Abstract
The BAM complex drives assembly of β-barrel proteins into the outer membrane of gram-negative bacteria. It is composed of five subunits: BamA, BamB, BamC, BamD and BamE. We find that the BAM complex isolated from the outer membrane of Escherichia coli consists of a core complex of BamA:B:C:D:E and in addition, a BamA:B module and a BamC:D module. In the absence of BamC, these modules are destabilized resulting in increased protease susceptibility of BamD and BamB. While the N-terminus of BamC carries a highly conserved region crucial for stable interaction with BamD, immunofluorescence, immunoprecipitation and protease-sensitivity assays show that the C-terminal domain of BamC, comprised of two helix-grip motifs, is exposed on the surface of Escherichia coli. This unexpected topology of a bacterial lipoprotein is reminiscent of the analogous protein subunits from the mitochondrial β-barrel insertion machinery, the SAM complex. The modular arrangement and topological features provide new insight into the architecture of the BAM complex, towards a better understanding of the mechanism driving β-barrel membrane protein assembly.
Keywords: β-barrel proteins, Omp85, outer membrane biogenesis, protein transport
INTRODUCTION
During cell growth bacteria assemble lipids and integral membrane proteins into the outer membrane. β-barrel proteins represent the largest subtype of proteins in the outer membrane, forming a β-barrel structure that traverses the lipid layer. These β-barrels are implicated in a myriad of cellular activities many of which are essential to cell viability. Examples include small molecule and substrate transporters, the Type V secretion systems responsible for protein translocation and, more recently, roles in outer membrane lipid and protein biogenesis 1. After translocation from the cytoplasm into the periplasm via the SecYEG complex, the targeting and integration of β-barrel proteins into the outer membrane requires the action of a molecular machine referred to as the β-barrel assembly machinery (BAM complex). In E. coli, this complex is composed of the integral β-barrel protein, BamA, along with four peripheral membrane lipoproteins: BamB, BamC, BamD and BamE. The four lipoproteins are attached to the outer membrane by virtue of a lipid moiety each has at their N-terminus, and by non-covalent interactions made with each other and with BamA.
BamA belongs to the evolutionary-conserved class of Omp85 proteins containing an N-terminal periplasmic domain comprised of five POTRA (POlypeptide TRansport-Associated) repeats and a C-terminal integral membrane β-barrel domain. The POTRA domains have been implicated in both outer membrane protein assembly via β-augmentation and provide the scaffold by which the remaining BAM lipoproteins associate with BamA. Structural analysis of the periplasmic domain of BamA gives detail into the topology of the five POTRA repeats. Hinges between the POTRA domains allow movement and extension of the periplasmic domain potentially allowing it to reach to the inner membrane.
Genetic deletion studies have established that while BamA and BamD are essential for viability in E. coli, individual deletions of bamB, bamC or bamE are tolerated with various degrees of growth phenotype and β-barrel protein assembly defects. BamB deletion mutants display significant defects whereas only minor changes are observed in a BamE deletion strain. Double deletion mutants lacking both BamB and BamE are lethal and result in changes to BamA conformation and stabilization, resulting in an increased sensitivity to proteolysis. The role of BamC however, has proven difficult to elucidate. The recent observation that BamC is highly conserved across diverse species of Gamma-proteobacteria 15 suggests this subunit is valuable to BAM complex function, yet BamC mutants have no obvious growth defects. Even double mutations (bamB,bamC mutants or bamC,bamE mutants) display a modest increase in phenotypic distinction compared to the single deletion strain, leaving the function of BamC enigmatic.
Characterization of the BAM complex has been enhanced by numerous atomic resolution structures now available for each component 1. BamB forms an eight bladed β-propeller that requires three BamA POTRA domains (2, 3 and 4) for association in the BAM complex. BamD forms an extended helical bundle comprised of tandem protein repeats, or TPRs, a motif commonly observed in forming protein-protein interactions. It also interacts with BamA, independently of BamB, binding to the last POTRA domain closest to the C-terminal β-barrel 8. BamC and BamE are of particular interest because they only assemble into the complex via interactions with BamD, displaying no affinity for BamA 20. BamE has a small compact domain that has two points of contact with the outer membrane: a binding interface for BamD and interaction with the lipid interface via a phosphatidylglycerol binding-site 21. Lastly, BamC is comprised of two compact helix-grip domains with a long ~75 residue N-terminal extension that is disordered in solution. Intriguingly, it is this N-terminal extension which provides the binding interface with BamD, leaving the role of the helix-grip domains wholly-uncertain 25.
Despite these large advances in characterizing individual subunits, we lack an understanding of the overall architecture and assembly requirements of the BAM complex. Previous studies on the BAM complex in Caulobacter crescentus revealed that it is built of modules that can be dissociated sequentially using non-ionic detergents 2. When resolved by blue-native polyacrylamide gel electrophoresis (BN-PAGE), BamA resides in a holo-complex of ~500 kDa that can be disassociated into a ~300 kDa core-complex (BamA:B:C:D:E) and a ~150 kDa sub-complex (BamA:B) with increasing amounts of detergent. Reconstitution experiments with purified components of the BAM complex from E. coli demonstrate a similar modular architecture whereby BamC:BamD and BamC:D:E modules can be formed, as can a BamA:B module, and the BamA:B and BamC:D:E modules can be docked to reconstitute a stable BAM complex. These two general lines of evidence lead to the proposition that dynamic interplay of the modules is important for the architecture and function of the BAM complex.
Here we show that the BAM complex isolated from the outer membrane of E. coli consists of a core-complex of ~250 kDa (BamA:B:C:D:E) that can be broken into a ~150 kDa BamA:B module and BamC:D and BamC:D:E modules. The BamC:D module can be over expressed and purified, and the interaction between BamC and BamD depends on a highly conserved segment in the N-terminus of BamC. Further analysis of BamC demonstrates the C-terminal domain of BamC is exposed on the surface of E. coli, accessible to antibodies and proteases. These findings challenge the current dogma for the architecture of the BAM complex and in doing so raise new prospects for understanding the mechanistic details of outer membrane protein assembly.
RESULTS
A modular BAM complex in E. coli
To investigate the architecture of the BAM complex and the role of BamC, we analyzed wild-type and ΔbamC mutants of E. coli by BN-PAGE. Membranes from isogenic strains were solubilized in dodecylmaltoside (DDM) and proteins detected with antibodies raised to each of the components of the BAM complex. In wild-type cells, BamA is found in a BamA:B:C:D:E core complex at ~250 kDa and the BamA:B module at ~150 kDa. In addition, a BamC:D module is present and migrates at ~80 kDa (Fig. 1a). Further analysis to test the effect of detergent concentration on the stability of the BAM complex confirmed that the dissociation of modules observed was not dependent on the increase of the DDM and hence supports the existence of multiple BAM modules (Fig. 1b).
Figure 1.
The BAM complex is modular. (a) Total membranes from BW25113 and ΔbamC strains were solubilized in 1.0 % DDM and analyzed by BN-PAGE followed by immunoblotting. (b) Detergent titration (0.1-2.0 % DDM) of BW25113 and ΔbamC membranes, analyzed by BN-PAGE and immunoblotting with antibodies recognizing BamB. (c) Proteinase K shaving of wild-type BW25113 and the isogenic ΔbamC mutant strain in the absence or presence of polymixin B. Optical densities between strains was normalised and samples were analyzed by SDS-PAGE and immunoblotting.
Analysis of the BAM complex in the ΔbamC mutants revealed two further findings. Firstly, the proportion of the BamA:B module is slightly increased in the mutants lacking BamC, suggesting a small role for BamC in promoting association of the BamD and BamE subunits (Fig. 1a). Secondly, BamA is found in two forms of the BAM complex, namely BamA:B:D and BamA:B:D:E, where the form containing BamE migrates more rapidly than the form without BamE. The apparent size of membrane protein complexes on BN-PAGE cannot always be used as a measure of actual size and stoichiometry 28. Here, either the stoichiometry of subunits in the BamA:B:D is increased, or this form of the complex has an overall shape change that retards its migration in the gel, perhaps by increasing the amount of associated detergent and/or lipid 28.
These striking changes to the BAM complex in ΔbamC mutants are surprising given the benign growth phenotype of the mutants and absence of any significant outer membrane damage as indicated by antibiotic sensitivity. Limited proteolysis of intact cells did not reveal any differences between wild-type and the ΔbamC deletion mutant with respect to protease sensitivity of BamA, BamB or BamD (Fig. 1c). BamA is partially degraded to a smaller fragment that correlates with cleavage from the C-terminus as the antiserum is raised to the N-terminal POTRA domains. Periplasmic BamB and BamD are protected from surface proteolysis. In contrast, after outer membrane damage was induced with the antibiotic polymyxin B, a significant change in protease susceptibility was observed for two components of the BAM complex. Under these conditions, BamB and BamD are more sensitive to proteinase K degradation in the presence of polymixin B. The amount of protease resistant BamB molecules has approximately halved in the ΔbamC strain, whereas BamD is completely degraded. To rule out any contribution of membrane permeability defects in ΔbamC that might contribute to the observed proteolysis of BAM, chemical sensitivity tests were performed on strains in the presence of Polymixin B which demonstrated no change in cellular sensitivity in ΔbamC compared to wild-type cells (results not shown). Collectively, these results would suggest the absence of BamC causes a destabilisation of the BAM complex rendering many of the components more vulnerable to proteolysis. This vulnerability might reflect a change in conformation of the subunit, a change in subunit-subunit interactions, or both.
BamC is exposed on the cell surface
To further characterise BamC we investigated its topology within the BAM complex by employing immunofluorscence microscopy of whole cells and permeabilized cells. When wild-type bacterial cells were probed with anti-BamC serum, fluorescence was observed, suggesting BamC is present on the surface of the cell (Fig. 2a). This signal was not detected in the ΔbamC strain confirming the specificity of the antibody. Outer membrane integrity was established by probing cells in parallel with antibodies against the periplasmic protein, SurA. As expected, SurA was not observed on the surface of intact bacterial cells, but was detected in the periplasm of permeabilized cells.
Figure 2.
BamC is exposed on the surface of intact E. coli cells. (a) Immunofluorescence and bright field images of intact or permeabilized (P) BW25113 (WT) and ΔbamC strains labelled with anti-BamC (green), anti-SurA (red) and DAPI (blue). (b) Indirect whole-cell ELISA of BW25113 (WT) and ΔbamC strains. The mean (n=8) and standard deviation error was used to graph absorbance405nm of wells incubated with anti-BamC serum followed by secondary antibodies (■), or secondary alone (□). To normalise the results between strains, the absorbance value of uncoated wells treated with primary or primary/secondary antibodies was subtracted. This experiment was independently repeated three times. (c) Co-immunoprecipitation of BamC ΔbamC in and BW25113 (WT) cells using anti-BamC sera. Whole cells (WC) were incubated with anti-BamC sera prior to cellular solubilisation to bind only cell surface exposed BamC, or, cells were solubilized (S) before adding anti-BamC sera. Co-immunopreciptation of WT cells using anti-SurA was also performed for WC and S samples. (d) Schematic representation of native BamC. After SDS-PAGE followed by western-blotting. Samples were analyzed by removal of the 24 residue signal sequence (SS), mature BamC contains an unstructured N-terminus (C25-G94) and two structured domains (G101-S212, PDB ID: 2YH6; T226-K344, PDB ID: 2YH5). (e) BL21(DE3) cells expressing BamCS were treated with trypsin and analyzed by SDS-PAGE and immunoblotting with anti-BamC, anti-SurA (periplasmic) and anti-BamA (outer membrane). Treatment of cells with trypsin results in the cleavage of two BamC fragments * and ** commencing at A225STTMD and T97QFTGD respectively as determined by N-terminal sequencing.
Indirect whole-cell ELISA was used to independently assess the surface-exposure of BamC (Fig. 2b). Wild-type and ΔbamC mutants were cultured to mid-log phase and cells were adhered to an ELISA plate. Incubation with anti-BamC antiserum and a secondary antibody-peroxidase reagent enabled antibody binding to be assessed by measuring the absorbance at 405 nm. Upon addition of anti-BamC sera to BW25113 cells a significant increase in signal (p<0.001) was observed, a change not detected in the ΔbamC strain, thus demonstrating BamC is present on the surface of wild-type cells.
To ascertain if the surface exposed BamC is present in the BAM complex, whole cells were incubated with anti-sera, then washed extensively prior to co-immunoprecipitation. In this way, only BAM components in complex with the surface-exposed BamC should be co-precipitated. The anti-BamC serum co-immunoprecipitated BamA, BamB and BamD, establishing that surface exposed BamC is assembled in the BAM complex (Fig. 2c). The periplasmic chaperone, SurA, was not recoginzed by anti-BamC sera consistent with previous studies of the BAM complex 29. As predicted, BW25113 cells incubated with antibodies recognizing BamC after detergent-solubilization co-precipitated BamC along with BamA, BamB and BamD. In parallel, anti-SurA sera was tested with both solubilized and whole cell samples. Of the proteins surveyed, only SurA was recognized by anti-SurA sera using solubilized cells, demonstrating the integrity of the whole cell experiments, the specificity of the pull-downs, and importantly, that SurA is not surface exposed.
To determine which domain(s) of BamC is exposed on the surface of E. coli, we attempted to use protease shaving. While a reliable method for topological analysis of proteins in the internal compartments of bacteria, “externally exposed” bacterial outer membrane proteins are often protected by tight-folding and their glycolipid environment. In wild-type E. coli we were unable to detect proteolysis of BamC (see Fig. 1c). We sought therefore to increase the proportion of BamC-containing modules that might be less compact, and therefore protease-sensitive. Overexpression of BamC resulted in a ladder of bands as observed by western, corresponding to full-length protein (36 kDa) serially degraded down to ~20 kDa in size (Fig. 2e). This protease-sensitivity is consistent with the previous observation that the N-terminal region of BamC is unstructured and therefore susceptible to proteolysis by endogenous periplasmic proteases.
When cells overexpressing BamC were treated with trypsin, two specific fragments of BamC were generated (Fig. 2e). Analysis of these fragments showed the N-terminal sequences commence with residues Thr97 and Ala225, corresponding to the helix-grip domains (Fig. 2d). As controls for the intact nature of the cells in these shaving assays, trypsin had no proteolytic effect on BamA or on the periplasmic protein SurA (Fig. 2e).
A conserved, N-terminal segment of BamC is required for interaction with BamD
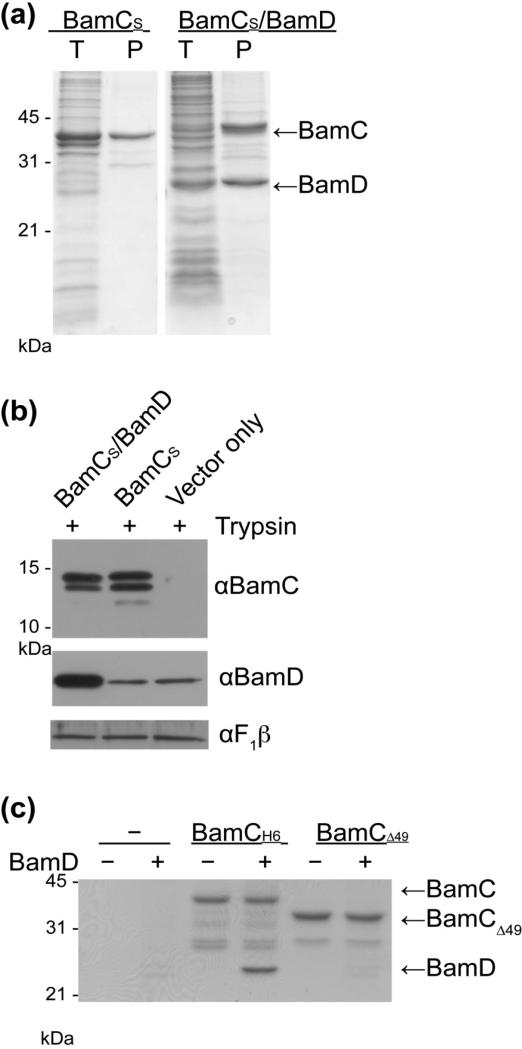
Recent work by Kahne and colleagues demonstrates the overexpression of BAM subunits can be used for reconstitution of a functional BAM complex in liposomes. Using a similar approach, we co-expressed native BamC (containing a C-terminal S-tag) with BamD (untagged) in BL21(DE3) E. coli. Membranes were isolated and the proteins solubilized in the presence of detergent. Total extracts (Fig. 3a, ‘T’) or affinity-purified samples (Fig. 3a, ‘P’) were analyzed by SDS-PAGE. BamC and BamD subunits form a genuine complex that can be purified with approximate 1:1 stoichiometry, as judged by Coomassie-staining intensities (Fig. 3a). Even when BamC is in complex with BamD, limited proteolysis showed again that the BamC helix-grip domains were surface-exposed (Fig. 3b). Given the location of BamD in the periplasm, the simplest explanation is that the unstructured N-terminus of BamC is in the periplasm and can interact with BamD. To test this hypothesis, the first 26 residues were truncated from the mature protein (BamCΔ49), and this truncated BamC assayed for interaction with BamD. After binding histidine-tagged BamC or BamCΔ49 to Ni2+ -affinity beads, detergent-solubilized membranes containing overexpressed BamD were added and protein binding analyzed by SDS-PAGE. BamD interacts with the mature form of BamC, but did not bind to the truncated BamC Δ49 (Fig. 3c).
Figure 3.
Interaction with BamD is mediated through a short N-terminal segment of BamC. (a) BL21(DE3) cells expressing BamCS or BamCS/BamD were cultured and fractionated by S-tag affinity chromatography into whole cell lysates (L) and affinity-purified samples (P), analyzed by SDS-PAGE and Coomassie staining. (b) BL21(DE3) cells expressing BamCS or BamCS/BamD or vector alone were incubated with trypsin. Samples were analyzed by SDS-PAGE and immunoblotting. (c) BamCH6 and BamCΔ49 were purified on Ni-NTA beads and then incubated with detergent-solubilized membranes containing BamD. Samples were analyzed by SDS-PAGE.
BamC is present in diverse Gamma-proteobacteria (such as E. coli) and Beta-proteobacteria. We analyzed 43 Gamma-proteobacteria BamC representatives, including E. coli, revealing three conserved regions in the unstructured N-terminal segment of BamC (Figure 4). Protein segments of intrinsic disorder tend to have conserved amino acid composition, but do not require highly conserved motifs in primary structure. The conserved region corresponding to BamC26-42 (Motif 1, E=2.6×10-172) is at the N-terminus of the mature protein and incorporates the segment we have demonstrated to be essential for binding to BamD. The second motif, BamC48-68 (Motif 2, E=9×10-137), further extends through the loop and together, these two motifs provide the majority of hydrogen bonds observed between BamC and BamD, as demonstrated in the recently published crystal structure of the BamC:BamD module (Fig. 4C) 25. A third motif, BamC74-102 (Motif 3, E=2.5×10-432), spans the region between the N-terminal BamD binding segment and the two structural domains that we have identified residing on the cell surface. According to the crystal structure, unlike Motif 1 and 2, which dominate in key BamC-BamD interactions, Motif 3 only contains a few residues at the N-termini that participate in BamD binding. Given that the primary sequence of intrinsically disordered regions is often poorly conserved, this level of sequence conservation hints at a fundamental role for this segment of BamC and we hypothesize it traverses the outer membrane, thereby reconciling surface exposure of the C-terminal helix-grip domains of BamC and its N-terminal interaction with BamD in the periplasm. Motif 2 and 3 are also present Beta-proteobacteria, with only slight variation in their boundaries, suggesting that the topology of BamC may be preserved between classes of proteobacteria.
Figure 4.
Conserved features in the intrinsically disordered N-terminal domain of BamC. (a) Motif analysis of the BamC identified three highly conserved regions in the unstructured N-terminus of BamC, Motif 1, Motif 2 and Motif 3. The height of each letter in the motifs indicates the degree of conservation of that residue across 43 distinct Gamma-proteobacterial (“γ-500proteobacteria”) BamC sequences. (b) Amino acid sequence of BamC (NlpB), residues 26-344, highlighting the regions corresponding to Motif 1 (cyan), Motif 2 (green), Motif 3 (orange) and the two helix-grip structured domains (underlined). Residue 49, corresponding to BamCΔ 49 is bold (c) Crystal structure (PDB ID: 3TGO) of soluble BamD (residues 26-242; purple) in complex with BamC (residues 28-213; blue, the structure does not include the second C-terminal helix-grip domain). The ribbon representation is shown in two views, after rotation by 90°. The regions corresponding to BamC Motif 1 (aqua), Motif 2 (green) and Motif 3 (orange) are highlighted.
DISCUSSION
The BAM complex is a molecular machine essential for the assembly of β-barrel proteins into the bacterial outer membrane. Determining the architecture and inter-subunit arrangements is paramount to understanding how the BAM complex catalyses protein assembly and integration. Here we have examined the BAM complex and identified stable modules present in E. coli. We have extended these findings by further characterizing BamC to show it is involved in the stabilization of not only BamD but also other BAM components and revealed a novel lipoprotein topology that includes a periplasmic N-terminus and a large folded surface domain.
To investigate the architecture of this multi-component assembly, we employed BN-PAGE. In addition to a stable BAM core complex comprised of five components, modules of BamA:B and BamC:D were also identified. While this is the first report of these sub-complexes, or “modules”, the results are in keeping with biochemical studies which have shown that equivalent modules can be formed from recombinantly-expressed subunits and reconstituted to form stable BAM complexes. The BAM complex assembled from purified BamA:B and BamC:D (or BamC:D:E) modules is functional in β-barrel assembly. We show that in cells lacking BamC, the BAM complex is destabilized such that there is increase in the amount of the BamA:B module. We suggest BamC may partially stabilize BamD facilitating binding to BamE and BamA:B. These results are consistent with previous affinity binding experiments in which BamE did not bind BamD in a BamC deletion strain 12. Furthermore, protease shaving experiments with permeabilized cells showed decreased BAM stability whereby BamD was increasingly susceptible to proteinase K proteolysis in the absence of BamC. BamB is also more susceptible to proteolysis, although to a lesser extent. Similar proteolysis experiments in a BamE deletion strain recently found BamA was completely degraded from the surface and the authors proposed BamE is responsible for stabilizing BamA via its interaction with BamD 13. Likewise, we propose BamC has an alternative yet somewhat complimentary role in stabilising the BAM complex, via its interaction with BamD.
Within the literature, BamC has been assumed to be as a periplasmic lipoprotein, so it was to our surprise that we detected BamC on the cell surface of E. coli. Moreover we could substantiate these findings using whole-cell ELISA experiments similarly demonstrating BamC was surface exposed. In keeping with this finding, BamC (NlpB) has been identified as a prospective vaccine candidate for meningitis, selected in a high-throughput screen for antibodies protecting against Neisseria meningitidis infection 32. Remarkably, this surface antigen incorporates both helix-grip domains as recognized by cell surface proteolysis. It is well established that all outermembrane lipoproteins are transported to the inner leaflet of the outer membrane by the LolA/LolB transport machinery 33. How BamC might further translocate and assemble a large folded domain onto the cell surface still remains to be elucidated.
Surface lipoproteins are by no means unprecedented in proteobacteria, although there are only a few examples in E. coli, including the well-characterized capsule-forming component, Wza, which extends across the outer membrane as an amphipathic helix displaying only a few residues on the cell surface 34. More recently, the ‘free’ form of Lpp, the most abundant protein in E. coli, was shown to be surface exposed and although the precise structure it forms for traversing the membrane remains unknown, the authors proposed it will likely be a trimer as observed for the ‘bound’ form 35. Neither Wza or Lpp contains a globular domain on the cell surface, hence BamC represents a novel topology for bacterial lipoproteins.
Despite efforts to identify a role for these domains, structural comparisons with other proteins have only hinted to possible functions. One recent study proposed a protein binding site in the C-terminal helix-grip domain which contains a conserved negatively charged groove that may serve to bind a positively charged helix reminiscent of the AP complex binding of arrestin 22. The surface location of these helix-grip domains alters the possible protein-protein interactions BamC may make. One hypothesis would be that the helix-grip domains may serve to stabilize surface-located inter-strand loops of BamA. BamC is resistant to cell surface proteolysis when assembled in BAM and was only accessible when overexpressed in amounts above the stoichiometry of the BAM complex. This would be consistent with the hinge region between the two helix-grip domains being protected at steady state levels: these interactions may only be obvious during dynamic steps in the assembly of β-barrel proteins into the membrane.
To extend the characterisation of the BamC:D module, we identified the binding interface of BamC required for a stable interaction with BamD. Previous experiments have shown deletion of the whole unstructured N-terminus (residues 26-94) result in the loss of BamC binding to BamD25. We have extended these findings to show that the first 25 residues of BamC are necessary for binding to BamD, and motif analysis of the unstructured N-terminal region shows this region to be conserved across diverse bacterial species (Motif 1; residues 28-42). These results are in keeping with the very recently published BamC:BamD complex crystal structure which shows even in the event of binding to BamD, the N-terminus of BamC forms a long unstructured loop interacting across the length of BamD 25. When Motif 1 is mapped onto the crystal structure, it becomes clear that these residues contribute a large majority of key protein-protein interactions between BamC and BamD; as do the residues of Motif 2 (Fig. 4c). The truncated protein BamCΔ49 would invariably result in destabilization of the BamC:D complex, as verified in biochemical experiments with the purified proteins.
BamD has been well defined as a periplasmic lipoprotein through its direct interactions with the periplasmic BamA POTRA domains. In order to reconcile the BamC N-terminus binding to BamD in the periplasm, and also containing a large folded C-terminus on the cell surface, BamC must cross the outer membrane. Sequence analysis suggests BamC is unlikely to contain an alpha-helical transmembrane segment, so an alternate structure would need to traverse the membrane. Conservation of Motif 3 does not appear to be essential for BamD binding, and its conserved sequence may instead reflect an importance for BamC topology. Intriguingly, Motif 3 (residues 74-102) contains a region of hydrophobic residues (P84PxQPLALLxG94) which could at least in theory cross a membrane. With respect to the BamC:D crystal structure, these residues pack neatly between the N-terminus of BamD and the first helix-grip domain (Fig. 4c) 25. Arguably, the BamC:D structure infers the first helix-grip domain of BamC resides adjacent to the N-terminus of BamD and according to current literature, the authors naturally assumed it to be periplasmic 25. These BamC:D crystals were obtained using soluble forms of BamC and BamD, lacking their native signal sequences and lipidated cysteine and we would argue that this arrangement may not necessarily depict what occurs in the native environment of a membrane.
The nature of a BamC transmembrane segment remains to be determined, but we note the similarity in amino acid composition in the conserved region defined by Motif 3, rich in proline and hydrophobic residues, with the “hydrophobic segments” in the mitochondrial surface exposed mitochondrial proteins Sam37/Tob37 and Sam35/Tob38. These externally-located subunits of the SAM complex function together with Sam50 (the mitochondrial homolog of BamA) in the assembly of β-barrel proteins into mitochondrial outer membranes 38. Dynamic protein-protein interactions within the plane of the outer membrane should be considered in future studies on the mechanism of β-barrel protein assembly in bacteria and mitochondria.
MATERIALS AND METHODS
Strains, Plasmids and Growth Conditions
E. coli parent strain BW25113 and ΔbamC::Kan (Keio collection) were obtained from NBRP (NIG, Japan) : E.coli 39. Protein overexpression was performed in E. coli BL21(DE3) Star cells (Invitrogen). All strains were routinely grown in Luria Broth supplemented with the appropriate antibiotics (kanamycin 30 μg/ml or chloramphenicol 34 μg/ml) and grown at 37 °C to mid-log density.
BamC (GenBank ID: CAQ32848.1) was amplified from E. coli strain BW25113 using primers BamC_N (5’ CGCGCCCATATG GCTTACTCTGTTCAAAAGTCG 3’) and BamC_C (5’ CGC CTC GAG CTT GCT AAA CGC AGC 3’), as was BamD (GenBank ID: CAQ32966.1) using primers BamD_N (5’ GCACCATGGGCACGCGCATGAAATATCTG 3’) and BamD_C (5’ GCGCAAGCTTCTATTATGTATTGCTGCTGTTTGC 3’). Inserts were cloned into the pACYC Duet vector (Novagen) using NcoI/HindIII restriction sites for BamD and NdeI/XhoI for BamC to form a coexpression plasmid containing both full-length BamC and BamD (BamCS/BamD). The resultant BamC protein incorporates a C-terminal linker of LeuGluSerGly followed by an S-Tag (Novagen). Respective pACYCDuet plasmids expressing BamCS and BamD individually were also constructed. A histidine-tagged version of BamC (BamCH6) was cloned in pET22b (Novagen) using NcoI/XhoI which introduces two C-terminal linker residues (Leu-Glu) and C-terminal hexa-histidine tag. This construct includes the PelB signal sequence followed by BamC (Cys25-Lys344).
Isolating cell membranes and membrane protein solubilisation
Prior to isolating total membranes, BW25113 and ΔbamC::Kan were grown to mid-log phase at 37 °C and BL21(DE3) Star cells harbouring overexpression BamC and BamD plasmids were grown to mid-log phase prior to induction with 0.2 mM IPTG for 2 h at 37 °C. All cells were harvested by centrifugation (5000 × g, 10 min, 4 °C) and membranes isolated as previously described 40. Total membranes were stored in aliquots at -80 °C until required.
For protein purification, total membranes were solubilized in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl (TBS) containing 5 % Elugent (Calbiochem) for 30 min spinning gently at 4 °C. Insoluble material was removed by ultracentrifugation (99,000 × g, 4 °C, 30 min). BamCH6 was purified on Ni-NTA (Qiagen) resin in the presence of 0.5 % Elugent and eluted with imidazole. BamCS and S-Tag BamCS/BamD were purified on S-Tag beads according to the manufacture's instructions (Novagen).
To obtain BamCΔ49, purified BamCH6 was incubated with V8 protease (1 U/20 μg of protein, 4 °C, 10 min). For affinity purification experiments, BamC or BamCΔ49, were prebound to Ni-NTA beads and incubated with solubilized membranes containing BamD for 30 min on ice prior to extensive washing with TBS/0.5 % Elugent. Samples were analyzed by SDS-PAGE.
Protease shaving experiments
Mid-log phase cells were resuspended in TBS and incubated in the absence or presence of polymixin B (20 μg/ml) before protease treatment with either proteinase K (500 μg/ml) or trypsin (100 μg/ml) for 30 min at 37 °C or room temperature respectively. Samples were precipitated with 10 % TCA followed by an acetone wash and then analyzed by SDS-PAGE and immunoblotting.
Electrophoresis
For BN-PAGE, BW25113 and ΔbamC::Kan cells were grown to mid-log phase, and the soluble fraction obtained as described earlier. Membranes were isolated by ultracentrifugation (99000 × g, 4 °C, 30 min). Total membranes (50 μg) were solubilized with 1 % DDM (Anatrace) at a 1:4 protein to detergent ratio, for 20 min on ice prior to analysis by BN-PAGE as previously described 2.
Antibody production
To generate BamC antibodies, a soluble form of BamC (residues 26-344) containing a C-terminal hexahistidine tag was expressed in Bl21(DE3) Star cells and purified by affinity chromatography and size exclusion chromatography to >95 % purity. The protein was used to generate polyclonal antibodies in rabbits by Davids Biotechnologie (Germany). Recombinant proteins corresponding to the mature form of each of the BamB, BamD and BamE lipoproteins or to the POTRA domain of BamA (residues 21-432) were expressed in BL21(DE3) cells, proteins were purified by affinity and size exclusion chromatography to > 99% purity. Antisera were raised in mice (Precision Antibody; Columbia, MD) by injecting protein at 1 mg/ml in phosphate buffered saline (PBS) using BamA POTRA domains, BamB, BamD and BamE. All other antisera used here have been previously described.
Immunofluorescence Microscopy and ELISA
E. coli parent strain BW25113 and ΔbamC::Kan were grown to mid-log and 1 mL of cells pelleted, washed and resuspended in PBS. Cells were fixed in 4 % paraformaldehyde for 30 min at room temperature followed by several washes with PBS. For permeabilized samples, cells were resuspended in Buffer P (PBS with 0.1 % Triton-X100, 10 mM EDTA) with 100 μg/ml lysozyme for 30 min at room temperature. Whole cell or permeabilized cell samples were resuspended in PBS or Buffer P respectively containing 2 % BSA prior to incubation with BamC anti-mouse and SurA anti-rabbit antibodies (1:500) for 1 h at 4 °C, followed by incubation with AlexFluor 488 goat anti-mouse IgG and AlexFluor 594 goat anti-rabbit IgG (1:300) for an 1 h. After washing cells were resuspended in DAPI (4 mg/ml in PBS) and slides made up. Images were obtained using an Olympus microscope and the data analyzed using Cell M (Olympus) and ImageJ.
Surface exposure of BamC was assessed by indirect whole cell ELISA as previously described with the following modification 41. BW25113 and ΔbamC::Kan were cultured to mid-log phase prior to dilution to OD600 of 0.2 and adhered to the wells of an ELISA plate at 65 °C for 2-3 h. After blocking in PBS containing 1 % fetal calf serum, the wells were incubated with the primary BamC anti-rabbit antibody (1:500 dilution) followed by Horseradish peroxidaseconjugated goat anti-rabbit secondary antibodies (1:2000 dilution). After washing, the substrate solution of ABTS [(NH4)2(2,2’-Azino-bis-3-ethylbenzothiazoline-6-sulfonicacid)] in 50mM phosphate-citrate buffer (1 mg/ml, pH 5) and hydrogen peroxide (10 μL in 12 mL ) was added to each well and absorbance measurements at OD405nm were taken after 15 and 30 min.
Co-immunoprecipitation
Co-immunoprecipitation were performed on the BW25113 and ΔbamC strains as previously described with the following exceptions 42. No cross-linkers were used in any of these experiments. For whole cell samples (WC), cells were washed in PBS and resuspended in 2 mL PBS with 2 % BSA. Cells were incubated with 5 μL BamC or SurA anti-rabbit antibodies for 1 h at RT, followed by three washes with PBS (1500 × g, 4 °C, 3 min) to remove any unbound antibodies. Cells were then solubilized as previously described 42. For the solubilized samples, 5 μL antibody was added to the cleared lysate after solubilization. The cleared lysate:antibody mixture was combined with 50 μL Protein A/G Plus Agarose beads (Santa Cruz Biotechnology), Samples were analyzed by SDS-PAGE followed by western immunoblotting.
Conserved sequences found in BamC
To define conserved regions of BamC: (i) A set of BamC homologs was compiled from the NCBI nr database (27-09-2011) with HHsenser 43, starting with E. coli BamC (UniProt ID: P0A903) as a query sequence, (ii) the sequence set was redundancy reduced such that no two sequences were greater than 90 % identical using CD-HIT 44, (iii) The collected sequences were truncated to remove the N-terminal signal sequence and lipid attachment Cys: in the case of E. coli BamC, the first residue used for motif discovery was S26, (iv) MEME v4.6.143 was used for motif discovery in ‘zoops’ (zero or one motif per sequence) and ‘oops’ (one motif per sequence) mode (-maxsize 1000000 -maxw 80 -nmotifs 5) on this BamC set (142 sequences from Gamma-and Beta-proteobacteria) and a more conservative set of 43 sequences restricted to Gamma-proteobacterial orders Enterobacteriales, Pasteurellales, Vibrionales (but excluding Pseudomonas, Cellvibrio, Oceanospirillales and Alteromonadales). This provided a further layer of sensitivity to establish highly conserved residues in a context of more highly conserved BamD (and other) partner proteins.
HIGHLIGHTS.
-The bacterial β-barrel assembly machinery, BAM, can dissociate into smaller stable modules
-The BamC:D module is stabilised via a highly conserved region at the N-terminus of BamC
-The large folded C-terminus of BamC is present on the bacterial cell surface
-BamC provides a novel topology for surface exposed proteins changing the current view of BAM assembly
ACKNOWLEDGEMENTS
We thank Yue Qu for expert advice on immunofluorescence microscopy. This work was supported by the National Health & Medical Research Council of Australia through an NHMRC Program Grant (606788). TL is an Australian Research Council Federation Fellow, CTW and AJP are NHMRC Biomedical Fellows and JS is supported by an NHMRC Postgraduate Research Scholarship. SKB and NN are supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- E. coli
Escherichia coli
- PDB
protein data bank
- DDM
dodecylmaltoside
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ricci DP, Silhavy TJ. The Bam machine: A molecular cooper. Biochimica et biophysica acta. 2011 doi: 10.1016/j.bbamem.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anwari K, Poggio S, Perry A, Gatsos X, Ramarathinam SH, Williamson NA, Noinaj N, Buchanan S, Gabriel K, Purcell AW, Jacobs-Wagner C, Lithgow T. A modular BAM complex in the outer membrane of the alpha-proteobacterium Caulobacter crescentus. PloS one. 2010;5:e8619. doi: 10.1371/journal.pone.0008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. The Journal of cell biology. 2004;164:19–24. doi: 10.1083/jcb.200310092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–5. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein HD. The double life of a bacterial lipoprotein. Molecular Microbiology. 2011;79:1128–31. doi: 10.1111/j.1365-2958.2011.07538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–45. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Ieva R, Tian P, Peterson JH, Bernstein HD. Sequential and spatially restricted interactions of assembly factors with an autotransporter beta domain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E383–91. doi: 10.1073/pnas.1103827108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Malinverni JC, Sliz P, Silhavy TJ, Harrison SC, Kahne D. Structure and function of an essential component of the outer membrane protein assembly machine. Science. 2007;317:961–4. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- 9.Gatzeva-Topalova PZ, Warner LR, Pardi A, Sousa MC. Structure and flexibility of the complete periplasmic domain of BamA: the protein insertion machine of the outer membrane. Structure. 2010;18:1492–501. doi: 10.1016/j.str.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson ES, Werner JN, Misra R. Differential effects of yfgL mutation on Escherichia coli outer membrane proteins and lipopolysaccharide. Journal of bacteriology. 2006;188:7186–94. doi: 10.1128/JB.00571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigel NW, Schwalm J, Ricci DP, Silhavy TJ. BamE Modulates the Escherichia coli Beta-Barrel Assembly Machine Component BamA. Journal of bacteriology. 2012;194:1002–8. doi: 10.1128/JB.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sklar JG, Wu T, Gronenberg LS, Malinverni JC, Kahne D, Silhavy TJ. Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6400–5. doi: 10.1073/pnas.0701579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigel NW, Schwalm J, Ricci DP, Silhavy TJ. BamE Modulates the Escherichia coli Beta-Barrel Assembly Machine Component BamA. Journal of bacteriology. 2011 doi: 10.1128/JB.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tellez R, Jr., Misra R. Substitutions in the BamA beta-Barrel Domain Overcome the Conditional Lethal Phenotype of a {Delta}bamB {Delta}bamE Strain of Escherichia coli. Journal of bacteriology. 2012;194:317–24. doi: 10.1128/JB.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anwari K, Webb CT, Poggio S, Perry AJ, Belousoff M, Celik N, Ramm G, Lovering A, Sockett RE, Smit J, Jacobs-Wagner C, Lithgow T. The evolution of new lipoprotein subunits of the bacterial outer membrane BAM complex. Molecular Microbiology. 2012 doi: 10.1111/j.1365-2958.2012.08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albrecht R, Zeth K. Structural basis of outer membrane protein biogenesis in bacteria. The Journal of biological chemistry. 2011;286:27792–803. doi: 10.1074/jbc.M111.238931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noinaj N, Fairman JW, Buchanan SK. The crystal structure of BamB suggests interactions with BamA and its role within the BAM complex. Journal of molecular biology. 2011;407:248–60. doi: 10.1016/j.jmb.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Andrea LD, Regan L. TPR proteins: the versatile helix. Trends in biochemical sciences. 2003;28:655–62. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Gatsos X, Perry AJ, Anwari K, Dolezal P, Wolynec PP, Likic VA, Purcell AW, Buchanan SK, Lithgow T. Protein secretion and outer membrane assembly in Alphaproteobacteria. FEMS microbiology reviews. 2008;32:995–1009. doi: 10.1111/j.1574-6976.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malinverni JC, Werner J, Kim S, Sklar JG, Kahne D, Misra R, Silhavy TJ. YfiO stabilizes the YaeT complex and is essential for outer membrane protein assembly in Escherichia coli. Molecular Microbiology. 2006;61:151–164. doi: 10.1111/j.1365-2958.2006.05211.x. [DOI] [PubMed] [Google Scholar]
- 21.Knowles TJ, Browning DF, Jeeves M, Maderbocus R, Rajesh S, Sridhar P, Manoli E, Emery D, Sommer U, Spencer A, Leyton DL, Squire D, Chaudhuri RR, Viant MR, Cunningham AF, Henderson IR, Overduin M. Structure and function of BamE within the outer membrane and the beta-barrel assembly machine. EMBO reports. 2011;12:123–8. doi: 10.1038/embor.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KH, Aulakh S, Tan W, Paetzel M. Crystallographic analysis of the C-terminal domain of the Escherichia coli lipoprotein BamC. Acta crystallographica. Section F, Structural biology and crystallization communications. 2011;67:1350–8. doi: 10.1107/S174430911103363X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knowles TJ, McClelland DM, Rajesh S, Henderson IR, Overduin M. Secondary structure and (1)H, (13)C and (15)N backbone resonance assignments of BamC, a component of the outer membrane protein assembly machinery in Escherichia coli. Biomolecular NMR assignments. 2009;3:203–6. doi: 10.1007/s12104-009-9175-3. [DOI] [PubMed] [Google Scholar]
- 24.Warner LR, Varga K, Lange OF, Baker SL, Baker D, Sousa MC, Pardi A. Structure of the BamC two-domain protein obtained by Rosetta with a limited NMR data set. Journal of molecular biology. 2011;411:83–95. doi: 10.1016/j.jmb.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KH, Aulakh S, Paetzel M. Crystal structure of beta-barrel assembly machinery BamCD protein complex. The Journal of biological chemistry. 2011;286:39116–21. doi: 10.1074/jbc.M111.298166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagan CL, Kahne D. The reconstituted Escherichia coli Bam complex catalyzes multiple rounds of beta-barrel assembly. Biochemistry. 2011;50:7444–6. doi: 10.1021/bi2010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagan CL, Kim S, Kahne D. Reconstitution of Outer Membrane Protein Assembly from Purified Components. Science. 2010;328:890–892. doi: 10.1126/science.1188919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wittig I, Schagger H. Features and applications of blue-native and clear-native electrophoresis. Proteomics. 2008;8:3974–90. doi: 10.1002/pmic.200800017. [DOI] [PubMed] [Google Scholar]
- 29.Bennion D, Charlson ES, Coon E, Misra R. Dissection of beta-barrel outer membrane protein assembly pathways through characterizing BamA POTRA 1 mutants of Escherichia coli. Molecular Microbiology. 2010;77:1153–71. doi: 10.1111/j.1365-2958.2010.07280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uversky VN. Intrinsically disordered proteins from A to Z. The international journal of biochemistry & cell biology. 2011;43:1090–103. doi: 10.1016/j.biocel.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Uversky VN, Dunker AK. Understanding protein non-folding. Biochimica et biophysica acta. 2010;1804:1231–64. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pajon R, Yero D, Niebla O, Climent Y, Sardi√±as G, Garc√≠a D. n., Perera Y, Llanes A, Delgado M, Cobas K, Caballero E, Taylor S, Brookes C, Gorringe A. Identification of new meningococcal serogroup B surface antigens through a systematic analysis of neisserial genomes. Vaccine. 2009;28:532–541. doi: 10.1016/j.vaccine.2009.09.128. [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama S, Yokota N, Tokuda H. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. The EMBO journal. 1997;16:6947–55. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong C, Beis K, Nesper J, Brunkan-Lamontagne AL, Clarke BR, Whitfield C, Naismith JH. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature. 2006;444:226–9. doi: 10.1038/nature05267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowles CE, Li Y, Semmelhack MF, Cristea IM, Silhavy TJ. The free and bound forms of Lpp occupy distinct subcellular locations in Escherichia coli. Molecular Microbiology. 2011;79:1168–81. doi: 10.1111/j.1365-2958.2011.07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gratzer S, Lithgow T, Bauer RE, Lamping E, Paltauf F, Kohlwein SD, Haucke V, Junne T, Schatz G, Horst M. Mas37p, a novel receptor subunit for protein import into mitochondria. The Journal of cell biology. 1995;129:25–34. doi: 10.1083/jcb.129.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lackey SW, Wideman JG, Kennedy EK, Go NE, Nargang FE. The Neurospora crassa TOB complex: analysis of the topology and function of Tob38 and Tob37. PloS one. 2011;6:e25650. doi: 10.1371/journal.pone.0025650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milenkovic D, Kozjak V, Wiedemann N, Lohaus C, Meyer HE, Guiard B, Pfanner N, Meisinger C. Sam35 of the mitochondrial protein sorting and assembly machinery is a peripheral outer membrane protein essential for cell viability. The Journal of biological chemistry. 2004;279:22781–5. doi: 10.1074/jbc.C400120200. [DOI] [PubMed] [Google Scholar]
- 39.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 inframe, single-gene knockout mutants: the Keio collection. Molecular systems biology. 2006;2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clements A, Bursac D, Gatsos X, Perry AJ, Civciristov S, Celik N, Likic VA, Poggio S, Jacobs-Wagner C, Strugnell RA, Lithgow T. The reducible complexity of a mitochondrial molecular machine. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15791–5. doi: 10.1073/pnas.0908264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konieczny MP, Suhr M, Noll A, Autenrieth IB, Alexander Schmidt M. Cell surface presentation of recombinant (poly-) peptides including functional T-cell epitopes by the AIDA autotransporter system. FEMS immunology and medical microbiology. 2000;27:321–32. doi: 10.1111/j.1574-695X.2000.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 42.Leyton DL, Sevastsyanovich YR, Browning DF, Rossiter AE, Wells TJ, Fitzpatrick RE, Overduin M, Cunningham AF, Henderson IR. Size and conformation limits to secretion of disulfide-bonded loops in autotransporter proteins. The Journal of biological chemistry. 2011;286:42283–91. doi: 10.1074/jbc.M111.306118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings / ... International Conference on Intelligent Systems for Molecular Biology ; ISMB. International Conference on Intelligent Systems for Molecular Biology. 1994;2:28–36. [PubMed] [Google Scholar]
- 44.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–9. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]