Abstract
We conducted a matched-cohort analysis of autologous transplant conditioning regimens for diffuse large cell lymphoma in 92 patients treated with either radioimmunotherapy (RIT) or total body irradiation (TBI)-based conditioning regimens. The RIT regimen consisted of 0.4 mCi/kg of 90Y-ibritumomab tiuxetan plus BEAM (BCNU, etoposide, cytarabine, melphalan). The TBI-based regimen combined fractionated TBI at 1200 cGy, with etoposide and cyclophosphamide. Five factors were matched between 46 patient pairs: age at transplant +/−5yrs, disease status at salvage, number of prior regimens, year of diagnosis +/−5yrs, and year of transplant +/−5yrs. Patients in the TBI group had higher rates of cardiac toxicity and mucositis, while Z-BEAM patients had a higher incidence of pulmonary toxicity. Overall survival at 4 years was 81.0% for the Z-BEAM and 52.7% for the TBI group (P = 0.01). The 4-year cumulative incidence of relapse/progression was 40.4% and 42.1% for Z-BEAM and TBI, respectively (P = 0.63). Non-relapse mortality was superior in the Z-BEAM group: 0% compared to 15.8% for TBI at 4 years (P < 0.01). Our data demonstrate that RIT-based conditioning had a similar relapse incidence to TBI, with lower toxicity, resulting in improved overall survival, particularly in patients with ≥2 prior regimens.
Keywords: radioimmunotherapy, diffuse large cell lymphoma, autologous hematopoietic cell transplantation, BEAM, TBI, DLCL
INTRODUCTION
While the Parma trial established the use of high dose chemotherapy with autologous hematopoietic cell transplantation (AHCT) as superior to conventional chemotherapy for relapsed chemotherapy-sensitive diffuse large cell lymphoma (DLCL) [1], relapsed disease remains the most common cause of treatment failure. To address this problem, various strategies have been used to reduce relapse rates including the use of novel conditioning regimens, and post-transplant immunotherapy with rituximab [2]. The regimens that have been studied as part of prospective clinical trials include: total body irradiation (TBI) plus combination chemotherapy with etoposide and cyclophosphamide [3], high-dose BEAM (BCNU, etoposide, cytarabine, melphalan) [4], BEAC (BCNU, etoposide, cytarabine, cyclophosphamide) [1], and CBV (cyclophosphamide, BCNU, etoposide) [5]. In a comparative study of TBI/etoposide/cyclophosphamide versus CBV, the relapse rate is lower in patients treated with the TBI-containing regimen [6]. An inverse relationship between recurrence rates and radiation doses is demonstrated in a phase III trial of 12Gy TBI versus 15.75Gy TBI; the relapse rate is lower in the higher dose radiation cohort, but also results in higher treatment related mortality [7]. In addition, the toxicity associated with a TBI-based conditioning regimen often precludes its use in older patients and even in some younger patients, as TBI is associated with substantial morbidity. A GEL/TAMO cooperative study of DLCL patients treated with a TBI-containing regimen shows a 2.5-fold higher (hazard) risk of death compared to those treated with chemotherapy alone [8].
Radioimmunotherapy (RIT) has been explored as a means of harnessing the anti-tumor effects of radiation while potentially reducing toxicity compared to fractionated TBI. The use of targeted antibodies to deliver radiation directly to the tumor and its microenvironment is intended to spare critical organs, thereby allowing treatment of older and more heavily-pretreated patients. Two different radio-labeled anti-CD20 antibodies have been used to treat B-cell lymphomas: Iodine-131 (I131)-tositumomab (Bexxar®) and yttrium-90 (Y90)-ibritumomab tiuxetan (Zevalin®). We previously reported the results of a phase I/II trial demonstrating the safety of combining standard dose 90Y-ibritumomab with high-dose BEAM followed by autologous transplant (Z-BEAM) [9]. The toxicity profile was similar to high-dose BEAM alone and the overall survival (OS) at 2 years was a promising 89.7% in the 20 DLCL patients. A randomized phase II comparison of BEAM versus Z-BEAM conditioning prior to autologous transplant for DLCL, reported at the 2010 American Society of Hematology Meeting, suggests improvements in both OS and PFS in the Z-BEAM arm [10]. However, while often assumed, it has never been demonstrated that outcomes for autologous transplant with the 90Y-ibritumomab Z-BEAM regimen are superior to TBI-based autologous conditioning regimens. In this study we performed a comparative analysis, of a consecutive case-series of DLCL patients prospectively treated with Z-BEAM, who were matched to patients receiving a TBI-based conditioning regimen. The goal of this retrospective study was to evaluate the impact of RIT-based conditioning on overall and progression-free survival.
PATIENTS AND METHODS
Patients
From January 1997 to January 2009, a matched series of 92 patients with DLCL (46 patients for each conditioning regimen) underwent AHCT at the City of Hope (COH); Z-BEAM patients were transplanted from 2002 to 2009, TBI patients were transplanted from 1997 to 2008. All DLCL patients treated on two phase I/II radioimmunotherapy (RIT) trials with myeloablative BEAM plus standard dose 90Y-ibritumomab tiuxetan (Zevalin®) were included in the analysis as part of the Z-BEAM treatment group. DLCL TBI patients were identified and paired/selected for analysis from a prospective observational research transplant database and were all treated based on a standard institutional operating procedure for Cy-TBI-VP-16 autologous transplant. In situations where more than one potential TBI patient was identified as a potential pair for a Z-BEAM patient, the best-matched patient was selected. Patients were matched on age (+/− 5 years), disease status at the time of salvage, number of prior regimens, year of diagnosis (+/− 5 years), and year of transplant (+/− 5 years). The COH Institutional Review Board (IRB) approved the analysis of these data. All pathology specimens were reviewed by the COH Department of Hematopathology to confirm diagnosis prior to transplant. Disease status was confirmed by clinical assessment including physical examination, laboratory evaluation, imaging by CT scans and nuclear imaging, and bone marrow biopsies per COH patient care standard operating procedures. Chemosensitivity was defined as at least a PR to salvage treatment, as determined by CT scanning, and resolution of all disease related symptoms that was maintained for at least 4 weeks. The IPI score was calculated as per the International Non-Hodgkin’s Lymphoma Prognostic Factors Project [11]. Patients in both treatment groups were managed similarly with respect to organ function screening, disease status assessments and follow-up. All patients were enrolled on prospective observational and long-term follow-up protocols.
Eligibility Criteria
ALL Patients
Patients with histologically confirmed CD20+ diffuse large cell lymphoma (DLCL) were eligible if they met any of the following conditions: 1) DLCL that required at least two different induction regimens to achieve either complete or partial remission, 2) high or high-intermediate age-adjusted international prognostic index (aaIPI) score at diagnosis, or 3) experienced a relapse event after initial response.
Z-BEAM
Patient exclusion criteria included: prior RIT, prior irradiation of more than 10Gy to the liver or lung, and/or active chronic hepatitis B or C. Organ function criteria was standard for AHCT. In addition, patients had to have less than 10% lymphomatous marrow involvement at the time of stem cell collection. After the initial trial consent and screening, patients were also determined to be ineligible if they were HAZA positive (human anti-Zevalin antibody) or if they had unfavorable biodistribution on pre-Zevalin imaging.
TBI
Patients between the ages of 18–65 years were eligible. The minimum organ function criteria followed institutional treatment guidelines for AHCT. Patient exclusion was primarily based on performance status, age, extent of prior radiation and other co-morbid conditions.
Debulking, Mobilization and Conditioning Regimens
ALL Patients
Salvage chemotherapy was given to debulk disease and to determine chemosensitivity before AHCT. Chemosensitivity was defined as at least a PR to salvage treatment and resolution of all disease related symptoms (based on CT scan) that was maintained for at least 4 weeks. Some patients received 1.5 – 2 gm/m2 cyclophopsphamide as part of mobilization, followed by filgrastim 10 µg/kg. Other patients were mobilized with filgrastim following debulking chemotherapy.
Z-BEAM
On day −21, patients were given an infusion of rituximab 250mg/m2 followed by Indium-111- labeled ibritumomab tiuxetan 185MBq. Starting in May 2008, patients were administered 250mg/m2 cold rituximab only if their serum rituximab levels were below 10 µg/ml prior to administration of either the imaging or treatment dose of radiolabeled antibody. 10/46 patients were accrued after May 2008 and had rituximab levels drawn; 2 of those 10 received rituximab 250mg/m2 prior to the imaging dose and 0/10 needed it prior to the therapeutic dose. Imaging studies were performed at 2, 24, 48 and 72 hours to determine the biodistribution of the Indium-111-labeled antibody. On day −14, patients with favorable imaging were given rituximab 250mg/m2 (except for those accrued post 5/2008), followed by 90Y-ibritumomab tiuxetan 14.8MBq (0.4mCi/kg); the dose was capped at 40mCi. One week later patients were admitted to the transplant unit and received BEAM; BCNU 150mg/m2 days −7 and −6, etoposide 100mg/m2 and cytarabine 100mg/m2 twice a day days−5 through −2 and melphalan 140mg/m2 day −1. On day 0 autologous stem cells were infused per institutional standard operating procedures and followed by filgrastim on day +5. All Z-BEAM patients with the exception of one (n=45) received rituximab therapy prior to transplant.
TBI
For all patients peripheral blood progenitor cells were mobilized with filgrastim 10 µg/kg with either cyclophosphamide or debulking chemotherapy. Radiation was delivered as three daily fractions starting on day −8 to a total dose of 1200 cGy. This was generally performed as an outpatient. On day −4 patients were admitted to the transplant unit and received etoposide 40 mg/kg, followed by cyclophosphamide 100 mg/kg on day −2. Stem cells were infused on day 0 followed by filgrastim on day +5. All patients received antibacterial, antiviral and antifungal prophylaxis as per standard institutional standard operating procedures. Just over half, 67% (n=31), of the TBI patients received prior rituximab therapy.
Disease Assessment
Response criteria for this analysis were from the International Working Group [12]. Complete response (CR) was defined as the complete resolution of all measurable disease, sustained for at least 4 weeks. Partial remission (PR) was defined as a 50% or more reduction in the sum of the products of the diameters of all measurable lesions. Induction failure (IF) was defined as failure to achieve at least a PR with first-line therapy, or progression from a CR or PR within 4 weeks of first-line treatment. Relapse was defined as a clinical or radiological progression at least 4 weeks after an initial CR or PR to first-line therapy.
Staging was performed at salvage chemotherapy, before AHCT and post-transplant at 100 days, 6-months, 12-months, 18 months, 24 months, and then every year thereafter or as clinically indicated. Staging included physical examination, complete blood counts, basic biochemical profile including renal and liver function tests, LDH, chest X-ray, computed topographies of the chest, abdomen and pelvis and unilateral or bilateral bone marrow biopsy if indicated.
Statistical Analysis
The primary endpoints were overall survival (OS) and progression-free survival (PFS); secondary endpoints included: early/late toxicities/complications, non-relapse mortality and relapse/progression incidence. Survival estimates were calculated based on the Kaplan-Meier product-limit method, 95% confidence intervals were calculated using the logit transformation and the Greenwood variance estimate [13]. Differences between Kaplan-Meier curves were assessed by the log-rank test. Patients who were alive at the time of analysis were censored at the last contact date. Overall survival (OS) was measured from transplant to death from any cause. Progression-free survival (PFS) was defined as time from transplant to recurrence, progression or death. Relapse/progression incidence (RP) was defined as time from transplant to recurrence or progression. Non-relapse mortality (NRM) was measured from transplant to death from any cause other than disease relapse or disease progression. Cumulative incidence curves were generated for NRM and RP in the competing risks setting, given that death and relapse/progression events were in competition. The cumulative incidence of NRM and RP were calculated using the method described by Gooley et al. [14]; differences between cumulative incidence curves in the presence of a competing risk were tested using the Gray method [15]. The significance of demographic and treatment features was assessed using stratified survival analysis and univariate, multivariable Cox proportional hazards regression analysis, or the corresponding hazard analysis for competing risks [16, 17].
Univariate and multivariable Cox regression models were used to assess the impact of patient, disease and treatment factors on OS and PFS. The factors studied were: disease status at the time of salvage (1CR/PR; induction failure, ≥ 1st relapse), bulky disease at diagnosis (≥ 5 cm, yes, no), bone marrow involvement at AHCT (yes, no), number of prior regimens (>2, ≤2), CD34 count (< or ≥ 5.2×106 cell dose), treatment regimen (RIT or TBI), and chemosensitive disease (yes, no). All calculations were performed using SAS® version 9.2 (SAS Institute, Cary, NC) or R 2.11.1. Generally, statistical significance was set at the P <0.05 level; all P values were two-sided. For multivariable Cox regression, factors shown to be significant at the P <0.10 level univariately, were included in the analysis. The data were locked for analysis on 03/31/2010 (analytic date). Modification of treatment-related effects by number of regimens received prior to AHCT (≤2, >2) and chemosensitivity status (sensitive, resistant), were evaluated by including interaction terms/stratification factors in the regression model [18].
RESULTS
Treatment Group Matching
Patient, disease, and treatment characteristics are shown in Table 1. The median age of the Z-BEAM cohort was 56 years (range: 19–78) and 53 years (range: 21–62) for TBI patients. While the groups showed no statistical differences on the match factors: age at transplant (+/− 5 years), disease status at salvage, number of prior regimens, year of diagnosis (+/− 5 years), and year of transplant (+/− 5 years). The two groups did show slight differences (not statistically significant) in prevalence of the following features: gender, chemosensitivity, bulky disease at transplant, median time from diagnosis to transplant, median time from first line therapy to transplant. Prior rituximab therapy in the Z-BEAM group was significantly higher than in the TBI group (p<0.01). Among the 92 patients, 76 patients had received prior rituximab: 45 Z-BEAM patients and 31 TBI patients. Of those patients receiving prior rituximab, we further stratified into those patients receiving ritumab for salvage therapy only, and those who received rituximab as part of induction therapy (+/− salvage therapy as well). In addition, 30 patients in the Z-BEAM and 20 in the TBI groups had failed rituximab induction (relapsed within 1 year of diagnosis). The breakdown of previous rituximab treatments is displayed in Table 1.
Table 1.
Patient Characteristics
| Variable | Z-BEAM (N=46) N (%) or Median (Range) |
TBI (N=46) N (%) or Median (Range) |
|---|---|---|
| Patient Gender N (%) | ||
| Female | 17 (37) | 21 (46) |
| Male | 29 (63) | 25 (54) |
| Age at Transplant (years)* median (range) | 56.5 (19 – 78) | 53 (21 – 62) |
| Months from Dx to HCT median (range) | 16.4 (0.6 – 130) | 12.8 (3.7 – 104) |
| Months from First-line Therapy to HCT | 14.9 (5.7 – 125) | 12.1 (3.6 – 54) |
| Year of transplant* median (range) | 2005 (2002–2009) | 2001 (1997–2008) |
| Disease Status at Time of Salvage N (%) | ||
| 1st CR | 6 (13) | 7 (15) |
| 1st PR | 7 (15) | 5 (11) |
| Induction Failure | 10 (22) | 12 (26) |
| ≥1st Relapse | 23 (50) | 22 (48) |
| Chemo Sensitivity N (%) | ||
| Resistant | 14 (30) | 8 (17) |
| Sensitive | 32 (70) | 38 (83) |
| Bone Marrow Involvement at Dx N (%) | ||
| No | 33 (72) | 35 (76) |
| Yes | 9 (19) | 10 (22) |
| Not Available | 4 (9) | 1 (2) |
| Bulky Disease at Dx N (%) | ||
| No | 11 (24) | 4 (9) |
| Yes | 26 (57) | 28 (61) |
| Not Available | 9 (19) | 14 (30) |
| CD34 Cell Dose median (range) | 5.4 (2.5 – 37) | 5.1 (1.3 – 30) |
| Number of Prior Regimens* median (range) | 2 (1 – 7) | 2 (1 – 5) |
| Prior Rituximab | ||
| No | 1 (2) | 15 (33) |
| Yes | 45 (98) | 31 (67) |
| Salvage only | 5 (11) | 7 (15) |
| Induction^ | 40 (87) | 24 (52) |
| Failed Rituximab at induction# | 30 (65) | 20 (43) |
Matched Factors, HCT – hematopoietic cell transplant, CR – complete remission, PR – partial remission, Dx – diagnosis.
Induction group indicates that all patients had rituximab induction, and may also have had rituximab during salvage therapy.
Failed rituximab at induction includes all patients who failed induction or relapsed within one year of diagnosis, and also had rituximab at induction.
Toxicity
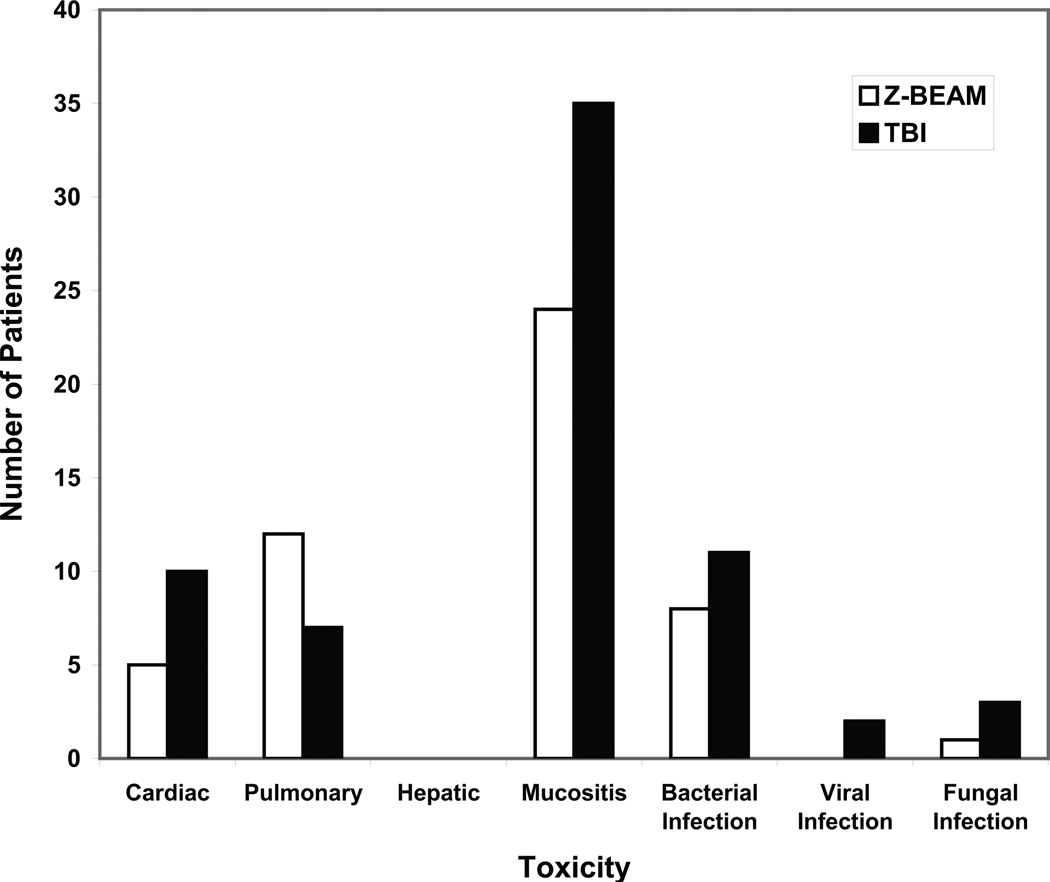
Toxicity data for the first 100 days post-transplant are illustrated in Figure 1. There was notably more cardiac toxicity in the TBI group, specifically ventricular and supraventricular arrhythmias, as well as more deaths attributable to cardiac disease. There were 8 episodes of documented bacterial infection in the Z-BEAM group and 11 in the TBI group. The 100-day mortality was 0% in the Z-BEAM group and 8.7% in the TBI group (4/46); of the four deaths in the TBI group, three were attributable to disease progression and one to infection. Patients continue to be followed for other long-term complications, including myelodysplasia and secondary malignancies as shown in Table 2. There was one case of myelodysplasia in the Z-BEAM group, and there were two cases of acute myelogenous leukemia in the TBI group.
Figure 1. Toxicities ≤ 100 Days.
The number of patients with grade 3 and above toxicity in the first 100 days is graphically depicted. Z-BEAM patients are white bars and TBI patients are black bars. Toxicities are NCI CTC v3.0.
Table 2.
Long-term toxicities > 100 days post-transplant
| Event | Z-BEAM (N=46) N (%)) |
TBI (N=46) N (%) |
|---|---|---|
| Grade ≥3 | ||
| Overall* | 18 (39)* | 32 (70)* |
| Cardiac | 3 | 4 |
| Pulmonary | 5 | 3 |
| Hepatic | 0 | 1 |
| Mucositis | 0 | 4 |
| Infection | 1 | 5 |
| Bacterial | 1 | 4 |
| Viral | 0 | 0 |
| Fungal | 0 | 1 |
| Secondary Malignancy | ||
| acute myelogenous leukemia | 0 | 2 |
| Basal cell carcinoma | 1 | 0 |
| Myelodysplasia | 1 | 0 |
| Squamous cell carcinoma | 2 | 0 |
All toxicities are NCI CTC v3.0.
Fisher’s Exact Test P = 0.006
Outcomes
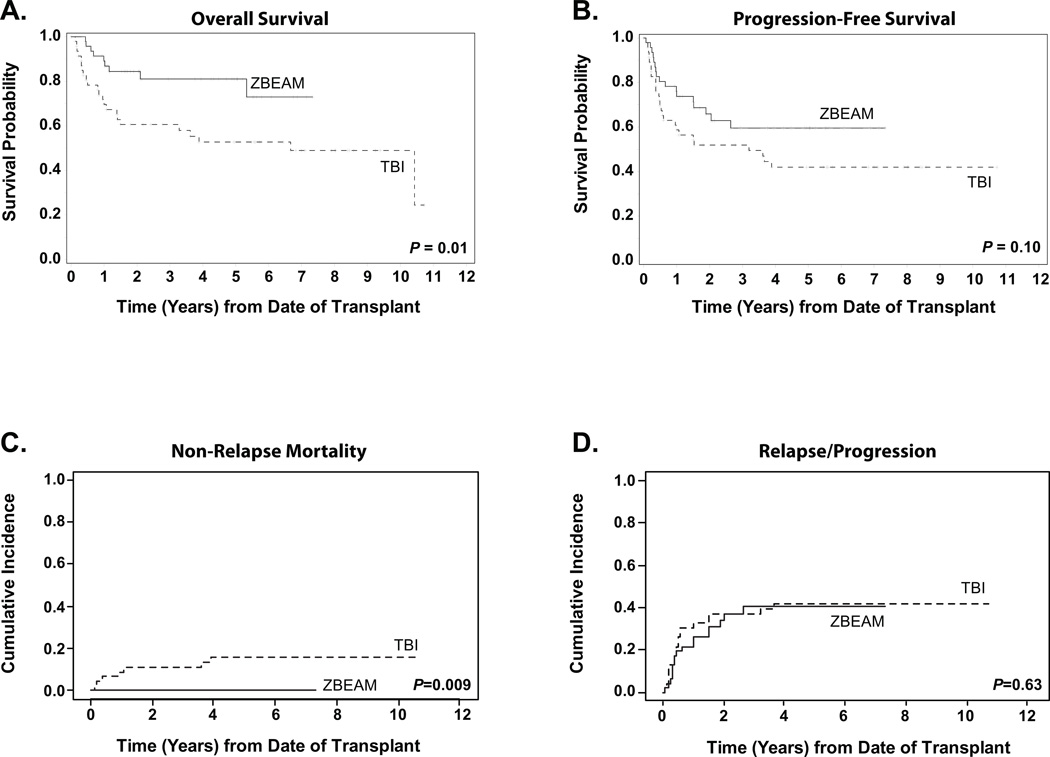
The median length of follow-up was 59.9 months (range: 11.3–128.7) for all surviving patients (n=60). The median follow-up for the 37 Z-BEAM patients was 51.0 months (range: 11.3–88.0) and 81.9 months (12.5–128.7) for the 23 TBI patients. As of the analytic date, there were 17 relapse/progression events in the Z-BEAM group and 19 in the TBI group. In the Z-BEAM group there were 9 total deaths and 23 in the TBI group; causes of death are listed in Table 3. Overall survival for the Z-BEAM group was significantly improved; 81% (95%CI: 68.8–88.8) for Z-BEAM versus 52.7% (95%CI: 44.6–60.2) for the TBI group at 4-years (P = 0.01) (Figure 2A). There was a trend towards improved progression free survival in the Z-BEAM group 59.6% versus 42% for the TBI group (P = 0.10) (Figure 2B). Results to date show that a plateau in PFS appears to have been achieved for both groups: at 2.6 years in the Z-BEAM group and 3.9 years for the TBI group. The poorer OS probability for the TBI cohort was primarily due to toxicity, with a 4-year cumulative incidence of non-relapse mortality of 0% for Z-BEAM and 15.8% (95%CI: 8.0–31.3) for TBI (p<0.009) (Figure 2C). The 4-year cumulative incidence of relapse/progression was very similar for both groups, as seen in Figure 2D, with 40.4 (95%CI: 27.7–59.0) for Z-BEAM and 42.1 (95%CI: 29.8–59.4) for TBI.
Table 3.
Relapse and Death Events
| Variable | Z-BEAM (N=46) N (%) |
TBI (N=46) N (%) |
|---|---|---|
| Number of Relapse/Progression Events | 17 (37) | 19 (41) |
| Number of Death Events | 9 (20) | 23 (50) |
| Cause of Death | ||
| Relapse/Disease Progression | 9 | 16 |
| Infection | 0 | 2 |
| Chronic heart failure, chronic renal insufficiency | 0 | 1 |
| Therapy-induced AML-relatedCNS bleed | 0 | 1 |
| Hypertensive hypertrophic cardiomyopathy with diastolic dysfunction | 0 | 1 |
| Left hemispheric infarct, autoimmune hemolytic anemia, pneumonia | 0 | 1 |
| Unknown | 0 | 1 |
Figure 2. Survival Outcomes Stratified by Treatment Regimen.
For all curves, solid lines represent Z-BEAM patients (n=46) and dashed lines represent TBI patients (n=46). Panel A shows the Kaplan-Meier estimate of overall survival probability. Panel B shows progression-free survival, defined as time from stem cell infusion to recurrence, progression, or death from any cause, whichever occurred first. Panel C shows the cumulative incidence of non-relapse mortality, and Panel D shows the cumulative incidence of relapse or progression. Relapse/progression and non-relapse mortality were calculated as competing risks.
Since the incidence of relapse/progression was similar for the two regimens, we decided to look at subsequent treatments and outcomes in the patients who relapsed following autologous transplant. In the Z-BEAM group, 8 of 17 relapsed patients were living (4 of the 8 survivng more than 3 years since relapse), while in the TBI group, only 3/19 were still alive at the analytic date. In the relapsed Z-BEAM (n=17) versus TBI patients (n=19), use of rituximab for salvage post-tranpslant was proportionally similar in the two groups: 8/17 for Z-BEAM, and 9/19 for TBI. A higher proportion of Z-BEAM patients were salvaged post-autologous relapse with agents such as gemcitabine (5/17 vs 3/19), lenalidomide (3/17 vs 0/19), bendamustine (2/17 vs 0/19), and bortezomib (2/17 vs 0/19), with several receiving more than one of the above-listed agents. Two of the Z-BEAM patients were salvaged post-autologous relapse using allogeneic transplant (both died) and two of the TBI patients were also salvaged post-autologous relapse with allo (1 died).
Predictors of Improved Survival: Multivariable Analysis
Using Cox regression modeling we further evaluated the independent effect of treatment group and other risk factors on overall survival and progression-free survival, analyzing factors that were identified as predictive in the univariate analysis at the p≤ 0.10 level. For overall survival, the factors found to be predictive by univariate analysis were: treatment type (two-fold increase in risk for TBI patients), disease status at salvage (4–8 fold increase in risk for patients beyond 1CR/PR), number of prior regimens (two-fold increase in risk for patients who received more than two regimens prior to AHCT), and prior rituximab therapy (two-fold increase in risk for patients who did not receive prior rituximab). Generally, the same list of risk factors was identified for progression-free survival, with the addition of chemosensitivity status. Patients with resistant disease showed a two-fold increase in relapse/progression or death when compared to patients with sensitive disease.
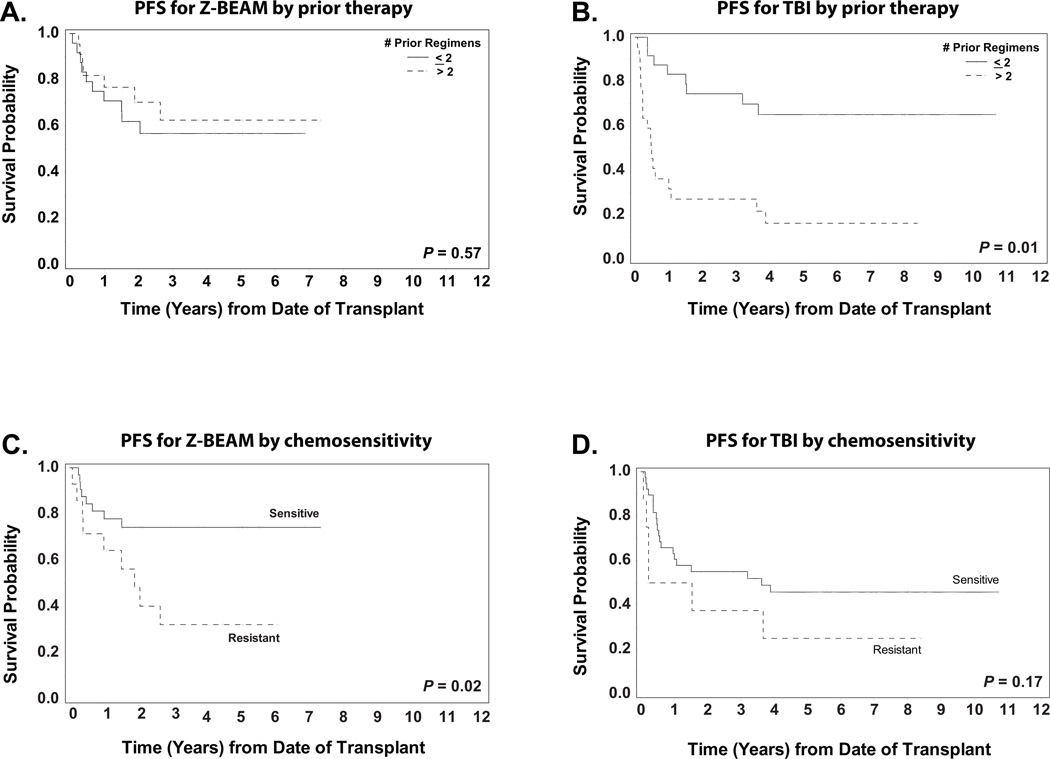
In our univariate and initial multivariable analysis, an interesting phenomenon occurred in which the impact of treatment arm on hazard risk did not attain significance in the univariate setting but was significant in the multivariable analysis. This uncharacteristic result prompted us to revise the Cox regression model to include an interaction term, which tested for possible interactions between treatment arm and other factors in the analysis. A significant interaction effect was seen between the variables of treatment arm and number of prior regimens (p< 0.01) for both overall and progression free survival. This interaction relationship was identified after stratified analyses revealed that the effect of one of the variables differed depending on the level of the other variable. As shown in figures 3A and 3B, patients treated with more than two prior regimens who underwent AHCT using a TBI-based regimen had significantly poorer PFS when compared to TBI patients who received two or fewer regimens (p< 0.01). This difference was not seen in the Z-BEAM group. A similar interaction trend (p=0.07) was seen between the variables of treatment regimen and chemosensitivity status, for progression-free survival but not for OS. When assessing the impact of treatment in the context of patient chemosensitivity status, the results showed that Z-BEAM patients who were chemosensitive had improved PFS outcomes when compared to those who were resistant (p= 0.02) (Figure 3C). Among TBI patients, however, this was not the case (p= 0.17) (Figure3D); patients who were chemosensitive did not show significantly improved PFS over chemoresistant patients.
Figure 3. Factors interacting with treatment regimen for Progression-free survival (PFS).
Panel A shows the PFS for the 46 Z-BEAM patients stratified by the number of prior regimens.
Panel B shows the PFS for the 46 TBI patients stratified by the number of prior regimens.
Panel C shows the PFS for the 46 Z-BEAM patients stratified by sensitivity to chemotherapy.
Panel D shows the PFS for the 46 TBI patients stratified by sensitivity to chemotherapy.
For overall survival, the multivariable model showed that, after controlling for the relationship between transplant conditioning regimen and number of prior regimens, TBI patients who received more than two regimens prior to AHCT were at a significantly increased risk for death post-transplant [HR: 3.46 (95%CI: 1.23–9.79), p=0.02] (Table 4). Patients who were classified as induction failures at AHCT were found to have a significant increase in risk of death post transplant compared to those in first CR or PR [HR: 6.66 (95%CI: 1.81–24.53), p<0.01]; this remained true after adjusting for the impact of treatment group and number of prior regimens. For progression-free survival, the multivariable results trended similar to overall survival (Table 4). The multivariable model showed that after controlling for the relationship between treatment group and number of prior regimens, TBI patients who received more than two regimens prior to AHCT had a trend toward increased risk for relapse/progression or death post transplant [HR: 1.89 (95%CI: 0.84–4.29), p<0.13] Patients who were classified as induction failures at AHCT were found to have a significant increase in risk of death or relapse/progression post transplant compared to those in first CR or PR [HR: 5.08 (95%CI: 1.69–15.31), p<0.01]; this remained true after adjusting for the impact of treatment group and number of prior regimens.
Table 4.
Multivariable Analysis: OS and PFS
| Overall Survival | Progression-free Survival | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio |
95% CI | P-value | Hazard Ratio |
95% CI | P-value |
| Treatment * Prior Regimens | ||||||
| RIT * ≤2 Regimens | 1.00 | ---- | ---- | 1.00 | ---- | ---- |
| RIT * >2 Regimens | 0.62 | 0.16–2.38 | 0.49 | 0.46 | 0.17–1.26 | 0.13 |
| TBI * ≤2 Regimens | 0.92 | 0.28–3.09 | 0.90 | 0.62 | 0.24–1.62 | 0.33 |
| TBI * >2 Regimens | 3.46 | 1.23–9.79 | 0.02 | 1.89 | 0.84–4.29 | 0.13 |
| Disease Status at HCT | ||||||
| 1st CR/ 1st PR | 1.00 | ---- | ---- | 1.00 | ---- | ---- |
| ≥1st Relapse | 3.20 | 0.87–11.72 | 0.08 | 3.57 | 1.28–9.90 | 0.01 |
| Induction Failure | 6.66 | 1.81–24.53 | <0.01 | 5.08 | 1.69–15.31 | <0.01 |
| Chemosensitive Status | ||||||
| Sensitive | NA | NA | NA | 1.00 | ---- | ---- |
| Resistant | NA | NA | NA | 1.51 | 0.78–2.92 | 0.22 |
indicates interaction between terms, CI – confidence interval, RIT – radioimmunotherapy, TBI – total body irradiation, CR – complete remission, PR – partial remission, NA – not analyzed
DISCUSSION
The last 20 years has seen a shift in research emphasis from standard radiotherapy and chemotherapy regimens toward inclusion of less toxic biologic, immunologic and targeted therapies. Radioimmunotherapy combines the potency of radiotherapy in the treatment of lymphoma, with the targeting capability and immunologic potency of cell-type specific monoclonal antibodies. 90Y-ibritumomab tiuxetan is more effective as a single-agent therapy than its unlabeled, monoclonal antibody counterpart, rituximab, for the treatment of B-cell lymphoma [19], and also increases the response rate when combined with CHOP chemotherapy [20]. RIT agents utilizing yttrium-90 as opposed to iodine-131 have potential advantages based on: 1) the longer path-length of the β-particle emission, allowing for crossfire killing of non-antigen bearing cells in the tumor microenvironment, and 2) the lack of γ-particle emissions that necessitate shielding of the patient from family members and friends. Standard-dose 90Y-ibritumomab tiuxetan can be administered in the outpatient setting and its ease of use and exportability has made yttrium the isotope of choice for RIT at City of Hope.
Addition of rituximab to frontline treatment regimens for DLCL has drastically improved responses and survival [21, 22], raising the 5-year EFS from 29% for CHOP to 47% for R-CHOP. Those selected DLCL patients who do fail rituximab-containing front-line therapy, have poorer outcomes following salvage therapy with autologous transplant, than do patients who have never been exposed to rituximab [23]. One potential method of improving response and survival rates for autologous transplant in relapsed DLCL patients is incorporation of radioimmunotherapy into the conditioning regimen.
Z-BEAM, the radioimmunotherapy plus chemotherapy conditioning regimen combining 90Y-ibritumomab tiuxetan plus high-dose BEAM, is demonstrated to be well tolerated and efficacious in the initial phase I/II studies [9, 24]. Median engraftment times in the initial trials are similar to conventional conditioning regimens. Rates of pulmonary and hepatic toxicity are also low, 12% grade 3 hepatic, 7% pulmonary toxicity [9]. Based on this initial data, we have performed subsequent trials targeting pre-RIT serum blood rituximab and have analyzed efficacy in various histologies such as mantle cell, follicular and diffuse large cell lymphoma. Based on preliminary data in diffuse large cell lymphoma patients, RIT-based conditioning had a particular benefit for DLCL patients, whose response to salvage BEAM plus autologous transplant is reduced in the post-rituximab era [23].
Our matched cohort analysis of diffuse large cell lymphoma patients supports the efficacy of RIT-based conditioning with Z-BEAM. The PFS (4-yr 60%) and OS (4-yr 81%) of Z-BEAM were similar to other RIT high dose therapy regimens. For instance, a phase II study from the Nebraska group of 40 chemosensitive diffuse large cell lymphoma patients yielded a 3-year PFS of 70% and OS of 81% [25]. The toxicity profile was favorable, especially considering that the cohort included older patients.
Nonetheless, a major concern regarding novel conditioning regimens is whether efficacy has been sacrificed in the name of minimizing toxicity. Historically, radiation has been extensively used in lymphoma conditioning, due to the radio-sensitivity of the disease. Use of fractionated radiation greatly reduced toxicity, allowing the delivery of higher radiation dosing in the context of total body irradiation. However, pulmonary toxicity especially in older patients remains a concern. Overall the incidence of pneumonitis after TBI-based conditioning for lymphoma is 22% [26]. The long- term toxicity of therapy-related myelodysplasia is also an ongoing issue, as radiation is a known risk factor for transplant-related MDS [27]. In many centers, such as our own, TBI has fallen out of favor because of these toxicities, and numerous novel therapies are under exploration. Nonetheless radiation-based conditioning remains a treatment modality for young, high-risk patients in many transplant centers, due to its long-term record of efficacy.
This matched comparative analysis suggests that the toxicity profile of total body irradiation-based conditioning for autologous transplant may outweigh its purported benefits. Cardiac toxicity was a major factor in the TBI-treated group; given the relatively older age of NHL patients, this is a major concern. Considering the fact that pre-HCT chest irradiation is a known risk factor for cardiovascular complications [28], the lower cardiac toxicity in the Z-BEAM population is particularly desirable. There were a higher number of pulmonary events in the Z-BEAM group but most were not clinically significant as they included coughing in four patients and temporary hypoxia during stem cell infusion in six; two patients had pneumonia and one pneumonitis that could be attributable to the conditioning regimen. Despite a higher incidence of fever and neutropenia in the Z-BEAM-treated group, survival was not impacted. It is possible the higher fever/neutropenia incidence was due to discontinuation of levofloxacin prophylaxis in 2005. Relapse and progression incidence was also not significantly different between the two groups, suggesting that RIT conditioning had equal disease control to TBI. Since follow-up on the TBI group is longer than for the Z-BEAM group, it is possible that the higher proportion of surviving relapsed patients in Z-BEAM compared to TBI (8/17 vs 3/19) may theoretically still die, bringing the OS curves closer together. However, of the 8 surviving relapsed patients in the Z-BEAM group, 4 of them have survived beyond 3 years post-relapse and are therefore beyond the high-risk period for disease recurrence. Thus, the use of RIT-based conditioning harnesses the efficacy of radiation while greatly reducing toxicity; non-relapse mortality was significantly lower for Z-BEAM at 0% compared to 15.8% for TBI (P < 0.009).
Our analysis of the interaction between treatment type (Z-BEAM vs. TBI) and number of regimens prior to AHCT also highlights the potential efficacy of Z-BEAM conditioning. Most striking were the vastly better results in Z-BEAM patients treated with multiple prior chemotherapy regimens (>2) compared to similar patients treated with TBI. While the number of prior regimens did not impact OS for Z-BEAM treated patients, the TBI patients with extensive prior therapy had significantly worse outcomes. This difference is likely attributable to the superior NRM of the RIT conditioning regimen. If these results are confirmed, patients with multiple prior regimens may derive benefit from Z-BEAM autologous transplantation and be spared the toxicity of allogeneic transplantion. On the other hand, when patients were stratified as chemosensitive versus resistant, PFS was significantly different in the RIT group compared to no difference in the TBI group. This suggests an improved efficacy of Z-BEAM in chemosensitive patients; i.e. the chemosensitive patients did strikingly well, whereas for TBI-treated patients, one could say both chemosensitive and chemoresistant patients did poorly.
We are aware that this study has some caveats related to its non-randomized nature; however, very few physicians would be likely to enroll such high-risk patients to a randomized comparison of these two regimens. One major difference in the treatment groups is related to an imbalance in the use of rituximab pre-transplant in the two treatment arms. Prior rituximab treatment was far more prevalent in the Z-BEAM arm (45 patients) compared to the rituximab arm (31 patients). Of those who received prior rituximab, there were 30 patients in the Z-BEAM arm who failed rituximab-containing induction therapy, and 20 patients in the TBI arm. Despite the negative prognostic impact of rituximab failure indicated by the CORAL study [23], the overall survival was better for Z-BEAM. Although more patients in the Z-BEAM arm received prior rituximab compared to the TBI arm (98% vs 67%), which would appear to favor prognosis in the Z-BEAM group, a larger percentage of the Z-BEAM patients (65%) had failed rituximab induction compared to TBI patients (43%), which puts the Z-BEAM arm at a prognostic disadvantage. It is possible that if there had been fewer rituximab failures in the Z-BEAM arm, it would have had an even greater survival advantage over TBI.
We have attempted to equalize as many variables as possible through factor matching, but there are some apparent differences (all non-significant except for rituximab-related) between the two treatment arms that may confound our results. For instance, some chemotherapy agents in the TBI arm, specifically cyclophosphamide, were not used in the RIT arm, and could therefore contribute to the higher toxicity rates. Also, as TBI is less frequently used in the past 5 years, the cases tend to be separated based on time, although we have limited this difference to 5 years in matched cases. In addition, the Z-BEAM patients were all treated on protocol, whereas the TBI patients were not. However, our review of supportive care standard operating procedures over the years included in the study does not reveal significant differences, with two exceptions. First was the cessation of routine levofloxacin prophylaxis in the Z-BEAM group, which may account for the higher rate of febrile neutropenia in that group. Secondly, many of the patients in the TBI arm were staged using only CT scan, while all Z-BEAM patients were staged pre-transplant using PET scan. Despite these issues, this study is as well-controlled as possible and, we believe, affirms the assumption that RIT provides radiotherapy as effectively as TBI with less morbidity.
The recently completed Bexxar®-BEAM versus BEAM trial is expected to further validate the RIT-based AHCT approach, if it demonstrates improved survival over BEAM alone. The small randomized phase II study comparing Zevalin®-BEAM to BEAM shows a trend toward improved progression-free survival in the RIT arm based on preliminary report of the data [10]. Updates of this abstract, presented orally at the American Society of Hematology meeting showed improvement in both OS and PFS for the Z-BEAM arm by multivariable analysis; providing support for a potential phase III study. Our comparison of Z-BEAM with TBI conditioning is another step toward the establishment of RIT conditioning as a new standard of care for AHCT conditioning for NHL. We demonstrate comparable efficacy of the two regimens, as evidenced by similar relapse incidence, with decreased toxicity and non-relapse mortality for Z-BEAM.
ACKNOWLEDGMENTS
We would like to thank the administrative, nursing and medical staff for their support and dedication, making this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: There are no conflicts of interest to disclose. This study was funded in part by grant # PO1 CA 30206.
Authorship: AK and JP designed the study, analyzed the data and wrote the manuscript. NT and JS collected and analyzed the data. ST assisted with data review and manuscript preparation. AN, AR and SJF offered critical review of study design and manuscript writing. All authors reviewed and approved the final version of the manuscript.
REFERENCES
- 1.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz SM, Negrin RS, Blume KG, et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103:777–783. doi: 10.1182/blood-2003-04-1257. [DOI] [PubMed] [Google Scholar]
- 3.Horning SJ, Negrin RS, Chao JC, Long GD, Hoppe RT, Blume KG. Fractionated total-body irradiation, etoposide, and cyclophosphamide plus autografting in Hodgkin's disease and non-Hodgkin's lymphoma. J Clin Oncol. 1994;12:2552–2558. doi: 10.1200/JCO.1994.12.12.2552. [DOI] [PubMed] [Google Scholar]
- 4.Santini G, Salvagno L, Leoni P, et al. VACOP-B versus VACOP-B plus autologous bone marrow transplantation for advanced diffuse non-Hodgkin's lymphoma: results of a prospective randomized trial by the non-Hodgkin's Lymphoma Cooperative Study Group. Journal of Clinical Oncology. 1998;16:2796–2802. doi: 10.1200/JCO.1998.16.8.2796. [DOI] [PubMed] [Google Scholar]
- 5.Haioun C, Lepage E, Gisselbrecht C, et al. Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor-risk aggressive non-Hodgkin's lymphoma: updated results of the prospective study LNH87-2. Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology. 1997;15:1131–1137. doi: 10.1200/JCO.1997.15.3.1131. [DOI] [PubMed] [Google Scholar]
- 6.Nademanee A, Molina A, Dagis A, et al. Autologous stem-cell transplantation for poor-risk and relapsed intermediate- and high-grade non-Hodgkin's lymphoma. Clin Lymphoma. 2000;1:46–54. doi: 10.3816/clm.2000.n.004. [DOI] [PubMed] [Google Scholar]
- 7.Fuks Z, Kaplan HS. Recurrence rates following radiation therapy of nodular and diffuse malignant lymphomas. Radiology. 1973;108:675–684. doi: 10.1148/108.3.675. [DOI] [PubMed] [Google Scholar]
- 8.Caballero MD, Perez-Simon JA, Iriondo A, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14:140–151. doi: 10.1093/annonc/mdg008. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan A, Nademanee A, Fung HC, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 10.Shimoni A, Avivi I, Rowe JM, et al. A Multi-Center Prospective Randomized Study Comparing Ibritumomab Tiuxetan (Zevalin) and High-Dose BEAM Chemotherapy (Z-BEAM) Vs. BEAM Alone as the Conditioning Regimen Prior to Autologous Stem-Cell Transplantation In Patients with Aggressive Lymphoma; Possible Advantage for Z-BEAM In Low-Risk Patients. ASH Annual Meeting Abstracts. 2010;116 686- [Google Scholar]
- 11.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A Predictive Model for Aggressive Non-Hodgkin's Lymphoma. New England Journal of Medicine. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 13.Breslow NE, Day NE. Statistical methods in cancer research: volume II, the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- 14.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1140–1154. [Google Scholar]
- 16.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 17.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 18.Cox DR. Interaction. International Statistical Review (Revue Internationale de Statistique) 1984;52:1–25. [Google Scholar]
- 19.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 20.Zinzani PL, Tani M, Fanti S, et al. A phase II trial of CHOP chemotherapy followed by yttrium 90 ibritumomab tiuxetan (Zevalin) for previously untreated elderly diffuse large B-cell lymphoma patients. Ann Oncol. 2008;19:769–773. doi: 10.1093/annonc/mdm560. [DOI] [PubMed] [Google Scholar]
- 21.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 22.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 23.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoni A, Zwas ST, Oksman Y, et al. Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin's lymphoma. Exp Hematol. 2007;35:534–540. doi: 10.1016/j.exphem.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Vose J, Bierman P, Bociek G, et al. Radioimmunotherapy with 131-I tositumomab enhanced survival in good prognosis relapsed and high-risk diffuse large B-cell lymphoma (DLBCL) patients receiving high-dose chemotherapy and autologous stem cell transplantation. J Clin Oncol (Meeting Abstracts) 2007;25 8013- [Google Scholar]
- 26.Chen CI, Abraham R, Tsang R, Crump M, Keating A, Stewart AK. Radiation-associated pneumonitis following autologous stem cell transplantation: predictive factors, disease characteristics and treatment outcomes. Bone Marrow Transplant. 2001;27:177–182. doi: 10.1038/sj.bmt.1702771. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–1593. [PubMed] [Google Scholar]
- 28.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]