Clinically important immune dysregulation is an early feature of leukaemia/small lymphocytic lymphoma (CLL) that often precedes other clinical manifestations of this disease. Defects in the adaptive immune system in patients with CLL results in quantitative and qualitative abnormalities in antibody production, and profound changes in T and Natural Killer (NK) cell numbers, ratios, and function (Palmer et al, 2008; Gonzalez-Rodriguez et al, 2010). Monocytes and macrophages, critical for adaptive and innate immune responses, also have an important role in the function of the CLL cell microenvironment, and this relationship continues to be explored (Caligaris-Cappio, 2011). However, little is known about the effects of CLL on monocyte/macrophage physiology and whether alterations in monocytes have any clinical role in CLL. We were especially interested in the possible role of immunosuppressive CD14+ monocytes with reduced HLA-DR expression in CLL as we have observed this phenomena in glioblastoma (Gustafson et al, 2010), non-Hodgkin lymphoma (Lin et al, 2011) and prostate cancer (Vuk-Pavlovic et al, 2010).
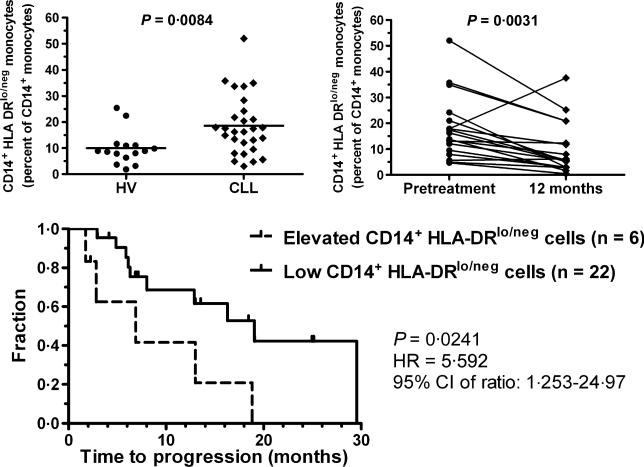
To identify potential monocyte alterations by CLL in patients, we performed flow cytometric analysis of peripheral blood leucocytes in patients with previously untreated early to intermediate stage CLL prior to therapy on a clinical trial of a novel therapy consisting of a 5-week regimen of alemtuzumab, rituximab and granulocyte-macrophage colony-stimulating factor and followed their progress over twelve months (ClinicalTrials.gov NCT00562328; Table 1). Antibody staining of whole blood and flow cytometry data collection was performed as previously described (Gustafson et al, 2010). In healthy individuals, about 90% of circulating CD14+ monocytes are positive for surface HLA-DR. The percentage of monocytes in leucocytes from CLL patients was similar to those seen in healthy volunteers (data not shown). However, the CD14+ monocytes in CLL patients were more likely to have reduced staining for HLA-DR than age-matched healthy volunteers (18·6% ± 11·5% vs. 9·9% ± 6·4%, P = 0·008) (Fig 1). The expression of monocyte activation markers CD16, TNFR2, and CD80 were similar in the CLL patients and healthy volunteers (data not shown). However, CD86 expression on monocytes was decreased in CLL patients versus healthy volunteers (83·9% ± 17·0%; n = 9 vs. 94·9% ± 1·2%; n = 5; P = 0·0290). The diminished expression of HLA-DR and CD86 molecules suggests that the monocytes in CLL patients could have decreased antigen-presenting capacity with reduced immune stimulatory capacity.
Table I.
Patient characteristics.
| Age, median (range) (years) | 59·0 (42·0–77·0) |
| Gender | |
| Female | 8 (27·6%) |
| Male | 21 (72·4%) |
| Follow up status | |
| Alive | 28 (96·5%) |
| Dead | 1 (3·5%) |
| Median follow-up (months) | 26·8 (10·1–38·8) |
| Progression status | |
| No progression | 11 (37·9%) |
| Progression | 18 (62·1%) |
| Rai stage | |
| 0 | 2 (6·9%) |
| 1 | 23 (79·3%) |
| 2 | 4 (13·8%) |
| CD38+ expression | |
| Positive | 14 (48·3%) |
| Negative | 15 (51·7%) |
| IGHV mutation | |
| Mutated | 3 (10·3%) |
| Unmutated | 26 (89·7%) |
| Zap-70 expression | |
| Positive | 21 (75%) |
| Negative | 7 (25%) |
| Fluorescence in situ hybridization, abnormal (24, 82·8%) | |
| 13q14 | 4 (13·8%) |
| 12+ | 6 (20·7%) |
| 11q22 | 10 (34·5%) |
| 17p13 | 3 (10·3%) |
| Other | 1 (3·5%) |
| Normal | 5 (17·2%) |
Fig 1.
CD14+HLA-DRlo/neg Monocytes in CLL. Blood from 29 CLL patients and 15 healthy volunteers were used for immune phenotyping. There was no age difference (median of 59 vs. 58 years, respectively; P = 0·2896). Patients were eligible for the clinical trial (ClinicalTrials.gov NCT00562328) if diagnosed with previously untreated high-risk CLL using standard criteria and did not meet guidelines for conventional treatment. Blood was collected before initiation of treatment and 6, 9, and 12 months after completion of treatment in patients who had a sustained response. The percentage of CD14+ cells with a loss of HLA-DR staining was determined and compared between CLL patients and healthy volunteers (HV; upper left). CLL patients with a sustained response to treatment had a decrease in the frequency of CD14+HLA-DRlo/neg monocytes 12 months after completion of treatment compared to measurement prior to treatment (upper right). Kaplan–Meyer survival curve comparing CLL patients with elevated ratios (>2·5 standard deviations) of CD14+HLA-DRlo/neg monocytes when compared to healthy volunteers (dashed line) or with ratios similar to those seen in healthy volunteers (solid line; bottom panel). HR, Hazard Ratio; 95% CI, 95% confidence interval.
We also studied the relationship between the pre-treatment monocytes, CD14+HLA-DRlo/neg cells, and CD19+ B cell (predominantly CLL cells) counts. For these comparisons we converted the percentage of CD14+HLA-DRlo/neg cells or monocytes into cell counts. There was a positive correlation between the B cell and both monocyte counts (P < 0·0001) and increased numbers of CD14+HLA-DRlo/neg cells (P = 0·0009). We investigated whether a sustained reduction in the absolute B cell count after treatment would be associated with a normalisation of the immune profile in terms of the monocyte pool. In patients who were in sustained remission at 12 months after completion of therapy (n = 18), the median frequency of CD14+HLA-DRlo/neg monocytes decreased (17·9% ± 12·3% pretreatment vs. 9·9% ± 10·0% post-treatment, P = 0·0046, Fig 1; upper right) to a value similar to healthy volunteers. This was not due to selection bias as there was no difference between the pretreatment frequency of CD14+HLA-DRlo/neg monocytes in patients with a sustained 12 month response compared to patients without this response (data not shown). This data supports a model also observed in glioblastoma, of tumour-mediated changes in monocyte phenotype, and suggests that loss of the tumour signal may lead to reversion to a normal phenotype (Gustafson et al, 2010).
To determine if monocyte phenotypes were prognostic, we compared the time to disease progression in patients with elevated pretreatment frequency of CD14+HLA-DRlo/neg monocytes (≥2·5 standard deviations above the healthy volunteer mean) to those with lower levels of CD14+HLADRlo/neg monocytes. The six patients with higher CD14+HLADRlo/neg monocytes had a shorter time to disease progression (median 6·9 months) compared to 19·1 months for patients with lower levels (n = 22; P = 0·024, Fig 1; lower panel). The number of total monocytes did not influence time to progression (data not shown). These data suggest loss of DR expression by CD14 cells is predictive of poorer prognosis.
CD14+HLA-DRlo/neg cells mediate immunosuppression through secretion of IL10 (Loercher et al, 1999; Vuk-Pavlovic et al, 2010) and/or transforming growth factor-β (Valenti et al, 2006), can induce T regulatory populations (Hoechst et al, 2008), inhibit responder T cells (Hoechst et al, 2008; Gustafson et al, 2010; Vuk-Pavlovic et al, 2010; Lin et al, 2011), and have defects in dendritic cell maturation (Gustafson et al, 2010; Lin et al, 2011). There is also growing evidence that ‘nurse-like cells’ are derived from CD14+ cells (Tsukada et al, 2002). Monocytes probably play a dual role in CLL, of promoting CLL survival and mediating CLL-induced immunosuppression, reflecting the importance of myeloid cell interaction on the pathogenesis of CLL. The elevated frequency of CD14+HLA-DRlo/neg monocytes and their association with increased tumour burden and poorer prognosis suggests an important role of monocytes in the pathogenesis of CLL.
Additionally, this data highlights their role as a biomarker and a potential important target for novel therapies.
Acknowledgements
The authors thank Timothy G. Call, Tait D. Shanafelt, Neil E. Kay, and Deborah A. Bowen for recruiting patients, Constance Sylak for her administrative assistance with patient recruitment; and Amy Mohr and Mary Maas for technical assistance. This work was supported in part by the University of Iowa/Mayo Clinic Lymphoma SPORE CA097274, Genentech, and Genzyme.
Footnotes
Conflict of interest
The authors have no conflict of interests to disclose.
References
- Caligaris-Cappio F. Inflammation, the microenvironment and chronic lymphocytic leukemia. Haematologica. 2011;96:353–355. doi: 10.3324/haematol.2010.039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez AP, Contesti J, Huergo-Zapico L, Fernandez-Guizan A, Acebes-Huerta A, Gonzalez-Huerta AJ, Gonzalez E, Fernandez-Alvarez C, Gonzalez S. Prognostic significance of CD8 and CD4 T cells in chronic lymphocytic leukemia. Leukaemia & Lymphoma. 2010;51:1829–1836. doi: 10.3109/10428194.2010.503820. [DOI] [PubMed] [Google Scholar]
- Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-Oncology. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–881. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian cancer that inhibits cytokine protien expression and proliferation of autologous T cells. Journal of Immunology. 1999;163:6251–6260. [PubMed] [Google Scholar]
- Palmer S, Hanson CA, Zent CS, Porrata LF, Laplant B, Geyer SM, Markovic SN, Call TG, Bowen DA, Jelinek DF, Kay NE, Shanafelt TD. Prognostic importance of T and NK-cells in a consecutive series of newly diagnosed patients with chronic lymphocytic leukaemia. British Journal of Haematology. 2008;141:607–614. doi: 10.1111/j.1365-2141.2008.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002;99:1030–1037. doi: 10.1182/blood.v99.3.1030. [DOI] [PubMed] [Google Scholar]
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Research. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]