Abstract
Photodynamic therapy (PDT) employs the combination of nontoxic photosensitizers and visible light that is absorbed by the chromophore to produce long-lived triplet states that can carry out photochemistry in the presence of oxygen to kill cells. The closed carbon-cage structure found in fullerenes can act as a photosensitizer, especially when functionalized to impart water solubility. Although there are reports of the use of fullerenes to carry out light-mediated destruction of viruses, microorganisms and cancer cells in vitro, the use of fullerenes to mediate PDT of diseases such as cancer and infections in animal models is less well developed. It has recently been shown that fullerene PDT can be used to save the life of mice with wounds infected with pathogenic Gram-negative bacteria. Fullerene PDT has also been used to treat mouse models of various cancers including disseminated metastatic cancer in the peritoneal cavity. In vivo PDT with fullerenes represents a new application in nanomedicine.
Keywords: antimicrobial photosensitizers, functionalized fullerenes, hydroxyl radicals, invasive wound infection, mouse models, peritoneal carcinomatosis, photodynamic therapy, singlet oxygen
Photodynamic therapy
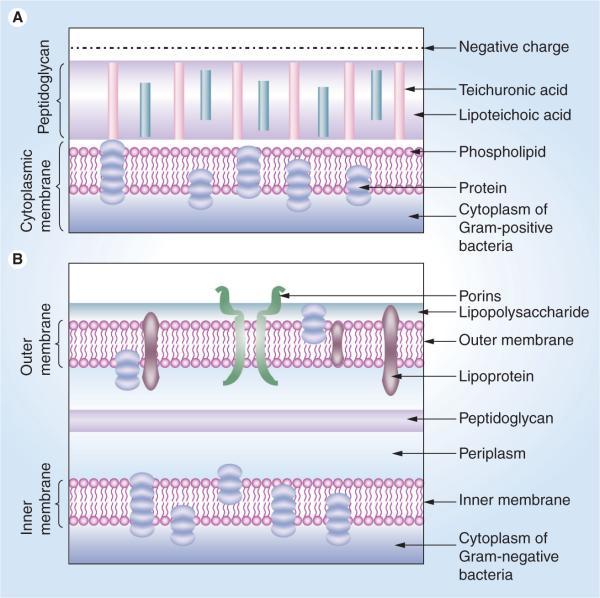
The fullerene molecule with its unique structure of 60 carbon atoms arranged in a soccer ball structure is a molecule of great potential for a variety of applications and has drawn the attention of lots of physicists, chemists and engineers. Recently these nanostructures have also been studied for their biological activities with a view towards using them for biomedical applications. One of the therapies for which fullerenes may have a medical application is the light-based therapy called photodynamic therapy (PDT) [1], which is a nonsurgical, minimally invasive approach that has been used in the treatment of solid tumors and many nonmalignant diseases [2]. PDT is a nonthermal photochemical reaction, which requires the simultaneous presence of a photosensitizing drug (photosensitizer [PS]), oxygen and visible light (Figure 1). It is a two-step procedure that involves the administration of a PS, followed by activation of the drug with the appropriate wavelength of light [3–5]. The photoactivation of the drug generates singlet oxygen and other reactive oxygen species (ROS), which cause a lethal oxidative stress and membrane damage in the treated cells and in the case of tumors, leads to cell death by direct cytotoxicity and a dramatic antivascular action that impairs blood supply to the area of light exposure [6]. It is known that depending on the parameters involved, in vitro PDT can kill cancer cells via apoptosis, necrosis or autophagy. The direct killing effect of PDT on malignant cancer cells that has been studied in detail in vitro also clearly applies in vivo, but in addition, two separate in vivo mechanisms leading to PDT-mediated tumor destruction have been described. They are the vascular shutdown effect mentioned above [7], and the PDT-induced activation of the host immune system [8]. In case of antimicrobial PDT Gram-positive bacteria are found to be more susceptible as compared with Gram-negative bacteria. This observation is explained by the difference in the structure of their cell walls (Figure 2) [9].
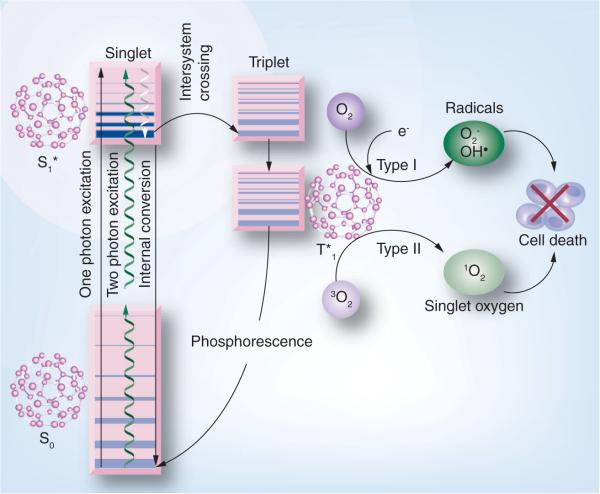
Figure 1. Jablonski diagram.
Initial absorption of a photon (or two photons of twice the energy) by the ground state of the singlet fullerene, which gives rise to the short-lived excited singlet state. This can lose energy by fluorescence (negligible in the case of fullerenes), internal conversion to heat or by intersystem crossing to the long-lived triplet state. Fullerene triplet states are efficiently quenched by molecular oxygen (a triplet state) to give type II (singlet oxygen) and type I (superoxide and hydroxyl radical) reactive oxygen species. In the absence of oxygen fullerene triplet states lose energy by phosphorescence.
Figure 2. Structures of the cell walls of two different classes of bacteria.
(A) Gram-positive bacterium showing a porous layer of peptidoglycan and single lipid bilayer. (B) Gram-negative bacterium showing a double lipid bilayer sandwiching the peptidoglycan layer and an outer layer of lipopolysaccharide.
The most common chemical structures that have been employed as PS for PDT purposes are derived from the tetrapyrrole aromatic nucleus found in many naturally occurring pigments such as heme, chlorophyll and bacteriochlorophyll. Tetrapyrroles usually have a relatively large absorption band in the region of 400 nm known as the Soret band, and a set of progressively smaller absorption bands as the spectrum moves into the red wavelengths known as the Q-bands. Another broad class of potential PS includes completely synthetic, non-naturally occurring, conjugated pyrrolic ring systems. These comprise such structures as texaphyrins [10], porphycenes [11] and phthalocyanines [12]. Other compounds that have been studied as PS are nontetrapyrrole-derived dyes, which may be either naturally occurring or totally synthetic, and these compounds have often been used as antimicrobial PS. Examples of the first group (naturally occurring dyes) are hypericin [13] and from the second group (synthetic dyes) are toluidine blue O [14] and Rose Bengal [15].
Some of the characteristics that the ideal PS should possess are the presence of low levels of dark toxicity and the presence of absorption bands that should be in the so-called optical window (600–900 nm) for sufficient tissue penetration of light. They should have relatively high absorption bands (>20,000–30,000 M−1cm−1) to minimize the dose of PS needed to achieve the desired effect. PS should ideally have high triplet and singlet oxygen quantum yields. The usefulness of various PS proposed for antimicrobial PDT has to be judged on different criteria. One of the requirements is that an antimicrobial PS should be able to kill multiple classes of microbes at relatively low concentrations and low fluences of light. PS should be reasonably nontoxic in the dark and should show selectivity for microbial cells over mammalian cells. To date there is no perfect PS that meets all the characteristics of an ideal PS. The reason why fullerenes are seen as potential PDT agents is that they possess some the characteristics that render them well suited as a photosensitizing drug as detailed below.
Fullerenes as PS
Due to rapidly growing interest in the medical application of fullerenes in nanotechnology [16], these molecules have gained considerable attention as possible PS for mediating PDT [17] of various diseases. Pristine C60 is highly insoluble in water and biological media and forms nano-aggregates that prevent its efficient photoactivity [18]. However, when fullerenes are derivatized by chemists who attach some functional groups to these molecules to make them more soluble in water and biological solvents, their biological usefulness is markedly improved [19]. Different hydrophilic or amphiphilic side chains or fused ring structures have been attached to the spherical C60 core. This functionalization imparts a higher ability to produce singlet oxygen, hydroxyl radicals and superoxide anion upon illumination, and these reactive species have been proposed as effective PDT mediators in several applications. Some of the advantages that fullerenes possess over the traditional PS used for PDT are:
-
■
Fullerenes are comparatively more photostable and demonstrate less photobleaching compared with tetrapyrroles and synthetic dyes;
-
■
Fullerenes show both kinds of photochemistry comprising type I (free radicals) and type II (singlet oxygen) while tetrapyrroles demonstrate largely type 2 photochemistry;
-
■
Fullerenes can be chemically modified for tuning the drug's partition coefficient (Log P or partition coefficient for [drug in n-octanol]/ [drug in H2O]) and pKa values for the variation of in vivo lipophilicity and the prediction of their distribution in biological systems;
-
■
To enhance the overall quantum yield and the ROS production and to extend their absorption spectrum further into the red wavelengths, a light-harvesting antennae can be chemically attached to C60;
-
■
Molecular self-assembly of fullerene cages into vesicles allows improved drug delivery and can produce self-assembled nanoparticles that may have different tissue-targeting properties.
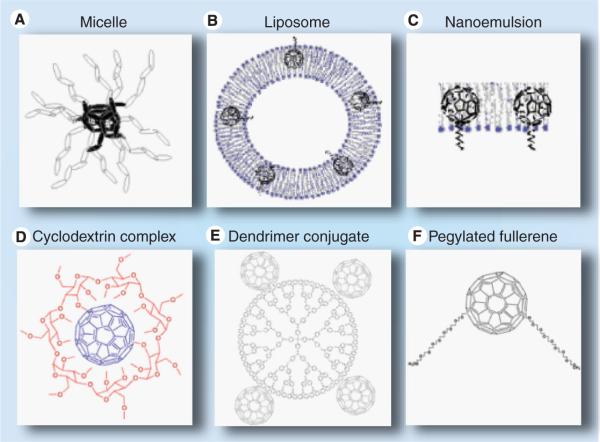
Besides these advantages, fullerenes show some disadvantages although they can be overcome by applying special strategies. One concern for the use of fullerenes is the question concerning biodegradability, as nanostructures such as fullerenes may conceivably accumulate in the environment [20]. However, studies have concluded that C60 itself is remarkably nontoxic [21]. Another concern was their extreme hydrophobicity and their innate tendency to aggregate, which renders them less promising for application as drugs in biomedicine. However, there have been many strategies applied for solubilization and drug delivery of fullerenes (e.g., liposomes; Figure 3B) [22–24], micelles (Figure 3A) [25,26], dendrimers (Figure 3e) [27,28], PEGylation (Figure 3F) [29–32], cyclodextrin encapsulation (Figure 3D) [33,34] and self-nanoemulsifying systems (Figure 3C) [35–38] to overcome this shortcoming of fullerenes. Figure 3 illustrates some of the strategies that have been used to solubilize fullerenes. Besides these disadvantages, the main optical absorption band of fullerenes is in the blue and green regions, whereas the absorption spectra of tetrapyrrole PS other than porphyrins (e.g., chlorins, bacteriochlorins and phthalocyanines) have been designed to have substantial absorption peaks in the red or far-red regions of the spectrum. For PS to be useful in vivo it is considered that the light used to excite them should be in the red/near-infrared spectral region where scattering and absorption of light by tissue is minimized. This unfavorable absorption spectrum of fullerenes can be overcome by various strategies such as covalent attachment of light-harvesting antennae to fullerenes [39–43] by using optical clearing agents [44–48] or by applying two-photon excitation where two near-infrared photons are simultaneously delivered to be equivalent to one photon of twice the energy (and half the wavelength, so that two 800 nm photons are equivalent to one 400 nm photon) [49–53].
Figure 3. Drug delivery vehicles for solubilizing fullerenes.
(A) Micelles; (B) liposomes; (C) nanoemulsion; (D) cyclodextrin complex; (E) dendrimer conjugate; and (F) pegylated fullerene.
Photophysics & photochemistry of fullerenes
On irradiation with visible light C60 is excited from the S0 ground state to a short-lived (~1.3 ns) S1 excited state. The S1 state rapidly decays to a lower lying triplet state T1 with a long lifetime of 50–100 μs (Equation 1). The S1→T1 decay is formally a spin-forbidden intersystem crossing, but is driven by an efficient spin-orbit coupling. In the presence of dissolved molecular oxygen (3O2), which exists as a triplet in its ground state, the fullerene T1 state is quenched (as a consequence of the quenching, its lifetime is reduced to ~330 ns) to generate singlet oxygen (1O1*) by energy transfer at a rate of 2 × 109 M −1s−1 (Equation 2). The singlet oxygen quantum yield, ϕΔ, for this process (at 532 nm excitation) has been reported to be near theoretical maximum, 1.0 [54].
It is being increasingly realized that as compared with the standard Type II ROS, (singlet oxygen) the ROS produced during PDT with fullerenes are inclined towards Type 1 photo-chemical products (superoxide, hydroxyl radical, lipid hydroperoxides, hydrogen peroxide). It is known that both pristine and functionalized C60 fullerenes are able to catalyze the formation of ROS after illumination [55]. However fullerenes dissolved in organic solvents in the presence of oxygen seem to preferentially produce reactive singlet oxygen (Equation 1) [54]. By contrast in polar solvents, especially those containing reducing agents (such as NADH at concentrations found in cells (Equation 2), illumination of various fullerenes will generate different Type 1 ROS, such as superoxide anion and hydroxyl radical [56].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Due to their triply degenerate low lying lowest unoccupied molecular orbital fullerenes are known to be excellent electron acceptors. They are capable of accepting as many as six electrons [57]. There is some evidence that fullerene excited states (in particular the triplet) are even better electron acceptors than the ground state [58,59]. It is thought that the reduced fullerene triplet or radical anion can transfer an electron to molecular oxygen forming superoxide anion radical O2−· (Equation 3). It has been shown that whereas 1O2 was generated effectively by photoexcited C60 in nonpolar solvents such as benzene and benzonitrile, while O2−· and OH− were produced in polar solvents such as water. Hydrogen peroxide is formed by dismutation of the superoxide anion (Equation 4). Fenton chemistry (using small amounts of ferrous iron found in cells) is able to produce hydroxyl radicals from hydrogen peroxide (Equation 5), while the Haber-Weiss reaction is able to reduce ferric iron back to ferrous iron in order to continue the cycle by regenerating the active divalent ferrous iron (Equation 6).
One apparent contradiction that arises in this area is the observation that fullerenes can act as antioxidants, and that C60 and derivatives can act as scavengers of ROS. These antioxidant effects of C60 have generally been studied in the absence of light [21,60–63]. How then can we reconcile the established ability of fullerenes to scavenge ROS and act as antioxidants, with the demonstrated ability of fullerenes to act as efficient producers of ROS under illumination with the correct light parameters? It was assumed that the double bonds of the fullerene cage reacted with ROS forming covalent bonds, thereby removing the ability of the ROS to react with sensitive biomolecules similar to those damaged during PDT. If this hypothesis were true it would be difficult to explain how fullerenes could act as efficient generators of ROS during PDT. A hypothesis that may explain this seeming contradiction was reported in 2009 by Andrievsky et al. [64]. They showed that the main mechanism by which hydrated C60 can inactivate the highly reactive ROS, hydroxyl radical, is not by covalently scavenging the radicals, but rather by action of the coat of `ordered water' that was associated with the fullerene nanoparticle [65]. Andrievsky et al. claimed that the ordered water coat could slow down or trap the hydroxyl radicals for sufficient time for two of the hydroxyl radicals to react with each other thus producing the less reactive ROS, hydrogen peroxide.
In vitro PDT with fullerenes
Phototoxicity using fullerenes combined with illumination to kill various cells in vitro has been demonstrated in many studies. It was Tokuyama et al. who first revealed the phototoxic potential of carboxylic acid functionalized fullerenes in HeLa (human cervical carcimoma) cells [66]. Burlaka et al. used pristine C60 at 10 μM with visible light from a mercury lamp to produce some phototoxicity in Ehrlich carcinoma cells or rat thymocytes [67]. In another study it was shown that tris-malonic acid fullerene derivative was found to be more phototoxic than a dendritic derivative in killing Jurkat cells when irradiated with UVA or UVB light [68]. Three C60 derivatives with two to four malonic acid groups (DMA C60, TMA C60 and QMA C60) were tested for their relative efficacy in HeLa cells and the results showed the following order for their efficacy DMA C60 > TMA C60 > QMA C60[69].
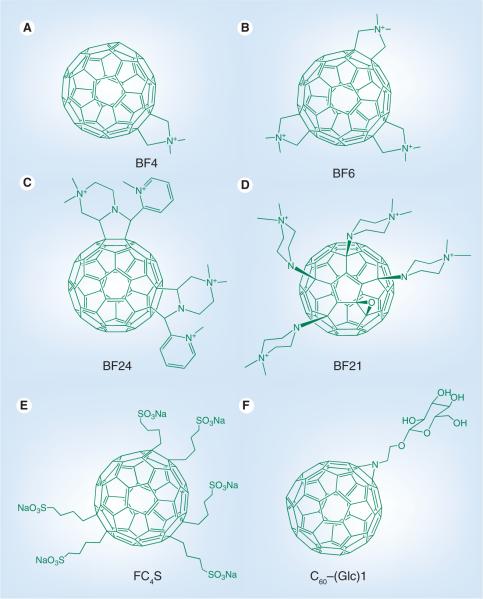
In our laboratory we have tested a group of six functionalized fullerenes that were prepared in two groups of three compounds [70]. We demonstrated that the C60 molecule mono-substituted with a single pyrrolidinium group (BF4; Figure 4a) was a very efficient PS which efficiently killed off a panel of mouse cancer cells at the low concentration of 2 μM on exposure to white light. Chiang et al. reported the synthesis of two new photoresponsive diphenylaminofluorene nanostructures and investigated their intramolecular photoinduced energy and electron transfer behavior [71]. They demonstrated that the large light-harvesting enhancement provided by the cyano-containing CPAF-OMe moiety led to a more efficient triplet state generator than the keto-containing DPAF-OMe. C60(>CPAF-OMe) was also significantly better than C60(>DPAF-OMe) at light-mediated killing of human cancer cells.
Figure 4. Chemical structures of functionalized fullerenes used for photodynamic therapy.
(A) Mono-pyrrolidinium fullerene, BF4 [70]; (B) tris-pyrrolidinium fullerene, BF6 [76]; (C) tetrakis-cationic fullerene, BF24 [78]; (D) tetrakis-cationic fullerene, BF21 [79]; (E) hexakis-anionic fullerene, FC4S [93]; and (F) mono-glycosylated fullerene C60–(Glc)1 [90].
Photodynamic reactions induced by photoactivated fullerenes have also been shown by many workers to inactivate enveloped viruses [72–75]. We have shown, in a series of reported experiments, that cationic fullerenes fulfill many of the desirable characteristics for efficient photoinactivation of bacteria and other pathogens. Our laboratory was the first to demonstrate that the soluble functionalized fullerenes, especially the tris-cationic compound BF6 (Figure 4B), were efficient and selective broad spectrum antimicrobial PS, and could mediate photodynamic inactivation of various classes of microbial cells [76]. In a study by Spesia et al. it was reported that a novel fulleropyrrolidinium iodide (DT-C602+ produced photodynamic inactivation in vitro of Escherichia coli [77]. Lee et al. showed that C60 derivatives were efficient in inactivating E. coli and MS-2 bacteriophage [75]. Recently we have demonstrated the use of innovative cationic fullerenes as broad-spectrum light-activated antimicrobials and carried out quantitative structure–function relationships to determine the optimal chemical structure of the fullerene derivatization [78]. The most effective compound overall against the various classes of microbial cells had the hexacationic structure illustrated as BF24 in Figure 4C. Another recent paper from our laboratory [79] looked at a further series of functionalized cationic fullerenes as antimicrobial PS and found a highly effective structure was the previously reported [80] tetrakis-cationic compound BF21 (Figure 4D).
Fullerenes have also been tested for their photodynamic efficacy by employing some of the delivery vehicles illustrated in Figure 3. Ikeda and coworkers used a series of liposomal preparations of C60 containing cationic or anionic lipids together. Illumination with 136 J/cm2 350–500 nm light killed 85% of cells in the case of cationic liposomes and apoptosis was demonstrated [81]. Akiyama et al. solubilized unmodified C60 with high stability using various types of poly(ethylene glycol) (PEG)-based block copolymer micelles, which showed cytotoxicity under photoirradiation in HeLa cells [26]. Another study demonstrated direct and short-time uptake of the fullerine into the cell membrane within 10 min using an exchange reaction from a fullerene-γ-cyclodextrin complex (Figure 3D) and PDT activity against cancer cells occurred [82]. Doi et al. compared the PDT activity of C60 and C70 encapsulated in dimyristoylphosphatidylcho-line liposomes (Figure 3B) against HeLa cells and found C70 was five times more active than C60 and attributed the difference to an improved absorption spectrum in the latter case [24]. In another publication [83] this group compared delivery of C70 encapsulated in surface cross-linked lipid vesicles called cerasomes with the above mentioned liposomes. They found additional stability of cerasomes coupled with equivalent PDT activity against HeLa cells suggesting the fullerene could mediate cell killing without being released from its delivery vehicle.
Although fullerenes have been widely used for PDT, little attention has been given to studying their interaction with different intracellular organelles and determining the cellular site of their potential action. The main reason for this lack of attention is that in contrast to the vast majority of other PS that are highly fluorescent, fullerenes are nonfluorescent; thus it is not feasible to use the common technique of fluorescence (confocal) microscopy to examine the intracellular uptake and subcellular localization of fuller-enes. Overcoming this limitation, Scrivens et al. were the first to demonstrate its uptake by human keratinocytes in tissue culture by preparing a radiolabeled fullerene [84]. In serum-free medium they found a time- (up to 6 h) and concentration-dependent uptake so that 50% of added fullerene was taken up. One group used indirect immunofluorescence staining with antibodies that recognize fullerenes and other organelle probes to show that a dicarboxylic acid derivative was localized in mitochondria and other intracellular membranes [85]. A recent paper described the use of energy-filtered transmission electron microscopy and electron tomography to visualize the cellular uptake of pristine C60 nanoparticulate clusters. When human monocyte-derived macrophages were examined C60 was found in the plasma membrane, lysosomes and nucleus [86]. In another study a pristine C60 preparation was obtained by sonication in methanol (toluene is more commonly used) and gave uniformly sized particles with photoluminescence detection at 750 nm after excitation at 488 nm. This emission was used to demonstrate cell uptake in normal and malignant breast cells after culturing them on fullerene-coated dishes [87].
Following on from the above in vitro studies the question arises whether these fullerene derivatives could be used to mediate PDT for in vivo applications either for antimicrobial use in infections or for anticancer use in tumor models.
In vivo PDT with fullerenes
As the previous section on in vitro studies demonstrated, fullerenes have the potential to act as efficient PS able to kill numerous classes of microbial cells as well as cancer cells. Now the key question is whether this high degree of in vitro activity could be translated into an in vivo anti-cancer effect or an antimicrobial effect in an appropriate animal model. In 1997, Tabata et al. first reported the use of fullerenes to carry out PDT of actual tumors [88]. In this study the fullerenes were chemically modified to make the molecules soluble in water as well as to enlarge the molecular size by pegylating C60 (Figure 3F). On injecting the PS intravenously in mice carrying a subcutaneous tumor on their backs, the C60–PEG conjugate demonstrated a higher accumulation and more prolonged retention in the tumor tissue than in normal tissue. The conjugate was excreted without being accumulated in any specific organ. Following intravenous injection of either C60–PEG conjugate or the Photofrin® (a recognized PS) to tumor-bearing mice, coupled with exposure of the tumor site to visible light, the volume increase of the tumor mass was suppressed and the C60 conjugate exhibited a stronger suppressive effect than Photofrin. Histological examination revealed that intravenous injection of the fullerene plus light irradiation strongly induced tumor necrosis without any damage to the overlying normal skin. The antitumor effect of the fullerene conjugate increased with increasing fluence and with increasing C60 dose. The antitumor effect was achieved by treatment with a dose of 424 μg/kg at a fluence of 107 J/cm2.
A novel PS was prepared from fullerene C60 that possessed additional MRI activity for efficient PDT of tumor. After chemical conjugation of PEG to C60 to form C60–PEG, diethylenetri diethylenetriaminepentaacetic acid (DTPA) was subsequently conjugated to the terminal group of PEG to form C60–PEG–DTPA. C60–PEG–DTPA was mixed with gadolinium acetate solution to obtain Gd3+-chelated C60–PEG–DTPA–Gd. Following intravenous injection of C60–PEG–DTPA–Gd into tumor-bearing mice, the PDT antitumor effect and the MRI tumor imaging were evaluated. Similar generation of superoxide upon illumination was observed with or without Gd3+ chelation. When intravenously injected into tumor-bearing mice, C60–PEG–DTPA–Gd maintained an enhanced MRI signal at the tumor tissue for a longer time period than Magnevist®. Injection of C60–PEG–DTPA–Gd plus light irradiation showed significant tumor PDT effect, although the effect was dependent on the timing of light irradiation. The PDT efficacy of C60–PEG–DTPA–Gd was observed at the time when the tumor accumulation was detected by the enhanced intensity of the MRI signal. This therapeutic and diagnostic hybrid system was found to be a promising tool to enhance the efficacy of PDT against tumors [31].
A preliminary in vivo study of PDT using hydrophilic nanospheres formed from hexa(sulfo-n-butyl)-C60 (FC4S; Figure 4e) was performed by Chiang and his coworkers [89]. This study was performed in imprinting control region mice bearing sarcoma 180 subcutaneous tumors. They were given either intraperitoneal or intravenous injection of water-soluble FC4S in PBS (5 mg/kg body weight). The tumor site was subsequently irradiated with an argon ion laser beam at a wavelength of 515 nm or an argon-pumped dye laser at 633 nm with the beam focused to a diameter of 7–8 mm with the total light dose adjusted to a level of 100 J/cm2 in each experiment. Consistently, inhibition of the tumor growth was found to be more effective using a lower wavelength (i.e., the 515 nm laser was bett absorbed than the 633 nm laser). Administration of FC4S to mice intraperitoneally demonstrated slightly better inhibition effectiveness than the intravenous method.
As cancer cells endocytose glucose more effectively than normal cells due to upregulation of glucose receptors, Otake and colleagues prepared a set of C60–glucose conjugates that also served the purpose of solubilizing the fullerene [90]. The glycoconjugated-C60 compounds produced selective phototoxicity (after irradiation with UVA1) towards cancer cells compared with normal fibroblasts showing the importance of targeting glucose receptors. Inhibition by sodium azide showed the involvement of singlet oxygen in the cell killing. In order to investigate the effect of PDT in vivo, human-melanoma (COLO679)-xenograft bearing mice were injected intratumor-ally with C60–(Glc)1 (Figure 4F; 0.1 or 0.2 mg/tumor) and irradiated with 10 J/cm2 UVA1 after a 4 h drug–light interval. PDT with C60–(Glc)1 was reported to suppress the tumor growth, with the higher dose performing better than the lower dose.
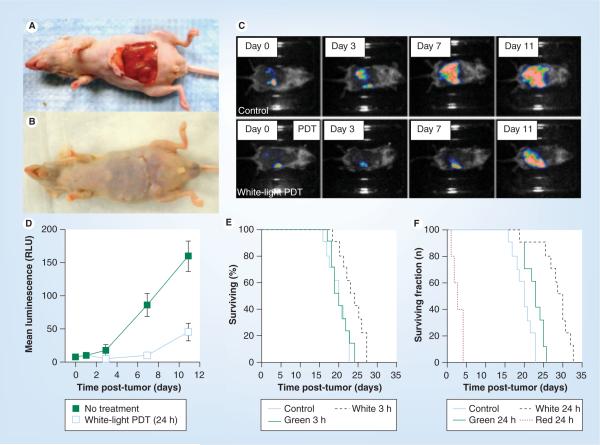
We have recently shown [91] that intraperitoneal PDT with a fullerene and white light was able to demonstrate significant therapeutic effects in a challenging mouse model of disseminated abdominal cancer. In humans this form of cancer is characterized as a thin covering of tumor nodules on the intestine and other abdominal organs, and responds poorly to standard treatment such as surgery or chemotherapy. In this study we formulated the monocationic BF4 (Figure 4A) in micelles composed of Cremophor EL (Figure 3A) to treat intraperitoneally disseminated colorectal cancer in a mouse model. We used a colon adenocarcinoma cell line (CT26) expressing firefly luciferase to allow monitoring of intraperitoneal tumor burden by noninvasive optical imaging. Intraperitoneal injection of a preparation of BF4 formulated in micelles (5 mg/kg), followed by white-light illumination (100 J/cm2) delivered through the peritoneal wall (after creation of a skin flap; Figure 5A & 5B) produced a statistically significant reduction in bioluminescence (Figure 5C & 5D) and a survival advantage in mice (Figure 5F). White light was more effective than green light (Figure 5F), while red light produced unacceptable toxicity to the mice due to excessive tissue penetration of the light into abdominal organs. A drug–light interval of 24 h was more effective than a 3 h drug–light interval (compare Figure 5e & 5F) showing the importance of allowing enough time for the fullerene to be taken up into the cancer cells. The intraperitoneal tumors were destroyed by the process of necrosis rather than the more usual apoptosis
Figure 5. Fullerene photodynamic therapy for disseminated peritoneal cancer in mice.
(A) Novel skin flap model to allow illumination of the abdominal cavity with white light. (B) Skin flap can be readily closed after illumination. (C) Noninvasive bioluminescence imaging of mouse colon carcinoma engineered to express luciferase after no treatment or after BF4 fullerene-mediated PDT. (D) Quantification of bioluminescence signals from (C). (E) Kaplan–Meier survival plot of mice treated with 100 J/cm2 of either green or white light or nothing 3 h after injection of 5 mg/kg BF4. (F) Similar to (E) but 24 h after injection and also including a red light treatment group.
PDT: Photodynamic therapy.
Data adapted with permission from [91].
Due to the aforementioned limitation of being nonfluorescent, the in vivo phamacokinetic characteristics of fullerenes such as the biodistribution and the organ/target specific binding has been less studied. It is considerably challenging to collect good biodistribution data of fullerenes. Approaches that could be applied for studying the biodistribution of fullerenes include radiolabeling fullerene with I125 or C14. In one study fullerenes were labeled with I125 and biodistribution studies were carried out. It was shown that after injection in to tumor-bearing mice the C60–PEG conjugate disappeared gradually from the blood circulation and 78% was excreted from the body within 24 h. This conjugate did not show any marked accumulation in any of the organs although the accumulation in the liver increased up to 24 h but decreased with time and was undetectable at 144 h after injection. The fullerene accumulated in the carcass and the GI tract in the early period but was eliminated thereafter in the same manner as the liver. This fullerene accumulated in the tumor tissue to a significantly higher extent than in the skin and the muscles and was also retained in the tumor tissue for a longer period than the normal tissue [32]. Liu and others conjugated PEG to C60 (C60–PEG), and DTPA was subsequently introduced to the terminal group of PEG to prepare C60–PEG–DTPA that was mixed with gadolinium acetate solution to obtain Gd3+-chelated C60–PEG–DTPA–Gd. Following intravenous injection of C60–PEG–DTPA–Gd into tumor-bearing mice, they observed tumor accumulation by enhanced intensity of the MRI signal [31].
Another study aimed to evaluate a fullerene as a therapeutic PS in the treatment of atherosclerosis. An atherosclerotic experimental rabbit model was prepared by causing intimal injury to bilateral external iliac arteries using balloon expansion. In four atherosclerotic rabbits and one normal rabbit, PEG-modified fullerene was infused into the left external iliac artery and illuminated by a light-emitting diode, while the right external iliac artery was only illuminated by light emitting diode. It was concluded that the infusion of a high concentration of fullerene–PEG followed by photoillumination did not result in suppression of atherosclerosis but rather in a progression of the atherosclerosis lesion. However, this intervention showed no adverse effects on the normal iliac artery [30].
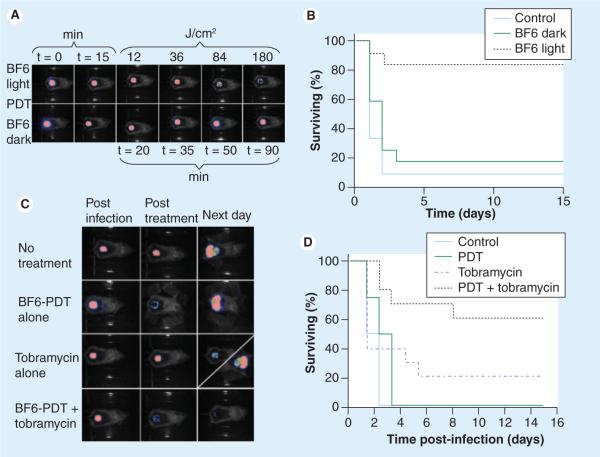
In addition to the in vivo anticancer application of fullerene PDT, we devised a study to test whether the high degree of light-mediated antimicrobial activity of fullerenes in vitro could produce an in vivo therapeutic effect in a mouse model of bacterial infection [92]. We used stable biolumi nescent bacteria and a low light imaging system to follow the progress of the infection noninvasively in real time in two potentially lethal mouse models of infected wounds. An excisional wound on the mouse back was contaminated with one of two bioluminescent Gram-negative species, Proteus mirabilis (2.5 × 107 cells) or Pseudomonas aeruginosa (5 × 106 cells). A solution of tris-cationic BF6 (Figure 4B) was placed into the wound followed by delivery of up to 180 J/cm2 of broadband white light (400–700 nm). Our results showed that in both cases there was a light- and dose-dependent reduction of bioluminescence from the wound not observed in control groups (light alone or BF6 alone;Figure 6A & 6C). Fullerene-mediated PDT of mice infected with P. mirabilis led to 82% survival compared with 8% survival without treatment (p < 0.001)(Figure 6B). PDT of mice infected with highly virulent P. aeruginosa did not lead to survival, but when PDT was combined with a suboptimal dose of the antibiotic tobramycin (6 mg/kg for 1 day) there was a synergistic therapeutic effect with a survival of 60% compared with a survival of 20% with tobramycin alone (p < 0.01;Figure 6D). In conclusion these data suggest that cationic fullerenes have clinical potential as an antimicrobial PS for superficial infections where red light is not needed to penetrate tissue.
Figure 6. Fullerene-mediated photodynamic therapy for Gram-negative bacterial wound infections in mice.
(A) Successive bioluminescence imaging of mice with wounds infected with bioluminescent Proteus mirabilis, treated with BF6 + light PDT (top row) or BF6 dark control (bottom row). (B) Kaplan–Meier survival curves for the groups of mice in (A); no treatment control (n = 12); BF6 in dark (n = 12); BF6 + light (n = 11). (C) Representative bioluminescence images of Pseudomonas aeruginosa infected mice (captured immediately postinfection, immediately post-treatment, and 24 h post-treatment), receiving: no treatment (top row); treated with BF6-PDT alone (180 J/cm2) (second row); treated with tobramycin alone (6 mg/kg for 1 day; third row, diagonal panel 24 h post-treatment shows two possible outcomes); treated with a combination of BF6-PDT and 1-day tobramycin (bottom row). (D) Kaplan–Meier survival curves for the groups of mice in Figure 6C; no treatment control (n = 10); PDT alone (n = 12); tobramycin alone (n = 12); PDT + tobramycin (n = 10). PDT: Photodynamic therapy.
Future perspective
Fullerenes have been widely studied in recent years as potential PS that could mediate PDT of diverse diseases. Most of these reports have been confined to in vitro studies where viruses, bacteria, fungi or cancer cells have been incubated with a diverse array of functionalized or solubilized fullerene compounds followed by illumination with light that is usually UVA, blue, green or white because the absorption spectrum of fullerenes is biased towards lower wavelengths. Since in vivo PDT usually uses red light for its improved tissue-penetrating properties, it was unclear whether fullerenes would mediate effective PDT in vivo. This question can now be answered in the affirmative. In fact, the report in which mice suffered toxicity after fullerene PDT with red light but exhibited a beneficial therapeutic effect after white light illumination suggests that this supposed drawback may actually be an advantage instead. Future studies will include synthesis of new fullerene derivatives particularly those with light-harvesting antennae to broaden the range of activating light that can be used, hence increasing light penetration depth into tissue. More experiments should be designed to increase understanding of the mechanisms that govern the balance between type I and type II reactive oxygen species. These studies will establish whether fullerenes can compete with more traditional PS in clinical applications of PDT.
Executive summary.
Photodynamic therapy
-
■
Photodynamic therapy (PDT) involves the combination of nontoxic photosensitizing dyes, harmless visible light and oxygen.
-
■
The photosensitizing dyes absorb light to form long-lived triplet states that can mediate photochemistry leading to generation of either free radicals (type 1) or singlet oxygen (type 2).
-
■
These reactive oxygen species can kill undesirable species that include cancer cells, pathogenic bacteria, fungi and viruses.
-
■
PDT can destroy tumors by direct tumor cell killing, vascular shutdown and activation of the host immune system.
Fullerenes as photosensitizers
-
■
Fullerenes have an absorption spectra that span the visible range, although they are biased towards the UVA and blue range.
-
■
Fullerenes have high photostability and resistance to photobleaching.
-
■
Fullerenes show both type I (free radicals) and type II (singlet oxygen) photochemistry.
-
■
Fullerenes can be chemically modified for tuning the drug's physical and chemical properties.
-
■
To extend their absorption spectrum further into the red wavelengths, they can be modified by attachment of light harvesting antennae.
-
■
Molecular self-assembly of fullerene cages into vesicles allows improved drug delivery.
Photophysics & photochemistry of fullerenes
-
■
Fullerenes efficiently generate singlet oxygen in organic solvents but switch to type 1 photochemistry in aqueous environments.
-
■
Electron transfer to a triplet fullerene can give a radical anion that produces superoxide from oxygen.
-
■
The highly toxic hydroxyl radical can be subsequently formed from superoxide.
-
■
Despite the demonstrated ability of fullerenes to quench reactive oxygen species in some circumstances, PDT can be efficiently mediated by illuminated fullerenes.
In vitro PDT with fullerenes
-
■
Several studies have shown that cancer cells such as HeLa can be killed after incubation with various fullerenes both pristine and functionalized, and illumination with various wavelengths of light.
-
■
Viruses can be inactivated and DNA breaks produced by photoactivated fullerenes.
-
■
Gram-positive bacteria, Gram-negative bacteria and fungal cells can all be killed by PDT mediated by fullerenes with cationic charges.
-
■
Determination of the subcellular localization of fullerenes in cancer cells is challenging due to a lack of fluorescence but some progress has been made.
In vivo PDT with fullerenes
-
■
There are reports that PDT with fullerenes can destroy or inhibit subcutaneous tumors growing in mice.
-
■
One report shows increased survival in a challenging disseminated abdominal cancer model.
-
■
An illuminated cationic fullerene can save the life of mice with an invasive bacterial wound infection.
-
■
Biodistribution and imaging studies may be carried out if the fullerene has a suitable label such as gadolinium.
Acknowledgments
This work was funded by the NIH (grants R01AI050875 to MR Hamblin; R01CA137108 to LY Chiang and R44CA103177 to Lynntech Inc.).
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Paper of special note have highlight as:
■ of interest
■ ■ of considerable interest
- 1.Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007;6(11):1139–1149. doi: 10.1039/b711141j. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ First review of the use of fullerenes as photosensitizers in photodynamic therapy (PDT).
- 2.Hamblin MR, Mroz P. Advances in Photodynamic Therapy: Basic, Translational and Clinical. Artech House; Norwood, MA, USA: 2008. [Google Scholar]; ■ Most recent and comprehensive textbook covering all aspects of PDT from chemistry, physics and biology to many different clinical applications of PDT for diverse diseases.
- 3.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 2011;61(4):250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ ■ Recent, comprehensive review of mechanisms and clinical application of PDT for cancer.
- 4.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 5.Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J. Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem. Photobiol. 1992;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 7.Abels C. Targeting of the vascular system of solid tumours by photodynamic therapy (PDT) Photochem. Photobiol. Sci. 2004;3(8):765–771. doi: 10.1039/b314241h. [DOI] [PubMed] [Google Scholar]
- 8.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Discusses the particular ability of PDT of cancer to activate the immune system in addition to destroying the tumor and therefore potentially changing a local treatment into a systemic therapy.
- 9.Hancock RE, Bell A. Antibiotic uptake into Gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 1988;7(6):713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- 10.Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004;47(16):3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 11.Szeimies RM, Karrer S, Abels C, et al. 9-Acetoxy-2,7,12,17-tetrakis-(beta-methoxyethyl)-porphycene (ATMPn), a novel photosensitizer for photodynamic therapy. uptake kinetics and intracellular localization. J. Photochem. Photobiol. B. 1996;34(1):67–72. doi: 10.1016/1011-1344(95)07275-6. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal I. Phthalocyanines as photodynamic sensitizers. Photochem. Photobiol. 1991;53(6):859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- 13.Agostinis P, Vantieghem A, Merlevede W, De Witte PA. Hypericin in cancer treatment: more light on the way. Int. J. Biochem. Cell Biol. 2002;34(3):221–241. doi: 10.1016/s1357-2725(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 14.Stockert JC, Juarranz A, Villanueva A, Canete M. Photodynamic damage to HeLa cell microtubules induced by thiazine dyes. Cancer Chemother. Pharmacol. 1996;39(1–2):167–169. doi: 10.1007/s002800050554. [DOI] [PubMed] [Google Scholar]
- 15.Bottiroli G, Croce AC, Balzarini P, et al. Enzyme-assisted cell photosensitization: a proposal for an efficient approach to tumor therapy and diagnosis. The rose bengal fluorogenic substrate. Photochem. Photobiol. 1997;66(3):374–383. doi: 10.1111/j.1751-1097.1997.tb03161.x. [DOI] [PubMed] [Google Scholar]
- 16.Cataldo F, Da Ros T. Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes. Springer Science+Business Media; NY, USA: 2008. [Google Scholar]; ■ Textbook covering a wide range of potential applications of fullerenes and carbon nanotubes in medicine, drug delivery and pharmaceutical science.
- 17.Duncan LK, Jinschek JR, Vikesland PJ. C60 colloid formation in aqueous systems: effects of preparation method on size, structure, and surface charge. Environ. Sci. Technol. 2008;42(1):173–178. doi: 10.1021/es071248s. [DOI] [PubMed] [Google Scholar]
- 18.Hotze EM, Labille J, Alvarez P, Wiesner MR. Mechanisms of photochemistry and reactive oxygen production by fullerene suspensions in water. Environ. Sci. Technol. 2008;42(11):4175–4180. doi: 10.1021/es702172w. [DOI] [PubMed] [Google Scholar]
- 19.Culotta L, Koshland DE., Jr. Buckyballs: wide open playing field for chemists. Science. 1991;254(5039):1706–1709. doi: 10.1126/science.254.5039.1706. [DOI] [PubMed] [Google Scholar]; ■ One of the first suggestions that fullerenes had an important future role to play in biomedicine and what was later to become known as the field of nanomedicine.
- 20.Oberdorster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J. Intern. Med. 2010;267(1):89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 21.Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;5(12):2578–2585. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- 22.Kato S, Aoshima H, Saitoh Y, Miwa N. Biological safety of liposome-fullerene consisting of hydrogenated lecithin, glycine soja sterols, and fullerene-C60 upon photocytotoxicity and bacterial reverse mutagenicity. Toxicol. Ind. Health. 2009;25(3):197–203. doi: 10.1177/0748233709106186. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Aoshima H, Saitoh Y, Miwa N. Fullerene-C60/liposome complex: defensive effects against UVA-induced damages in skin structure, nucleus and collagen type I/IV fibrils, and the permeability into human skin tissue. J. Photochem. Photobiol. B. 2009;98(1):99–105. doi: 10.1016/j.jphotobiol.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Doi Y, Ikeda A, Akiyama M, et al. Intracellular uptake and photodynamic activity of water-soluble [60]- and [70]fullerenes incorporated in liposomes. Chemistry. 2008;14(29):8892–8897. doi: 10.1002/chem.200801090. [DOI] [PubMed] [Google Scholar]
- 25.Yan A, Von Dem Bussche A, Kane AB, Hurt RH. Tocopheryl polyethylene glycol succinate as a safe, antioxidant surfactant for processing carbon nanotubes and fullerenes. Carbon N Y. 2007;45(13):2463–2470. doi: 10.1016/j.carbon.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiyama M, Ikeda A, Shintani T, et al. Solubilisation of [60]fullerenes using block copolymers and evaluation of their photodynamic activities. Org. Biomol. Chem. 2008;6(6):1015–1019. doi: 10.1039/b719671g. [DOI] [PubMed] [Google Scholar]
- 27.Kojima C, Toi Y, Harada A, Kono K. Aqueous solubilization of fullerenes using poly(amidoamine) dendrimers bearing cyclodextrin and poly(ethylene glycol) Bioconjug. Chem. 2008;19(11):2280–2284. doi: 10.1021/bc8001503. [DOI] [PubMed] [Google Scholar]
- 28.Pan B, Cui D, Xu P, et al. Synthesis and characterization of polyamidoamine dendrimer-coated multi-walled carbon nanotubes and their application in gene delivery systems. Nanotechnology. 2009;20(12):125101. doi: 10.1088/0957-4484/20/12/125101. [DOI] [PubMed] [Google Scholar]
- 29.Hooper JB, Bedrov D, Smith GD. Supramolecular self-organization in PEO-modified C60 fullerene/water solutions: influence of polymer molecular weight and nanoparticle concentration. Langmuir. 2008;24(9):4550–4557. doi: 10.1021/la703057y. [DOI] [PubMed] [Google Scholar]
- 30.Nitta N, Seko A, Sonoda A, et al. Is the use of fullerene in photodynamic therapy effective for atherosclerosis? Cardiovasc. Intervent. Radiol. 2008;31(2):359–366. doi: 10.1007/s00270-007-9238-8. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Ohta S, Sonoda A, et al. Preparation of PEG-conjugated fullerene containing Gd3+ ions for photodynamic therapy. J. Control. Release. 2007;117(1):104–110. doi: 10.1016/j.jconrel.2006.10.008. [DOI] [PubMed] [Google Scholar]; ■■ Important study of PDT of a tumor in vivo with a fullerene derivatized to be both soluble and also to contain gadolinium ions that allow MRI and facilitate biodistribution studies.
- 32.Tabata Y, Murakami Y, Ikada Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn J. Cancer Res. 1997;88(11):1108–1116. doi: 10.1111/j.1349-7006.1997.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ First report of the use of a functionalized fullerene to mediate PDT of a tumor in vivo.
- 33.Filippone S, Heimann F, Rassat A. A highly water-soluble 2:1 beta-cyclodextrin-fullerene conjugate. Chem. Commun. (Camb.) 2002;14:1508–1509. doi: 10.1039/b202410a. [DOI] [PubMed] [Google Scholar]
- 34.Zhao B, He YY, Bilski PJ, Chignell CF. Pristine (C60) and hydroxylated [C60(OH)24] fullerene phototoxicity towards HaCaT keratinocytes: type I vs type II mechanisms. Chem. Res. Toxicol. 2008;21(5):1056–1063. doi: 10.1021/tx800056w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bansal T, Mustafa G, Khan ZI, Ahmad FJ, Khar RK, Talegaonkar S. Solid self-nanoemulsifying delivery systems as a platform technology for formulation of poorly soluble drugs. Crit. Rev. Ther. Drug Carrier Syst. 2008;25(1):63–116. doi: 10.1615/critrevtherdrugcarriersyst.v25.i1.20. [DOI] [PubMed] [Google Scholar]
- 36.Shakeel F, Faisal MS. Nanoemulsion: a promising tool for solubility and dissolution enhancement of celecoxib. Pharm. Dev. Technol. 2009;15(1):53–56. doi: 10.3109/10837450902967954. [DOI] [PubMed] [Google Scholar]
- 37.Bali V, Ali M, Ali J. Novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. J. Drug Target. 2010;18(7):506–519. doi: 10.3109/10611860903548362. [DOI] [PubMed] [Google Scholar]
- 38.Amani A, York P, Chrystyn H, Clark BJ. Factors affecting the stability of nanoemulsions – use of artificial neural networks. Pharm. Res. 2010;27(1):37–45. doi: 10.1007/s11095-009-0004-2. [DOI] [PubMed] [Google Scholar]
- 39.Boyd PD, Reed CA. Fullerene-porphyrin constructs. Acc. Chem. Res. 2005;38(4):235–242. doi: 10.1021/ar040168f. [DOI] [PubMed] [Google Scholar]
- 40.El-Khouly ME, Araki Y, Ito O, et al. Spectral, electrochemical, and photophysical studies of a magnesium porphyrin-fullerene dyad. Phys. Chem. Chem. Phys. 2005;7(17):3163–3171. doi: 10.1039/b507673k. [DOI] [PubMed] [Google Scholar]
- 41.Imahori H. Porphyrin-fullerene linked systems as artificial photosynthetic mimics. Org. Biomol. Chem. 2004;2(10):1425–1433. doi: 10.1039/b403024a. [DOI] [PubMed] [Google Scholar]
- 42.Schuster DI, Cheng P, Jarowski PD, et al. Design, synthesis, and photophysical studies of a porphyrin-fullerene dyad with parachute topology; charge recombination in the marcus inverted region. J. Am. Chem. Soc. 2004;126(23):7257–7270. doi: 10.1021/ja038676s. [DOI] [PubMed] [Google Scholar]
- 43.Vail SA, Schuster DI, Guldi DM, et al. Energy and electron transfer in beta-alkynyl-linked porphyrin-[60]fullerene dyads. J. Phys. Chem. B. 2006;110(29):14155–14166. doi: 10.1021/jp061844t. [DOI] [PubMed] [Google Scholar]
- 44.Gebhart SC, Lin WC, Mahadevan-Jansen A. In vitro determination of normal and neoplastic human brain tissue optical properties using inverse adding-doubling. Phys. Med. Biol. 2006;51(8):2011–2027. doi: 10.1088/0031-9155/51/8/004. [DOI] [PubMed] [Google Scholar]
- 45.Tuchin VV, Wang RK, Yeh AT. Optical clearing of tissues and cells. J. Biomed. Opt. 2008;13(2):021101. doi: 10.1117/1.2903745. [DOI] [PubMed] [Google Scholar]
- 46.Hirshburg J, Choi B, Nelson JS, Yeh AT. Correlation between collagen solubility and skin optical clearing using sugars. Lasers Surg. Med. 2007;39(2):140–144. doi: 10.1002/lsm.20417. [DOI] [PubMed] [Google Scholar]
- 47.Jiang J, Boese M, Turner P, Wang RK. Penetration kinetics of dimethyl sulphoxide and glycerol in dynamic optical clearing of porcine skin tissue in vitro studied by Fourier transform infrared spectroscopic imaging. J. Biomed. Opt. 2008;13(2):021105. doi: 10.1117/1.2899153. [DOI] [PubMed] [Google Scholar]
- 48.Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006;123–126:369–385. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Goeppert-Mayer M. Über elementarakte mit zwei Quantensprüngen. Ann. Phys. 1931;9:273–295. [Google Scholar]
- 50.Bhawalkar JD, Kumar ND, Zhao CF, Prasad PN. Two-photon photodynamic therapy. J. Clin. Laser Med. Surg. 1997;15(5):201–204. doi: 10.1089/clm.1997.15.201. [DOI] [PubMed] [Google Scholar]
- 51.Karotki A, Khurana M, Lepock JR, Wilson BC. Simultaneous two-photon excitation of photofrin in relation to photodynamic therapy. Photochem. Photobiol. 2006;82(2):443–452. doi: 10.1562/2005-08-24-RA-657. [DOI] [PubMed] [Google Scholar]
- 52.Samkoe KS, Clancy AA, Karotki A, Wilson BC, Cramb DT. Complete blood vessel occlusion in the chick chorioallantoic membrane using two-photon excitation photodynamic therapy: implications for treatment of wet age-related macular degeneration. J. Biomed. Opt. 2007;12(3):034025. doi: 10.1117/1.2750663. [DOI] [PubMed] [Google Scholar]
- 53.Samkoe KS, Cramb DT. Application of an ex ovo chicken chorioallantoic membrane model for two-photon excitation photodynamic therapy of age-related macular degeneration. J. Biomed. Opt. 2003;8(3):410–417. doi: 10.1117/1.1577117. [DOI] [PubMed] [Google Scholar]
- 54.Arbogast JW, Darmanyan AP, Foote CS, et al. Photophysical properties of C60. J. Phys. Chem. A. Mol. Spectrosc. Kinet. Environ. Gen. Theory. 1991;95(1):11–12. [Google Scholar]
- 55.Foote CS. Photophysical and photochemical properties of fullerenes. Top. Curr. Chem. 1994;169:347–363. [Google Scholar]
- 56.Yamakoshi Y, Umezawa N, Ryu A, et al. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2. J. Am. Chem. Soc. 2003;125(42):12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]; ■■ Provides convincing evidence that fullerenes operate via a type I photochemical mechanism producing superoxide especially in aqueous biological environments.
- 57.Koeppe R, Sariciftci NS. Photoinduced charge and energy transfer involving fullerene derivatives. Photochem. Photobiol. Sci. 2006;5(12):1122–1131. doi: 10.1039/b612933c. [DOI] [PubMed] [Google Scholar]
- 58.Guldi DM, Prato M. Excited-state properties of C(60) fullerene derivatives. Acc. Chem. Res. 2000;33(10):695–703. doi: 10.1021/ar990144m. [DOI] [PubMed] [Google Scholar]
- 59.Arbogast JW, Foote CS, Kao M. Electron-transfer to triplet C-60. J. Am. Chem. Soc. 1992;114(6):2277–2279. [Google Scholar]
- 60.Lens M, Medenica L, Citernesi U. Antioxidative capacity of C(60) (buckminsterfullerene) and newly synthesized fulleropyrrolidine derivatives encapsulated in liposomes. Biotechnol. Appl. Biochem. 2008;51(Pt 3):135–140. doi: 10.1042/BA20080007. [DOI] [PubMed] [Google Scholar]
- 61.Spohn P, Hirsch C, Hasler F, Bruinink A, Krug HF, Wick P. C60 fullerene: a powerful antioxidant or a damaging agent? The importance of an in-depth material characterization prior to toxicity assays. Environ. Pollut. 2009;157(4):1134–1139. doi: 10.1016/j.envpol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Cai X, Jia H, Liu Z, et al. Polyhydroxylated fullerene derivative C(60)(OH)(24) prevents mitochondrial dysfunction and oxidative damage in an MPP+-induced cellular model of Parkinson's disease. J. Neurosci. Res. 2008;86(16):3622–3634. doi: 10.1002/jnr.21805. [DOI] [PubMed] [Google Scholar]
- 63.Dugan LL, Gabrielsen JK, Yu SP, Lin TS, Choi DW. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol. Neurosci. 1996;3(2):129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 64.Andrievsky GV, Bruskov VI, Tykhomyrov AA, Gudkov SV. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radic. Biol. Med. 2009;47(6):786–793. doi: 10.1016/j.freeradbiomed.2009.06.016. [DOI] [PubMed] [Google Scholar]; ■ Provides a possible explanation of the seeming paradox that on the one hand fullerenes can generate cytotoxic reactive oxygen species on illumination, and on the other hand, that fullerenes can quench reactive oxygen species and act as powerful antioxidants.
- 65.Weiss DR, Raschke TM, Levitt M. How hydrophobic buckminsterfullerene affects surrounding water structure. J. Phys. Chem. B. 2008;112(10):2981–2990. doi: 10.1021/jp076416h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tokuyama H, Yamago S, Nakamura E. Photoinduced biochemical activity of fullerene carboxylic acid. J. Am. Chem. Soc. 1993;115:7918–7919. [Google Scholar]
- 67.Burlaka AP, Sidorik YP, Prylutska SV, et al. Catalytic system of the reactive oxygen species on the C60 fullerene basis. Exp. Oncol. 2004;26(4):326–327. [PubMed] [Google Scholar]
- 68.Rancan F, Rosan S, Boehm F, et al. Cytotoxicity and photocytotoxicity of a dendritic C(60) mono-adduct and a malonic acid C(60) tris-adduct on Jurkat cells. J. Photochem. Photobiol. B. 2002;67(3):157–162. doi: 10.1016/s1011-1344(02)00320-2. [DOI] [PubMed] [Google Scholar]
- 69.Yang XL, Fan CH, Zhu HS. Photo-induced cytotoxicity of malonic acid [C(60)]fullerene derivatives and its mechanism. Toxicol. In Vitro. 2002;16(1):41–46. doi: 10.1016/s0887-2333(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 70.Mroz P, Pawlak A, Satti M, et al. Functionalized fullerenes mediate photodynamic killing of cancer cells: type I versus type II photochemical mechanism. Free Radic. Biol. Med. 2007;43(5):711–719. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Shows that functionalized fullerenes are efficient mediators of PDT killing of cancer cells via a type I photochemical mechanism
- 71.Chiang LY, Padmawar PA, Rogers-Haley JE, et al. Synthesis and characterization of highly photoresponsive fullerenyl dyads with a close chromophore antenna-C(60) contact and effective photodynamic potential. J. Mater. Chem. 2010;20(25):5280–5293. doi: 10.1039/C0JM00037J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasermann F, Kempf C. Photodynamic inactivation of enveloped viruses by buckminsterfullerene. Antiviral Res. 1997;34(1):65–70. doi: 10.1016/s0166-3542(96)01207-7. [DOI] [PubMed] [Google Scholar]
- 73.Hirayama J, Abe H, Kamo N, et al. Photoinactivation of vesicular stomatitis virus with fullerene conjugated with methoxy polyethylene glycol amine. Biol. Pharm. Bull. 1999;22(10):1106–1109. doi: 10.1248/bpb.22.1106. [DOI] [PubMed] [Google Scholar]
- 74.Lin CP, Lynch MC, Kochevar IE. Reactive oxidizing species produced near the plasma membrane induce apoptosis in bovine aorta endothelial cells. Exp. Cell Res. 2000;259(2):351–359. doi: 10.1006/excr.2000.4979. [DOI] [PubMed] [Google Scholar]
- 75.Lee I, Mackeyev Y, Cho M, et al. Photochemical and antimicrobial properties of novel C60 derivatives in aqueous systems. Environ. Sci. Technol. 2009;43(17):6604–6610. doi: 10.1021/es901501k. [DOI] [PubMed] [Google Scholar]
- 76.Tegos GP, Demidova TN, Arcila-Lopez D, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2005;12(10):1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ First demonstration that photoactivated cationic functionalized fullerenes are highly active, selective, broad-spectrum antimicrobials.
- 77.Spesia MB, Milanesio ME, Durantini EN. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives. Eur. J. Med. Chem. 2008;43(4):853–861. doi: 10.1016/j.ejmech.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Huang L, Terakawa M, Zhiyentayev T, et al. Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials. Nanomedicine. 2009;6(3):442–452. doi: 10.1016/j.nano.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizuno K, Zhiyentayev T, Huang L, et al. Antimicrobial photodynamic therapy with functionalized fullerenes: quantitative structure-activity relationships. J. Nanomedic. Nanotechnol. 2011;2(2):100109–100117. doi: 10.4172/2157-7439.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Isobe H, Tomita N, Nakamura E. One-step multiple addition of amine to [60]fullerene. synthesis of tetra(amino)fullerene epoxide under photochemical aerobic conditions. Org. Lett. 2000;2(23):3663–3665. doi: 10.1021/ol006573y. [DOI] [PubMed] [Google Scholar]
- 81.Ikeda A, Doi Y, Nishiguchi K, et al. Induction of cell death by photodynamic therapy with water-soluble lipid-membrane-incorporated [60]fullerene. Org. Biomol. Chem. 2007;5(8):1158–1160. doi: 10.1039/b701767g. [DOI] [PubMed] [Google Scholar]
- 82.Ikeda A, Matsumoto M, Akiyama M, Kikuchi J, Ogawa T, Takeya T. Direct and short-time uptake of [70]fullerene into the cell membrane using an exchange reaction from a [70]fullerene-gamma-cyclodextrin complex and the resulting photodynamic activity. Chem. Commun. (Camb.) 2009;12:1547–1549. doi: 10.1039/b820768b. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda A, Nagano M, Akiyama M, et al. Photodynamic activity of C70 caged within surface-cross-linked liposomes. Chem. Asian J. 2009;4(1):199–205. doi: 10.1002/asia.200800271. [DOI] [PubMed] [Google Scholar]
- 84.Scrivens JMT, Creek KE, Pirisi L. Synthesis of C-14-labeled C-60, its suspension in water, and its uptake by human keratinocytes. J. Am. Chem. Soc. 1994;116:4517–4518. [Google Scholar]
- 85.Foley S, Crowley C, Smaihi M, et al. Cellular localisation of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002;294(1):116–119. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 86.Porter AE, Gass M, Muller K, Skepper JN, Midgley P, Welland M. Visualizing the uptake of C60 to the cytoplasm and nucleus of human monocyte-derived macrophage cells using energy-filtered transmission electron microscopy and electron tomography. Environ. Sci. Technol. 2007;41(8):3012–3017. doi: 10.1021/es062541f. [DOI] [PubMed] [Google Scholar]
- 87.Levi N, Hantgan RR, Lively MO, Carroll DL, Prasad GL. C60-Fullerenes: detection of intracellular photoluminescence and lack of cytotoxic effects. J. Nanobiotechnol. 2006;4:14–25. doi: 10.1186/1477-3155-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tabata Y, Murakami Y, Ikada Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn J. Cancer Res. 1997;88(11):1108–1116. doi: 10.1111/j.1349-7006.1997.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chi Y, Canteenwala T, Chen HC, Chen BJ, Canteenwala M, Chiang LY. Hexa(sulfobutyl) fullerene-induced photodynamic effect on tumors in vivo and toxicity study in rats. Proc. Electrochem. Soc. 1999;99:234–249. [Google Scholar]
- 90.Otake E, Sakuma S, Torii K, et al. Effect and mechanism of a new photodynamic therapy with glycoconjugated fullerene. Photochem. Photobiol. 2010;86(6):1356–1363. doi: 10.1111/j.1751-1097.2010.00790.x. [DOI] [PubMed] [Google Scholar]
- 91.Mroz P, Xia Y, Asanuma D, et al. Intraperitoneal photodynamic therapy mediated by a fullerene in a mouse model of abdominal dissemination of colon adenocarcinoma. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.04.007. doi:10.1016/j.nano.2011.04.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Shows that a photoactivated, functionalized fullerene was able to produce tumor response and increased survival in a new and challenging mouse model of disseminated metastatic cancer.
- 92.Lu Z, Dai T, Huang L, et al. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond.) 2010;5(10):1525–1533. doi: 10.2217/nnm.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ First study to show that PTD with a functionalized fullerene is able to save the life of mice at risk of dying from a highly aggressive and invasive bacterial infection.
- 93.Chen HH, Yu C, Ueng TH, et al. Acute and subacute toxicity study of water-soluble polyalkylsulfonated C60 in rats. Toxicol. Pathol. 1998;26(1):143–151. doi: 10.1177/019262339802600117. [DOI] [PubMed] [Google Scholar]