small KW. Crystalline retinopathies. Surv Ophthalmol. 2006;51(6):535–549. doi: 10.1016/j.survophthal.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Nadim F, Walid H, Adib J. The differential diagnosis of crystals in the retina. Int Ophthalmol. 2001;24(3):113–121. doi: 10.1023/a:1021189215498. [DOI] [PubMed] [Google Scholar]
- 17.van der Veen RL, Fuijkschot J, Willemsen MA, et al. Patients with Sjogren-Larsson syndrome lack macular pigment. Ophthalmology. 117(5):966–971. doi: 10.1016/j.ophtha.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Fuijkschot J, Cruysberg JR, Willemsen MA, et al. Subclinical changes in the juvenile crystalline macular dystrophy in Sjogren-Larsson syndrome detected by optical coherence tomography. Ophthalmology. 2008;115(5):870–875. doi: 10.1016/j.ophtha.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 19.Lima LH, Freund KB, Klancnik JM, Jr, Spaide RF. Intraretinal crystalline deposits in neovascular age-related macular degeneration. Retina. 30(4):542–547. doi: 10.1097/IAE.0b013e3181c713e4. [DOI] [PubMed] [Google Scholar]
- 20.Franze K, Grosche J, Skatchkov SN, et al. Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci U S A. 2007;104(20):8287–8292. doi: 10.1073/pnas.0611180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarthy V, Ripps H. The retinal Muller cell : structure and function. xiv. New York: Kluwer Academic/Plenum Publishers; 2001. p. 278. 1 leaf of plates. [Google Scholar]
- 22.Hogan MJ, Alvarado JA, Weddell JE. Histology of the human eye; an atlas and textbook. xiii. Philadelphia: Saunders; 1971. p. 687. [Google Scholar]
- 23.Powner MB, Gillies MC, Tretiach M, et al. Perifoveal Muller Cell Depletion in a Case of Macular Telangiectasia Type 2. Ophthalmology. doi: 10.1016/j.ophtha.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]