Abstract
This protocol has been designed to generate neural precursor cells (NPCs) from human embryonic stem cells (hESCs) using a physiological oxygen (O2) level of 3% and chemically defined conditions. The first stage involves suspension culture of hESC colonies at 3% O2, where they acquire a neuroepithelial identity over two weeks. This timescale is comparable to that at 20% O2, but survival is enhanced. Sequential application of retinoic acid (RA) and purmorphamine (PM), from day 14 to 28, directs differentiation towards spinal motor neurons. Alternatively, addition of FGF-8 and PM generates midbrain dopaminergic neurons. OLIG2 induction in motor neuron precursors is 2-fold greater than at 20% O2, whereas EN1 is 5-fold enhanced. 3% NPCs can be differentiated into all three neural lineages, and such cultures can be maintained long-term in the absence of neurotrophins. The ability to generate defined cell types at 3% O2 should represent a significant advance for in vitro disease modelling and potentially cell-based therapies.
Keywords: Dopaminergic neurons, human embryonic stem cells, hypoxia, low oxygen, motor neurons, neural precursor cells, neural stem cells, normoxia
INTRODUCTION
Human embryonic stem cells (hESC) can be manipulated to generate defined neuronal and glial lineages, offering a major opportunity to study neurodevelopment and model neurological disease in vitro, as well as having potential direct therapeutic applications in the field of regenerative neurology. However, certain challenges remain before the promise of hESCs for neurological diseases can be fully realised, including the need to optimise survival, fate and function of neural derivatives upon both neural conversion and long-term differentiation in vitro and in vivo1-4. Current protocols for the derivation of neural precursor cells (NPCs) from ESCs recommend defined conditions; however, these conditions lead to significant cell death, in which the production of reactive oxygen species (ROS) plays a central role5-9. Thus neuralisation protocols often contain antioxidants, which may increase the propensity to accumulate genetic mutations, involve co-culture with stromal feeder layers or use B27 and/or N2 supplements2, 6, 8-13.
ROS generation can potentially be decreased by culture at a low, physiological oxygen level (3%), that is closer to that found in the developing embryo and the adult brain9, 14, 15. There are a number of reports of the beneficial effects of low oxygen tensions in enhancing the survival, proliferation and long-term maintenance of various stem cell and neuronal populations, and oxygen also appears to be a critical component of the stem cell niche8, 9, 12, 15-30. Furthermore, low oxygen promotes the generation of dopaminergic neurons from midbrain NPCs and oligodendrocyte differentiation from human fetal NPCs25-29, leading to the suggestion that oxygen acts as a developmental morphogen16. The cellular response to low oxygen is co-ordinated by three hypoxia inducible factors (HIFs), and both HIF-1α and HIF-2α have been implicated in NPC and neuronal survival at low oxygen1, 18, 29-31. However, it is likely that HIF-independent pathways also play a role9, 32.
By contrast, it is becoming increasingly clear that the more traditional stem cell systems employing oxygen levels approximating room air (20%) are far from optimal, particularly in regards to neural specification and differentiation8, 9. Thus this protocol has been designed to generate NPCs and their regionally specified derivatives, from human embryonic stem cells (hESCs) using a physiological oxygen level of 3% (normoxia)31.
Overview of this protocol
The protocol involves the induction of NPCs from hESCs, maintained either in feeder-based or feeder-free conditions, in a chemically-defined, serum-free medium. hESC colonies are enzymatically detached and transferred to a low oxygen incubator (3% O2) where they are grown in suspension culture in the absence of pluripotency maintaining factors activin and FGF-2. Cells acquire a neuroepithelial identity within 2 weeks, and can then be expanded with FGF-2 or directed to differentiate towards midbrain dopaminergic neurons or spinal motor neurons by the sequential application of Shh agonists and either FGF-8 or RA7, 10, 33, 34. NPCs are terminally differentiated on poly-d-lysine (pdl) / laminin coated coverslips in a basic medium of DMEM, 2% B27 and 1% PSF, in which they can be maintained for many months without the addition of growth factor supplements such as BDNF or GDNF. Confirmation of functional neuronal maturity can be demonstrated with electrophysiological recordings.
Comparison to other protocols
Standard neuralising protocols established at 20% O2 have reported a two week timecourse for the acquisition of a neuroepithelial identity7, 33-35. Reports of an interaction between HIF-2α and Oct4 might suggest that this would be delayed at low O2 19, but we report NPC generation over 14 days31. Furthermore, survival of NPCs is significantly greater when neural conversion is performed at 3% O2, reducing the technical difficulties of the procedure9, 31. This effect is particularly marked when using a fully chemically-defined and serum-free medium as described here, which at 20% oxygen typically results in the death of >90% of NPCs9. Other protocols circumvent this survival challenge by including antioxidants (increasing the propensity to acquire genetic mutations), supplements such as B27 or N2 or undefined components such as knockout-serum replacement, stromal feeder layers or matrigel (an animal product derived from a mouse sarcoma line)2, 7, 10, 11, 36.
Our protocol has the added advantage of using feeder-free hESCs, maintained on MEF-coated plates44, thereby removing potential contamination from MEFs themselves and once again avoiding the use of Matrigel. It works equally well for hESCs maintained on feeders.
Unlike several other protocols, our method of neural conversion does not involve an embryoid body step, where, in the absence of defined conditions (serum is often present) large, often cystic bodies containing all three germ layers are formed, requiring a later, somewhat technically challenging, mechanical selection step for neural rosettes2, 7, 10. This protocol is suspension culture based throughout (until terminal differentiation). In order to avoid the need for daily pipetting, advised in some protocols, we recommend the use of an orbital shaker to prevent adherence to the base of the culture dish10, 35.
A further refinement of our protocol is the use of a McIlwain tissue chopper to passage the NPCs. This generates many small, similarly sized fragments in a short period of time (5-10mins per plate), avoiding the genetic instability associated with enzymatic passaging (although this tendency is also decreased in cultures at low oxygen) and replacing the potentially time consuming use of flame-pulled pasteur pipettes7, 37, 38.
Finally, we demonstrate that directed differentiation towards spinal motor neurons and midbrain dopaminergic neurons is markedly enhanced at 3% compared to 20% O2 31. Whilst this does not appear to have been studied in motor neurons, this effect is entirely consistent with studies on rat, mouse and human mesencephalic neural precursors, reporting enhanced survival and improved generation of dopaminergic neurons at 3% oxygen25-28.
Applications
This protocol enables the generation of human NPCs for in vitro and in vivo experiments, encompassing a wide range of neural developmental studies as well as neurodegenerative disease modelling and cell based therapies. The key feature is the use of a low O2 environment, which provides a novel and more physiologically relevant system. Furthermore, there is broad scope to adapt this method to allow disease modelling, using induced-pluripotent stem cells (iPS) derived from patient specific fibroblasts as the starting material. This will be of particular relevance to neurodegenerative diseases in which ROS are proposed to play a key role, because it will allow investigation of the mechanisms of neural degeneration and testing of potential neuroprotective agents to be undertaken in a more appropriate environment30, 39, 40. We also predict that this protocol will have applications for cell-based therapies, overcoming the oxygen challenge represented by culture at 20% O2 and transplantation into a much lower oxygen environment, which can be as low as <1% for dopaminergic neurons in the midbrain16.
Limitations
The main limitation of this protocol is the requirement for a low oxygen environment in which to undertake the cultures. We have used a low oxygen, triple gas incubator, in which oxygen is displaced by nitrogen, but it should also be possible to use an oxygen depletion flush box or chamber, filled with a pre-made gas mixture with the desired composition of O2, N2 and CO2 and sealed before placing inside a standard incubator41. One advantage of the low oxygen incubator over the oxygen depletion chambers is that it allows room for an orbital shaker, which removes the need for daily pipetting to release spheres that have adhered to the culture vessel10, 35. Both of these low oxygen system can be used in conjunction with an enclosed glove box when removing cells for feeding, passaging and other standard cell culture routines. Alternatively, full ‘hypoxic’ workstations are now available, which maintain a constant gaseous environment throughout, but incur a considerable expense. However, changes in oxygen tension during cell culture routines are not a significant problem when using a low oxygen incubator because, whilst oxygen equilibration in the gaseous phase above the media is complete within 15mins for a 10cm dish (55mins for a T75), it takes around 3hrs for oxygen to dissolve in the media 42. The fact that the cells are unaffected by periodic exposure of the culture vessels to room air is reflected in our observations of enhanced survival of NPCs at 3% O2; downregulation of HIF-1α followed by maintenance of HIF-2α (and no reappearance of HIF-1α); stable electrophysiological properties of neurons removed from the low oxygen environment for several hours of recordings and establishment of long-term differentiated neural cultures in a basic medium of DMEM and 2% B27, without the requirement for supplements such as GDNF, BDNF, IGF, cAMP or ascorbic acid. The possibility of changes in oxygen tension can be further minimised by allowing the media to equilibrate in the low oxygen incubator for several hours prior to feeding43.
Experimental Design
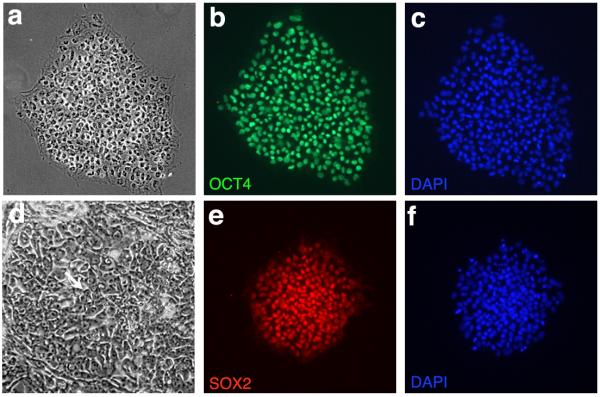
The most important factor determining the efficiency of neural conversion is the quality of the starting material – the hESC colonies. Following thawing, we recommend culturing the cells for at least two passages before attempting the neural induction protocol. Obtaining cells with low passage numbers is less important than the quality of the colonies, which should have well defined edges and an undifferentiated appearance, confirmed by uniform staining for pluripotency markers such as Oct4 and Sox2 (fig 1). Detailed procedures for culturing hESCs are discussed elsewhere44, 45.
Figure One.
hESC Culture: Colonies demonstrate a uniform, undifferentiated appearance with well defined borders (a) and the characteristic double nucleoli present within individual cells (arrow, d). hESCs label with the pluripotency markers OCT4 (b, c) and SOX2 (e,f).
The neural induction process is based on the default model of neural conversion5, through which the removal of pluripotency maintaining factors and maintenance in a defined environment (the absence of BMP signalling), allows the acquisition of a neural fate. The addition of BMP antagonists such as SB431542 (activin inhibitor), which increases the speed of neural conversion at 20% oxygen46, results in smaller numbers of NPCs at low oxygen (Stacpoole and Bilican, unpublished observations), and is therefore not included in the protocol. The method is suspension culture-based throughout (fig 2), with the first step being enzymatic detachment of hESC colonies with liberase (highly purified collagenase I and II plus dispase) which are then propagated as spheres in defined conditions, acquiring a neural identity (98.7±0.5% SOX1, 97.4±0.3% NESTIN and 1.1±0.7% OCT4 positive; fig 3a) over 2 weeks; this timescale is comparable to that seen at 20% O2 7, 33, 35, 46. This contrasts with embryoid body based differentiation protocols, requiring a later mechanical selection of neural rosettes. Equally, neural conversion involving adherent culture also typically requires a selection step for neural rosettes2, 7, 10, 11.
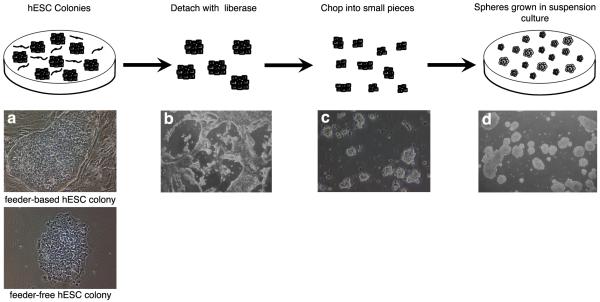
Figure Two.
Procedure for Neural Conversion: Feeder-free or feeder-based hESC colonies (a) are detached by incubation with liberase for 10-30 mins (b). The colonies are chopped into small pieces at 120 μm distances in two directions (c) which then form spheres when grown in suspension culture (d).
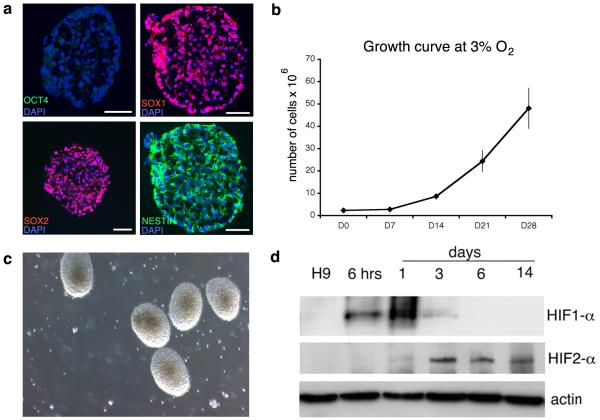
Figure Three.
NPC Generation at 3% O2: After 14 days in culture, NPCs are generated, expressing SOX2, SOX1 (98.7±0.5%) and NESTIN (97.4±0.3%) with loss of OCT4 (1.1±0.7%) (a). NPCs grow rapidly at 3% O2 (b) and, by D14, look healthy, with a phase-bright appearance, well-rounded borders and few dead cells in the background (c). HIF-1α expression is only transiently observed on transfer to the low oxygen environment, whilst HIF-2α appears later but expression persists (d). Scale bar = 50 μm. Figure 3d reproduced from Stacpoole et al, 2011, Cell Death and Differentiation.
By performing neural induction in a low, more physiological oxygen environment, cells are protected from the significant stresses associated with neural conversion, reported in defined conditions at 20% O2 6, 8, 9. Over the first 7-10 days, the number of cells remains fairly static, reflecting adaptation to the low oxygen environment since the ES cultures are maintained at 20% O2; this is supported by the appearance and subsequent disappearance of HIF-1α protein over 6 days (fig 3b, d). Coinciding with the stabilisation of HIF-2α, and following the addition of FGF-2, the spheres grow rapidly and should now appear phase-bright with well-defined edges and few dead cells (fig 3b-d). The original seeding density at 200,000 cells/ ml is important - if it is too low the cells will die, whereas if it is too high, paracrine signalling may interfere with neural conversion. Equally, the initial chopping of the ES colonies into small pieces is required, because larger spheres tend to demonstrate a lower efficiency of neural conversion35. As the spheres grow, it is necessary to break them into smaller ones, so that all cells are accessible to nutrients and growth factors; passaging is performed mechanically to reduce the chance of genetic mutations developing, which is a recognised risk in enzymatically propagated cells and is further decreased by expanding the NPCs at low oxygen12.
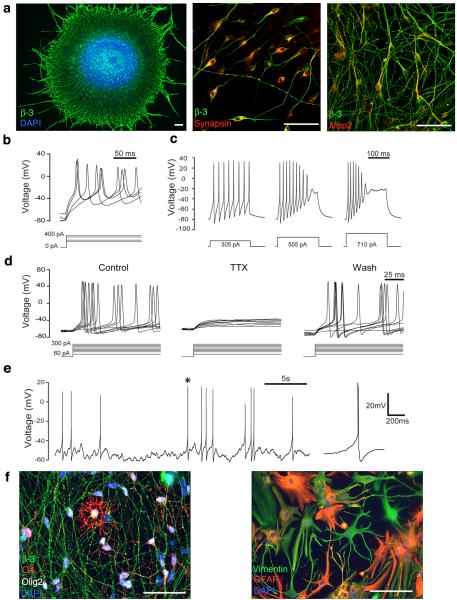
NPCs can be terminally differentiated from D28 onwards (fig 4). Projections emerge from the spheres overnight and express β-III TUBULIN. SYNAPSIN can be detected by 48hrs and the more mature neuronal marker MAP2 by 5 days. Neuronal maturity can be further investigated with electrophysiology (whole cell recordings in current-clamp mode), demonstrating immature appearing action potentials at 10 days, and spontaneous action potentials by 30 days, along with the expected relationship between current injection and action potential frequency that is characteristic of functional neurons47. Similar to early reports of NPC differentiation at 20% O2 35, 48, early NPCs (before D50) differentiated at 3% O2 generate mainly neurons, whereas those plated after longer periods in cultures tend to yield fewer neurons and more astrocytes, which resembles in vivo development where neurogenesis precedes gliogenesis . A few O4 positive oligodendrocytes are also observed at later timepoints (fig 4f).
Figure Four.
Functional Differentiation at 3% O2: β-III TUBULIN positive processes emerge from spheres plated at day 30 for 24 hrs. SYNAPSIN can be detected after 48 hrs of terminal differentiation and MAP2 after 5 days (a). Action potentials can be evoked by current injection, as early as 10 days after plating (b). After 30 days of terminal differentiation, increasing frequency of action potential firing can be observed with increasing current injection, as expected for functional neurons (c), and these are reversibly blocked by the sodium channel blocker TTX (30 days post plating, n=16)(d). Spontaneous action potentials can also be detected, after terminal differentiation for 30 days (e). Asterisk denotes the action potential which is magnified on the right. At later time points, O4 positive oligodendrocytes as well as GFAP and VIMENTIN positive astrocytes are also observed (f). Scale bar = 50 μm. Part of figure 4a reproduced from Stacpoole et al, 2011, Cell Death and Differentiation.
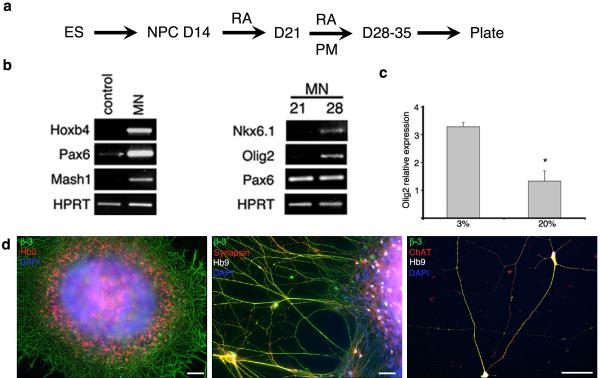
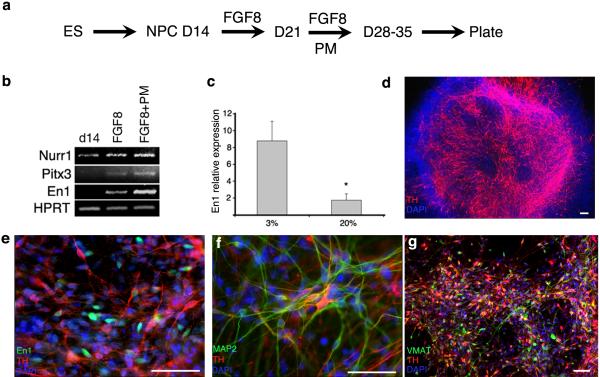
D14 NPCs generated at 3% O2 can be specified into spinal motor neurons or midbrain dopaminergic neurons, by adapting previously published protocols developed at 20% O2 33. Thus, the sequential addition of RA to caudalise the NPCs, followed by the combination of RA and the Shh agonist PM to ventralise them, results in the induction of OLIG2 expressing cells that differentiate into HB9 positive motor neurons, and mature to express ChAT (fig 5). OLIG2 induction is two-fold greater at 3% than 20% O2. Similarly, sequential application of FGF-8 then FGF-8 plus PM, results in the induction of EN1 and TH positive neurons that mature to express MAP2 and VMAT (fig 6). It should be noted that the TH and EN1 antibodies vary across suppliers and we recommend using the EN1 sourced from DSHB and TH from either Chemicon or Pelfreeze. (A full list of primary antibodies used is provided in table 2.) A positive staining control of human embryonic midbrain dopaminergic cells can be sought.
Figure Five.
Spinal Motor Neurons: Sequential application of 0.1 μM RA for 7 days, followed by RA and 1 μM PM for 7-14 days (a) generates spinal motor neuron precursors expressing HOXB4, PAX6, OLIG2 and NKX6.1 (b). OLIG2 induction is 2-fold more efficient than at 20% O2 (c). HB9 and β-III positive motor neurons are present after 48 hrs of terminal differentiation, and mature to express SYNAPSIN and ChAT (d). Scale bar = 50 μm. Figure 5b reproduced from Stacpoole et al, 2011, Cell Death and Differentiation.
Figure Six.
Midbrain Dopaminergic Neurons: Sequential application of 100 ng/ ml FGF-8 for 7 days followed by FGF-8 and 1 μM PM for 7-14 days (a) generates cells which express the midbrain marker EN1 and NURR1 and PITX3, which are required for the development of dopaminergic neurons of the substantia nigra (b). EN1 induction is 5-fold greater than at 20% O2 (c). Large numbers of TH-positive neurons appear after 10days (d), with co-staining of EN1 and TH apparent after 48hrs of terminal differentiation (e). These TH-positive neurons mature to express MAP2 (f) and VMAT (g). Scale bar = 50 μm Figure 6b & c reproduced from Stacpoole et al, 2011, Cell Death and Differentiation.
Table 2.
Primary Antibodies
| Primary Antibody | Isotype | Concentration | Manufacturer |
|---|---|---|---|
| Β-III tubulin | IgG2b | 1 in 750 | Sigma T8660 |
| Chat | Rabbit1 | 1 in 500 | Chemicon AB143 |
| Engrailed-1 | IgG1 | 1 in 25 | DSHB 4G11-S |
| GFAP | Rabbit | 1 in 750 | Dako Z0334 |
| Hb9 (MNR2) | IgG1 | 1 in 200 | DSHB 81.5C10 |
| Map2a+b | IgG1 | 1 in 250 | Sigma M2320 |
| MBP | Rat | 1 in 25 | Abcam AB7349 |
| Musashi | Rabbit | 1 in 500 (cells) | Chemicon AB5977 |
| Nestin | IgG1 | 1 in 500 | Chemicon MAB5326 |
| Oct4 | IgG2b | 1 in 100 (cells) | Santa Cruz SC-5279 |
| O4 | IgM | 1 in 1000 | R&D systems MAB1326 |
| Olig2 | Rabbit | 1 in 500 | Chemicon AB9610 |
| Sox1 | Rabbit | 1 in 500 | Chemicon AB15766 |
| Sox2 | IgG2b | 1 in 250 (cells) 1 in 250 (Flow cytometry) |
Chemicon MAB4343 |
| Synapsin | Rabbit | 1 in 500 | Calbiochem 574777 |
| Tyrosine Hydroxylase | IgG1 | 1 in 500 | Chemicon MAB318 |
| Tyrosine Hydroxylase | Rabbit | 1 in 1000 | Pelfreeze P40101 |
| VMAT | Rabbit | 1 in 500 | Pelfreeze P40601 |
MATERIALS
REAGENTS
Accutase (PAA, cat. no. L11-007)
Activin A (Peprotech, cat. no. 120-14 )
Antibodies (various): please see table 2 for details
B27 supplement 50x (Invitrogen, cat. no. 17504044)
BDNF (Peprotech; 450-02)
Bovine serum albumin (BSA), Fraction V (PAA, cat. no. K51-001) Critical: The quality of the BSA is key to maintaining undifferentiated feeder-free hESCs. New supplies should be batch tested in good time prior to use on all cultures.
Collagenase IV (Gibco, cat.no. 17194-019)
DMEM (Invitrogen, cat. no 41966052)
DMSO (Sigma, cat. no. D-8779) Caution: Handle with care because DMSO readily penetrates the skin.
DPBS (Invitrogen, cat. no. 14190169)
Fibroblast growth factor-2 (FGF-2), recombinant human (Peprotech, cat. no. 100-18B)
Fibroblast growth factor-8 (FGF-8), recombinant mouse (R&D, cat. no. 423-F8-025)
F12 Nutrient Mixture (Ham) liquid with Glutamax (Invitrogen, cat. no. 31765068)
GDNF (Peprotech, cat. no. 450-10)
Heparin (Sigma, cat. no. H-3149)
hESC lines H9 (WiCell Research Institiute, Madison, USA) and Hues-9 (hES facility, Harvard University, USA)
Iscove’s Modified Dulbeccos Medium (IMDM) with L-Glutamine and 25 mM HEPES (Invitrogen, cat. no. 21980065)
Insulin, human recombinant (Roche, cat. no. 11376497001)
Laminin (Sigma, cat. no. L2020)
Liberase DH Research Grade (Roche Diagnostic, cat. no. 05401054001)
Lipid concentrate, chemically defined (Invitrogen, cat. no. 11905)
Monothioglycerol (Sigma, cat. no. M6145) CAUTION:Toxic, irritating to the skin, mutagenic and malodorous; laboratory protective equipment should be worn.
PDL (Sigma, cat. no. P7405)
PFA (Sigma, cat. no. 158127) CAUTION: PFA is toxic if inhaled and can be absorbed through the skin. Use PFA in a fume hood and wear appropriate laboratory protective equipment.
PSF (Invitrogen, cat. no. 15240062)
Purmorphamine (PM) (Calbiochem, cat. no. 540220)
Retinoic Acid (R.A) (Sigma, cat. no. R2625)
Tetrodotoxin (Tocris, cat. no. 1069) CAUTION: TTX is an extremely potent neurotoxin so should be handled with great care; laboratory protective equipment should be worn.
Transferrin; from human serum (Roche, cat. no. 10652202001)
Trypsin-EDTA 0.05% (Invitrogen; cat. no. 25300-054)
Water, sterile (Sigma, W1503)
EQUIPMENT
Conical tubes 15 ml (Cellstar cat. no. 188271)
Conical tubes 50ml (Cellstar cat. no. 210261)
Corning 6 cm culture dishes (Fisher Scientific, cat no TKV-160-034S)
Corning 10 cm culture dishes (Fisher Scientific, cat no TKV-160-049F)
Glass coverslips 13 mm ( VWR, cat. no. 631-0148)
Humidified tissue culture incubator (37 °C, 5% CO2, 20% O2)
Humidified tissue culture incubator (37 °C, 5% CO2, 3% O2)
McIlwain Tissue Chopper (Mickle Engineering, Gomshall, Surrey, U.K.)
Nunc low adherence T75 culture flasks (Fisher Scientific, cat. no. TKT-300-020G)
Nunc 4 well plates (Fisher Scientific, cat. no. TKT-190-130V)
Nunc 24 well plates (Fisher Scientific, cat. no. TKT-190-010Y)
Orbital Shaker (Fisher Scientific, cat. no. SGM-250-030K) with large platform (Fisher Scientific, cat. no. SGM-250-510T)
Sterilin 9 cm petri-dishes (Fisher Scientific PDS-140-050F)
Superfrost plus slides (VWR, cat. no. 631-0108)
Unplugged Pasteur Pipettes 230 mm (Fisher Scientific, cat. no. FB50253)
Vacuum Filter (0.22 μm) Unit 500 ml (Fisher Scientific, cat. no. FDR-120-090W)
Wilkinson sword blades (Scientific Laboratory Supplies, cat. no. BH10)
REAGENT SET UP
Chemically Defined Medium for hESC and NPC culture (500 ml) 44
Measure out 2.5 g BSA and, in a sterile environment, dissolve in 250 ml of IMDM and 250 ml of F12. Add 5 ml lipids (1%), 350 μl insulin (7μg/ ml), 250 μl transferrin (15 μg/ ml), 20 μl monothioglycerol (450 μM) with or without 5 ml PSF (1%). Filter through a 0.22 μm filter and store at 4 °C for up to 10 – 14 days.
Plating Medium for terminal differentiation of NPCs (50 ml)
In a sterile environment, prepare 48.5 ml DMEM, 1 ml B27 (2%) and 0.5 ml PSF (1%). This can be stored at 4 °C for 7-10 days.
Electrophysiology Solutions
Internal solution consists of 130 mM potassium gluconate, 4 mM NaCl, 10 mM HEPES, 10 mM BAPTA, 4 mM MgATP, 0.5 mM Na2GTP, 0.5 mM CaCl2 and 2 mMK-Lucifer yellow (pH adjusted to 7.3 with KOH). This can be stored at −20 °C for up to two months.
External solution contains 144 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 1 mM NaH2PO4, 2.5 mM CaCl2, 10 mM glucose, 2 mM MgCl2, pH set to 7.35 with NaOH. This should be prepared fresh before each recording session.
Activin (10 μg / ml)
Dissolve 10 μg in 1ml PBS with 0.1% BSA. Store at − 80 °C in 50 μl aliquots for up to 6 months. Once thawed, store at 4 °C and use within a week.
Collagenase (50 ml)
Dissolve 50 mg of collagenase IV in 50 ml of CDM to make 1 mg/ ml. This can be warmed at 37 °C to speed up the dissolving process. Filter through a 0.22 μm filter. The solution can be stored at 4 °C for up to 7 days, but the activity is best when freshly prepared.
FGF-2 (20 μg / ml stock)
Dissolve 100 μg of FGF-2 in 5 ml sterile PBS with 0.1% BSA. Store in 100 μl aliquots for up to 6 months at −80 °C; once thawed, store at 4 °C and use within a week.
FGF-8 (25 μg / ml stock)
Reconstitute 25 μg of FGF-8 in 1ml sterile PBS containing 0.1 % BSA. Store in 40 μl aliquots at −80 °C for up to 6 months. Once thawed, aliquots can be kept at 4 °C for a week.
Heparin (5 mg / ml)
Dissolve 5 mg of heparin in 5ml sterile PBS, aliquot and store at −80 °C for up to 6 months.
Insulin (10 mg / ml)
Resuspend the 100 mg lysophilizate in 10 ml sterile water. Store in 1 ml aliquots at −20 °C.
Laminin 10 μg/ ml
In a sterile environment, add 99 ml DMEM to the 1 ml of 1mg/ ml stock and mix thoroughly. Prepare 1-5 ml aliquots and store at −20 °C for up to 6 months.
Liberase
Dissolve 5 mg of liberase in 1 ml of sterile water (5mg/ ml). Store in 100 μl aliquots at −20 °C for up to a month or −80 °C for 6 months. When ready to use, dilute 1 in 40 – 1 in 80 (125 μg/ ml to 62.5 μg/ ml) in CDM.
Critical
Liberase activity varies between batches; therefore the optimal working dilution will need to be redetermined for each batch.
4% PFA (100 ml)
Measure out 4g of PFA into a 100 ml glass bottle. Add 100 ml of 1x PBS. Place on a hot plate at 60 °C and add a magnetic stirrer. Add 1-2 drops of 5 M sodium hydroxide, cover with foil (do not screw on the lid), label and leave for 10-30 mins to dissolve. Use HCL (2 M) to adjust the pH of the solution to between 7.2-7.4. Filter (0.45 μm) into two 50 ml conical tubes and, once cool, store at 4 °C. Use within 7 days.
Caution
PFA is toxic and should be prepared in a fume hood, wearing a lab coat, gloves and eye protection.
Critical
do not allow the solution to boil.
Poly-D-Lysine
In a sterile environment, add 5 ml autoclaved water to 5 mg PDL (1 mg/ ml) and mix thoroughly. Prepare 100-200 μl aliquots and store at −20 °C for up to 6 months. Dilute 1 in 50 with autoclaved water to make a working solution of 20 μg/ ml which can be stored for 7-10 days at 4 °C.
Purmorphamine (10 mM stock)
Dissolve 5 mg of purmorphamine in 960 μl DMSO, mix thoroughly and store in 20 μl aliquots at −20 °C for up to 6 months. Once thawed, use within 7 days.
Caution
Do not expose to light. Wear appropriate laboratory protective equipment including gloves.
Retinoic Acid
(100 mM stock): Dissolve 50 mg of RA in 1.67 ml of DMSO. Store in 50 μl aliquots at −80 °C, in a container protected from the light (eg. cover with tin foil). 1 mM working stock can be stored in the dark at −20 °C for a month: dilute a 100 mM aliquot with 100% ethanol. Once thawed for use, an individual 1 mM aliquot can be stored (in a dark container) at 4 °C for up to a week.
Caution
RA is a powerful morphogen and can be absorbed through the skin, therefore special care should be taken in pregnancy. Gloves should be worn and any spillages cleared up immediately.
Critical
RA activity is destroyed by exposure to the light, so aliquots should be prepared with the lights off in the hood and stored in dark containers.
EQUIPMENT SET UP
Poly-L-ornithine and laminin coated coverslips
First, prepare the coverslips. Place the 13 mm glass coverslips into a glass beaker and cover fully with methanol; leave overnight on a shaker to gently agitate the coverslips. Pour off the methanol and rinse thoroughly with water a number of times, then arrange the coverslips on filter paper in a stack in a glass dish and autoclave; the coverslips can then be stored until required. In a sterile hood, place one coverslip into each well of a 4- or 24-well plate. This can be done using sterile tweezers or with a glass Pasteur pipette attached to suction. Next, cover each coverslip with 300 μl of 20 μg/ ml PDL and check that none of the coverslips are floating. Cover and leave in the hood overnight. Aspirate the PDL and rinse 3 times with sterile water. Leave in the hood with the lid off until completely dry (2-4 hrs). Carefully add 50-60 μl of laminin to the centre of each coverslip and gently transfer to the incubator at 37 °C, for a minimum of 1 hour.
Critical step
be sure to wash the coverslips with sterile water rather than PBS, as the latter forms crystals when dry.
Critical step
do not allow the Laminin to spill off the edge of the coverslip or dry out.
PROCEDURE
Culture of hESCs
-
1
Grow hESCs in feeder-free conditions on 6 cm / 10 cm Corning plates with 3 ml / 6 ml CDM supplemented with 12 ng / ml FGF-2 and 10 ng / ml activin A. Alternatively, grow hESCs on irradiated mouse embryonic fibroblasts, supplementing the CDM with 10 ng / ml FGF-2, 10 ng / ml activin and 10 ng / ml insulin. Exchange media daily.
-
2
Passage, approximately every 5-7 days by incubating with 1 mg / ml collagenase for 5-10 mins. Replace collagenase with CDM and remove colonies by gently scraping with the tip of a 5 ml pipette. Centrifuge at 132g for 3 mins, remove supernatant and resuspend in FGF-2 and activin-supplemented CDM; split 1:5 to 1:10 depending on confluence at time of passaging. Alternatively, passage Hues ES cells as single cells, with 0.05% trypsin-EDTA for 3-5 mins, inactivate with serum and complete passage as above.
Preparation of ES cells for Neural Conversion (Fig 2): Timing 1.5-4 hrs
-
3
Allow the hESC colonies to grow almost to confluence; this is usually a day longer than the point at which they would be passaged.
-
4
Prepare the chopper by sterilising a fresh blade and the stage by flaming with 100% ethanol.
-
5
Aspirate the ES medium and add 2 ml of freshly prepared liberase (62.5 μg/ ml to 125 μg/ ml) to each 10 cm plate; 1 ml for a 6 cm plate. Return to the incubator at 37 °C for 10-30 mins. Review every 5 mins. Critical step: the liberase must be freshly prepared. Troubleshooting?
-
6
Once the colonies begin to float, swirl the plate to aid detachment and transfer contents to a 15 ml conical tube. Combine the colonies from two 10 cm plates or three 6 cm plates into one 15 ml conical tube.
-
7
Tilt the plate and rinse off remaining colonies by forcefully pipetting 2-3 ml CDM once across the plate and add to conical tube. This step can be repeated once or twice until all or almost all of the colonies have been collected. (Sometimes all the colonies come off together as one sheet; simply transfer to the conical tube.) Critical step: Do not pipette the colonies more than once on any one occasion because this breaks them up into single cells which show poor survival on neural conversion.
-
8
The majority of colonies will settle to the bottom of the conical tube by gravity. Allow 3-5 mins for this and aspirate as much of the supernatant (containing the liberase) as possible, leaving around 1 ml. Critical step: be very cautious if considering centrifuging the cells at this point because it causes cell lysis; if necessary, try 0.5g for 2 mins.
-
9
Rinse the cells gently, to remove the liberase, with a further 6-8 ml of CDM per conical tube and allow to settle by gravity. Critical step: it is important not to miss out this step or residual enzymatic activity will remain and the neuralising procedure will fail.
-
10
Remove all but 1 ml of the supernatant and transfer the colonies from one 15 ml tube onto the upturned lid of one of the 6 cm Corning plates, forming a circle 1-2 cm across. Use a rubber bulb and glass Pasteur pipette for this, and transfer as little media as possible. Then remove as much media from the edges of the circle as possible, using a P10. Spread the cells out a little. Critical step: the cells must be as dry as possible prior to chopping, but should not be allowed to dry out.
-
11
Place the colonies on the chopper and chop at 120 μm distances.
-
12
Rotate the plate by 90° and chop a second time.
-
13
Use a P1000 and 1 ml of CDM to transfer the chopped colonies to a 50 ml conical tube. Rinse the plate several times until almost all of the colonies have been transferred.
-
14
Repeat steps 10 - 13 with the settled colonies in the other conical tubes.
-
15
Transfer the cells to low adherence T75s, and place flat in the 3% O2 incubator. As a rough guide, cells from two 10 cm plates or three 6 cm plates can be combined into one T75 containing 10 ml CDM. For greater accuracy, take a proportion of cells (eg. 2 ml of 20 ml chopped colony suspension), dissociate with accutase and perform a cell count; then seed the remaining cells at approximately 200,000 cells / ml of CDM.
Neural Conversion of ES cells (Fig 3)
-
16
Record the day that the neural cultures are set up as day 0.
-
17
Leave the cultures for 48hrs (day 2) and then add 9 ml warm CDM to each flask (equivalent to a 50% media change).
-
18
On day 4, tap the flasks to dislodge any attached spheres and transfer the media and spheres from each flask into a 15 ml conical tube and allow to settle. If the spheres are very small, they can be centrifuged at 1.2g for 1min. Aspirate down to 3-4 ml. Resuspend in 10 ml of CDM and transfer to fresh T75s.
-
19
Add 9 ml warm CDM on day 6.
-
20
On day 8, tilt the T75s and allow the spheres to settle in one corner. Remove most of the medium (taking care not to suction the spheres) and add 8 ml fresh CDM.
-
21
On day 10, settle the spheres in 15 ml conical tubes. Chop at 120 μm in three directions and transfer in 10 ml CDM to 9 cm Sterilin petri dishes on an orbital shaker to prevent aggregation. The orbital shaker should be set to around 40 rotations per minute.
-
22
On day 11 or 12, supplement the medium directly with 20 ng / ml FGF-2 plus heparin 5 μg / ml, to promote proliferation.
-
23
Feed the cells every 2-3 days, by transferring the spheres to a 15 ml conical tube, allowing them to settle by gravity and performing a 70% media change with CDM supplemented with 20 ng/ ml FGF-2 plus heparin 5 μg / ml. NPC cultures can be easily maintained in this way for over 100 days.
-
24
Mechanical passaging should be performed every 7-10 days, when the spheres are large (often appearing darker in the middle). Place the spheres to settle and use a glass Pasteur pipette to transfer to the upturned lid of a 6 cm Corning plate with as little medium as possible. Chop at 120μm in three directions. Split in a 1:2 -1:4 ratio depending on the size of the cell pellet. Critical step: Be sure to use the upturned lid of a 6 cm Corning plate; other plastics have different electrostatic properties, resulting in sub-optimal passaging. Troubleshooting?
-
25
Sphere samples can be taken at any stage for sectioning and immunocytochemistry to characterise the cells and establish the efficiency of neural conversion (box 1 and fig 3). Troubleshooting?
-
26
NPCs can be frozen, in serum-free conditions, at any stage after day 14, and thawed when required (see box 2).
Box 1: SECTIONING SPHERES FOR IMMUNOSTAINING.
First, transfer the spheres to a 15 ml conical tube and spin at 8.4g for 2mins. The bigger the pellet, the easier it will be to find them in the sections later; typically 1/3 to ½ of the contents of a 10 cm plate is required.
Remove the medium and resuspend in 4 ml of 4% PFA. Fix at room temperature for 45 mins.
Rinse 3 times with PBS, allowing the spheres to settle between each rinse.
Remove the PBS and cryoprotect by replacing with 30% sucrose for at least 24 hours (until the spheres have sunk). Spheres can be stored in sucrose for up to 2 weeks.
Prepare a foil container, about 2 cm deep with a flat base around 0.5 cm2.
Remove the sucrose and gently suspend the spheres in 400 μl OCT and transfer to the container, taking care not to form air bubbles.
Freeze on dry ice.
Remove foil and section on a cryostat at 12-14 μm intervals onto charged, superfrost plus slides.
Store at −20 °C until ready to stain. Troubleshooting?
Box 2: FREEZING NPCs.
First, transfer the spheres to a 15 ml conical tube and spin at 8.4g for 2 mins.
Remove the medium and resuspend in 1ml of pre-cooled (4 °C) serum-free freezing medium consisting of CDM and 10% DMSO.
Transfer to a cryovial and place in a freezing pot, precooled at −20 °C.
Place at −80 °C for between 24 and 48hrs.
Store in liquid nitrogen. Frozen cells can be stored in this way for several years.
When required, thaw rapidly in a waterbath at 37 °C and add to 9 ml CDM.
Centrifuge at 132g for 2 mins and remove all the supernatant (containing DMSO).
Resuspend in 10 ml fresh CDM with 20 ng/ ml FGF-2 plus 5 μg/ml heparin, transfer to a 9 cm sterilin petri-dish and place on the shaker in the low oxygen incubator.
Exchange medium the following day to remove any dead cells. Then feed every 2-3 days and passage mechanically as required. Troubleshooting?
Terminal Differentiation of NPCs (Fig 4)
-
27
Plate spheres for neural differentiation from D28 onwards. Aspirate the laminin from the coverslips (see equipment set up) and place 1-2 spheres in 40 μl plating medium in the centre of each coverslip. Replace in the incubator and after 60-90 mins add 500 μl plating medium down the side of the wells, taking care not to disturb the spheres. Troubleshooting?
-
28
Alternatively, spheres can be dissociated into single cells by incubation with 1 ml accutase for 5-10 mins followed by gentle trituration with a P1000. Resuspend in plating medium and count the cells. Plate between 40,000 (low density) and 100,000 in 30 μl of plating medium. Add a further 500 μl after 30-40 mins, once the cells have visibly adhered to the coverslips.
-
29
Feed every 2-3 days by removing 200 μl and adding 300 μl of warm plating medium, which can be supplemented with 10 ng/ ml BDNF +/- 10 ng/ ml GDNF. For high density cultures, up to 1 ml medium may be required, with daily exchanges.
-
30
Differentiating cultures can be maintained for many weeks. Cells can be fixed at any stage by replacing plating medium with 300 μl PFA at room temperature for 10−20mins, followed by 3 rinses with PBS. Plates can be stored at 4 °C in PBS containing 0.1% sodium azide (to prevent microbial growth) prior to immunostaining.
Neuronal Electrophysiology (Fig 4)
-
31
Neurons can be patched at any stage after plating, but optimal recordings are obtained after around 30 days of differentiation.
-
32
Prepare internal and external solutions (see reagent set up) and glass micro-electrodes with 3–6 MΩ resistance, in order to record from neurons in whole-cell current-clamp mode.
-
33
A detailed description of the procedures involved in performing whole-cell current clamp recordings from neurons is provided elsewhere49, 50.
Directed Differentiation – Spinal Motor Neurons (Fig 5)
-
34
Follow steps 1 – 22 as above, and on day 14, settle the spheres, suction off the medium and resuspend in 10 ml of warm CDM (without FGF-2). Turn off the lights to the hood, add 1 μl of 1 mM RA (0.1 μM) directly into the plate and replace on the orbital shaker. Critical step: RA is unstable in the light, so light exposure should be minimised.
-
35
Perform a 70% media change every 2-3 days, adding 1 μl of 1 mM RA directly into the 10 ml CDM in the plate.
-
36
On day 21, supplement with 1 μl of 10 mM PM (1 μM) as well as 1 μl of 1 mM RA. Critical step: use a well calibrated P2 pipette because the optimal concentration range of PM is relatively narrow.
-
37
Perform a 70% media change every 2-3 days, adding 1 μl of 1 mM RA and 1 μl of 10 mM PM directly to the plate.
-
38
On day 24, mechanically passage the spheres, as described in point 24 above. Critical step: ideally, spheres should be mechanically passaged 3-5 days prior to plating for differentiation, so that they are reasonably small when plated as whole spheres, to allow room for proliferation following plating.
-
39
Plate spheres for differentiation at any point between D28 and D35, as described in points 27-30 above.
Directed Differentiation – Midbrain Dopaminergic Neurons (Fig 6)
-
40
Follow steps 1 – 22 as above and on day 14, settle the spheres, suction off the medium and resuspend in 10 ml of warm CDM (without FGF-2). Add 40 μl of 25 μg / ml FGF-8 stock (i.e. 100 ng / ml) directly to the plate and replace on the orbital shaker.
-
41
Perform a 70% media change every 2-3 days, adding 40 μl of 25 μg / ml FGF-8 directly to the plate on each occasion.
-
42
On day 21, supplement with 1 μl of 10 mM PM (1 μM) as well as 40 μl of 25 μg / ml FGF-8.
-
43
Perform a 70% media change every 2-3 days, adding 1 μl of 10 mM PM and 40 μl of 25 μg/ ml FGF-8 directly to the plate.
-
44
On day 24, mechanically passage the spheres, as described in point 24 above. Critical step: ideally, spheres should be mechanically passaged 3-5 days prior to plating for differentiation, so that they are reasonably small when plated as whole spheres, to allow room for proliferation following plating.
-
45
Plate cells for differentiation at any point between D28 and D35, as described in points 27-30 above.
TIMING
Preparation of ES cells for neural conversion: 1.5-4hrs; steps 3-15
Neural conversion of ESCs: 14 days; steps 16-26
Directed differentiation towards spinal motor neurons or midbrain dopaminergic neurons: 14 - 21 days plus 2-30 days terminal differentiation; steps 34-45
Terminal Differentiation of NPCs: 2 to 90 days; steps 27-30
TROUBLESHOOTING
Please refer to table 1.
Table 1.
Troubleshooting
| Step | Problem | Potential Explanation | Solution |
|---|---|---|---|
| 5 | Colonies do not detach within 30 mins |
Insufficient liberase activity; enzyme activity varies between batches so the recommended 1 in 60 working dilution may need to be adjusted. |
Increase the concentration and ensure there is adequate volume to cover the base of the whole culture dish |
| 5 | Colonies detach within a few minutes and strings of lysed material appear to which the spheres attach |
Excessive liberase activity |
Decrease the working concentration of liberase and be sure to rinse with CDM prior to chopping |
| 24 | Large spheres are still present after mechanical passaging |
Insufficient medium removed or chopper incorrectly set up |
Remove as much medium as possible when preparing for passaging, and ensure that the blade force is adjusted so that it marks the plate when chopping |
| 25 | Decreased efficiency of neural conversion as assessed by Sox1, Nestin expression of sectioned spheres |
Poor starting material i.e. hESC cultures contain differentiated colonies |
Hand select any differentiated colonies prior to step 3 and remove with a pipette tip |
| 27 | Spheres do not attach to the coverslips |
The original 40 μl spills off the coverslip on transfer into the incubator |
Try not to allow the original 40 μl to spill off the edge of the coverslip, but if it does, add 500 μl plating medium immediately and leave undisturbed for 48-72 hours |
| Box 1 | Unable to locate spheres following sectioning |
Too few spheres in too large a volume of OCT |
Resuspend the sphere sample in as small a volume of OCT as practical. It helps to cut the end off the P1000 tip due to the viscosity of OCT |
| Box 2 | Low yield of NPCs following thawing |
Too rapid temperature change on freezing, or too slow on thawing |
Take care to freeze gradually, via −20 °C, then −80 °C then liquid nitrogen. Thaw rapidly – it should take no longer than 5mins from collecting the vial to full thaw |
ANTICIPATED RESULTS
Neural conversion of ES cells into NPCs should be achieved by day 14, as exemplified by loss of OCT4, maintenance of SOX2 and upregulation of NESTIN and SOX1 (98.7±0.5% SOX1, 97.4±0.3% NESTIN and 1.1±0.7% OCT4 positive; fig 3). Differentiation of day 30 NPCs should yield neurons expressing β-III TUBUIN and maturing to express SYNAPSIN and MAP2 that, after 30 days of differentiation, demonstrate electophysiological properties of functional neurons (fig 4).
Regional specification of day14 NPCs with RA should induce expression of caudal genes including HOXB4, and subsequent application of Shh agonists should direct these spinal NPCs towards motor neuron precursors, expressing OLIG2 and NKX6.1. Terminal differentiation should generate HB9 and β-III TUBULIN positive motor neurons by 48hrs, and by 10 days, ChAT will start to be expressed (fig 5).
Directed differentiation of day 14 NPCs by sequential application of FGF-8 and Shh agonist should induce expression of the midbrain dopaminergic genes NURR1, PITX3 and EN1. Terminal differentiation should generate EN1 and TH co-positive neurons after 2-10 days, with the number of TH positive neurons increasing with time in culture. TH-positive neurons mature to express MAP2 and VMAT (fig 6).
Acknowledgements
We are grateful to Ludovic Vallier for kind provision of feeder-free ES cells, Morgan Alexander for valuable technical assistance and Roger Barker for the use of the low oxygen incubator. This work was supported by the MS Society UK, Evelyn Trust, MRC, Wellcome Trust (AL) and Royal Society (RK). SS is supported by a Sir David Walker Fellowship, a joint Medical Research Council and Multiple Sclerosis Society Clinical Research Training Fellowship (no. G0800487) and a Raymond and Beverly Sackler Studentship.
Footnotes
These authors contributed equally to this work
Competing Financial Interests:
The authors declare that they have no competing financial interests.
References
- 1.Zhao T, et al. Hypoxia-driven proliferation of embryonic neural stem/progenitor cells--role of hypoxia-inducible transcription factor-1alpha. FEBS J. 2008;275:1824–1834. doi: 10.1111/j.1742-4658.2008.06340.x. [DOI] [PubMed] [Google Scholar]
- 2.Erceg S, Ronaghi M, Stojkovic M. Human embryonic stem cell differentiation toward regional specific neural precursors. Stem Cells. 2009;27:78–87. doi: 10.1634/stemcells.2008-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson’s disease. J. Intern. Med. 2009;266:358–371. doi: 10.1111/j.1365-2796.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 4.Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat. Rev. Neurosci. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- 5.Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- 6.Smukler SR, Runciman SB, Xu S, van der KD. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J. Cell Biol. 2006;172:79–90. doi: 10.1083/jcb.200508085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cimadamore F, et al. Nicotinamide rescues human embryonic stem cell-derived neuroectoderm from parthanatic cell death. Stem Cells. 2009;27:1772–1781. doi: 10.1002/stem.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke L, van der KD. Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem Cells. 2009;27:1879–1886. doi: 10.1002/stem.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho MS, Hwang DY, Kim DW. Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat. Protoc. 2008;3:1888–1894. doi: 10.1038/nprot.2008.188. [DOI] [PubMed] [Google Scholar]
- 11.Perrier AL, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li TS, Marban E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178–1185. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly CM, et al. Neonatal desensitization allows long-term survival of neural xenotransplants without immunosuppression. Nat. Methods. 2009;6:271–273. doi: 10.1038/nmeth.1308. [DOI] [PubMed] [Google Scholar]
- 14.Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 15.Csete M. Oxygen in the cultivation of stem cells. Ann. N. Y. Acad. Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 16.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol. Cell Neurosci. 2007;35:424–435. doi: 10.1016/j.mcn.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Chen HL, et al. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- 19.Covello KL, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafsson MV, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2009 doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Morrison SJ, et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Studer L, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J. Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milosevic J, et al. Low atmospheric oxygen avoids maturation, senescence and cell death of murine mesencephalic neural precursors. J. Neurochem. 2005;92:718–729. doi: 10.1111/j.1471-4159.2004.02893.x. [DOI] [PubMed] [Google Scholar]
- 27.Storch A, et al. Long-term proliferation and dopaminergic differentiation of human mesencephalic neural precursor cells. Exp. Neurol. 2001;170:317–325. doi: 10.1006/exnr.2001.7706. [DOI] [PubMed] [Google Scholar]
- 28.Maciaczyk J, Singec I, Maciaczyk D, Nikkhah G. Combined use of BDNF, ascorbic acid, low oxygen, and prolonged differentiation time generates tyrosine hydroxylase-expressing neurons after long-term in vitro expansion of human fetal midbrain precursor cells. Exp. Neurol. 2008;213:354–362. doi: 10.1016/j.expneurol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Akundi RS, Rivkees SA. Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells. PLoS. One. 2009;4:e4739. doi: 10.1371/journal.pone.0004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, Marks JD, Schumacker PT, Young RM, Brorson JR. Physiological hypoxia promotes survival of cultured cortical neurons. Eur. J. Neurosci. 2005;22:1319–1326. doi: 10.1111/j.1460-9568.2005.04335.x. [DOI] [PubMed] [Google Scholar]
- 31.Stacpoole SR, et al. Derivation of neural precursor cells from human ES cells at 3% O(2) is efficient, enhances survival and presents no barrier to regional specification and functional differentiation. Cell Death. Differ. 2011 doi: 10.1038/cdd.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiq A, et al. Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB-independent pathways. J. Neurosci. 2009;29:8828–8838. doi: 10.1523/JNEUROSCI.1779-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Y, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XJ, et al. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joannides AJ, et al. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells. 2007;25:731–737. doi: 10.1634/stemcells.2006-0562. [DOI] [PubMed] [Google Scholar]
- 36.Li TS, Marban E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178–1185. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho MS, et al. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svendsen CN, et al. A new method for the rapid and long term growth of human neural precursor cells. J. Neurosci. Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 39.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol. Sci. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 40.Behl C, Moosmann B. Oxidative nerve cell death in Alzheimer’s disease and stroke: antioxidants as neuroprotective compounds. Biol. Chem. 2002;383:521–536. doi: 10.1515/BC.2002.053. [DOI] [PubMed] [Google Scholar]
- 41.Wright WE, Shay JW. Inexpensive low-oxygen incubators. Nat. Protoc. 2006;1:2088–2090. doi: 10.1038/nprot.2006.374. [DOI] [PubMed] [Google Scholar]
- 42.Allen CB, Schneider BK, White CW. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am. J. Physiol Lung Cell Mol. Physiol. 2001;281:L1021–L1027. doi: 10.1152/ajplung.2001.281.4.L1021. [DOI] [PubMed] [Google Scholar]
- 43.Wion D, Christen T, Barbier EL, Coles JA. PO(2) matters in stem cell culture. Cell Stem Cell. 2009;5:242–243. doi: 10.1016/j.stem.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Vallier L. Serum-free and feeder-free culture conditions for human embryonic stem cells. Methods Mol. Biol. 2011;690:57–66. doi: 10.1007/978-1-60761-962-8_3. [DOI] [PubMed] [Google Scholar]
- 45.Hartung O, Huo H, Daley GQ, Schlaeger TM. Clump passaging and expansion of human embryonic and induced pluripotent stem cells on mouse embryonic fibroblast feeder cells. Curr. Protoc. Stem Cell Biol. 2010 doi: 10.1002/9780470151808.sc01c10s14. Chapter 1, Unit. [DOI] [PubMed] [Google Scholar]
- 46.Patani R, et al. Activin/Nodal inhibition alone accelerates highly efficient neural conversion from human embryonic stem cells and imposes a caudal positional identity. PLoS. One. 2009;4:e7327. doi: 10.1371/journal.pone.0007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moe MC, et al. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain. 2005;128:2189–2199. doi: 10.1093/brain/awh574. [DOI] [PubMed] [Google Scholar]
- 48.Bouhon IA, Joannides A, Kato H, Chandran S, Allen ND. Embryonic stem cell-derived neural progenitors display temporal restriction to neural patterning. Stem Cells. 2006;24:1908–1913. doi: 10.1634/stemcells.2006-0031. [DOI] [PubMed] [Google Scholar]
- 49.Cummins TR, Rush AM, Estacion M, Dib-Hajj SD, Waxman SG. Voltage-clamp and current-clamp recordings from mammalian DRG neurons. Nat. Protoc. 2009;4:1103–1112. doi: 10.1038/nprot.2009.91. [DOI] [PubMed] [Google Scholar]
- 50.Molleman A. Patch clamping: An introductory guide to patch clamp electrophysiology. John Wiley; West Sussex, UK: 2003. [Google Scholar]