Summary
Starvation activates MAPK in the pharyngeal muscles of C. elegans through a muscarinic acetylcholine receptor, Gqα, and nPKC as shown by the following results: (1) Starvation causes phosphorylation of MAPK in pharyngeal muscle. (2) In a sensitized genetic background in which Gqα signaling cannot be downregulated, activation of the pathway by a muscarinic agonist causes lethal changes in pharyngeal muscle function. Starvation has identical effects. (3) A muscarinic antagonist blocks the effects of starvation on sensitized muscle. (4) Mutations and drugs that block any step of signaling from the muscarinic receptor to MAPK also block the effects of starvation on sensitized muscle. (5) Overexpression of MAPK in wild-type pharyngeal muscle mimics the effects of muscarinic agonist and of starvation on sensitized muscle. We suggest that, during starvation, the muscarinic pathway to MAPK is activated to change the pharyngeal muscle physiology to enhance ingestion of food when food becomes available.
Introduction
In C. elegans, starvation causes a variety of changes in development, longevity, and behavior. Starvation of newly hatched larvae causes arrest in the L1 (first larval) stage. If starvation happens in the L2 stage, worms can choose an alternative form called the dauer, which can survive long-term starvation (Riddle et al., 1981). Thermotaxis and chemotaxis studies show that worms learn to associate environmental cues such as temperature or smell not only with presence of food but also with absence of food (starvation), so as to actively avoid starvation (Hedgecock and Russell, 1975; Saeki et al., 2001).
The C. elegans feeding organ, the pharynx, also changes its behavior during starvation. In wild-type worms, feeding occurs when the pharyngeal muscle contracts and relaxes (pumps) to take in bacteria as food. This feeding behavior, pumping, is controlled by the worm’s internal feeding status. During short periods (e.g., 6 hr) of starvation, the pumping rate gradually increases, as if worms sample the environment in order to increase their chance of ingesting food (Avery and Horvitz, 1990). Food quality also controls pumping rate. Food quality is defined operationally by two criteria: high-quality food supports growth better than poor quality food, and given a choice, worms prefer the food that best supports growth (Shtonda and Avery, 2006). Low-quality food that starves worms increases pumping rate, while high-quality food decreases pumping rate (data not shown). These findings suggest that there is a signal generated in response to starvation to change the animal’s behavior. But neither the identity of the signal nor the pathway through which it acts has been fully defined.
One starvation-induced signal in other systems is activation of the MAPK (mitogen-activated protein kinase) pathway (Malone, 1990; Roberts and Fink, 1994). In yeast, starvation induces pseudohyphal growth, allowing them to invade the substrate so as to forage actively for nutrients. Constitutive activation of the MAPK pathway enhances this growth (Gimeno et al., 1992; Roberts and Fink, 1994), suggesting a fundamental role of the pathway in response to starvation.
Muscarinic receptors are G protein-coupled acetylcholine receptors, whose roles in smooth muscle contractibility, heart hypertrophy, and neuronal plasticity, as well as in controlling food intake, are known (Bartus et al., 1982; Wess et al., 2003). One of the widely studied downstream pathways is the MAPK pathway (Berkeley et al., 2001; Hamilton and Nathanson, 2001). The C. elegans pharyngeal muscle contracts upon acetylcholine release from the MC motor neurons (McKay et al., 2004; Raizen et al., 1995). Fast and precisely coordinated pumping is critical for the competition in the natural environment, allowing them to eat as much as possible of the available food, so as to grow faster and leave more progeny than worms that pump slowly or with poor coordination. Our previous studies showed that acetylcholine is the critical neurotransmitter not only to effect fast pumping through nicotinic receptors but also to regulate pumping through muscarinic receptors (Raizen et al., 1995; Steger and Avery, 2004).
In this study, we show that starvation activates muscarinic signaling to MAPK and that this starvation signal alters pharyngeal muscle function. We propose that one purpose of the signal is to change pharyngeal muscle physiology to enhance ingestion of food when it becomes available again.
Results
Starvation and a muscarinic agonist activate MPK-1 in pharyngeal muscle
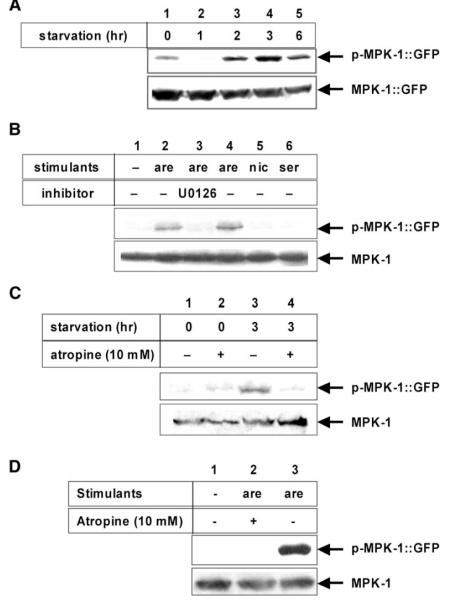
MPK-1 is the C. elegans ortholog of mammalian ERK1/2. It shares 88% sequence identity with ERK2 (Wu and Han, 1994). In order to study MPK-1 function in pharyngeal muscle, we generated transgenic worms that expressed GFP fused to MPK-1 under the control of the pharyngeal muscle specific myo-2 promoter (Okkema et al., 1993). To block the harmful effects of MPK-1 overexpression (described below), the mpk-1::gfp transgene carried a K57R mutation to inactivate the kinase. Pharyngeal MPK-1::GFP, which could be distinguished from endogenous MPK-1 by its size, was activated after 3 hr of starvation (Figure 1A).
Figure 1.
Starvation and a muscarinic agonist activate pharyngeal muscle MPK-1
A) Activation of pharyngeal muscle MPK-1 in synchronized wild-type young adults starved for the indicated times. Three hours of starvation activated pharyngeal muscle MPK-1 as detected by phospho-specific anti-MPK-1 antibody (p-MPK-1::GFP). MPK-1::GFP is shown as loading control.
B) Activation of pharyngeal muscle MPK-1 in synchronized wild-type young adults treated with various chemicals. Thirty minute treatment with 1 mM and 5 mM arecoline activated pharyngeal muscle MPK-1 (lanes 2 and 4), but neither 5 mM nicotine (lane 5) nor 10 mM serotonin (lane 6) did. Cotreatment with 100 μM U0126 blocked activation of MPK-1 by 1 mM arecoline (lane 3).
C) Starvation-induced pharyngeal muscle MPK-1 activation was inhibited by 10 mM atropine treatment during starvation. Lane 1: no starvation, lane 2: no starvation + 10 mM atropine, lane 3: 3 hr of starvation, lane 4: 3 hr of starvation + 10 mM atropine.
D) Arecoline-induced pharyngeal muscle MPK-1 activation was inhibited by cotreatment with 10 mM atropine. Lane 1: control, lane 2: 1 mM arecoline + 10 mM atropine, lane 3: 1 mM arecoline.
The muscarinic agonist arecoline activated pharyngeal muscle MPK-1. Two other drugs that affect feeding, nicotine and serotonin, did not (Figure 1B). Activation of MPK-1 by either starvation or arecoline was blocked by the muscarinic antagonist atropine, suggesting that starvation might cause muscarinic signaling to activate MPK-1 in the pharyngeal muscle (Figures 1C and 1D). Starvation and arecoline also activated endogenous MPK-1, indicating that the activation is physiological (Figure S1 in the Supplemental Data available with this article online).
gpb-2 mutants are sensitive to starvation and to excessive muscarinic signaling
To test the hypothesis that starvation might cause muscarinic signaling, we used gpb-2 mutants as a sensitized background. gpb-2 mutants are hypersensitive to excessive muscarinic signaling, due to the lack of a negative regulator of Gqα signaling (Robatzek et al., 2001). Steger and Avery (2004) have shown that gar-3, a muscarinic receptor coupled to Gqα, is upstream of gpb-2 for this hypersensitivity: treatment of gpb-2 with 5 mM arecoline causes hypercontraction of the pharyngeal muscle and subsequent lethality through gar-3 and Gqα.
If starvation activates muscarinic signaling, then starvation, like muscarinic agonists, should kill gpb-2 worms. In fact, after 3 days of starvation, 70% of gpb-2 L1s couldn’t recover in the presence of food (Figure 2A). They failed to grow and eventually died. In contrast, 99% of wild-type worms survived and grew. Addition of food during incubation completely blocked the lethality, excluding the possibility that liquid cultivation affected the viability of gpb-2 mutants and confirming that starvation was the cause of the defect. gpb-2 mutants have a mild feeding defect, but this doesn’t explain their inability to recover from starvation. eat-4 mutants, which have a feeding defect of similar severity (Lee et al., 1999a), didn’t show sensitivity to starvation (data not shown).
Figure 2.
gpb-2 mutants are sensitive to starvation due to defect in pharyngeal muscle
A) Percent of worms recovering (shown as “% that grow”) on E. coli seeded plates after incubation in M9 buffer for the indicated time (see Experimental Procedures): gpb-2 with (◆) or without (▴) food were compared to wild-type without food (∎). After 3 days of starvation, about 70% of gpb-2 mutants were not able to recover. This lethality is caused by starvation, because it was rescued by addition of food during incubation. Error bars represent SEM.
B) Effect of bacterial size during recovery on the survival rate of starved gpb-2 mutants. Small size bacteria (Comamonas) worsened survival of gpb-2 compared to larger size bacteria (E. coli).
C) Pharyngeal muscle targeted GPB-2 rescued starvation sensitivity of gpb-2 mutants. About 86% of transgenic worms expressing wild-type GPB-2 only in pharyngeal muscle recovered on Comamonas seeded plates after 3 days of starvation. About 2% of nontransgenic siblings recovered. Data were obtained from two independent experiments. Error bars represent SEM.
D) Pharyngeal muscle targeted GPB-2 rescued arecoline sensitivity of gpb-2 mutants. About 83% of transgenic worms expressing wild-type GPB-2 only in pharyngeal muscle recovered from treatment with 5 mM arecoline. None of nontransgenic siblings recovered. Data were obtained from two independent experiments. Error bars represent SEM.
Starvation and arecoline treatment result in the identical pharyngeal phenotypes in gpb-2 mutants
gpb-2 mutants recovering on food after 3 days of starvation showed the same phenotype (Figures 3B and 3C) as gpb-2 after 24 hr of arecoline treatment (Figures 3D and 3E): the grinders (indicated by arrows Figures 3A–3E) were stuck open and there were unground bacteria in the intestine, as indicated by fluorescence from GFP-expressing E. coli (compare Figures 3G, 3J, and 3M). This open conformation is only seen very briefly and transiently in the wild-type during pharyngeal muscle contraction. Thus gpb-2 pharyngeal muscle was hypercontracted and couldn’t relax. In contrast, the wild-type grinder was in the fully relaxed and closed conformation (Figure 3A) and there were no unground bacteria in the intestine. Because the grinder mechanically breaks bacteria, the presence of unground bacteria in the intestine is consistent with the grinder defect of gpb-2 mutants after starvation or arecoline treatment. Thus, both arecoline and starvation caused identical pharyngeal muscle hypercontraction, which could cause lethality.
Figure 3.
Starvation and arecoline treatment cause the identical defect in pharyngeal muscles in gpb-2 mutants
A) A wild-type pharynx after 3 days of starvation as an L1 followed by recovery on E. coli seeded plates for 5 hr. The arrow indicates the grinder, a set of three wing-shaped plates in the terminal bulb. (Only two of the plates are visible in a single focal plane.) In this picture, the wild-type grinder shows a closed conformation. The plates of the grinder were fully relaxed. (A–E) Each inset is a schematic drawing of the grinder shape.
B and C) Terminal bulb and grinder plates (arrow) of gpb-2 mutants after 3 days of starvation as L1s followed by recovery on E. coli seeded plates for 5 hr. As illustrated in the insets, the grinders were hypercontracted and open. There is a range of extent of hypercontraction from full opening (B) to persistent contraction (C).
D and E) Terminal bulb and grinder plates (arrow) of gpb-2 mutants after 24 hr exposure to 5 mM arecoline on E. coli seeded plates. The same types of hypercontraction were observed. Full opening (D) and persistent contraction (E) of grinder muscle morphology are almost identical to those of 3 day-starved gpb-2 after refeeding. (Compare [D] to [B] and [E] to [C]).
F–H) A gpb-2 mutant after 3 days of starvation as an L1 followed by recovery on GFP-expressing E. coli seeded plates for 5 hr. (F) Differential interference contrast image of the whole worm. (G) Green fluorescence image of (F). Fluorescence indicates unground bacteria in the pharynx and intestine. (H) Overlay of (F) and (G).
I–K) A gpb-2 mutant after 24 hr of exposure to 5 mM arecoline on GFP-expressing E. coli seeded plates. (I) Differential interference contrast image of the whole worm. (J) Green fluorescence image of (I). Fluorescence indicates unground bacteria in the pharynx and intestine. (K) Overlay of (I) and (J).
L–N) A wild-type control after 3 days of starvation as L1 followed by recovery on GFP-expressing E. coli seeded plates for 5 hr. (L) Differential interference contrast image of the whole worm. (M) Green fluorescence image of (L). Very little fluorescence was detected from trapped GFP bacteria in the anterior terminal bulb of the pharynx and auto-fluorescence. (N) Overlay of (L) and (M).
Small bacteria aggravate the lethality
To test whether pharyngeal muscle abnormality and unground bacteria cause the starvation-induced lethality of gpb-2 mutants, we used two different sizes of bacteria as food during recovery. For normal worms, Comamonas bacteria are easy to eat because of their small size (Avery and Shtonda, 2003). Mutants with grinder defects, in contrast, do not grow better on Comamonas (S. Straud, personal communication). Since the grinder can’t grind bacteria efficiently when it is either malformed or hypercontracted, small bacteria would be passed more easily into the intestine unground, leading to failure to absorb nutrients, which could contribute to lethality. Unstarved gpb-2 mutants grow on Comamonas as well as on E. coli (data not shown). However, after 3 days of starvation, less than 5% of gpb-2 mutants grew on small bacteria (Comamonas), while on the larger bacteria (E. coli), about 30% of gpb-2 mutants grew (Figure 2B). The anterior intestine of the worms that failed to grow on Comamonas was full of unground bacteria (data not shown). This result indicates that the size of food affects the viability, suggesting that the defect in pharyngeal function is the primary cause of the lethality. We suspect that the failure of starved gpb-2 mutants to survive is due to their inability to extract nutrients from unground bacteria, not to pathogenic effects of unground bacteria in the intestine, because UV-killed E. coli or Comamonas didn’t enhance the recovery rate (Figure S3).
Pharyngeal muscle targeted gpb-2 rescues the lethality
To confirm that a defect in pharyngeal muscle is the cause of the lethality, we generated a transgenic line that expressed wild-type GPB-2 only in the pharyngeal muscle using myo-2 promoter (Okkema et al., 1993). The transgenic line rescued both starvation sensitivity and arecoline sensitivity of gpb-2 mutants (Figures 2C and 2D). After 3 days of starvation, about 87% of transgenic worms survived on Comamonas, while only 2% of nontransgenic siblings survived (Figure 2C). For arecoline treatment (5 mM), about 83% of transgenic worms survived while 0% of nontransgenic siblings did (Figure 2D).
Overexpression of MPK-1 in the pharyngeal muscle phenocopies the feeding defect of starved gpb-2 mutants
In Figure 1, we showed biochemically that pharyngeal muscle MPK-1was a downstream target of both starvation and muscarinic signaling. Not surprisingly, arecoline caused more activation of endogenous MPK-1 in gpb-2 mutants than in wild-type (Figure 4A). Could hyperactivation of MPK-1 in gpb-2 pharyngeal muscle be the cause of hypercontraction and lethality caused by starvation and muscarinic agonist? To test this idea, we overexpressed wild-type MPK-1 in pharyngeal muscle. Five independent transgenic lines showed symptoms of a severe feeding defect, such as L1 arrest, slow growth rate, and a severely starved appearance. Moreover, video recording showed that MPK-1 overexpression phenocopied the abnormal grinder movement and unground bacteria phenotypes of starved or arecoline treated gpb-2 (compare Figures 4B and 4C). Similar transgenic lines carrying a K57R mutation to inactivate the kinase activity of MPK-1 did not show this feeding defect (data not shown). Thus the feeding defect, probably caused by grinder hypercontraction, is caused by the kinase activity of MPK-1.
Figure 4.
Overexpressing MPK-1 in the pharyngeal muscle phenocopies the grinder defect in starved gpb-2 mutants
A) Hyperactivation of MPK-1 in gpb-2 mutants. Young adults of wild-type (lane 1–3) and gpb-2 worms (lane 4–6) were treated with no arecoline (0 mM: lanes 1 and 4) or arecoline at two different concentrations, 0.1 mM (lanes 2 and 5) or 1 mM (lane 3 and 6), as indicated. In gpb-2 mutants, MPK-1 was more activated by treatment with either 0.1 mM or 1 mM arecoline (lanes 5 and 6, respectively), compared to wild-type (lanes 2 and 3, respectively). Quantitation indicated 2.9-fold more increase in the band intensity in gpb-2 mutants compared to in wild-type (data not shown).
B) Terminal bulb and grinder muscle (arrow) of a wild-type worm. The grinder was closed and there were no unground bacteria in the intestine.
C) Terminal bulb and grinder muscle (arrow) of a MPK-1 overexpressing worm. The grinder stayed open and there were unground bacteria in the intestine. Unground bacteria in the anterior intestine are visible by their textured appearance (circled region). (For comparison, the dotted circle outside the worm also shows unground bacteria.) Unground bacteria are more clearly visible in the video segment (see Supplemental Data).
Blocking muscarinic signaling rescued gpb-2 sensitivity to starvation as well as to arecoline
We took pharmacological and genetic approaches to further test whether the muscarinic signal is a starvation signal. Treatment with the muscarinic antagonist atropine during starvation rescued the starvation sensitivity of gpb-2, confirming that muscarinic receptors mediate the signal (Figure 5B: gpb-2 + atropine). In this experiment, we blocked muscarinic signaling only during starvation, not during recovery. Thus, the muscarinic signaling that causes lethality occurs during starvation, not during recovery. To find out which muscarinic receptor is responsible for the starvation-induced lethality in gpb-2, we took a genetic approach. C. elegans has three known muscarinic receptor genes, gar-1, gar-2 and gar-3 (Lee et al., 1999b). gar-2; gar-3; gar-1 triple knock out mutants rescued starvation-induced lethality of gpb-2 to a similar extent as a gar-3 single mutant (Figure 5A). This result is consistent with our previous results that GAR-3, but not GAR-1 or GAR-2, is responsible for the arecoline hypersensitivity of gpb-2 mutants (Steger and Avery, 2004). Also, this result shows that both starvation and arecoline kill gpb-2 mutants at least in part through a muscarinic receptor GAR-3, thus confirming that a muscarinic receptor mediates the starvation signal. Gqα mutation egl-30 fully suppressed the effects of starvation of gpb-2 both on E. coli and on Comamonas (Figure 5B), confirming the interaction between the muscarinic receptor and Gqα in starvation signaling.
Figure 5.
Blocking muscarinic signaling rescues gpb-2 sensitivity to starvation
A) Percent recovery of gpb-2; gar-3 or gpb-2; gar-1; gar-2; gar-3 on E. coli seeded plates after 3 days of starvation. The triple-knockout mutant did not show enhanced rescue effect compared to the gar-3 single mutant.
B) Percent recovery of double mutants of gpb-2 with muscarinic receptor mutations gar-3 or Gqα mutation egl-30, or of single mutants treated with atropine during starvation. Worms of the indicated genotypes were plated either on E. coli seeded plates (∎) or on Comamonas seeded plates (◻) after 3 days of starvation. Introduction of gar-3 or egl-30 mutations into gpb-2 mutants rescued the sensitivity, as did treatment with atropine (10 mM) during starvation.
Atropine rescued gpb-2 on Comamonas more effectively than muscarinic receptor mutants (Figure 5B). This might suggest that GAR-3 muscarinic receptor function during recovery helps to adjust the pharyngeal muscle to different sizes of bacteria, or that an uncharacterized receptor other than GAR-1, GAR-2, or GAR-3 contributes to starvation-induced lethality (Putrenko et al., 2005).
Starvation and muscarinic signaling activate pharyngeal MPK-1 through the nPKC- MAPK pathway
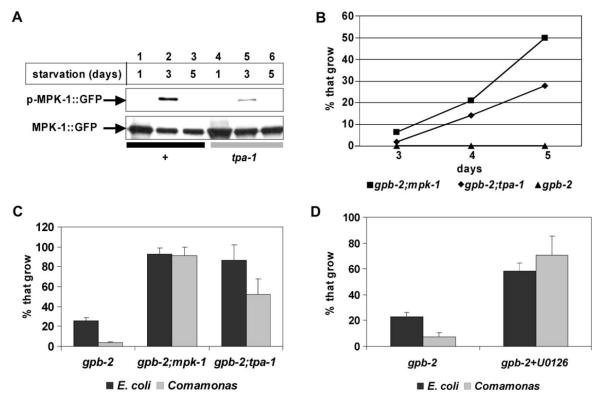
We attempted to find the components of the pathway from the muscarinic signal to MAPK. Protein kinase Cs (PKCs) can be activated downstream of Gqα and are known upstream activators of the MAPK pathway (El-Shemerly et al., 1997; Hamilton et al., 2001; Robbins et al., 1992). In fact, TPA-1, the C. elegans ortholog of the δ/ε form of novel protein kinase C (Tabuse et al., 1995), was required for muscarinic activation of MPK-1 (Figure S2). Under the conditions that induce lethality in gpb-2 mutants (3 days of starvation at the L1 stage), starvation activated pharyngeal muscle MPK-1, and the activation was greatly reduced in tpa-1 mutants (Figure 6A). Thus, nPKC is necessary for full MPK-1 activation by starvation in pharyngeal muscle.
Figure 6.
Starvation and muscarinic signal activates pharyngeal MPK-1 through the nPKC- MAPK pathway and mutations in the pathway rescue gpb-2 sensitivity to starvation and arecoline
A) Activation of pharyngeal muscle MPK-1 after indicated days of starvation at the L1 stage. Worms in lanes 1–3 had a wild-type genetic background; and lanes 4–6 were tpa-1 mutants. Three days of starvation activated pharyngeal muscle MPK-1 (p-MPK-1::GFP). In the tpa-1 mutant background, the activation was greatly reduced.
B) mpk-1 and tpa-1 mutations rescued gpb-2 sensitivity to arecoline. The number of worms that reached L4 or adult stage on the indicated day were counted and accumulated for the next time point. While gpb-2 mutants couldn’t grow at all for 5 days in the presence of 5 mM arecoline, about 30% of gpb-2;tpa-1 and 50% of gpb-2;mpk-1 grew and reached adulthood.
C) mpk-1 and tpa-1 mutations rescued gpb-2 sensitivity to starvation, as shown by improved recovery on both E. coli and Comamonas.
D) Treatment with the MEK inhibitor U0126 (25 μM) during starvation rescued gpb-2 sensitivity to starvation on E. coli and Comamonas.
nPKC and MAPK mutants rescue the starvation sensitivity and arecoline sensitivity of gpb-2
Starvation and muscarinic signal activated MPK-1 through nPKC. To find out if this pathway also mediates gpb-2 sensitivity to arecoline and starvation, gpb-2; mpk-1 and gpb-2; tpa-1 double mutants were tested. mpk-1 and tpa-1 mutations suppressed gpb-2 sensitivity to arecoline (Figure 6B) and to starvation, allowing recovery both on E. coli and on Comamonas (Figure 6C). Treatment with the MEK inhibitor U0126 during starvation also rescued the sensitivity of gpb-2 mutants, confirming that the hyperactivation of the MAPK pathway during starvation contributed to the starvation sensitivity of gpb-2 (Figure 6D).
Pumping rate changes during starvation in a muscarinic signaling dependent manner
To understand the purpose of the muscarinic signaling to MAPK pathway during starvation, we measured pumping rate (Figure 7A). Wild-type worms initially pumped slowly when they were taken off food, but they increased pumping rate gradually in the first 2 hr of starvation. When the pharyngeal muscle GAR-3 → MPK-1 pathway was blocked with a gar-3 mutation, the increase in pumping rate was reduced. Conversely, in gpb-2 mutants, in which the pathway is hyperactive, pumping rate increased more on starvation. These data suggest that activation of the muscarinic receptor during starvation contributed to the increase in starvation-induced pharyngeal activity.
Figure 7.
Starvation induces pumping in a muscarinic signaling-dependent manner
A) Starvation induced pumping in a muscarinic signaling-dependent manner. Pumping rates increased for first 2 hr in the absence of food. Two mutants defective in muscarinic signaling showed altered pumping rates compared to wild-type. gpb-2 mutants, which have upregulated muscarinic signaling, showed a higher pumping rate, and gar-3 mutants, which have downregulated muscarinic signaling, showed a lower pumping rate than wild-type.
B) A suggested pathway for function of the muscarinic receptor → MAPK pathway in starvation.
Discussion
We showed that starvation activated MAPK in C. elegans feeding muscle through a muscarinic receptor → Gqα → nPKC pathway. Based on the observation that both starvation and a muscarinic agonist activated pharyngeal muscle MPK-1, we hypothesized starvation activated muscarinic signaling to MPK-1. Using the sensitized muscle of a gpb-2 mutant that is hypersensitive to muscarinic signaling due to unrestrained Gqα signaling, we were able to demonstrate the pathway biochemically and genetically, identifying the muscarinic signal as starvation signal. We showed that both starvation and muscarinic signaling caused an identical specific pharyngeal muscle phenotype: grinder malfunction and unground bacteria. Our results strongly suggest that this defect in the pharyngeal muscle was the primary cause of the lethality in gpb-2 mutants because: (1) the lethality was dependent on the size of food, (2) pharyngeal muscle-specific expression of GBP-2 rescued the lethality, and (3) overexpressing MPK-1 only in the pharyngeal muscle phenocopied the starved gpb-2 phenotype. Using pharmacological, biochemical, and genetic approaches, we were able to identify components of the muscarinic receptor → Gqα → nPKC → MAPK pathway. gpb-2 sensitivity both to a muscarinic agonist and to starvation was rescued by inhibition of this pathway, strongly suggesting that the same pathway mediates starvation sensitivity of gpb-2 mutants.
This discovery raises interesting questions. For instance, what is the purpose of muscarinic signal in response to starvation? We propose that pharyngeal muscle, one of the biggest muscles of C. elegans as well as the most important organ for feeding, must undergo changes in order to survive starvation and perhaps to prepare for recovery, and that the muscarinic signal brings about these changes. The increase of pumping rate during starvation (Figure 7A) suggests that one change might be enhanced muscle responsiveness to food. Well-fed worms decrease the pumping rate when the food concentration is lowered (Avery and Horvitz, 1990). If starvation persists, however, the pumping rate increases again even at continued low food concentration. Increased feeding muscle responsiveness might be one mechanism that causes this change in behavior. When starved worms were transferred to food plates, they showed an enhanced feeding response (a great increase in pumping rate) compared to worms that hadn’t experienced starvation (Avery and Horvitz, 1990). This suggests that starvation causes changes in feeding responsiveness so that when they encounter food, they can eat faster. Considering that acetylcholine is the major neurotransmitter to effect fast pumping at the pharyngeal neuromuscular junction (McKay et al., 2004; Raizen et al., 1995), it is plausible that a G protein coupled muscarinic signal in the muscle might fine tune muscle contractility under conditions like starvation. Based on these observations, we suggest a simplified pathway (Figure 7B), summarizing how muscarinic signaling to MAPK might be activated by starvation in order to change muscle physiology.
How does this finding link to muscarinic signaling in other organisms? In mammals, most muscarinic receptors are involved in muscle function. For instance, they control heart muscle and smooth muscle in the digestive tract. The mammalian muscarinic receptor with highest similarity to GAR-3, the M3 muscarinic receptor, is expressed predominantly in brain and in muscles of the gastrointestinal tract. Moreover, when the expression of the M3 receptor is genetically disrupted, the mice show a lean phenotype due to decreased food intake (Yamada et al., 2001), analogous to the effect of gar-3 disruption in C. elegans (Figure 7A). This suggests that muscarinic receptor function in controlling food intake could be conserved among animals.
During long-term starvation, autophagy is induced to supply energy (Lum et al., 2005). Our preliminary data show that during starvation, autophagy is induced in wild-type pharyngeal muscle, and more rapidly and strongly in the pharyngeal muscle of gpb-2 mutants (C. Kang, personal communication). Muscarinic signal-induced autophagy could contribute both to the ability of the wild-type to survive starvation and to gpb-2 lethality. We found that 5 days of starvation killed gpb-2 mutants even before they were fed, and that their extrapharyngeal bodies appeared severely damaged. This suggests that there could be more than one mechanisms or places to make gpb-2 mutants sensitive to starvation. We are currently pursuing this in search of an integrated view of starvation signaling.
Despite the prevalence of feeding disorders from obesity to anorexia, the identity and mechanism of action of starvation signals are largely unknown. Our study of starvation sensitivity of gpb-2 mutants and the downstream signaling pathways in feeding muscles suggests that feeding disorders may result from inappropriate behavioral responses to starvation signals.
Experimental procedures
General methods and strains
Worms were cultured and handled as described previously (Sulston and Hodgkin, 1988) with the following modifications: worms were routinely grown on NGMSR plates (Avery, 1993) with nystatin. All worms were maintained at 20°C on E. coli strain HB101 (Boyer and Roulland-Dussoix, 1969) unless indicated otherwise. The wild-type strain was C. elegans variant Bristol, strain N2. Mutant strains used were DA541 gpb-2(ad541) I, JD283 gpb-2(ad541) I; gar-3(lg1201) V, DA2050 gpb-2(ad541) I; mpk-1(ku1) III, DA2051 gpb-2 (ad541) I; tpa-1(k530) IV, DA803 egl-30(ad803sd) gpb-2(ad541) I, DA2038 adIs2038[myo-2p::MPK-1K57R::GFP], DA2052 adEx2052[myo-2p::mpk-1:: GFP], MJ563 tpa-1(k530) IV, JD276 gpb-2(ad541) I; gar-2(by124) III; gar-3 (lg1201) V; gar-1(ad1676) X.
PCR construction of mpk-1::GFP fusion with myo-2 promoter, myo-2p::mpk-1::GFP construct
myo-2 promoter region (PCR #1) was amplified from pPD96.48 vector (a gift from A. Fire) using primers 5′-GTGGTGGACAGTAACTGTCTG-3′ and 5′-AACCGCTTCTCCGTCGGCCATTTTTTCTACCGGTACCGT-3′. cDNA clone yk531h7 from Y Kohara, was used to amplify the mpk-1 coding region using 5′-GTACCGGTAGAAAAAATGGCCGACGGAGAAGCGGTTATC-3′ and 5′-GGTCCTTTGGCCAATAACAGGATTCTGCCCTCCATTATT-3′ (PCR #2). gfp coding DNA containing the unc-54 3′ UTR region was amplified from pPD95.79 using 5′-GGTCCTTTGGCCAATAACAGGATTCTGCCCTCCATTATT-3′ and 5′-GTACGGCCGACTAGTAGGA-3′ (PCR #3). PCR #2 and # 3 were fused using 5′-GTACCGGTAGAAAAAATGGCCGACGGAGAAGCGGTTATC-3′ and 5′-GGAAACAGTTATGTTTGGTATATTGGG-3′ (PCR #4). Finally, myo-2 promoter (PCR #1) was fused to the mpk-1::GFP translational fusion (PCR #4) using 5′-TCCTGACCAGGTTGCAATTC-3′ and 5′-GGAAACAGTTATGTTTGGTATATTGGG-3′. A kinase-dead mutation (K57R) of mpk-1 was introduced to primers 5′-ACTCGTGATCGCGTTGCTATCAGAAAGATTTCTCCA-3′ (backward primer) and 5′-ATGTTCGAATGGAGAAATCTTTCTGATAGCAACGCG-3′ (forward primer). Substituted nucleotides are underlined.
PCR construction of wild-type gpb-2 with myo-2 promoter, myo-2p::gpb-2 construct
1.3 kb of myo-2 upstream sequence was obtained using primers, 5′-TCCTGACCAGGTTGCAATTC-3′ and 5′-TGGCTGAGAGTTTTCTGGCATTTTTTCTACCGGTACCGT-3′. 3.4 kb of gpb-2 genomic DNA was obtained using, 5′-GTACCGGTAGAAAAAATGCCAGAAAACTCTCAGCCAACA-3′ and 5′-TCAATCTTACTCTATCTGCTAC-3′. Finally these two PCR products were fused using, 5′-GGGTTTTGTGCTGTGGACG-3′ and 5′-TAGTTGCAGTAATCCGATTCAA-3′.
Transgenic and integration lines
adEx2052[myo-2p::mpk-1::GFP], carrying an extrachromosomal array of mpk-1 (cDNA) fused with GFP under the control of the myo-2 promoter, was obtained by injecting PCR products (20 μg/ml) into wild-type worms.
A myo-2p::mpk-1::GFP DNA construct carrying a K57R mutation in the mpk-1 gene was injected into wild-type worms and the transgenic lines were irradiated with γ rays (6500 rad) to produce an integration line, adIs2038[myo-2p::mpk-1K57R::GFP].
For rescuing gpb-2 mutants, a myo-2p::gpb-2 DNA construct was injected into gpb-2 mutants at 10 μg/ml with 10 μg/ml of a myo-2p::mpk-1K57R::GFP DNA construct as a coinjection marker.
For Figure 6A, adIs2038 was introduced into the tpa-1 mutant background by crossing.
Chemicals
Arecoline hydrobromide, atropine sulfate salt, nicotine and serotonin creatinine sulfate complex were purchased from Sigma. U0126 (a MEK inhibitor) was purchased from Promega.
Antibodies
ERK1/2 antibody (M5670) and phospho-specific ERK1/2 (p-ERK) antibody (M8159) were purchased from Sigma. Goat anti-rabbit (sc-2004) and goat anti-mouse (sc-2055) conjugated to horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology.
Arecoline sensitivity assay
As described in Steger and Avery, 2004.
Western blot assay
Sample buffer was made and electrophoresis of proteins was performed as described (Sambrook et al., 1989) with the following changes. After trans-blotting, membranes were incubated in blocking buffer (10% nondry milk and 1% BSA in 0.5% TBST) overnight. Membranes were incubated with antibody in 0.5% TBST (1% milk, 0.1% BSA) at 1:2500 dilution for the primary antibody and 1:4000 for the secondary antibody. Enhanced chemiluminescence (ECL) was used as the method of detection.
Sample preparation of starved worms for Western blot assay
All worms were synchronized by egg preparation (Lewis and Fleming, 1995). For long-term starvation test (days of starvation), eggs were incubated at 20°C for 24 hr in M9 buffer. At 24 hr of incubation, approximately 10,000 L1s in 0.2 ml of M9 buffer (50 L1s/μl) were distributed into each of several 1.5 ml microcentrifuge tubes. The sample for ‘day 1 starvation’ was harvested immediately. The tubes for further starvation test were placed on a rocker at 20°C for indicated times of starvation. After incubation, samples were harvested by 30 s of centrifugation and kept on ice for 10 min. After supernatant was removed, samples were kept at −80°C until western blot assay. For short-term starvation tests (hours of starvation), worms were synchronized and grown on E. coli seeded plates until they were young adults. Well-fed worms were washed off the plates, and then washed 2 times with M9 buffer to remove remaining bacteria. Approximately, 500 adults in 0.2 ml of M9 buffer (2.5 adults/μl) were incubated for the indicated times (0, 1, 2, 3, and 6 hr). After incubation, samples were harvested and handled as described above.
Sample preparation of drug-treated worms for Western blot assay
All worms were synchronized and grown until they were young adults as described above. Well-fed worms were washed from plates with M9 butter and further washed twice with the buffer. For each lane approximately 500–1000 adults were incubated for 30 min at the indicated concentration of a chemical dissolved in M9 butter. After drug treatment, samples were harvested and handled as described above.
Starvation survival (= starvation recovery) assay
After synchronization by egg preparation, approximately 30,000 L1s in 3 ml of sterilized M9 buffer were placed in a 15 ml conical tube and left on a rocker at 20°C for the indicated time. Days were counted from egg preparation: day 1 is 24 hr after egg preparation, day 3 is 72 hr after egg preparation, and so on. At each time point, a 20 μl aliquot (approximately 200 worms) from each sample tube was plated on each of three plates seeded with either E. coli or Comamonas. The tube was returned to the rocker for further days of starvation. Plated worms grew for 3 days, and the number of the worms that reached L4 or adult stage was counted from each plate. The numbers from three plates were averaged and recorded. This number from day 1 of starvation was used as control and as the denominator to calculate the percentage of worms recovering after 3 days of starvation. All results shown are representative of at least three independent experiments.
Bacteria preparation for liquid cultivation
A saturated E. coli culture in LB broth was washed three times with M9 buffer and resuspended in 1/20 volume of the original culture volume of M9 buffer. This concentrated E. coli culture was added to the liquid culture of gpb-2 mutants at 1:1 dilution in order to provide food during incubation.
Drug treatment during starvation test
Atropine treatment: After egg preparation, atropine was added to the samples during starvation at a final concentration of 10 mM. After that, the worms were handled identically to the worms in the starvation survival assay.
MEK inhibitor treatment: After egg preparation, U0126 dissolved in DMSO (0.1%) was added to the samples during starvation at a final concentration of 25 μM. The same amount of DMSO was added to the control starvation sample. After that, the worms were handled identically to the worms in the starvation survival assay.
Photography
Pictures were taken on a Zeiss Axiophot using a MaxCam CM7-2E CCD camera (Finger Lakes Instrumentation).
Supplementary Material
Acknowledgments
We thank Kate Steger for helpful discussion and strains, Daniel Omura for a GFP expressing E. coli strain, Yuji Kohara for providing the mpk-1 cDNA and Dr. Min Han for DNA constructs. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). This research was supported by National Institutes of Health research grants HL46154 (L.A.) and DK34128 (M.H.C) and Korea Research Foundation Grant KRF-2005-070-C00118 (J.K.).
Footnotes
Supplemental data Supplemental data include three figures and a movie and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/3/4/237/DC1/.
References
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Avery L, Shtonda B. Food transport in the C. elegans pharynx. J. Exp. Biol. 2003;206:2441–2457. doi: 10.1242/jeb.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Gomeza J, Wess J, Hamilton SE, Nathanson NM, Levey A. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol. Cell. Neurosci. 2001;18:512–524. doi: 10.1006/mcne.2001.1042. [DOI] [PubMed] [Google Scholar]
- Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- El-Shemerly MY, Besser D, Nagasawa M, Nagamine Y. 12-O-Tetradecanoylphorbol-13-acetate activates the Ras/extracellular signal-regulated kinase (ERK) signaling pathway upstream of SOS involving serine phosphorylation of Shc in NIH3T3 cells. J. Biol. Chem. 1997;272:30599–30602. doi: 10.1074/jbc.272.49.30599. [DOI] [PubMed] [Google Scholar]
- Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Hamilton M, Liao J, Cathcart MK, Wolfman A. Constitutive association of c-N-Ras with c-Raf-1 and protein kinase C epsilon in latent signaling modules. J. Biol. Chem. 2001;276:29079–29090. doi: 10.1074/jbc.M102001200. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Nathanson NM. The M1 receptor is required for muscarinic activation of mitogen-activated protein (MAP) kinase in murine cerebral cortical neurons. J. Biol. Chem. 2001;276:15850–15853. doi: 10.1074/jbc.M011563200. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J. Neurosci. 1999a;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Park YS, Chang DJ, Hwang JM, Min CK, Kaang BK, Cho NJ. Cloning and expression of a G protein-linked acetylcholine receptor from Caenorhabditis elegans. J. Neurochem. 1999b;72:58–65. doi: 10.1046/j.1471-4159.1999.0720058.x. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Fleming JT. Basic Culture Methods. Methods Cell Biol. 1995;48:3–29. [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat. Rev. Mol. Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Malone RE. Dual regulation of meiosis in yeast. Cell. 1990;61:375–378. doi: 10.1016/0092-8674(90)90517-i. [DOI] [PubMed] [Google Scholar]
- McKay JP, Raizen DM, Gottschalk A, Schafer WR, Avery L. eat-2 and eat-18 are required for nicotinic neurotransmission in the Caenorhabditis elegans pharynx. Genetics. 2004;166:161–169. doi: 10.1534/genetics.166.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics. 1993;135:385–404. doi: 10.1093/genetics/135.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putrenko I, Zakikhani M, Dent JA. A family of acetylcholinegated chloride channel subunits in Caenorhabditis elegans. J. Biol. Chem. 2005;280:6392–6398. doi: 10.1074/jbc.M412644200. [DOI] [PubMed] [Google Scholar]
- Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Swanson MM, Albert PS. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Robatzek M, Niacaris T, Steger K, Avery L, Thomas JH. eat-11 encodes GPB-2, a Gbeta(5) ortholog that interacts with G(o)alpha and G(q)alpha to regulate C. elegans behavior. Curr. Biol. 2001;11:288–293. doi: 10.1016/s0960-9822(01)00074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D, Cheng M, Zhen E, Vanderbilt CA, Feig LA, Cobb MH. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc. Natl. Acad. Sci. USA. 1992;89:6924–6928. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Fink GR. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J. Exp. Biol. 2001;204:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A laboratory manual. Second Edition Volume 3. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J. Exp. Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger KA, Avery L. The GAR-3 muscarinic receptor cooperates with calcium signals to regulate muscle contraction in the Caenorhabditis elegans pharynx. Genetics. 2004;167:633–643. doi: 10.1534/genetics.103.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Hodgkin JA. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 587–606. others. [Google Scholar]
- Tabuse Y, Sano T, Nishiwaki K, Miwa J. Molecular evidence for the direct involvement of a protein kinase C in developmental and behavioural susceptibility to tumour-promoting phorbol esters in Caenorhabditis elegans. Biochem. J. 1995;312:69–74. doi: 10.1042/bj3120069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, Duttaroy A, Zhang W, Gomeza J, Cui Y, Miyakawa T, Bymaster FP, McKinzie L, Felder CC, Lamping KG, et al. M1–M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Receptors Channels. 2003;9:279–290. [PubMed] [Google Scholar]
- Wu Y, Han M. Suppression of activated Let-60 ras protein defines a role of Caenorhabditis elegans Sur-1 MAP kinase in vulval differentiation. Genes Dev. 1994;8:147–159. doi: 10.1101/gad.8.2.147. [DOI] [PubMed] [Google Scholar]
- Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.