ABSTRACT
Background:
Pelvic radiotherapy with concurrent 5-fluorouracil-based chemotherapy is a component of standard therapy for patients with T3/T4 or node-positive rectal cancer and may be associated with acute gastrointestinal toxicity. In this retrospective study, we sought to compare patient-reported outcomes (PROs) with clinician reports of acute symptoms experienced by rectal cancer patients receiving chemoradiation.
Patients and Methods:
Charts of 199 patients with rectal cancer who received chemoradiation at some point from November 2006 through February 2011 were reviewed. Clinicians assessed toxicity weekly using Common Terminology for Clinical Adverse Events version 3.0, and, beginning in September 2009, the patients reported symptoms weekly, using the 7-item Bowel Problems Scale. One hundred ninety-seven patients with at least 1 clinician or patient assessment were eligible for the study. We used descriptive statistics to compare patient and clinician assessments in a subgroup of 65 patients (paired group) who had at least 1 patient and clinician assessment on the same day. Cohen's κ coefficient was used to evaluate agreement between the patients and the clinicians.
Results:
The patients reported diarrhea and proctitis more often than clinicians reported them throughout treatment. Uncorrected agreement for diarrhea and proctitis was 82% and 72%, respectively. Cohen's κ was .64 for diarrhea, indicating moderate agreement, and .22 for proctitis, indicating only slight agreement.
Conclusions:
Our findings suggest a discrepancy between clinician and PRO reports. Further study may discern potential benefits of collecting PROs in prospective studies and in clinical practice.
Pelvic radiotherapy with concurrent 5-fluorouracil-based chemotherapy (chemoradiation) is a component of standard treatment for patients with T3/T4 or node-positive rectal cancer. Pelvic radiation can be associated with both acute and long-term toxicities due to the radiosensitivity of bowel, bladder, and bone. Clinician-assessed toxicity is commonly captured in prospective studies, but patient-reported outcomes (PROs) may provide additional data1–3 that, in some cases, may be of prognostic value.4–7 Studies of various cancer types indicate that patients may report a greater prevalence of cancer- and treatment-related symptoms than clinicians report.8–12 The National Cancer Institute has made PROs a priority area for research13; however, there remains a paucity of studies on PROs during rectal cancer treatment.
At the Massachusetts General Hospital, Chen et al14 established the feasibility of collecting PROs during chemoradiation for rectal cancer and described the trajectory of acute gastrointestinal (GI) symptoms as reported by physicians and patients. In that study, physician assessments of toxicity were graded by Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity Scoring Criteria.15 Use of the RTOG criteria allows assignment of a single global value for lower GI symptoms, whereas the Common Terminology for Clinical Adverse Events (CTCAE) allows for separate evaluation of each symptom. The first purpose of this study was to validate the use of a PRO assessment tool to describe and compare patient and clinician reporting of acute symptoms experienced by rectal cancer patients receiving chemoradiation in a subgroup of 65 patients. The second purpose was to compare patient-reported symptom assessment with reporting of symptoms by physicians using the CTCAE.
PATIENTS AND METHODS
Patients and Therapy
Medical records were reviewed for 199 consecutive patients who received concurrent 5-FU-based chemoradiation therapy for rectal adenocarcinoma, predominately in a preoperative approach, at Memorial Sloan-Kettering Cancer Center (MSKCC) at some point from November 2006 through February 2011. A waiver of authorization was obtained from the MSKCC institutional review board.
The patients received standard fractionation radiation therapy at a dose of 180 cGy to 200 cGy daily, 5 times per week, to a median total dose of 5000 cGy to the primary tumor and 4500 cGy to the pelvic nodes. Standard treatment therapy was delivered over 5 to 6 weeks, and the patients underwent approximately 5 weekly clinician symptom assessments. In the cohort treated after September 2009, the Bowel Problems Scale (BPS) questionnaire was collected at each weekly clinic visit.
Symptom Assessment
The patients were evaluated at least once weekly in the clinic while receiving chemoradiation therapy during on-treatment visits. A nurse specializing in GI radiation oncology (E.B.L.) graded toxicity severity in each patient by using the CTCAE version 3.016 and documented the findings on a standardized form listing grades for the following symptoms: fatigue, dermatitis, mucositis, nausea, vomiting, diarrhea, proctitis, and cystitis. The attending physician (K.A.G.) verified the CTCAE grading each week following the nursing assessment.
Beginning in September 2009, PRO assessments were conducted weekly in the clinic, with the 7-item BPS.17 The questionnaire asks the following:
In the past week, have you had diarrhea or loose watery stools?
Have you had a sense of urgency that you move your bowels?
Have you had any tenderness or pain when you move your bowels?
Have you had bleeding with your bowel movements?
Have you had any abdominal cramping or pain?
Have you passed mucus from your rectum?
Have you had the feeling that you have an urge to move your bowels but have nothing to pass?
The patients reported the frequency of each symptom on a 5-point Likert-type scale, as follows:
Score 1: “not at all”;
Score 2: “occasionally” (1–2 times/week);
Score 3: “fairly frequently” (3–4 times/week);
Score 4: “frequently” (1–2 times/day);
Score 5: “very frequently” (≥3 times/day).
The patients completed the questionnaire before the weekly toxicity status checks in the clinic, giving clinicians the opportunity to review the results before completing their own assessments.
Analysis
The grades on the CTCAE are associated with the degree of medical intervention indicated, whereas the BPS assesses the frequency of symptoms experienced. Therefore, the analysis focused on the prevalence of symptoms in the study sample at each time point. We described the proportion of the patients with each symptom via clinician (CTCAE) and patient (BPS) reporting. The proportion of patients reporting clinically meaningful symptom severity (score, ≥3) was also described for each symptom in the BPS.
Among the 65 patients who had at least 1 treatment date with both a clinician- and patient-reported assessment (paired group), we compared the prevalence at each time point of diarrhea and proctitis, defined as CTCAE grade ≥1 and BPS score ≥2. Agreement between patient and clinician assessments was evaluated by Cohen's κ coefficient, in which the clinician was specified as rater 1 and the patient as rater 2.
RESULTS
Patient Characteristics
A total of 199 consecutive patients received concurrent 5-FU-based chemoradiation therapy from November 2006 through February 2011 for rectal adenocarcinoma. Of these, 2 patients who were treated before the introduction of the BPS did not have a recorded clinician symptom assessment and were excluded from analysis. A total of 197 patients had at least 1 clinician- or patient-reported symptom assessment and were included in the analysis. Of these, 42% were women, with an average age of 58 years. Most of the patients (91%) presented with primary, locally advanced rectal adenocarcinoma; 9% had locally recurrent disease. Ninety percent received neoadjuvant (n = 173) or definitive (n = 5) chemoradiation therapy, and 10% received adjuvant (postoperative) chemoradiation therapy. The majority (84%) of the patients underwent surgery with definitive intent (Table 1). Demographic characteristics, disease status, and course of treatment were well balanced between all 197 patients with rectal cancer and the 65 patients in the paired group, with the exception of the use of intensity-modulated radiotherapy (IMRT). IMRT was first used in this cohort in April 2007 and has been used increasingly for rectal cancer over time. Therefore, since the BPS was introduced in September 2009, IMRT was used in a larger proportion of the paired group vs. all the study patients (77% vs. 51%, respectively; Table 1).
Table 1.
Characteristics of all 197 patients studied and of 65 patients with both clinician and patient symptom assessments on ≥1 treatment date (paired group)
| Characteristic | All rectal patients (11/06–2/11) (n = 197) |
Paired group (9/09–2/11) (n = 65) |
||
|---|---|---|---|---|
| n | %/Mean | n | %/Mean | |
| Demographics | ||||
| Median age, y | 58.9 | 58.3 | ||
| Age range, y | 18–93 | 24–89 | ||
| Gender, female | 82 | 42% | 32 | 49% |
| Presentation | ||||
| Primary tumor | 181 | 92% | 59 | 91% |
| Recurrent tumor | 16 | 8% | 6 | 9% |
| Stage I | 13 | 7% | 5 | 8% |
| Stage II | 31 | 16% | 8 | 12% |
| Stage III | 125 | 63% | 41 | 63% |
| Stage IV | 13 | 7% | 4 | 6% |
| Average tumor distance from anal verge, cm | 6.9 | 6.5 | ||
| Chemoradiation therapy intent | ||||
| Preoperative | 173 | 88% | 58 | 89% |
| Postoperative | 19 | 10% | 4 | 6% |
| Definitive | 5 | 2% | 3 | 5% |
| Radiation modality | ||||
| Conventional 3-field RT | 97 | 49% | 15 | 23% |
| IMRT | 100 | 51% | 50 | 77% |
| Therapy completion | ||||
| Experienced treatment break | 10 | 5% | 2 | 3% |
| Completed surgery | 166 | 84% | 58 | 89% |
| Surgical pathology | ||||
| Pathologic complete response | 24 | 12% | 12 | 18% |
| Positive margins | 6 | 3% | 1 | 2% |
| Outcomes | ||||
| Follow-up time, mos | 23.6 | 12.5 | ||
| Deceased | 22 | 11% | 1 | 2% |
IMRT = intensity-modulated radiotherapy; RT = radiotherapy.
Questionnaire Completion
Completion in this study was defined as having at least 1 pair of assessments (both patient and clinician) performed on the same day. Among 78 patients with at least 1 treatment visit after the introduction of the BPS, 65 patients had at least 1 pair of assessments to compare for analysis, for a completion rate of approximately 86%. The number of paired assessments per week of treatment was 47, 49, 50, 47, 45, and 20 for weeks 1 through 6, respectively.
In our cohort, 89% of patients completed 4 or more assessments, the level used by Chen et al14 to define a participant, which is comparable to the 95% completion rate among their patients.
Patient- vs. Clinician-Reported Symptoms
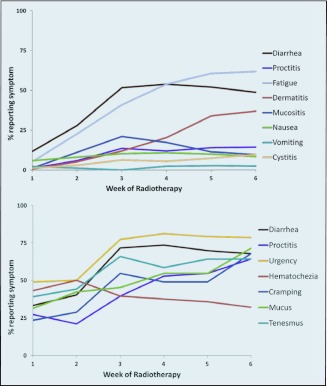
The prevalences of acute symptoms as reported by the clinicians and patients are illustrated in Figures 1a and 1b, respectively. During the first week of treatment, fewer than half of the patients reported experiencing each symptom, apart from rectal bleeding. All symptoms worsened by week 5, with the exception of rectal bleeding, which improved over the course of treatment (Figure 1b).
Figure 1.
Prevalence of acute side effects of chemoradiation as assessed by (a) the clinicians and (b) the patients.
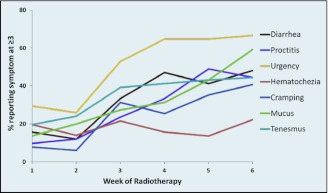
Patient reporting of symptoms defined as clinically meaningful (score, ≥3) is shown in Figure 2. The proportion of patients with diarrhea, bowel urgency, and tenesmus increased most sharply between weeks 2 and 3 of treatment, with more gradual increases continuing until the end of treatment (Figure 2). The trajectory of patient-reported proctitis scores demonstrated that pain developed more slowly, with greater increases later in treatment, between weeks 4 and 5 (Figure 2).
Figure 2.
Prevalence of clinically relevant acute side effects (score ≥3) of chemoradiation as reported by the patients.
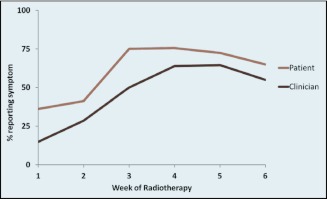
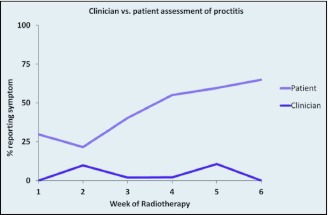
In the subgroup of patients with both clinician assessments and patient-reported symptoms (n = 65), we found that both diarrhea and proctitis were reported more frequently by patients than by clinicians throughout the chemoradiation treatments (Figures 3 and 4). Uncorrected agreement for diarrhea and proctitis was 82% and 72%, respectively. Corrected for chance, Cohen's κ was .64 for diarrhea, indicating moderate agreement between clinicians and patients, and .22 for proctitis, indicating only slight agreement (Table 2).
Figure 3.
Clinician- vs. patient-reported prevalence of diarrhea during chemoradiation.
Figure 4.
Clinician- vs. patient-reported prevalence of proctitis during chemoradiation.
Table 2.
Cohen's κ coefficient of agreement for diarrhea and proctitis between patients and clinicians
| Observed agreement | Expected agreement | κ | SE | Z | P > Z | |
|---|---|---|---|---|---|---|
| Diarrhea | 82% | 49% | 0.64 | 0.06 | 10.71 | <.001 |
| Proctitis | 72% | 64% | 0.22 | 0.10 | 2.28 | .01 |
DISCUSSION
In our study, throughout chemoradiation treatment, the patients were more likely than the treating clinicians to report diarrhea and proctitis. In the case of proctitis, there was only minimal agreement in reporting between the clinicians and patients. While physician-reported CTCAE grades may better predict serious adverse events, such as hospitalization or death, PROs may be more sensitive in describing subjective symptoms than standard clinician toxicity-assessment tools.9 Patient-reported quality-of-life measures have been shown to be associated with toxicity in other cancers.18 In addition to their usefulness as a potential prognosticator, the collection of PROs has been shown to improve physician–patient communication about symptoms,19 which can enable clinicians to make better informed decisions about symptom management.
In line with the results of another study of patient-reported outcomes,10 our findings show that clinicians and patients may not agree on symptoms experienced during cancer treatment. In a study of patients with lung or genitourinary cancer, Basch et al8 found that clinicians and patients were more likely to agree on directly observable events and less likely to agree on the presence of those that were not as observable. Our findings were in accordance with theirs, in that there was more agreement among clinicians and patients on the presence of diarrhea and less agreement on the presence of the more subjective symptom, proctitis.
One possible reason for the discrepancy in symptom reports is that clinicians may report only those symptoms attributable to treatment, while patients may describe any symptom they are experiencing.20 Symptoms that are not probable side effects of treatment may nevertheless be important for the patient,21 and by reporting only symptoms that are likely to be due to treatment, clinicians may miss information about the effect of those symptoms on patient function.22
To elucidate the difference between patient- and clinician-reported symptoms, Chen et al14 described the range of patient-reported severity by each RTOG grade of lower GI symptoms in their cohort of rectal cancer patients. In our study, we examined the difference in reports of the incidence of side effects. It is important to note, however, that we did not compare differences in the severity of side effects as reported by clinicians vs. patients, because, apart from describing incidence, the scales do not provide comparable end points. CTCAE scores describe whether a symptom is present and whether intervention is indicated or death will ensue. The BPS questionnaire allows patients to describe the frequency of symptoms. In addition to our finding that the patients reported a greater incidence of symptoms than the clinicians reported, we note that the scale used by the patients may enable clinicians to better distinguish gradations of symptoms that are not well elucidated by standard CTCAE grading.
As Chen et al14 described, the BPS is feasible to incorporate into clinical practice, with a high rate of patient completion. As electronic and web-based platforms further improve clinicians' ability to assess a patient's experience,23,24 PROs may be useful in setting appropriate expectations for patients undergoing chemoradiation for rectal cancer. Collecting PROs during treatment may also enhance clinician–patient communication and aid in effective symptom management. In addition, PROs may assist in the follow-up period to document the trajectory of improvements in symptoms after therapy and to determine whether patients have recovered to baseline levels after treatment. These data may also help practitioners counsel patients on the long-term effects of their therapy.25 Further study is warranted to determine the optimal process for incorporating PRO collection into both routine clinical practice and clinical trials to complement clinician toxicity scoring.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Trotti A, Colevas AD, Setser A, et al. : Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol 25:5121–5127, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Gondek K, Sagnier PP, Gilchrist K, et al. : Current status of patient-reported outcomes in industry-sponsored oncology clinical trials and product labels. J Clin Oncol 25:5087–5093, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Garcia SF, Cella D, Clauser SB, et al. : Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol 25:5106–5112, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Langendijk H, Aaronson NK, de Jong JM, et al. : The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol 55:19–25, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Fang FM, Liu YT, Tang Y, et al. : Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 100:425–432, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Efficace F, Biganzoli L, Piccart M, et al. : Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. Eur J Cancer 40:1021–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Grande GE, Farquhar MC, Barclay SI, et al. : Quality of life measures (EORTC QLQ-C30 and SF-36) as predictors of survival in palliative colorectal and lung cancer patients. Palliat Support Care 7:289–297, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Basch E, Iasonos A, McDonough T, et al. : Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol 7:903–909, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Basch E, Jia X, Heller G, et al. : Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 101:1624–1632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fromme EK, Eilers KM, Mori M, et al. : How accurate is clinician reporting of chemotherapy adverse effects?—a comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 22:3485–3490, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Butler L, Bacon M, Carey M, et al. : Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. J Clin Oncol 22:2461–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Stephens RJ, Hopwood P, Girling DJ, et al. : Randomized trials with quality of life endpoints: are doctors' ratings of patients' physical symptoms interchangeable with patients' self-ratings? Qual Life Res 6:225–236, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Clauser SB, Ganz PA, Lipscomb J, et al. : Patient-reported outcomes assessment in cancer trials: evaluating and enhancing the payoff to decision making. J Clin Oncol 25:5049–5050, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Chen RC, Mamon HJ, Chen YH, et al. : Patient-reported acute gastrointestinal symptoms during concurrent chemoradiation treatment for rectal cancer. Cancer 116:1879–1886, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity Scoring Criteria. Available at: http://www.rtog.org/ResearchAssociates/AdverseEventReporting/AcuteRadiationMorbidityScoringCriteria.aspx Accessed August 9, 2011
- 16. National Cancer Institute (NCI) Cancer Therapy Program, Common Terminology Criteria for Adverse Events, Version 3.0. August 9, 2006. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed August 9, 2011
- 17. Clark JA, Talcott JA: Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care 39:1118–1130, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Osoba D, Zee B, Pater J, et al. : Determinants of postchemotherapy nausea and vomiting in patients with cancer. Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 15:116–123, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Velikova G, Booth L, Smith AB, et al. : Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 22:714–724, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Cirillo M, Venturini M, Ciccarelli L, et al. : Clinician versus nurse symptom reporting using the National Cancer Institute-Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient's self-reported questionnaire. Ann Oncol 20:1929–1935, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Cleeland CS: Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr 2007:16–21, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Osoba D: Translating the science of patient-reported outcomes assessment into clinical practice. J Natl Cancer Inst Monogr 2007:5–11, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Movsas B, Hunt D, Watkins-Burner D, et al. Electronic web-based technology significantly improves quality of life (QOL) data collection: analysis of RTOG 0828. Proc Am Soc Radiat Oncol 81:S111, 2011. (abstr 221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fromme EK, Kenworthy-Heinige T, Hribar MR, et al. A novel computerized system makes patient reported symptom and QOL outcomes available in time for radiotherapy office visits. Proc Am Soc Radiat Oncol 81:S111–S112, 2011. (abstr 222) [Google Scholar]
- 25. Pucciarelli S, Del Bianco P, Efficace F, et al. : Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg 253:71–77, 2011 [DOI] [PubMed] [Google Scholar]