Abstract
The present study examined risk factors for depression during pregnancy in a very large population sample. Two research questions have been addressed: first, the association between demographic factors and past negative obstetrical outcomes on depression severity scores, and second, the differences in these factors between women recruited at a university medical center and maternal health centers (MHC). The study included more than 5,000 pregnant women attending regular appointments at the University Obstetrics and Gynecology Clinic or at several MHCs in Eastern Iowa. Participants completed a Beck depression inventory (BDI) and a demographic questionnaire. We performed a statistical analysis on the association between risk factors and depression severity scores. Regression analysis revealed that week of pregnancy, site of recruitment, years of education, income, marital status, employment, and number of miscarriages and stillbirths were significant predictors of total BDI score. Compared to their university counterparts, participants at MHCs had more depressive symptoms, were younger, mostly single, and had lower socioeconomic status and more past negative obstetrical outcomes. Our study can inform providers about some of the risk factors during depression screening in pregnancy to increase diagnostic vigilance and tailor the level of prenatal care accordingly.
Keywords: Depression, Pregnancy, Antenatal, Risk factors
Introduction
Recent data strongly suggest that depression during pregnancy is common and may be of greater clinical impact than postpartum depression. The prevalence of depression during pregnancy is relatively high with estimates between 7% and 13% (Bennett et al. 2004; Johanson et al. 2000). Moreover, there is solid evidence that depression during pregnancy can lead to negative obstetrical and neonatal outcomes (Marcus 2009; Bonari et al. 2004; Rahman et al. 2004; Orr and Miller 1995; Zuckerman et al. 1989); it has been hypothesized that the stress of depression in utero may also impact fetal development directly in addition to its known effect on diminishing perinatal obstetrical care (Field et al. 2002).
Despite the clinical recognition that antenatal depression is common (Evans et al. 2001), research literature on depression during pregnancy is limited, and most epidemiologic studies have relatively small samples. Though two decades previously O’Hara (1986) had suggested that factors associated with depression in the antenatal period differ from predictors of depression postpartum, only a few studies to date have examined the risk factors for antenatal depression. In an earlier study of 360 pregnant women, Gotlib et al. (1989) reported that depressed pregnant women had a significantly younger age, lower education, and higher number of children (all p values <0.05) than their non-depressed counterparts. A more recent study of similar sample size (Leigh and Milgrom 2008) concluded that poor social support (p<0.001), negative cognitive style (p<0.001), major life events (p=0.01), and low income (p=0.04) were significant predictors of depression in women in the second trimester of pregnancy. In a sample of about 1,600 pregnant women, Rich-Edwards et al. (2006) suggested that the strongest predictors of antenatal depressive symptoms were history of depression (odds ratio (OR)=4.07) and young maternal age (OR=2.71), the latter of which they attributed largely to association with financial hardship, unwanted pregnancy, and lack of a partner.
Recently, Bunevicius et al. (2009) prospectively examined the prevalence of depressive disorders by trimester in a sample of 230 pregnant women in Lithuania. The study indicated that factors like unwanted and unplanned pregnancy (OR=6.07–15.35) and high neuroticism (OR=3.89–7.73) were independent determinants of antenatal depressive disorders throughout the entire pregnancy; however, others, like previous history of depression (first trimester OR=6.28), low education (first trimester OR=3.68), and psychosocial stressors (third trimester OR=5.23), were trimester specific.
A new review article examined 57 studies that included information on the association between antenatal depressive symptoms and risk factors (Lancaster et al. 2010). The authors summarized that some of the most important risk factors, indicated by the literature, are life stress, history of depression, lack of social support, unintended pregnancy, Medicaid insurance, domestic violence, lower income, lower education, smoking, and single status.
Most studies on risk factors for antenatal depression have focused on depression as a diagnostic category and have not examined depressive symptoms on a continuum. Notably, depressive symptoms are commonly reported by pregnant women both with and without clinical depression (Klein and Essex 1995). Hence, it is critical to know what risk factors are predictive of the range of depressive symptoms in pregnancy; these data should also inform screening for depression in pregnancy, particularly collecting demographic or historical information which would enhance the detection of risk.
While demographic risk factors have been specifically examined, minimal research has addressed the role of adverse obstetrical experiences on vulnerability for depression in subsequent pregnancies (Hughes et al. 1999; Bernazzani and Bifulco 2003).
The present study involves a very large population sample of more than 5,000 pregnant women screened for depression at two distinct types of sites—an academic medical clinic (the Obstetrics and Gynecology Outpatient Clinic at the University of Iowa) and several maternal health centers (MHC) in Iowa. The university clinic serves a wide range of women in private practice and training settings as well as serving women with high-risk pregnancies. In contrast, the State of Iowa MHCs serve women and children who have no other access to care, i.e., those that have no insurance.
We addressed the following in this large group of women: First, we examined the association between demographic and obstetrical factors and depression severity scores. Second, we studied the differences in demographic and obstetrical factors between women screened at the University Medical Center and those screened at the MHCs.
In addition to the large sample size and the comparison of distinct demographic samples, a unique contribution of our study is the inclusion of past obstetrical outcomes as potential predictors for depressive symptoms in pregnancy. The factors included in this category were the number of full-term births, premature births, stillbirths, miscarriages, and abortions.
Methods
Participants and procedure
The participants were 5,404 women between 4 and 41 weeks of pregnancy recruited from a university hospital ob-gyn clinic (N=4,077) or one of six maternal health care centers in Eastern Iowa (N=1,327). The participants’ ages were 18–47 [mean=27.7, standard deviation (SD)=5.7]. The mean number of years of education was 14.5 (SD=2.9). The majority of the sample was employed (62%), married or living with a romantic partner (77%), and self-identified as Caucasian (83%). Almost half (48%) the participants reported annual family incomes of US$30,000 or less and about a quarter (24%) had relatively higher incomes (US$60,000 or more).
Pregnant women attending regular appointments at the clinics were approached and asked to participate in the study. Data were collected between January 2000 and December 2004. After completing a signed consent approved by the University of Iowa IRB, the women completed the Beck depression inventory (BDI) (Beck et al. 1961) and a demographic questionnaire. Data are not available regarding the number or characteristics of women invited to participate in the study who chose not to do so.
The BDI is a commonly used 21-item self-report inventory used to measure severity of depression. Each question consists of four statements describing increasing intensities of symptoms of depression. Items are rated on a scale of 0–3, reflecting how participants have felt over the last week. Total scores range from 0 to 48 with higher scores reflecting more severe depressive symptomatology. Cronbach’s alpha in the current sample was 0.89.
Demographic information included age, ethnicity, years of education, marital status, employment, income, and a number of items regarding obstetrical history (Tables 1, 2).
Table 1.
Characteristics of participants and comparison between university and MHCs for interval variables
| Descriptive factor (interval variables) | Mean (SD) | MHC vs. University (F) |
Significance (p value) |
|
|---|---|---|---|---|
| Age | MHC | 23.69 (4.58) | 963.857 | <0.001 |
| University | 28.99 (5.55) | |||
| Week of pregnancy | MHC | 14.38 (5.72) | 260.031 | <0.001 |
| University | 17.36 (5.87) | |||
| Years education | MHC | 12.28 (1.62) | 1,323.822 | <0.001 |
| University | 15.26 (2.87) | |||
| Years education | MHC | 1.98 (1.10) | 1,285.465 | <0.001 |
| University | 4.73 (2.42) | |||
| Number of children living in home | MHC | 0.93 (1.14) | 7.336 | 0.007 |
| University | 0.84 (1.00) | |||
| Number of previous pregnancies | MHC | 2.08 (1.41) | 4.045 | 0.044 |
| University | 2.21 (1.63) | |||
| Number of full-term births | MHC | 1.21 (1.03) | 12.188 | <0.001 |
| University | 1.07 (1.01) | |||
| Number of premature births <36 weeks | MHC | 0.10 (0.33) | 29.357 | <0.001 |
| University | 0.20 (0.53) | |||
| Number of stillbirths | MHC | 0.02 (0.16) | 5.130 | 0.024 |
| University | 0.04 (0.23) | |||
| Number of miscarriages | MHC | 0.54 (0.87) | 8.740 | 0.003 |
| University | 0.66 (1.13) | |||
| Number of abortions | MHC | 0.22 (0.53) | 3.311 | 0.069 |
| University | 0.26 (0.63) |
Table 2.
Characteristics of participants and comparison between university and MHCs for categorical variables
| Descriptive factor (categorical variables) |
University cases (%) |
MHC cases (%) |
University vs. MHC (Pearson’s Chi-square) |
Significance (p value) |
|
|---|---|---|---|---|---|
| Marital status | Single | 9.6 | 38.4 | 1,077.311 | <0.001 |
| Married | 76.6 | 28.2 | |||
| Widowed | 0.1 | 0.3 | |||
| Separated | 1.3 | 4.8 | |||
| Divorced | 2.9 | 5.5 | |||
| Cohabitating | 9.1 | 22 | |||
| Other | 0.4 | 0.8 | |||
| Living with partner | 89.7 | 59.0 | 635.204 | <0.001 | |
| Ethnicity | Am. Indian | 1.1 | 1.5 | 83.648 | <0.001 |
| Asian or Pacific Islander | 5.4 | 1.2 | |||
| African American | 3.9 | 6.2 | |||
| Hispanic | 4.2 | 8.2 | |||
| Caucasian (not Hispanic) | 83.6 | 81.2 | |||
| Other | 1.8 | 1.7 | |||
| Employed | 65.9 | 46.1 | 165.052 | <0.001 | |
| Head of household | Self | 20.2 | 54.2 | 6,888.2 | <0.001 |
| Partner | 50.8 | 29.7 | |||
| Parents | 2.0 | 7.5 | |||
| Both self and partner | 26.2 | 4.2 | |||
| Other | 0.8 | 4.4 | |||
| Income<20,000 | 23.6 | 74.2 | 904.5 | <0.001 | |
| First time pregnant | 32.1 | 34.5 | 2.496 | 0.114 | |
Statistics
First, we performed correlation and analysis of variance (ANOVA) on the demographic and obstetrical variables in relation to BDI total scores. Factors that emerged as significant were included in a subsequent regression analysis. Second, we compared participants recruited at the university and MHC by the same factors.
Results
BDI scores
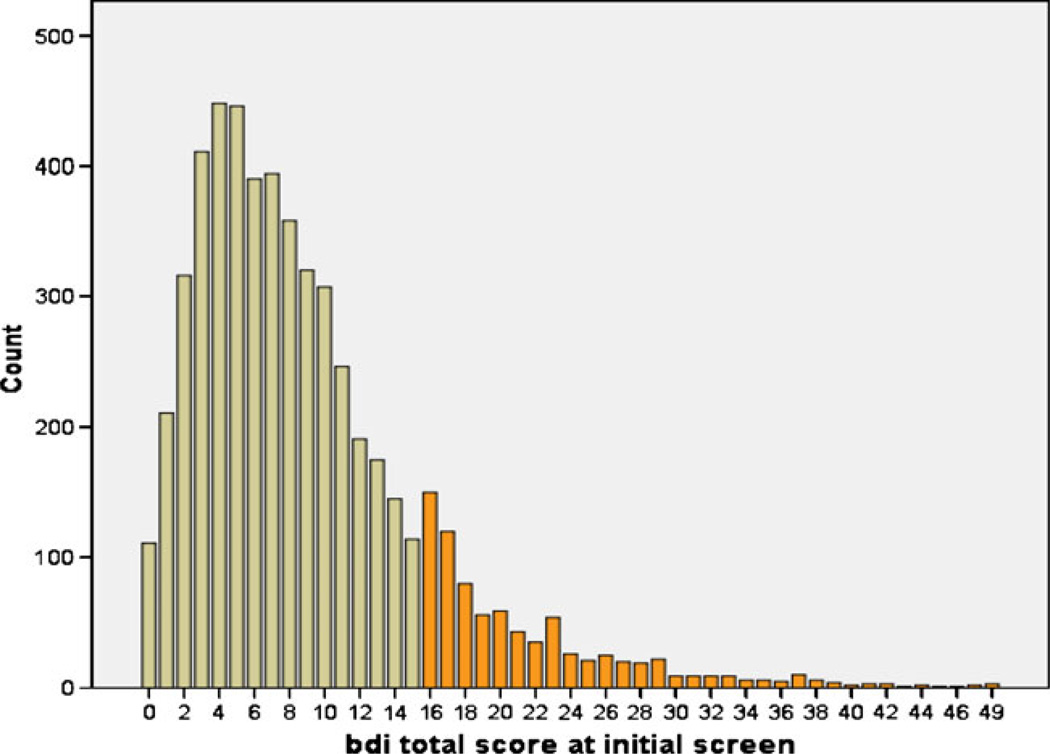
The mean total BDI score for the whole sample was 9.04 (SD=6.88; Min=0; Max=49). The distribution of total BDI scores (see Fig. 1) shows a normal distribution of scores below 16 and a separate descending trend for scores above 16. The use of a score of 16 has been accepted as the threshold for clinical depression in pregnancy by several authors (Salamero et al. 1994; Holcomb et al. 1996). Patients evaluated at the university OB clinic had significantly lower mean total BDI scores compared to patients screened at community MHCs (University BDI mean=7.99; MHC mean=12.27; p=0.000).
Fig. 1.
Distribution of BDI scores in the sample (count is equal to the number of cases). Darker color indicates BDI scores >16 (above threshold for clinical depression)
Analysis of associations between demographic factors and total BDI score
Correlation analysis was performed for interval variables in the whole sample (see Table 3). Given the large number of cases in the sample, it was not surprising that many of the factors were significantly correlated with total BDI scores. Those factors included age, week of pregnancy, years of education, income, number of children, and number of previous pregnancies, miscarriages, premature births, and stillbirths. The only factors that were not significantly correlated with total BDI score were number of full-term births and number of abortions. Most correlations were weak (r<0.3) except for income, which showed a modest negative correlation with BDI score (r=−0.318).
Table 3.
Correlations between BDI total score and risk factors (interval variables)
| Risk factor (interval variables) |
BDI total score (Spearman correlation) |
Significance (two-tailed) p value |
|---|---|---|
| Age | −0.206 | <0.001 |
| Week of pregnancy | −0.084 | <0.001 |
| Years of education | −0.271 | <0.001 |
| Income | −0.317 | <0.001 |
| Number of children living in home | 0.030 | 0.028 |
| Number of previous pregnancies | 0.080 | <0.001 |
| Number of full-term births | 0.011 | 0.528 |
| Number of premature births <36 weeks | 0.038 | 0.024 |
| Number of stillbirths | 0.043 | 0.010 |
| Number of miscarriages | 0.057 | 0.001 |
| Number of abortions | 0.014 | 0.392 |
ANOVA analysis of differences between groups defined by risk factor was conducted on categorical variables (predictors) with total BDI score as dependent variable for the whole sample (Table 4). As in the correlation analysis above, the majority of factors examined were found to be significantly associated with total BDI score. Those factors included marital status, ethnicity, employment, head of household, income <20,000, and previous pregnant status. BDI scores did not differ in participants whether they were living with a partner or not (p=0.16). BDI scores were the highest in widowed women followed by separated, single, divorced, cohabitating, and married, all being significantly different between each other, except for the contrast between single and divorced. With regard to ethnicity, BDI scores were highest in American Indian followed by African American, Hispanic, white, and Asian Americans with the only significant difference noticed between African American and Hispanic and white and Asian Americans; however, caution should be used when interpreting these results since many ethnic groups were insufficiently represented.
Table 4.
ANOVA analysis of differences in BDI total scores between groups defined by risk factor (categorical variables)
| Risk factor (categorical variables) | Type III sum of squares |
df | Mean square | F | Significance p value |
|---|---|---|---|---|---|
| Marital status | 1,456.612 | 5 | 291.322 | 7.546 | <0.001 |
| Living with partner | 77.187 | 1 | 77.187 | 1.999 | 0.157 |
| Ethnicity | 649.128 | 4 | 162.282 | 4.204 | 0.002 |
| Employment | 1,249.653 | 1 | 1,249.653 | 32.370 | <0.001 |
| Head of household | 786.367 | 4 | 196.592 | 5.092 | <0.001 |
| Income<20,000 | 624.579 | 1 | 624.579 | 16.178 | <0.001 |
| Previously pregnant | 342.246 | 1 | 342.246 | 8.865 | 0.003 |
Regression analysis of risk factors and total BDI score
A linear regression analysis was performed, including the demographic factors significant in the preliminary analysis (above) and total BDI score as dependent variable. Categorical variables were re-coded for linear regression. Collinearity diagnostics did not reveal significant collinearity problems among the factor variables (VIF<2.5). The whole regression model explained only 15.31% of the variance in total BDI scores (R square, 0.153) and the whole model was statistically significant (F=42.443; p=0.000). Several factors remained significant independent predictors of BDI score in this analysis (see Table 5): week of pregnancy, years of education, income, recruitment at MHC site, being married or living with a partner, employment status, and number of miscarriages and stillbirths. The other variables did not reach statistical significance in this regression model.
Table 5.
Linear regression analysis of risk factors (regression model values: R square=0.153; adjusted R square=0.149, standard error=6.393; F=42.443; p=0.000)
| Predictor | B | Standard error |
Beta | t | Significance (p value) |
|---|---|---|---|---|---|
| Age | −0.047 | 0.028 | −0.039 | −1.707 | 0.088 |
| Week of pregnancy | −0.074 | 0.021 | −0.063 | −3.588 | <0.001 |
| Years of education | −0.168 | 0.059 | −0.069 | −2.830 | 0.005 |
| Income | −0.405 | 0.073 | −0.143 | −5.561 | <0.001 |
| Number of children living in home | 0.023 | 0.128 | 0.003 | 0.179 | 0.858 |
| MHCa | 0.054 | 0.009 | 0.115 | 5.820 | <0.001 |
| Married or living with partnera | −1.704 | 0.341 | −0.099 | −4.991 | <0.001 |
| Caucasiana | −0.428 | 0.329 | −0.023 | −1.299 | 0.194 |
| Employeda | −0.912 | 0.267 | −0.065 | −3.412 | 0.001 |
| Number of premature births <36 weeks | 0.361 | 0.258 | 0.025 | 1.395 | 0.163 |
| Number of stillbirths | 1.800 | 0.580 | 0.054 | 3.104 | 0.002 |
| Number of miscarriages | 0.283 | 0.117 | 0.043 | 2.415 | 0.016 |
B unstandardized regression coefficients, beta standardized regression coefficients
Categorical variables were re-coded for linear regression
Comparison between demographic factors at university and MHC
Continuous variables were compared using ANOVA (Table 1). Participants recruited at the MHCs were of younger age, at an earlier week of pregnancy, had lower education and income, fewer previous pregnancies, and more premature births, stillbirths, and miscarriages. Participants at the university clinic had fewer children living at home and correspondingly lower number of previous full-term births. The number of abortions was the only factor that did not significantly differ between groups.
Categorical variables were compared between groups with Chi-square tests (Table 2). Patients at the university clinic were more likely to be married, living with a partner, and employed as well as have their partner as head of the household or share household responsibilities with him. Patients at MHCs had lower income, were more likely to be unemployed, single, living without a partner, and be the sole head of the household. The majority of participants in both groups were Caucasian, which is consistent with the demographic profile of the state of Iowa, with slightly higher number of Caucasians at the university clinic than MHCs and more Hispanic patients at the MHCs.
Conclusions
Total BDI scores in this sample were significantly associated with age, pregnancy week, years of education, income, marital status, and employment status. In addition, patients in this study receiving treatment at community MHC exhibited more depressive symptoms, were younger, were more likely to be single and have lower socioeconomic status, and have a higher number of past negative obstetrical outcomes than pregnant women at the university clinic.
Preliminary analysis of associations between depression scores and demographic factors revealed many significant correlations most of which were fairly weak with the highest being income (r>0.3) and employment (F=32.37); however, when all of these factors were included as independent variables in a regression analysis, most, but not all of them, remained statistically significant predictors of severity of depressive symptoms. These were (in a descending order of strength of association) site, income, married or living with a partner, week of pregnancy, employment status, number of stillbirths, years of education, and number of miscarriages. This is consistent with previous studies in which younger age, lower education, low income, and lack of a partner were significant predictors of clinical depression (Gotlib et al. 1989; Rich-Edwards et al. 2006; Leigh and Milgrom 2008; Bunevicius et al. 2009). Also, several other studies have found that stillbirths are a risk factor for depression and anxiety during subsequent pregnancies (Hughes et al. 1999; Bernazzani and Bifulco 2003).
In contrast to previous reports, our study did not find younger age to be a significant predictor for depressive symptoms when other demographic factors were included in the predictive model. Since age is highly correlated with years of education and income, it is likely that the effect of age on depression scores is largely explained by the latter two factors. Our analysis revealed that unemployment, lower income, and less years of education can be viewed as risk factors independent of each other. It is important to note that in reality these variables are highly correlated, as indicated by the distinct group of patients recruited by site.
As suggested by others (Bolton et al. 1998) screening for depression in pregnancy is critical in socially and financially disadvantaged women. Our data concur that women evaluated at MHC tend to present with lower socioeconomic characteristics, and thus may carry a higher risk for depressive symptoms during pregnancy.
Our results uniquely contribute to the literature with the study of some obstetrical factors that are commonly reported during routine pregnancy screening. Notably, history of miscarriages and stillbirths may increase vulnerability to depressive symptoms in successive pregnancies, while abortions and premature births may not imply such a high risk; however, it is possible that, given the rarity of these events, we did not have sufficient power to detect the effect of the latter two factors.
In summary, this study suggests that women who are unmarried, early in pregnancy and of lower socioeconomic status and those who have experienced miscarriages and stillbirths may be more vulnerable to depressive symptoms during pregnancy.
A limitation of the current study is the lack of information on past psychiatric and family history of depression with the focus placed solely on demographic and obstetrical risk factors. Indeed, previous reports indicate that history of depression is one of the strongest predictors of antenatal depression (Rich-Edwards et al. 2006). This can explain why the compilation of risk factors in our study predicts only 15% of the variance in depressive symptoms in pregnancy. The remaining variance may be due to biologically determined factors like past history or family history of depression, hormonal factors, or psychosocial factors like personality traits and social stressors, as suggested by the literature (Séguin et al. 1995).
A significant strength of our study is the very large population sample size with diverse socioeconomic background. The examination of symptoms of depression in pregnancy from a dimensional perspective, rather than a categorical one using a depression diagnosis, makes it highly relevant to clinical screening for depression during pregnancy. Screening instruments such as the BDI are used in clinical practice—hence the correlation with symptoms is critical.
Our results can inform clinicians about some of the risk factors for depression in pregnant women early on and can help in the development of specific questionnaires to identify patients at risk. This will prompt providers to employ higher vigilance to detect depressive symptoms in pregnant women and adjust antenatal care accordingly with more frequent follow-up visits and referral for mental health consultation as necessary.
Acknowledgements
This research was supported in part by NIMH research grant MH59688.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Hristina Koleva, Email: hristina-koleva@uiowa.edu, Department of Psychiatry, University of Iowa, Iowa City, IA 52242-1007, USA; 200 Hawkins Drive, Iowa City, IA 52242-1007, USA.
Scott Stuart, Email: scott-stuart@uiowa.edu, Department of Psychiatry, University of Iowa, Iowa City, IA 52242-1007, USA; Department of Psychology, University of Iowa, Iowa City, IA 52242, USA; 1-293 Medical Education Building, Iowa City, IA 52242, USA.
Michael W. O’Hara, Department of Psychology, University of Iowa, Iowa City, IA 52242, USA
Jennifer Bowman-Reif, Department of Psychology, University of Iowa, Iowa City, IA 52242, USA.
References
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. (Apr.) Review. Erratum in: Obstet Gynecol. 2004 103(6):1344 (Jun) [DOI] [PubMed] [Google Scholar]
- Bernazzani O, Bifulco A. Motherhood as a vulnerability factor in major depression: the role of negative pregnancy experiences. Soc Sci Med. 2003;56:1249–1260. doi: 10.1016/s0277-9536(02)00123-5. [DOI] [PubMed] [Google Scholar]
- Bolton HL, Hughes PM, Turton P, et al. Incidence and demographic correlates of depressive symptoms during pregnancy in an inner London population. J Psychosom Obstet Gynaecol. 1998;19:202–209. doi: 10.3109/01674829809025698. [DOI] [PubMed] [Google Scholar]
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735. doi: 10.1177/070674370404901103. Review. [DOI] [PubMed] [Google Scholar]
- Bunevicius R, Kusminskas L, Bunevicius A, Nadisauskiene RJ, Jureniene K, Pop V. Psychosocial risk factors for depression during pregnancy. Acta Obstet Gynecol Scand. 2009;88(5):599–605. doi: 10.1080/00016340902846049. [DOI] [PubMed] [Google Scholar]
- Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. Br Med J. 2001;323:257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the foetus and neonate in different ethnic and socio-economic status groups. J Reprod Infant Psychol. 2002;20:149–157. [Google Scholar]
- Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269–274. doi: 10.1037//0022-006x.57.2.269. [DOI] [PubMed] [Google Scholar]
- Holcomb WL, Jr, Stone LS, Lustman PJ, Gavard JA, Mostello DJ. Screening for depression in pregnancy: characteristics of the Beck Depression Inventory. Obstet Gynecol. 1996;88:1021–1025. doi: 10.1016/s0029-7844(96)00329-8. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Turton P, Evans CDH. Stillbirth as risk factor for depression and anxiety in the subsequent pregnancy: cohort study. Br Med J. 1999;318:1721–1724. doi: 10.1136/bmj.318.7200.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson R, Chapman G, Murray D, Johnson I, Cox J. The North Staffordshire Maternity Hospital prospective study of pregnancy-associated depression. J Psychosom Obstet Gynaecol. 2000;21:93–97. doi: 10.3109/01674820009075614. [DOI] [PubMed] [Google Scholar]
- Klein MH, Essex MJ. Pregnant or depressed? The effect of overlap between symptoms of depression and somatic complaints of pregnancy on rates of major depression in the second trimester. Depression. 1995;2:308–314. [Google Scholar]
- Lancaster C, Gold K, Flynn H, Yoo H, Marcus S, Davis M. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol. 2010;202(1):5–14. doi: 10.1016/j.ajog.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry. 2008;8:24. doi: 10.1186/1471-244X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM. Depression during pregnancy: rates, risks and consequences. Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e15–e22. Epub 2009 Jan 22. Review. [PubMed] [Google Scholar]
- O’Hara MW. Social support, life events, and depression during pregnancy and the puerperium. Arch Gen Psychiatry. 1986;43:569–573. doi: 10.1001/archpsyc.1986.01800060063008. [DOI] [PubMed] [Google Scholar]
- Orr ST, Miller CA. Maternal depressive symptoms and the risk of poor pregnancy outcome. Epidemiol Rev. 1995;17(1):165–171. doi: 10.1093/oxfordjournals.epirev.a036172. [DOI] [PubMed] [Google Scholar]
- Rahman A, Iqbal Z, Bunn J, Lovel H, Harrington R. Impact of maternal depression on infant nutritional status and illness: a cohort study. Arch Gen Psychiatry. 2004;61:946–952. doi: 10.1001/archpsyc.61.9.946. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, Joffe H, Gillman MW. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health. 2006;60(3):221–227. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamero M, Marcos T, Gutierrez F, Rebull E. Factorial study of the BDI in pregnant women. Psychol Med. 1994;24:1031–1035. doi: 10.1017/s0033291700029111. [DOI] [PubMed] [Google Scholar]
- Séguin L, Potvin L, St-Denis M, Loiselle J. Chronic stressors, social support, and depression during pregnancy. Obstet Gynecol. 1995;85(4):583–589. doi: 10.1016/0029-7844(94)00449-N. [DOI] [PubMed] [Google Scholar]
- Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 Pt 1):1107–1111. doi: 10.1016/0002-9378(89)90170-1. [DOI] [PubMed] [Google Scholar]