Abstract
Contemporary human females have long life expectancy (81y US), especially relative to age at menopause (51y US). Menopause is a consequence of reproductive aging and follicular depletion (ovarian failure), yielding very low circulating estrogen* serum concentrations and biologically disadvantageous metabolic alterations. Stated in terms of antagonistic pleiotropy, the ongoing hypoestrogenic endocrine environment, beneficial during lactation, results in acceleration of several age-related health conditions following menopause (i.e. late postmenopausal osteoporosis, cardiovascular disease and cognitive decline). In contrast, the complex hypoestrogenic hormonal milieu present during postpartum lactation provides biologic advantages to both mother and newborn. The lactational hormonal milieu causes symptoms similar to those of the late perimenopause and early postmenopause, prompting theories for their biologic selective advantage. The precepts of evolutionary medicine encourage a reassessment of hormone therapy. Based on data presented, the authors propose additional opportunities for disease prevention and morbidity reduction in postmenopausal women.
Keywords: evolution, menopause, aging, lactation, hypoestrogenism, osteoporosis, heart disease
Introduction
Efforts to understand the evolutionary origin and significance of human menopause have engaged anthropologists and evolutionary biologists for decades. The adaptive hypothesis views programmed ovarian failure among humans as offering fitness by favoring females who become infertile years before death.2 An alternate explanation, the epiphenomena hypothesis, considers menopause a product of antagonistic pleiotropy, favoring early fertility at the expense of late fertility or as the byproduct of an increase in life span or life expectancy.3 The emergence of significant post-reproductive life expectancy in humans occurred during an evolutionary time when overall life expectancy was short, with death from acute, not chronic, conditions. Paleodemographic data from Australopithecines, Neanderthals, hunter-gatherers and proto-agricultural populations underscore the likelihood of menopause evolving with minimal life expectancy thereafter.4 Although evolutionary medicine generally seeks to unify emerging health issues and their genetic antecedents, here we focus on diseases appearing during the post-reproductive period that are no longer subjected to the fitness-selecting forces of evolutionary biology.5,6
The long post-reproductive life span of women appears to be an evolutionary trait of long standing, although experts interpreting the available paleodemographic data disagree.4,6–8 Debate centers on when, during the course of human evolution, menopause occurred, but it is likely to have evolved since the time of the last common ancestor.9 From a Darwinian perspective, the long life spans of women after menopause are not easily explained by the energetic costs of reproduction alone, revealing a capacity for somatic aging to be uncoupled from reproductive aging to some degree.10 Natural selection would favor females who become infertile many years before death if offspring require exceptional duration and intensity of parental care, as is the case with humans.2 This adaptation allows extension of maternal nurturing energies well beyond the direct energy costs of pregnancy and lactation. This adaptation is also necessary to enhance survival of human offspring with a prolonged period of dependency (as compared with all other primates).2,5,10
The trend toward ever-increasing percentages of individuals reaching life spans beyond 80 or 90 years is a recent phenomenon.11–13 Current data from more developed nations reveal no survival advantage conferred to younger populations with increasing numbers of octogenarians. Since 1900, achieving very old age is no longer an indicator of greater reproductive fitness, as it was in earlier centuries. Long life spans result from public health measures, socioeconomic factors and cultural customs, with medical and scientific interventions accounting for a small percentage.14 In the 21st century, the largest population expansion in US women is in those older than 65 years13, with survival not coupled to prior reproductive or ongoing biologic fitness.
Lactation, Endocrine Milieu and Physiology
Nearly all states of endocrine reproductive fitness are estrogen replete, with lactation the only low-estrogen condition associated with successful reproductive effort. Mammalian lactation fulfills the precepts of evolutionary biologic selective pressure, providing mobile, adequate nutrition to enable survival of suckling offspring.15 Lactation physiology is of particular interest because it is the only low-estrogen state that has resulted from selective genetic evolutionary pressure.16 In contrast, the menopause has no process of natural selection operating upon it.2 While the mechanism of lactational hypoestrogenism is different (lactation vs. menopause); many physiologic changes resulting from the estrogen deficiency are similar.
During pregnancy, estrogen, progesterone and prolactin concentrations prepare breast tissue for lactation. Immediately after delivery of the infant, expulsion of the placenta results in a sudden drop in both estrogen and progesterone. Abrupt withdrawal of progesterone in the presence of high prolactin levels initiates lactation. With suckling, prolactin levels continue to be elevated. These high prolactin levels, through their inhibitory impact on gonadotropin secretion, inhibit ovarian function to greater or lesser degrees throughout lactation. Ovarian suppression results in sustained low estrogen levels until the frequency of suckling diminishes and maternal energy supplies become adequate for further reproduction.17
Many maternal symptoms and physiologic alterations result from these lactational hormone shifts that are biologically advantageous for nursing infants at a maternal cost. The low estrogen concentrations during lactation induce many metabolic alterations. We will focus on the metabolism of serum lipoproteins, calcium balance and the resorption of bone, as well as on some alterations of the central nervous system. Although we are concentrating on these specific metabolic alterations, our hypothesis is more complete encompassing other metabolic alterations, menopausal symptoms (e.g. genital atrophy), and immune function. These are included in Table 1 for completeness, but are not discussed further in this manuscript.
Table 1.
Responses to Falling Sex Steroids
| Adaptive During Reproductive Era | Affected Cell Systems | Maladaptive in Menopause |
|---|---|---|
| Maintenance of capillary blood flow to critical organs | Arterial tone increased | Cardiovascular disease, hypertension |
| High blood fat or milk | Lipoprotein lipase (?) | Cardiovascular disease, arteriosclerosis |
| Mobilization of calcium for milk | Remodeling cycle favors bone loss | Osteoporosis, fracture |
| Discarding of genital skin, bulk and elasticity | Organ turgor (genital and sexual skin) | Scarring and genital atrophy |
| Radiant warming of suckling newborn | Vasomotor control, changes in thermoneutral zone set-point | Hot flushes, night sweats |
| Increased vigilance | Sleep pattern alterations | Sleep disorders |
| Affective and cognitive changes, decreased immune brain barrier | Central nervous system alterations | Memory deficiency, impaired cognition, dystrophies |
| Pro-inflammatory (?) anti-inflammatory during puerperium (reversed when estrogen is high) | Immune response to insult and foreign antigens | Failure of immune privilege (?), CNS vulnerability and cancer |
Hormonal Effects on Serum Lipoproteins During Lactation
Circulating low estrogen levels during lactation are associated with an increase in lipoprotein lipase enzyme activity18 resulting in an increase in serum lipoproteins. During lactation, such changes likely serve to increase the fat content of maternal milk, with energetic enrichment being an advantage to suckling infants.
Hormonal Effects on Calcium Balance and Resorption of Bone During Lactation
Approximately 200 mg/day of elemental calcium is lost from the maternal skeleton because of milk production in lactating women.19 Ample calcium concentrations in human breast milk enhance bone mineralization of offspring at the cost of maternal calcium stores when calcium intake is insufficient.20 This transfer of calcium results in a 3%–9% loss of bone mineral density (BMD) at the lumbar spine during a 6-month period.21 Loss of BMD during lactation is greater than the average loss of 1%–2%/yr. observed during the late postmenopause, but approximates the rapid early loss in the late perimenopause and early postmenopause.21 BMD losses appear greatest during the first 5 months of lactation, with recovery to normal BMD levels after weaning.21
Bone density does not always return to pre-pregnant levels, even after resumption of menstruation.22 Women in whom lower bone mass is likely to persist following lactation are those nursing multiple babies, adolescent mothers, women with inadequate nutrition and women in the later childbearing years who may not be able to regain lost skeletal mass before menopause onset.23
Lactation may be a contributing factor for postmenopausal osteoporosis in women whose bone density does not completely recover to pre-pregnancy levels upon weaning, although studies on this topic are inconclusive.19 Some recent epidemiologic research suggests a higher bone density in women who breastfed their infants compared with women who did not. This is observed in the presence of adequate nutrition, providing there is a sufficient length of time with resumption of menses prior to the onset of menopause.24
If lactational bone loss is compared with that occurring during menopause, several variables must be considered. Many researchers fail to define the stage of menopause being studied. For example, late perimenopause and early postmenopause, as defined in the STRAW staging system,1 are associated with more rapid bone loss than is the late postmenopause. Whereas the normal premenopausal bone remodeling cycle (resorption, reversal, formation, quiescence) occurs over a period of 4–8 months, the remodeling cycle during lactation occurs at twice that frequency: every 3–4 months.23 During lactation, high prolactin levels prevent gonadotropin-induced ovulation and maintain low estrogen levels, which promote bone resorption. At 6 months postpartum, exclusively breastfeeding women have prolactin levels that remain elevated,25 supporting continued transfer of calcium from bone to breast milk.
Once menses return and estrogen levels rise, bone loss abates.26,27 Data from nutritionally healthy women corroborate this phenomenon of BMD restoration, showing that multiparity, as compared with low parity or nulliparity, is not associated with post-menopausal low bone mass.28 Unlike lactation-associated bone loss, postmenopausal bone loss has no restorative phase. In addition, menopause is of longer duration than lactation and results in lower bone mass. Lowered bone mass, along with other changes associated with aging, results in measurable increases in fracture risk over time.29
Vasomotor Symptoms During Lactation
The low estrogen state is frequently accompanied by vasomotor symptoms (VMS; hot flashes, hot flushes [used interchangeably in this text], and night sweats) in women during the puerperium and postpartum lactation. In this setting, VMS may be integral to positively adaptive endocrinology (as opposed to the negative implications of VMS in post reproductive women). Hot flashes occurring postpartum or during menopause result from alterations in the temperature-regulatory centers of the hypothalamus. The so-called thermoneutral zone narrows in a low serum estrogen environment, making both hot flashes (an exothermic warming phenomenon) and sweating (ultimately a host surface-temperature evaporative cooling phenomenon) more likely.30 During the hypoestrogenic milieu of lactation, VMS could serve a biologic purpose by elevating maternal skin surface temperature.31 Such temperature elevations provide radiant heat for infant warming.31b Lowering caloric demands for self-warming conserves infant energy for weight gain and reduces metabolic stress on the newborn.32,33 Therefore, VMS would likely contribute a survival advantage in terms of warming of offspring, and convey some evolutionary biologic advantage.7
Insomnia During Lactation
Lactation-associated sleep disorders likely confer biologic advantage. Easy wakefulness and heightened alertness during awakenings result in enhanced nighttime attention to the newborn, increase the frequency of feedings, and promote greater early infant weight gain, especially in the high-mortality neonatal interval. Additional maternal endocrine changes and blunted stress responses serve to enhance lactation adequacy and prevent maladaptive sensitization of infant central nervous system (CNS) programming.32,34 This stabilization of both basal and stress-induced hypothalamic-pituitary-adrenal activity may be important for maintaining a constant endocrine environment, thereby preventing negative programming effects on the developing offspring. Other aspects of the lactational endocrine milieu likely serve to enhance nurturing behaviors and maternal calm during the low-estrogen state.35
Longevity, Demography and Changing Patterns of Disease
Whether a product of natural selection advantage, chance, or constraint as the cause of design complexity, menopause confers some advantages toward survival.36 In view of suggestions by others, one should consider any claim of adaptation against constraint and pathology as alternatives.37 The mechanism by which the naturally occurring state of menopause (constraint or pathology) accelerates somatic aging and advances disease risks over time will be explored.
Survival beyond age 65 of a large percentage of the female population is a very recent phenomenon in terms of evolutionary time. The earliest census data for the United States are from Massachusetts, where life expectancy in 1850 (free-living white women) at birth was 40, biased by high rates of infant mortality. If a woman reached age 40, the data predicted 28 additional years of life expectancy, indicating that if one survived infancy and the high mortality of childbearing, then surviving until age 68 was common.38 This estimate is similar to the maximum 70- to 74-year life expectancy observed in the most fertile women, as reported by Emery Thompson et al.39 Demographic data do not support life spans beyond the eighth decade as a frequent naturally occurring event.
We can expect the population of US women older than 80 years to increase by more than 15 million over the next three decades.40 Considering the large number of nursing home-dwelling women at present, these statistics stimulate thoughts regarding possible measures to slow the pace of somatic aging in menopausal women.41 In less developed nations, women who lived to be very old were a relatively small percentage of those who were biologically fittest.42 Hunter-gatherer octogenarians were subjected to tests of reproductive fitness and were the most vigorous with respect to later-life somatic fitness.43 With medical and scientific advances today, surviving to a very old age no longer represents evidence of fitness selection.44
Comparative Endocrine Milieu of Reproductive and Post-reproductive Women
The principles of low estrogen menopausal physiology help explain diseases that appear to increase in frequency and severity during the late postmenopause. Low serum estrogen levels in menopause, compared with low levels during lactation, result in very different metabolic and CNS effects.45 It does not seem logical that VMS and sleep disorders serve any beneficial purpose later in life, although VMS in infant-caring menopausal grandmothers could potentially offer similar warming advantages for grand-offspring survival.
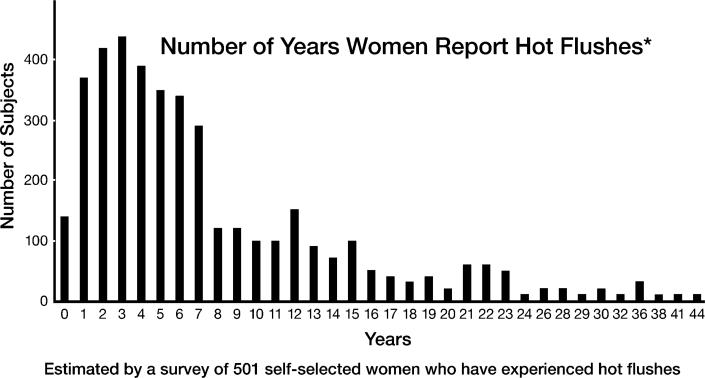
Vasomotor symptoms affect a large majority of post-industrial menopausal women, with some estimates as high as 90%. Although all women are not equally affected, about one-third of affected women have very severe symptoms causing multiple nighttime awakenings, severe night sweats and disturbed cognitive function (Figure 1).46,47 These studies are reported from present-day sedentary populations and are not representative of hunter-gatherers.48 Menopausal symptoms at every stage are decreased in very physically active women as compared with those who are less active.46,48
Figure 1.
Hot Flushes May Continue Years After Menopause
Because hunter-gatherers are extremely vigorous and active even through the late menopause years, they are likely to have been among the least bothered by menopausal symptoms, as evidenced by data on sleep disorders in women from less developed nations as compared with sedentary women in post-industrial countries.48 Compensatory mechanisms that diminish VMS occur in 80% of postmenopausal women, and appear to occur in most women 10 years after the final menstrual period.46,46b Although hypoestrogenic symptoms of menopause (urogenital atrophy, vaginal dryness) are highly prevalent, these symptoms seldom are associated with severe disease or disability. Recent assessments are divided as to whether VMS may signal the presence of cardiovascular disease (CVD) in menopausal women. Recent data from the Women's Health Initiative (WHI) suggest that persistent VMS are a marker of subclinical CVD.49 This analysis showed that women most likely to suffer a cardiovascular event (heart attack or stroke) when given either oral estrogen or oral estrogen/progestin therapy were those in late menopause with persistent VMS.49 Women whose symptoms abated after the typical 4–5 years (Figure 1) had much lower risk when similarly treated. CVD is the major cause of mortality for US women, equal in number to the sum of the next four causes of death, thus refining our risk factor analyses may assist in efforts aimed at CVD risk reduction.50
Lipids and Cardiovascular Disease
In contrast to the Paleolithic-era diet of hunter-gatherer populations, the high-glycemic-index and high-fat diets characteristic of populations in more developed nations result in adverse cardiovascular risk factors and higher rates of disease.51 In menopausal women, a more sedentary lifestyle and higher body-fat content result in higher circulating serum lipoprotein concentrations.50 Plasma lipoprotein elevations caused by increased lipoprotein lipase activity in the presence of low serum estrogen levels, common in menopausal women, further increase risk.52–54 In the menopause, low serum estradiol levels are associated with a sustained rise in total cholesterol, low-density lipoprotein cholesterol, and triglycerides and a concomitant fall in high-density lipoprotein cholesterol—a more atherogenic lipid/lipoprotein profile.52–54
Although not universally demonstrated in cross-sectional studies, longitudinal data document a small but statistically significant elevation in diastolic blood pressure (BP), in the range of 0.48 mmHg for diastolic BP across the menopause transition in conjunction with low serum estrogen levels.54 Other adverse effects of low serum estrogen are a rise in cardiospecific C-reactive protein (C-CRP), a marker of vascular inflammation in the coronary circulation. Elevated C-CRP is recognized as a marker for increased risks for CVD and thrombus formation. Low serum estrogen levels cause increases in arterial tone, with a rise in BP in both primates and humans.45 Increases in arterial tone also result in vascular luminal narrowing, requiring greater pressures to maintain adequate blood flow to vital organs. Increased arterial pressures over time increase vascular injury and formation of atheromata, and contribute to higher rates of plaque rupture, myocardial infarcts and peripheral vascular disease.55,56
Primates rendered menopausal in the laboratory exhibit adverse changes in CVD risk markers that are similar to those in human females with premature, surgical or spontaneous menopause.45,52–59 A comparison of premenopausal and postmenopausal women aged 40–55 showed that, regardless of age stratum, CVD was more common in the postmenopausal group than in the premenopausal group.57 Similarly, the degree of CVD increases the earlier hysterectomy/oophorectomy is performed.58,59 The cumulative effects of dyslipidemia, when compounded by abnormal serum inflammatory markers and hypertension, result in elevated risks for atherosclerotic CVD, stroke, renal disease and dementia in menopausal women.
Bone Metabolism and Chronic Disease
In late postmenopause, the prolonged hypoestrogenic milieu causes a decline in BMD over time, with no rebound compensatory phase of bone formation as occurs after lactation ends in reproductively fit women. Resultant late postmenopausal skeletal fractures, particularly those of the hip, cause higher rates of disability and death. One-year mortality rates after this injury range from 15% to 20%.60 A prospective study on the socioeconomic aspects of proximal femur fracture demonstrated that approximately 50% of patients who lived independently before hip fracture were unable to regain their independent lifestyle subsequent to the fracture.60,61 The inexorable demineralization of bone following diminished ovarian estrogen synthesis, as in late postmenopause, is biologically disadvantageous.
Low serum estrogen concentrations lead to accelerated rates of bone resorption, with reductions in bone mass; this phenomenon occurs in both lactating and menopausal women. Lactation-related fractures are rare. Multiple factors may account for this phenomenon, but the precise mechanisms are not known. Younger age, higher bone density, a shorter duration of accelerated bone resorption (compared with menopause) and other neuromuscular factors are likely protective, rendering the nursing mother's skeleton relatively resistant to falls and fracture.62 Frailty, which increases risk for falls and fractures, is very rare in lactating women but quite common in elderly women with osteoporosis.63 The human costs of osteoporosis due to prolonged low serum estrogen levels are substantial. Approximately 50% of women older than 50 will have an osteoporosis-related fracture at some time during their remaining lifetime.
The sustained loss of muscle mass, collagen and elastin seen in late postmenopause results in frailty, a second phase of accelerated bone loss.63 These underlying alterations in menopausal bone, muscle and connective tissue physiology are disadvantageous, decreasing self-sufficiency and independence as women age. Growing demands for assisted living services reflect the cumulative impact of loss of vigor in aging sedentary women.41
With an evolutionary medical perspective, one might organize the data regarding lactational and menopausal physiology, and their disparate short- and long-term effects on cardiovascular and skeletal health, as shown in Table 1.
Paradigm Reassessment
There is a paucity of evidence to demonstrate that menopause has a genetic evolutionary contribution for humans over time. Women living beyond their own reproductive fitness may improve maternal-infant care by providing additional hunter-gatherer labor and reliable food and water and by playing midwifery roles that lower infant and maternal mortality rates. There does not appear to be a genomic advantage to menopause per se, other than shortening the reproductive life span to allow for more rapid generation times, enhancing adaptability and lengthening the time of protection and provisioning support of grand-offspring. Selective advantage depends on traits carried by offspring and on the “grandmother effect.”64 In these cases, the selective advantage is not related to post-reproductive adaptation and does not contribute inheritable genetic alterations or novel metabolic responses to the loss of estrogen.2,16
Sociological and scientific advances allow humans to experience new challenges and opportunities with respect to longevity. With current life expectancy approximating 80 years, we ask the question: Is it possible for a large percentage of women to be very old and also be very healthy without specific “normalizing” physiologic interventions? The human genome was selected in past environments far different from those of the present. Cultural evolution now proceeds too rapidly for genetic accommodation—resulting in dissociation between our genes and our lives. The mismatch between inherent biology and sedentary lifestyle fosters increasing prevalence of degenerative diseases and chronic illness.65 We must develop an overall conceptual framework to better characterize differences between ancient and modern life histories and relationships to the emergence of disease patterns. Through such analysis, one can create a unifying approach upon which to base consistent, compelling and effective recommendations for reduction in disease burdens for individuals and across populations of women.7
Conclusion
From these examples, we conclude that low serum estrogen's “natural” biologic advantage—its selective evolutionary purpose—is likely restricted to postpartum lactation. Decades of sustained estrogen deprivation, with attendant disadvantageous cardiovascular and musculoskeletal physiology, result in greater rates of diseases and declining vigor in women. As Austad stated, “Assuming the human body has been physiologically adapted to the conditions extant during the vast majority of human history, it may be well worth pursuing how the signs and symptoms of menopause are affected by dietary, exercise, and reproductive hormone regimes mimicking those of the late Paleolithic era.”4 Adopting evolutionary premises in our approach to menopause, we view it as a state of human existence upon which evolutionary biologic pressures no longer operate. An evolutionary medicine perspective creates a radical shift in our thinking on the biodemography of this aspect of aging in women. Through the study of evolutionary medicine, we are called upon to create new approaches to menopausal health care in order to lower morbidity rates associated with accelerated somatic aging.
US physicians and public health officials specializing in post-reproductive health care are concerned about the expanding number of aging women whose longevity will lead to exponential growth in the population of dependent elderly. Analyzing the fundamental underlying physiology that characterizes the postmenopause reveals many characteristics that are biologically disadvantageous when sustained over time. Correcting the underlying cause of the pathophysiology could lessen morbidity during late postmenopause in modern Western cultures. An evolutionary medicine perspective calls for reassessing the current approach that discourages the use of estrogen support in menopause. A methodical evolutionary medical inquiry may generate options that not only lengthen disease-free life spans but also improve quality of life.7
Future Directions and Research Opportunities
Careful examination of the inherent self-preserving physiologic and evolutionarily selected mechanisms for homeostasis during and following lactation may provide new insight into the prevention of disease following menopause. Furthering our understanding of energy conservation during lactation may provide opportunities for reduced caloric consumption and its attendant longevity benefits following menopause, with similar salutary effects. Perhaps the hormonal milieu present during and immediately following lactation, an environment that restores skeletal integrity, could be harnessed later in life to prevent osteoporosis, fracture and other infirmities so prevalent in aged women.
The profound similarities between the metabolic changes during lactation and those following menopause bring several research opportunities into focus. Lactation evokes rapid, self-regulating, compensatory mechanisms for its metabolic changes (typically within a few years), with few, if any, attendant long-term health effects. Similar metabolic changes following the final menstrual period result in well-documented adverse lifelong health consequences in late postmenopause (e.g. CVD, osteoporosis and fractures). Hormone therapy has been shown to reduce risk for many of these hypoestrogenic consequences when started early in the menopausal transition. These principles could stimulate a research agenda and, ultimately, a shift in public policy. We need to better characterize differences between ancient and modern women's life histories, identify which of these factors affect the development of disease and ultimately integrate epidemiologic, mechanistic and genetic data with evolutionary principles to create a unifying concept on which to base persuasive, consistent and effective recommendations.
Acknowledgment
The authors wish to gratefully acknowledge the assistance and encouragement of Adrienne Zihlman, PhD, Professor of Anthropology, University of California, Santa Cruz.
Funded in part by a grant from the National Institute of Health, Institute on Aging Workshop on Aging, June 2009
Conflicts of interest/disclosures: Ricki Pollycove: Advisory Board for Teva/Duramed/Barr and Corcept Therapeutics, Inc. Speaker Bureau for Aventis, Novo Nordisk, Ther-Rx, Upsher-Smith, Noven. Author, Pocket Guide to BioIdentical Hormones, 2010, Alpha Press.
Fred Naftolin: Member of the SAB for Se-Cure Inc. who market Femarelle James A Simon: consultant or on the advisory boards of: Allergan (Irvine, CA), Alliance for Better Bone Health (Cincinnati, OH), Amgen Inc. (Thousand Oaks, CA), Ascend Therapeutics (Herndon, VA), Bayer (Leverkusen, Germany), BioSante (Lincolnshire, IL), Boehringer Ingelheim (Ingelheim, Germany), Concert Pharmaceuticals (Lexington, MA), Corcept Therapeutics, Inc. (Menlo Park, CA), Depomed, Inc. (Menlo Park, CA), GlaxoSmithKline (Philadelphia, PA), KV Pharmaceutical Co. (St. Louis, MO), Lipocine, Inc. (Salt Lake City, UT), Meditrina Pharmaceuticals (Ann Arbor, MI), Merck (Whitehouse Station, NJ), Merrion Pharmaceuticals (Wilmington, NC), Nanma/Tripharma/Trinity (Glen Arm, MD), NDA Partners LLC (Lakewood Ranch, FL), Novo Nordisk (Bagsvrerd, Denmark), Novogyne (East Hanover, NJ), Pear Tree Pharmaceuticals (Cambridge, MA), QuatRx Pharmaceuticals (Ann Arbor, MI), Roche (Basel, Switzerland), Schering-Plough (Kenilworth, NJ), Sciele (Atlanta, GA), Solvay (Marietta, GA), Teva Pharmaceutical Industries Ltd (Jerusalem, Israel), Ther-Rx (Bridgetown, MO), Warner Chilcott (Rockaway, NJ), and Wyeth (Madison, NJ).
He has received grant/research support from BioSante (Lincolnshire, IL), Boehringer Ingelheim (Ingelheim, Germany), FemmePharma (Wayne, PA), GlaxoSmithKline (Philadelphia, PA), Nanma/Tripharma/Trinity (Glen Arm, MD), Novartis (Basel, Switzerland), Proctor and Gamble (Cincinnati, OH), QuatRx Pharmaceuticals (Ann Arbor, MI), and Teva Pharmaceutical Industries Ltd (Jerusalem, Israel),
He has also served on the speakers bureaus of: Amgen Inc. (Thousand Oaks, CA), Ascend Therapeutics (Herndon, VA), Bayer (Leverkusen, Germany), Boehringer Ingelheim (Ingelheim, Germany), GlaxoSmithKline (Philadelphia, PA), KV Pharmaceutical Co. (St. Louis, MO), Merck (Whitehouse Station, NJ), Novartis (Basel, Switzerland), Novogyne (East Hanover, NJ), Sciele (Atlanta, GA), Teva Pharmaceutical Industries Ltd (Jerusalem, Israel), Ther-Rx (Bridgeton, MO), Warner Chilcott (Rockaway, NJ), and Wyeth (Madison, NJ).
Footnotes
To avoid unnecessary complexity that would obscure the main thrust of our argument, we have used the terms estrogen and estrogenic throughout, rather than specifying the different compounds involved. For the interested reader, estrogenic refers to the net action of the following estrogens at any time: classical estrogens (estradiol, estrone and estriol), conjugates (e.g. lipoidal estrogens), nonestrogens active at the estrogen receptor level (e.g. progesterone, testosterone, 27 OH-cholesterol) and xenoestrogens (e.g. isoflavonoids, SERMs [selective estrogen receptor modified compounds] and some pesticides such as DDT). Serum estradiol is the primary estrogen measured in human menopause research. Terms for the stages of menopause are those defined by the 2001 Stages of Reproductive Aging Workshop.1
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–879. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 2.Peccei JS. A critique of the grandmother hypothesis: old and new. Am J Hum Biol. 2001;13:434–452. doi: 10.1002/ajhb.1076. [DOI] [PubMed] [Google Scholar]
- 3.Pavard S, E Metcalf CJ, Heyer E. Senescence of reproduction may explain adaptive menopause in humans. Am J Phys Anthropol. 2008;136:194–203. doi: 10.1002/ajpa.20794. [DOI] [PubMed] [Google Scholar]
- 4.Austad SN. Menopause: an evolutionary perspective. Exp Gerontol. 1994 May-Aug;29(3–4):255–63. doi: 10.1016/0531-5565(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 5.Hawkes K. Grandmothering and Evolution of Human Longevity. Am J Hu Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- 6.Eaton SB. Evolutionary health promotion. Prev Med. 2002 Feb;34(2):109–18. doi: 10.1006/pmed.2001.0876. [DOI] [PubMed] [Google Scholar]
- 7.Naftolin F, Whitten P, Keefe D. An evolutionary perspective on the climacteric and menopause. Menopause. 1994;4:223–225. [Google Scholar]
- 8.Sturdee DW, MacLennan AH. Evolution and revolution at the menopause. Climacteric. 2004 Dec;7(4):325–6. doi: 10.1080/13697130400014656. [DOI] [PubMed] [Google Scholar]
- 9.Fedigan LM, Pavelka MSM. Is there adaptive value to reproductive termination in Japanese macaques? A test of maternal investment hypotheses. Int J Primatol. 2001;22:109–125. [Google Scholar]
- 10.Hall R. An energetics-based approach to understanding the menstrual cycle and menopause. Human Nature. 2004;15(1):83–99. doi: 10.1007/s12110-004-1005-9. [DOI] [PubMed] [Google Scholar]
- 11.Eurostat European Commission Statistics in focus. Office for Official Publications of the European Communities. Catalogue number: KS-SF-08-072-EN-N. 2008 [cited]. Available at: http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-SF-08-
- 12.Years of healthy life—selected states, United States, 1993–1995. MMWR Morb Mortal Wkly Rep. 1998 Jan 16;47(1):5–7. [PubMed] [Google Scholar]
- 13.US Census Bureau US Population Projections. 2008 [updated 2008 August 28, 2008; cited July 15,2009]; Available at: http://www.census.gov/population/www/projections/projectionsagesex.html.
- 14.Perls TT, Fretts RC. The evolution of menopause and human life span. Ann Hum Biol. 2001 May-Jun;28(3):237–45. doi: 10.1080/030144601300119052. [DOI] [PubMed] [Google Scholar]
- 15.Zihlman A. Women's bodies, women's lives: an evolutionary perspective. In: Morbeck ME, Galloway A, editors. The Evolving Female: A Life History. Princeton Press; 1997. pp. 185–197. [Google Scholar]
- 16.Kirkwood T. The origins of human ageing. Philos Trans R Soc Lond B Biol Sci. 1997 Dec 29;352(1363):1765–72. doi: 10.1098/rstb.1997.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valeggia C, Ellison P. Interactions between metabolic and reproductive functions in the resumption of postpartum fecundity. Am J Hum Biol. 2009 Jul-Aug;21(4):559–66. doi: 10.1002/ajhb.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004 Nov;5(4):197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 19.Prentice A. Calcium in pregnancy and lactation. Annu Rev Nutr. 2000;20:249–72. doi: 10.1146/annurev.nutr.20.1.249. [DOI] [PubMed] [Google Scholar]
- 20.Neumann C, Harrison G. Onset and evolution of stunting in infants and children: examples from the Human Nutrition Collaborative Research Support Program. Kenya and Egypt studies. Eur J Clin Nutr. 1994;48(Suppl 1):S90–S102. [PubMed] [Google Scholar]
- 21.Kalkwarf HJ, Specker BL. Bone mineral changes during pregnancy and lactation. Endocrine. 2002;17:49–53. doi: 10.1385/ENDO:17:1:49. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67:693–701. doi: 10.1093/ajcn/67.4.693. [DOI] [PubMed] [Google Scholar]
- 23.Kalkwarf HJ. Hormonal and dietary regulation of changes in bone density during lactation and after weaning in women. J Mammary Gland Biol Neoplasia. 1999;4:319–29. doi: 10.1023/a:1018780425600. [DOI] [PubMed] [Google Scholar]
- 24.Paton LM, Alexander JL, Nowson CA, et al. Pregnancy and lactation have no longterm deleterious effect on measures of bone mineral in healthy women: a twin study. Am J Clin Nutr. 2003;77:707–14. doi: 10.1093/ajcn/77.3.707. [DOI] [PubMed] [Google Scholar]
- 25.Hayslip CC, Klein TA, Wray HL, Duncan W. The effects of lactation on bone mineral content in healthy postpartum women. Obstet Gynecol. 1989;73:588–92. [PubMed] [Google Scholar]
- 26.Sowers MF, Corton G, Shapiro B, et al. Changes in bone density with lactation. JAMA. 1993;269:3130–5. [PubMed] [Google Scholar]
- 27.Kalkwarf HJ, Specker BL. Bone mineral loss during lactation and recovery after weaning. Obstet Gynecol. 1995;86:26–32. doi: 10.1016/0029-7844(95)00083-4. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int. 2001;12(10):828–34. doi: 10.1007/s001980170033. [DOI] [PubMed] [Google Scholar]
- 29.Kanis JA, Black D, Cooper C, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002 Jul;13(7):527–36. doi: 10.1007/s001980200069. [DOI] [PubMed] [Google Scholar]
- 30.Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005 May;23(2):117–25. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- 31.Kronenberg F, Downey JA. Thermoregulatory physiology of menopausal hot flashes: a review. Can J Physiol Pharmacol. 1987 Jun;65(6):1312–24. doi: 10.1139/y87-208. [DOI] [PubMed] [Google Scholar]
- 31b.Marshall WM, Cumming DC, Fitzsimmons GW. Hot flushes during breast feeding? Fert Steril. 1992;57(6):1349–50. doi: 10.1016/s0015-0282(16)55101-2. [DOI] [PubMed] [Google Scholar]
- 32.Walker CD. Nutritional aspects modulating brain development and the responses to stress in early neonatal life. Prog Neuropsychopharmacol Biol Psychiatry. 2005 Dec;29(8):1249–63. doi: 10.1016/j.pnpbp.2005.08.010. Epub 2005 Oct 25. [DOI] [PubMed] [Google Scholar]
- 33.Gjerdingen DK, Froberg DG, Chaloner KM, McGovern PM. Changes in women's physical health during the first postpartum year. Arch Fam Med. 1993 Mar;2(3):277–83. doi: 10.1001/archfami.2.3.277. [DOI] [PubMed] [Google Scholar]
- 34.Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2001;133:111–29. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- 35.Brunton PJ, Russell JA, Douglas AJ. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J Neuroendocrinol. 2008 Jun;20(6):764–76. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 36.Hawkes K, O'Connell JF, et al. Grandmothering, menopause and the evolution of human life histories. Proc Natl Acad Sci. 1998;95:1336–9. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellison PT, Jasienska G. Constraint, pathology, and adaptation: how can we tell them apart? Am J Hum Biol. 2007 Sep-Oct;19(5):622–30. doi: 10.1002/ajhb.20662. [DOI] [PubMed] [Google Scholar]
- 38.Historical Census Browser. 2004 Retrieved April25, 2010, from the University of Virginia, Geospatial and Statistical Data Center: http://fisher.lib.virginia.edu/collections/stats/histcensus/index.html.
- 39.Emery Thompson M, Jones JH, Pusey AE, et al. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007 Dec 18;17(24):2150–6. doi: 10.1016/j.cub.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. http://www.cdc.gov/MMWR/preview/mmwrhtml/00050833.htm.
- 41. [Accessed April 30, 2010]; >http://www.cdc.gov/nchs/pressroom/00facts/nurshome.htm.
- 42.Bengtsson T, Merlo J, Mineau GP. IUSSP Scientific Committee on Historical Demography, International Seminar on Longevity 6 Report. Lund University; 2006. Longevity:Early life conditions, social mobility and other factors trhat influence survival at old age. [Google Scholar]
- 43.Jasienska G. Reproduction and lifespan: trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. Am J Hum Biol. 2009;21(4):524–32. doi: 10.1002/ajhb.20931. [DOI] [PubMed] [Google Scholar]
- 44.Williams GC, Nesse RM. The dawn of Darwinian medicine. Quart Rev Biol. 1991;66:1–22. doi: 10.1086/417048. [DOI] [PubMed] [Google Scholar]
- 45.Clarkson TB, Mehaffey MH. Coronary heart disease of females: lessons learned from nonhuman primates. Am J Primatol. 2009 Sep;71(9):785–93. doi: 10.1002/ajp.20693. [DOI] [PubMed] [Google Scholar]
- 46.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–33. [DOI] [PubMed] [Google Scholar]
- 46b.Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: a longitudinal study. Menopause. 2009 May-Jun;16(3):453–7. doi: 10.1097/gme.0b013e31818d414e. [DOI] [PubMed] [Google Scholar]
- 47.Simon JA, Reape KZ. Understanding the menopausal experiences of professional women. Menopause. 2009 Jan-Feb;16(1):73–6. doi: 10.1097/gme.0b013e31817b614a. [DOI] [PubMed] [Google Scholar]
- 48.Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10:197–214. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- 49.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007 Apr 4;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 50.Anderson RN, Smith BL. Deaths: leading causes for 2001. Natl Vital Stat Rep. 2003 Nov 7;52(9):1–85. [PubMed] [Google Scholar]
- 51.Jonsson T, Granfeldt Y, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35–49. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carr MC, Kim KH, Zambon A, Mitchell ES, Woods NF, Casazza CP, et al. Changes in LDL density across the menopausal transition. J Investig Med. 2000 Jul;48(4):245–50. [PubMed] [Google Scholar]
- 53.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993 Jan 4;98(1):83–90. doi: 10.1016/0021-9150(93)90225-j. [DOI] [PubMed] [Google Scholar]
- 54.Do KA, Green A, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000 Mar 15;151(6):584–93. doi: 10.1093/oxfordjournals.aje.a010246. [DOI] [PubMed] [Google Scholar]
- 55.Burke AP, Farb A, et al. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J. 2001 Feb;141(2 Suppl):S58–62. doi: 10.1067/mhj.2001.109946. [DOI] [PubMed] [Google Scholar]
- 56.ESHRE Capri Workshop Group Hormones and cardiovascular health in women. Hum Reprod Update. 2006 Sep-Oct;12(5):483–97. doi: 10.1093/humupd/dml028. [DOI] [PubMed] [Google Scholar]
- 57.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976 Oct;85(4):447–52. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 58.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol. 2009 May;113(5):1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol. 1981 Jan;139(1):47–51. doi: 10.1016/0002-9378(81)90410-5. [DOI] [PubMed] [Google Scholar]
- 60.Schurch MA, Rizzoli R, Mermillod B, Vasey H, Michel JP, Bonjour JP. A prospective study on socioeconomic aspects of fracture of the proximal femur. J Bone Miner Res. 1996 Dec;11(12):1935–42. doi: 10.1002/jbmr.5650111215. [DOI] [PubMed] [Google Scholar]
- 61.Rao SS, Singh M, Parkar M, Sugumaran R. Health maintenance for postmenopausal women. Am Fam Physician. 2008 Sep 1;78(5):583–91. [PubMed] [Google Scholar]
- 62.Henderson PH, 3rd, Sowers M, Kutzko KE, Jannausch ML. Bone mineral density in grand multiparous women with extended lactation. Am J Obstet Gynecol. 2000 Jun;182(6):1371–7. doi: 10.1067/mob.2000.107468. [DOI] [PubMed] [Google Scholar]
- 63.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):744–51. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 64.Shanley DP, Sear R, Mace R, Kirkwood TB. Testing evolutionary theories of menopause. Proc Biol Sci. 2007 Dec 7;274(1628):2943–9. doi: 10.1098/rspb.2007.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stearns SC. Evolution in health and disease: Work in progress. Quarterly Rev Biol. 2001;76:417–32. doi: 10.1086/420539. [DOI] [PubMed] [Google Scholar]
- 65.Stearns SC. Evolution in health and disease: Work in progress. Quarterly Rev Biol. 2001;76:417–32. doi: 10.1086/420539. [DOI] [PubMed] [Google Scholar]