Abstract
Although cancer cells can be immunogenic, tumour progression is associated with the evasion of immunosurveillance, the promotion of tumour tolerance and even the production of pro-tumorigenic factors by immune cells. Cytotoxic T lymphocyte-associated antigen 4 (CTLA4) represents a crucial immune checkpoint, the blockade of which can potentiate anti-tumour immunity. CTLA4-blocking antibodies are now an established therapeutic approach for malignant melanoma, and clinical trials with CTLA4-specific antibodies in prostate cancer have also shown clinical activity. This treatment may provide insights into the targets that the immune system recognizes to drive tumour regression, and could potentially improve both outcome and toxicity for patients with prostate cancer.
Prostate cancer remains the most common cancer in men in the United States, with an estimated incidence of 240,890 new cases (29% of all new cancer cases) in 2011, and it is the second most common cause of death from cancer in men, with 33,720 estimated deaths (11% of all estimated deaths) in 2011 (REF. 1). First-line therapies for early stage localized prostate cancer are surgery and radiotherapy, and the 5-year relative survival rate is essentially 100% based on 2001–2007 statistics2. However, for patients with prostate cancer that has metastasized, the 5-year relative survival rate is 28.8%. Androgen ablation by surgical or chemical castration is used to treat men with recurrent prostate cancer, as prostate epithelial cells are dependent on androgens for survival3. Initially, prostate cancer cells respond to androgen deprivation but they eventually become resistant. There have been numerous clinical trials examining androgen deprivation combined with other treatments in men with metastatic prostate cancer, but most of these trials have shown no significant improvement in the survival rate4.
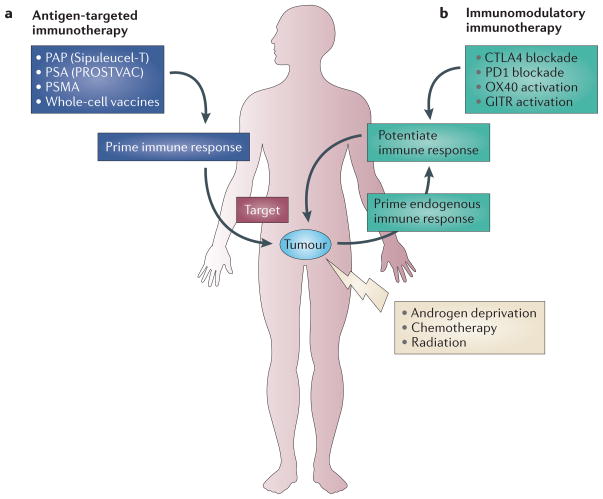
Cancer immunotherapy, whereby a patient’s immune system is stimulated to create an anti-tumour effect, has emerged as a novel form of therapy for men with metastatic, androgen-insensitive prostate cancer (mAIPC)5. Immunotherapy for prostate cancer can be divided into two approaches: antigen-targeted therapies and immunomodulatory therapies (FIG. 1). In antigen-targeted immunotherapy, exogenous tumour-associated antigen or antigens (that is, a vaccine) are introduced into the patient to elicit an immune response. Ideally, the targeted antigen is typically expressed in the tumour or its microenvironment and not in other tissues. Prostate-specific antigens that have been targeted in immunotherapy include prostate-specific antigen (PSA)6, prostatic acid phosphatase (PAP)7, prostate-specific membrane antigen (PSMA; also known as FOLH1)8 and antigens derived from whole-tumour-cell lysis. An example of an approach using whole-tumour-cell lysis is GVAX, which consists of irradiated allogeneic prostate cancer cell lines transduced with a transgene so that they express granulocyte–macrophage colony stimulating factor (GM-CSF), a cytokine that may target and promote the maturation of certain antigen-presenting cells (APCs), such as dendritic cells (DCs)9. As most tumour-specific antigens are self-antigens (that is, the immune system has developed so that it does not react to these antigens), the antigen must be delivered in a way that promotes T lymphocyte activation and that breaks tolerance to the antigen, as well as tumour-associated immunosuppression. One such approach is sipuleucel-T7, which is a US Food and Drug Administration (FDA)-approved immunotherapy for prostate cancer. In this treatment, total peripheral blood mono-nuclear cells, comprising of T cells, B cells and antigen-presenting cells, are isolated from a patient and co-cultured ex vivo with a fusion protein of PAP and GM-CSF10, and then re-infused into the patient. An alternative approach that is being studied in a Phase III trial involves a prime-boost vaccination strategy using the PSA-expressing PROSTVAC vaccine6. PROSTVAC uses an initial immunization with vaccinia virus-expressing PSA to prime the patient’s immune system and then subsequent vaccinations with fowlpox-expressing PSA to boost an adaptive immune response to the targeted antigen.
Figure 1. Immunotherapy for prostate cancer.
Immunotherapy methodologies fall into two major camps: antigen-targeted immunotherapy and immunomodulatory immunotherapy. In antigen- targeted immunotherapy, tumour-associated antigens, such as prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), prostate-specific membrane antigen (PSMA) and whole-cell vaccines, are introduced into the patient as a vaccine to elicit an immune response that targets the tumour. In immunomodulatory immunotherapy, the immune system, presumably primed by endogenous tumour-associated antigens, is potentiated either by blocking inhibitory immune effectors such as cytotoxic T lymphocyte-associated antigen 4 (CTLA4) and programmed cell death protein 1 (PD1) or by triggering immune activators such as OX40 and glucocorticoid-induced TNF receptor-related gene (GITR) using antibodies or agonists. This enables immune cells to become activated and to target the tumour. Conventional therapies such as radiation, chemotherapy or androgen deprivation contribute to the antigenic pool by increasing tumour cell death. Antigen-targeting and immunomodulatory immunotherapies have also been used in combinations in current clinical trials.
In immunomodulatory therapy, the immune system itself is targeted for modulation. For example, blocking the activity of the immune checkpoint protein cytotoxic T lymphocyte-associated antigen 4 (CTLA4) removes one of the crucial ‘brakes’ on the immune system, either by lowering the threshold of T cell activation and allowing normally unreactive effector T (Teff) cells to become activated, or by removing inhibitory signals that attenuate strongly reactive Teff cells. Immune checkpoint inhibitors such as CTLA4 and programmed cell death protein 1 (PD1)11 can be blocked with antibodies, thus activating the immune system. Conversely, co-stimulatory receptors — such as OX40 (also known as CD134), which is currently in clinical trials (NCT01303705; see the ClinicalTrials.gov website (see Further information)), and glucocorticoid-induced TNF receptor-related protein (GITR; also known as TNFRSF18)12 — can be activated with chemical agonists or agonistic antibodies. Combinations of immunomodulatory therapies to target different components of the immune system have also been investigated. In theory, immunomodulatory approaches can activate the immune system to the entire antigenic range of a patient’s tumour cells rather than to a specific tumour antigen. Therefore, immunomodulatory therapy could be described as patient-specific because endogenous antigens are recognized. Immunomodulatory therapy has also been combined with antigen-targeted therapy, which should drive the immune response towards the introduced antigens, as is intended with the combination of CTLA4-specific antibodies and GVAX13.
One antibody that targets CTLA4 (ipilimumab (Yervoy; Bristol-Myer Squibb)) has recently obtained FDA approval for the treatment of melanoma14. Two Phase III clinical trials using ipilimumab are currently underway in men with mAIPC (NCT00861614 and NCT01057810). However, how CTLA4 blockade mediates its anti-tumour effects is not entirely clear. This Opinion article describes the mechanistic data from recent clinical trials on CTLA4-specific antibodies in the treatment of prostate cancer. These data are beginning to indicate precisely how blocking CTLA4 increases the endogenous and the vaccine-induced immune response and are also beginning to reveal the antigens that might mediate the anti-tumour effects.
CTLA4: the molecule
CTLA4 was first cloned by differential screening using a mouse cytotoxic T lymphocyte (CTL) cDNA library that was enriched for genes that are preferentially expressed in CTLs and multiple subtractions with non-CTL mRNA15. CTLA4 belongs to the CD28 immunoglobulin superfamily, with one variable (V)-like domain, a hydrophobic transmembrane domain and a cytoplasmic domain. The genes that encode CTLA4, and the closely related molecule CD28, are present on human chromosome 2q33 and mouse chromosome 1. CTLA4 and CD28 also have an MYPPPY motif that enables them to bind the same B7 ligands16, and they signal to the same downstream molecules, such as PI3K17 and serine/threonine phosphatase PP2A18. Importantly, CTLA4 is highly conserved between mice and humans (76% amino acid homology and 100% conservation in the cytoplasmic tail)19.
Activated T lymphocytes or Teff cells mediate the adaptive immune response against pathogens and tumours. The adaptive immune response is generated sequentially. First, APCs are activated in the presence of a target antigen, and these antigens are taken up, processed and presented by APCs to T cells in the form of antigenic peptides. This results in the activation and the proliferation of T cells and the targeting of antigens that are expressed by infected cells or tumour cells. Finally, this response is downregulated through inhibitory feedback pathways that involve CTLA4. Activation of T lymphocytes by APCs requires two signals, in a process that is known as co-stimulation20. The first signal is T cell receptor (TCR) engagement of antigenic peptides that are presented by major histocompatibility complex (MHC) molecules that are expressed on APCs (MHC I and MHC II), and other nucleated cells (MHC I only), in the body. The second co-stimulatory signal is provided by the interaction of CD28 receptors expressed on the surface of T lymphocytes, with B7 ligands (CD80 and CD86) expressed on APCs21.
The expression of CTLA4 on the cell surface is very low compared with the expression of CD28 (REF. 22). Whereas CD28 is constitutively expressed on resting and activated T cells, CTLA4 resides in endosomal vesicles and becomes readily detectable only on T cell activation23. Transcription of CTLA4 mRNA occurs rapidly after T cell activation, and surface protein levels are upregulated 24–36 hours after activation. The level of CTLA4 on the surface is also tightly regulated by endocytosis — the unphosphorylated cytoplasmic tail of CTLA4 interacts with the mu-1 subunit of the clathrin-associated adaptor complex, AP2 (AP2M1), which regulates the internalization of CTLA4 into clathrin-coated vesicles24. Tyrosine phosphorylation of the YVKM motif inhibits AP2M1 binding, thereby increasing and stabilizing CTLA4 on the cell surface. Microtubule reorganization during TCR engagement with peptide–MHC complexes also focuses intracellular and surface levels of CTLA4 towards sites of T cell–APC engagement. In essence, these mechanisms provide geographically oriented and temporal components for providing negative feedback after CD28 has been activated.
In addition, CTLA4 binds with significantly greater avidity than CD28 to B7 ligands25. CTLA4 homodimers form stable multimeric complexes with B7 molecules, creating a lattice-like network that can disrupt CD28–B7 ligand association. Therefore, CTLA4 can out-compete CD28 for binding to B7 ligands and thus serves as a negative-feedback regulator of T lymphocyte activation. Indeed, mice that are deficient for CTLA4 develop lymphoproliferative disorders, resulting in early lethality26, and further studies have shown that CTLA4 inhibits interleukin-2 (IL-2) cytokine production and cell cycle progression27.
However, these mechanisms cannot fully explain how CTLA4 mediates negative regulation. Evidence from mice expressing CTLA4 that lacks a cytoplasmic tail indicates that competition alone does not negate T cell activation and proliferation28. CTLA4 has also been shown to interfere with lipid raft formation at the plasma membrane, thereby disrupting the micro-platforms that are required for CD28 co-signals29. CTLA4 can also increase T cell motility, reducing the time of contact between T cells and APC, and raising the ‘threshold’ for T cell activation30.
In addition to regulating the activation of Teff cells, CTLA4 also has a role in the induction of peripheral tolerance to self-antigens31. Self-tolerance is the process by which the immune system is prevented from attacking tissues in the body and it prevents autoimmunity. CD4+CD25+ regulatory T (TReg) cells that express FOXP3 represent a group of T lymphocytes that is essential for maintaining self-tolerance32. The transcription factor FOXP3 represses IL2 transcription and upregulates expression of CTLA4. Thus, FOXP3+CD25+CD4+ TReg cells constitutively express cell surface CTLA4 (REF. 33). Several studies in mice have shown that CTLA4-deficient TReg cells have impaired suppressive activity34,35. By mixing cells from wild-type mice with cells that have transgenic expression of human CTLA4, and by using monoclonal antibodies to block mouse CTLA4 only, blockade of CTLA4 on TReg cells and Teff cells has a synergistic effect on an anti-tumour response36. Blocking Teff cells alone demonstrated some protection, but blocking TReg alone had no effect on an anti-tumour response, showing that Teff cells are essential for the anti-tumour effects; whereas, TReg cells have a supporting role. In summary, CTLA4 maintains immune system homeostasis by functioning as a major feedback inhibitor of T cell activation.
Preclinical studies of CTLA4 blockade
Antibodies to CTLA4 that block its interaction with B7 ligands remove this inhibitory signal, increase the co-stimulatory effect of CD28, and enhance T cell activation in vitro37 and in vivo in mice38. The ability to target CTLA4 with antibodies made it an attractive target for cancer immunotherapy. Administration of CTLA4-specific antibodies was first shown to result in the rejection of pre-implanted 51BLim10 colorectal carcinoma and Sa1N fibrosarcoma tumours in syngeneic mice39. The curative response was almost 100% and the rejection resulted in immunity to secondary exposure to tumour cells, suggesting that tumour rejection that is mediated by CTLA4 blockade results in immunological memory.
To investigate prostate cancer in mice, syngeneic transplantable epithelial prostate cancer cell lines were developed from prostate tumours that arose from transgenic adenocarcinoma mouse prostate (TRAMP) mice as a result of the expression of the SV40 large T antigen oncoprotein under a prostate-specific promoter40. These cell lines do not express the SV40 large T antigen in vitro or in vivo, making them suitable for immunotherapeutic studies. TRAMP cells were injected subcutaneously into the backs of male wild-type C57BL/6 mice, the syngeneic host for TRAMP cells, and CTLA4-specific antibodies and control-irrelevant hamster antibodies were administered41. Of the mice injected with CTLA4-specific antibodies, 42% exhibited complete rejection of their tumours and most of the remaining mice demonstrated delayed tumour growth. Conversely, the control mice had uniform tumour growth. In addition, CTLA4-specific antibodies administered immediately after primary tumour resection reduced metastatic relapse from 97.4% to 44%, suggesting that CTLA4 blockade may be used as an adjunctive therapy after surgery to eliminate residual metastatic prostate cancer cells42.
The effect of CTLA4-specific blockade on spontaneous tumour formation has also been examined in TRAMP mice43. CTLA4-specific antibody treatment alone had no significant effect on tumour incidence, and neither did treatment with a γ-irradiated TRAMP cell vaccine either expressing or not expressing GM-CSF. However, mice that received a combination of CTLA4-specific antibodies and either of the TRAMP cell vaccines had a significant reduction in tumour incidence, with a slightly lower incidence in the mice that received the TRAMP cells transduced with GM-CSF (33% incidence for anti-CTLA4 plus TRAMP plus GM-CSF, and 43% incidence for anti-CTLA4 plus TRAMP). Similarly, a combination of anti-CTLA4 blockade and a cellular tumour vaccine expressing GM-CSF resulted in the regression of tumours in a weakly immunogenic mammary carcinoma (SM1) mouse model: these tumours are refractory to CTLA4-specific antibodies alone. This synergistic effect was shown to be dependent on both CD4+ and CD8+ T lymphocytes44. These studies suggest that weakly or non-immunogenic tumours in mice require co-administration of a tumour vaccine with CTLA4-specific antibodies to mount an immune response and to mediate an anti-tumour effect. In these studies, however, the mice receiving the combination therapy also exhibited a higher incidence of an auto-immune response, which was shown by the accumulation of inflammatory cells in normal tissues, compared with mice receiving only CTLA4-specific antibodies or vaccine, and no inflammation was observed in the control group.
Blocking CTLA4 was also shown to enhance the anti-tumour effect that is mediated by low-dose chemotherapy (melphalan) in mice with MOPC-315 plasmacytoma45. Survival was 73% in the combination of melphalan plus CTLA4-specific antibodies, compared with 44% for melphalan plus a control antibody, and was 0% for CTLA4-specific antibodies alone. Low-dose melphalan was shown to promote the accumulation of CD8+ T cells in the tumour nodules. In addition, CTLA4 blockade also increased the efficacy of radiation in mice with 4T1 metastatic mammary carcinomas46. The ionizing irradiation increased the secretion of CXCL16, a chemokine that binds to CXCR6 on T helper 1 (TH1) cells and activated CD8 Teff cells, and increased the migration of CD8+ CXCR6+-activated T cells into breast tumours47. Furthermore, the addition of chemotherapy or radiation therapy was able to promote antigen presentation through killing tumour cells, resulting in the release of tumour-specific antigens48. Finally, androgen ablation in an autochthonous mouse prostate cancer model is able to mitigate specific CD4+ T cell tolerance to the prostate gland49, and reverse ageing-associated thymic involution, leading to increased output of naive T cells50. Although the combination of androgen ablation and CTLA4-specific antibody therapy has not been carried out in mice with prostate cancer, these and other findings in human studies (see below) provide a rationale for combining androgen ablation with CTLA4 blockade.
Blocking CTLA4 has also been associated with enhancing immune autoreactivity and can exacerbate autoimmune disease in experimental allergic encephalomyelitis51 and autoimmune diabetes in mice52. Blocking CTLA4 with antibodies in mouse cancer models demonstrated anti-tumour responses, as well as autoimmune responses, such as depigmentation in melanoma models53. However, preclinical evaluation of CTLA4-specific antibody in cynomolgus monkeys demonstrated no autoimmune responses or toxicity54, cumulating in the testing of CTLA4-blocking antibodies in clinical trials.
Clinical trials of CTLA4 blockade
Although ipilimumab is a fully human CTLA4-specific IgG1 monoclonal antibody that has been approved in the United States for the treatment of unresectable advanced melanoma, it was in fact first administered to patients with prostate cancer. In this initial trial, a single dose of ipilimumab at 3 mg per kg given to patients with hormone-refractory prostate cancer resulted in a ≥50% decline in PSA levels in two of 14 patients, and a <50% decline in eight others55. The two responders did not have measurable disease, but one developed grade 3 toxicity of an inflammatory nature (rash). Of the two other patients who had measurable disease at baseline who were evaluated by repeat radiographic imaging, neither demonstrated an objective response as defined by Response Evaluation Criteria in Solid Tumours (RECIST). This trial showed that a single 3 mg per kg dose of ipilimumab is tolerated, but it also indicated the risk of treatment-related immune events.
Overall, there have been ten clinical trials that either have explored or are exploring the use of CTLA4-specific antibodies in the setting of prostate cancer (TABLE 1). As a result of the data from TRAMP mice that were treated with CTLA4-specific antibodies combined with other treatments, six clinical trials have explored combinations with other immune adjuvants (GM-CSF), cancer vaccines (GVAX and PROSTVAC), chemotherapy, radiation and anti-androgen therapy. Although these studies are too small to be definitive, these trials have noted overall declines in PSA levels and a low frequency of radiographic responses at doses of ipilimumab ≥3 mg per kg. Radiographic responses in prostate cancer can be difficult to detect because bone metastases, which are common with prostate cancer, are difficult to measure. In a Phase I trial combining GM-CSF with escalating doses of ipilimumab, 22% of patients receiving ipilimumab ≥3 mg per kg experienced a significant PSA response56,57. At the time of this report, one patient had a partial objective response in liver metastases in accordance with RECIST. Combining cancer vaccines and ipilimumab would presumably improve anti-tumour activity by amplifying immune responses that are focused on relevant antigens, as demonstrated in mice. In a trial investigating ipilimumab with GVAX, five of 28 patients who completed treatment had a ≥50% decline in PSA levels, and 12 had stabilization of metastatic bone disease for extended durations (12–21 months)13,58,59. PROSTVAC has also been combined with ipilimumab. In a randomized Phase II trial, PROSTVAC alone significantly improved overall survival compared with a placebo in healthy, well-performing patients with mAIPC60. In the combination trial, five of nine chemo-naive patients with mAIPC who received 3 or 5 mg per kg of ipilimumab plus PROSTVAC had ≥50% declines in PSA levels61. Overall, 14 of 30 patients at any dose level of ipilimumab (1, 3, 5 and 10 mg per kg) had some decline in PSA levels. Physical tumour shrinkage, however, was infrequent. Of the previous nine patients, four had stable disease at ≥6 months, and two had unconfirmed partial responses. Nine of 15 receiving 10 mg per kg of ipilimumab had stable disease at ≥6 months. Despite limited numbers of noticeable tumour shrinkage, both the GVAX and the PROSTVAC with ipilimumab trials showed a significant number of patients with stabilization of their disease under combination therapy that can last for several months, and these treatments resulted in manageable side effects. However, further studies are required to see whether the combination is more effective than either ipilimumab or vaccine alone.
Table 1.
Summary of anti-CTLA4 clinical trials in prostate cancer
| Study trial (identifier) | Phase | Population (numbers enroled)* | End points | Clinical results‡ | Immune events‡ |
|---|---|---|---|---|---|
| Ipilimumab55 (CA184-009) | I | Advanced AIPC; pre-chemotherapy and post-chemotherapy (14) | Safety, PK, PSA response and objective responses | PSA declines of ≥50%: 2 pts | Grade 3 pruritis: one pt |
| Ipilimumab plus GM-CSF 56, 57 (NCT00064129 and CA184-098) | I/II | mAIPC (36) |
|
PSA declines of ≥50%. At 3 mg per kg: three of six pts. At 10 mg per kg: one of six pts |
irAEs are observed in a dose-dependent manner (≥3 mg per kg) |
| Ipilimumab plus GVAX13, 58 (CA184-119) | I | Chemo-naive mAIPC (12 escalation and 16 expansion) | Primary end points were MTD and safety; secondary end points were PSA response, TTP, immune response and survival | PSA declines of ≥50%. Escalation cohort: 5 pts. Expansion cohort: 1 pt |
irAEs at doses ≥3 mg per kg. Escalation cohorts: five pts. Expansion cohort: three pts |
| Ipilimumab plus PROSTVAC plus GM-CSF 61, 69 (NCT00124670 and CA184-100) | I | mAIPC, with six chemo-pretreated and 24 chemo-naive (30) | Primary end points were MTD and safety; secondary end points were PSA response in HLA-A2+ pts and immune response | Median TTP among chemo-naive pts: 6.1 months. Median OS among all pts: 31.8 months |
Grade ≥2 irAEs are seen in dose-dependent manner. 1 mg per kg: none of three pts. 3 mg per kg: two of six pts. 5 mg per kg: five if six pts. 10mg per kg: 13 of 15 pts |
| Ipilimumab plus XRT62,63 (NCT00323882 and CA184-017) | I/II | mAIPC, pre-docetaxel and post-docetaxel (Phase I: 26 and Phase II: 45) | Primary end point was safety; secondary end points were PSA response and metabolic bone activity | PSA declines of ≥50% with and without XRT. Phase I: 6 of 26 pts. Phase II: 10 of 45 pts | Grade ≥3 irAEs are seen in chemo-treated and chemo-naive pts. Phase I: nine pts. Phase II: 11 pts |
| Ipilimumab plus docetaxel64 (NCT00050596 and CA184-019) | II | mAIPC (43) | Safety and PSA response | Confirmed PSA responses + and − docetaxel: 3 pts | Three of 18 pts experienced SAEs consistent with irAEs |
| Ipilimumab plus AA with leuprolide acetate and bicalutamide65 (NCT00170157 and CA184-118) | II | Advanced chemo-naive prostate cancer (108) | Primary end point was proportion without progression at 18 months; secondary end point was PSA response | 55% of ipilimumab + AA versus 38% AA alone had undetectable PSA by 3 months | Grade ≥3 irAEs in ipilimumab + AA group. Colitis: 4.5%. Diarrhoea: 4.5% |
| Tremelimumab plus AA with bicalutamide (NCT00702923) | I | PSA-recurrent non-metastatic prostate cancer (estimated 24) | Primary end point is safety; secondary end points are PSA response and prostate-associated immune responses | Ongoing | Ongoing |
| Ipilimumab compared with placebo (NCT01057810) | III | Chemo-naive mAIPC (estimated 600) | Primary end point is OS; secondary end points are PFS, time to pain progression and safety | Ongoing | Ongoing |
| Ipilimumab plus XRT compared with placebo plus XRT (NCT00861614) | III | Advanced metastatic prostate cancer with prior treatment with docetaxel (estimated 800) | Primary end point is OS; secondary end points are PFS, time to pain progression and safety | Ongoing | Ongoing |
AA, androgen ablation; AIPC, androgen-insensitive prostate cancer; CTLA4, cytotoxic T lymphocyte-associated antigen 4; DLT, dose-limiting toxicity; GM-CSF, granulocyte–macrophage colony-stimulating growth factor; HLA, human leukocyte antigen; irAEs, immune-related adverse events; mAIPC, metastatic AIPC; MTD, maximum tolerated dose; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PK, pharmacokinetics; PR, partial response; PSA, prostate-specific antigen; pt, patient; SAEs, serious (grade ≥3) adverse events; TTP, time to progression; XRT, radiotherapy.
Population descriptors are listed as defined in publication of data.
As reported in publicly available materials.
In a Phase II trial that combined ipilimumab with radiotherapy, no difference in the number of patients who had a decline in PSA levels of ≥50% was seen between patients treated with ipilimumab alone (five of 16 chemo-naive patients) versus the ipilimumab and radiotherapy (four of 15 chemo-naive patients), and fewer patients in the post-chemotherapy group had a decline in PSA levels of ≥50% when treated with ipilimumab and radiation (one of 14)62,63. A randomized Phase II trial comparing ipilimumab at 3 mg per kg alone with ipilimumab plus a single dose of docetaxel showed a limited number of PSA responses: two of 23 patients treated with ipilimumab alone, and one of 20 patients treated with the combination therapy64. There were no patients who showed an objective radiographic response. However, in a randomized Phase II trial of ipilimumab and androgen ablation, patients treated with ipilimumab and androgen ablation were more likely to have undetectable levels of PSA by 3 months (55% versus 38%)65. Another human IgG2 monoclonal antibody (tremelimumab) has been tested in a Phase I trial in combination with androgen deprivation in patients with PSA-recurrent non-metastatic prostate cancer but the results are not yet available (NCT00702923)66.
Blocking CTLA4 in patients with prostate cancer revealed tumour response patterns that had not previously been appreciated by using inbred mouse models. For example, a decline in PSA levels can occur immediately after treatment but also after a period of stable disease or even after disease progression. For early phase trials, which are designed to capture early end points, such as safety or PSA responses, not enough time may have elapsed to see objective tumour responses by RECIST. Even so, more patients achieve disease stabilization rather than tumour shrinkage by radiographic assessment. One potential explanation is that AIPC mostly metastasizes to the bone, and that bone lesions, whether regressing or progressing, are notoriously difficult to measure with current radiographic techniques. Conversely, these response patterns have been consistently observed in patients with measurable metastases in advanced melanoma67, but objective responses still remain low overall (~10%)68. If tumour control is sustained, however, patients may live longer. Indeed, the median overall survival in the PROSTVAC combination trial is 31.8 months69, a significant duration in patients with mAIPC, and in pretreated metastatic melanoma, CTLA4 blockade significantly improves survival by 4 months over gp100 vaccine, despite no improvement in time to progression or progression-free survival70.
Given these experiences with CTLA4 blockade in mAIPC, the two ongoing Phase III trials investigating ipilimumab in mAIPC will address overall survival as the primary end point. The first trial will compare ipilimumab following radiation therapy versus placebo following radiation therapy in patients who have already been treated with docetaxel chemotherapy for mAIPC. A second study will compare ipilimumab versus placebo in individuals with mAIPC who have not already been treated with chemotherapy. Both studies will address as their primary end point whether patients live longer with CTLA4 blockade than patients treated in the control group.
These treatments have also been associated with immune-related adverse events (irAEs) affecting the colon (colitis), skin (pruritis and dermatitis), liver (hepatitis) and, less commonly, the eye and endocrine organs (thyroid and pituitary). Severe inflammatory events attributed to CTLA4 blockade have been described in ~10–24% of early Phase I/II prostate cancer trials, and these events are generally responsive to immunosuppressive corticosteroids. One concern is whether the concordant use of steroids will be detrimental to CTLA4-blocking antibodies. As CTLA4 blockade nonspecifically potentiates T cell activation to endogenous antigens (self and non-self), developing an irAE could correlate with clinical benefit, but no trial has been powered to address that hypothesis.
Immune effects with human treatment
The markers that have been used to detect T cell activation during and after treatment with CTLA4-specific antibodies in cancer patients include CD45RO71, inducible co-stimulator (ICOS)72, CD69 (REF. 56) and major histocompatibility complex, class II, DRα (HLA-DRA)73. CD45RO is an established marker for memory T cells. ICOS is structurally related to CD28 and CTLA4, and CD4+ and CD8+ T cells express ICOS on their cell surface following activation. ICOS functions as a co-stimulatory molecule on activated T cells and has been associated with the prolonged survival of Teff cells. CD69 and HLA-DRA are expressed on activated T cells.
In patients with mAIPC, the expansion of circulating activated CD69+CD8+ Teff cells occurred at a higher frequency with increased doses of ipilimumab (threshold at 3 mg per kg) and GM-CSF, and at a greater magnitude compared with either treatment at the same dose alone56. The combination treatment also induced an increase in the number of activated Teff cells, CD4+ Teff cells and the number of CD4+ FOXP3+ TReg cells that possessed suppressor function. The increases in the levels of Teff cells were greater at higher doses of CTLA4-specific antibody74. These initial results are interesting in the context of observations in mouse models that shifting of the ratio between Teff cells and TReg cells in favour of Teff cells may be an important effect of CTLA4 blockade75. A clinical trial in patients with melanoma that combines CTLA4-specific antibody treatment with an antibody to GITR to block TReg cell function is currently underway76 (NCT01216436).
As prostate cancer can be more challenging than melanoma to biopsy, the effect of CTLA4 blockade on the tumour microenvironment has also not been assessed, despite the complex immune response that is often present. No studies have yet been carried out to determine the levels of immunosuppressive cytokines compared with levels of proinflammatory cytokines at the tumour sites or the numbers of tumour-infiltrating lymphocytes (TILs). It is possible that immunological changes within the tumour might better correlate with clinical outcomes.
Antigen recognition with CTLA4 blockade
Determining the antigens that the T cells can recognize on CTLA4 blockade should reveal novel antigenic cancer effectors and pathways. Some of the antigens the T cells respond to are likely to be modified self-antigens, as prostate cancer can possess genetic mutations and translocations leading to novel proteins that are altered from normal self proteins that T cells tolerate. T cells that recognize these mutant proteins could be potentiated by CTLA4 blockade. These tumour antigens can be identified either by isolating tumour-specific T cells or by examining the induced humoral responses mediated by B cells (described below).
Tumour-specific T cells have been generated from mice vaccinated with a TRAMP cell line expressing GM-CSF and treated with a CTLA4-specific antibody77. To identify the corresponding antigens, the T cells were fused with cells containing an inducible NFAT promoter lacZ reporter construct to generate T cell hybridomas. These cells can measure TCR-mediated ligand-specific activation and were used to screen a cDNA library that was generated from a TRAMP tumour. A SPAS-1 epitope was identified that contained a point mutation found in TRAMP cells but not in normal mice. The corresponding non-mutated epitope is found in the human orthologue endophilin B1 (SH3GLB2), and specific T cell activity to the peptide can be generated with human T cells. SH3GLB2 overexpression has been reported in prostate cancer metastases78. T cell responses to PSA by enzyme-linked immunosorbent spot (ELISPOT) have been reported in patients with mAIPC treated with ipilimumab plus PROSTVAC6, but in the case of ipilimumab plus GM-CSF without a vaccine, antigen-specific T cell immune responses against known antigens (such as PAP, PSA, PSMA, ephrin type A receptor 2 (EPHA2) and survivin (also known as BIRC5)) could not be detected56. These results suggest that the endogenous immune responses potentiated by CTLA4 blockade recognize other unknown tumour antigens in mediating the anti-tumour response.
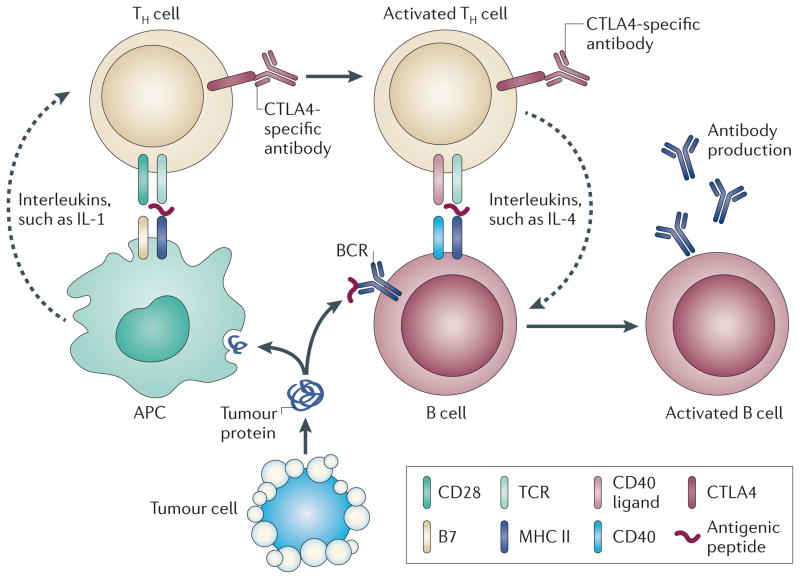
We and others have observed that a B cell (humoral) response to NY-ESO-1, a known cancer testis antigen, is increased in patients with either metastatic prostate cancer56 or melanoma who have been treated with CTLA4 antibodies79. Antibodies to NY-ESO-1 in the prostate group were identified using bacteria that expressed a phage display library of currently known cancer testis antigens spotted onto nitrocellulose membranes80. Antibodies to NY-ESO-1 in the melanoma group were detected using enzyme-linked immunosorbent assay (ELISA), and NY-ESO-1-specific T cells were detected by stimulating T cells to a pool of overlapping peptides spanning the entire NY-ESO-1 sequence and assaying for T cell activity through the production of interferon-γ (IFNγ), tumour necrosis factor (TNF) and macrophage inhibitory protein 1β (MIP1β). An antibody response resulting from CTLA4 blockade indicates that antigen-specific B cells are also activated, probably through TH cell activation (FIG. 2). The focus of T cell response in anti-tumour immunity has been on TH1-type immune responses; that is, the production of IFNγ by CD4+ and CD8+ Teff cells. However, a TH1-type immune response may not be exclusive in mediating the anti-tumour effect. TH2 and follicular T helper cell responses enable B cells to proliferate and differentiate into plasma cells in response to antigen. TH2 cells also promote isotype switching, so that IgG, IgA and IgE antibodies can be produced in addition to IgM and IgD antibodies. As yet, these TH responses have not been thoroughly examined in patients treated with CTLA4-specific antibodies.
Figure 2. CTLA4-specific antibodies potentiate TH cell-dependent B cell activation.
Helper T (TH) cells (TH2 and follicular T helper cells) are activated through the co-stimulation of two signalling receptor complexes. The first signal involves the presentation of antigenic peptides on a T cell receptor (TCR) on TH cells to major histocompatibility complex (MHC) II on antigen-presenting cells (APCs). The second signal occurs through the binding of CD28 molecules on TH cells to the B7 molecules on APCs. TH cells are also activated by interleukins (ILs) that are secreted by APCs. The B cell is also activated by activated TH cells through co-stimulation. The first signal consists of the binding of antigenic peptide that is presented by the TCR on a TH cell to MHC II expressed by the B cell. The second signal is given by the binding of a CD40 ligand on a TH cell to a CD40 molecule that is expressed on the B cell. B cell-activating interleukins are also secreted by activated TH cells. Activation of the B cell also requires the binding of the B cell receptor (BCR) to an antigenic peptide that is recognized by B cells. As cytotoxic T lymphocyte-associated antigen 4 (CTLA4)-specific antibodies increase TH cell activation, TH cells may subsequently increase B cell activation, resulting in the production of antibodies that recognize the targeted antigen.
In patients with melanoma, five of 15 patients treated with an anti-CTLA4 at a dose of >10 mg per kg produced antibodies to NY-ESO-1 that correlated with a therapeutic response79. However, in a different study, no correlation was found between a clinical response and the presence of NY-ESO-1 antibodies in patients with metastatic melanoma treated with CTLA4 antibodies81, although an integrated NY-ESO-1 and CD8+ T cell responses did correlate with clinical benefit in patients with metastatic melanoma in another study82. However, in nine patients with prostate cancer who were treated with an anti-CTLA4 at a dose of >3 mg per kg, the production of NY-ESO-1 antibodies in two of these patients did not correlate with a clinical response56. Other tumour-associated antigens may better correlate with clinical outcomes and have yet to be determined.
Serum from a patient with melanoma who responded to vaccination with a GM-CSF-secreting tumour cell vaccine and anti-CTLA4 treatment has been used to screen a cDNA expression library constructed from melanoma metastases83. Several antigens were identified, including MHC class I chain-related protein A (MICA). Consequently, additional patients that responded to either vaccination with GM-CSF-secreting tumour vaccine alone or combined with anti-CTLA4 treatment were found to have antibodies to these antigens83. However, the frequency of induced antibodies to any one antigen is fairly low, reflecting the heterogeneity of tumours. MICA is a ligand of NKG2D, an activating receptor on natural killer (NK) cells and CD8+ T lymphocytes, which contributes to immune-mediated tumour destruction. Increased shedding of soluble MICA by tumour cells is thought to be another mechanism of evasion of the immune system, and this increased expression may increase the immunogenicity of MICA. Antibodies to MICA detected in the sera of patients correlated with decreased levels of soluble MICA, increased levels of NKG2D, and restoration of NK cell and CD8+ lymphocyte functions. In addition, the investigators also found that treatment with GM-CSF-secreting tumour cell vaccines and CTLA4 blockade also induced a humoral reaction against multiple angiogenic cytokines, such as macrophage migration inhibitory factor (MIF)84. MIF is a cytokine that has been implicated in tumour blood vessel formation in mice85. Serum from a patient who responded to treatment with anti-CTLA4 and that was found to contain antibodies to MIF was able to antagonize in vitro angiogenesis assays. At concentrations similar to a MIF-specific antibody, this serum inhibited the production of matrix metalloproteinase 9 (MMP9) and the expression of MIF-induced angiopoietin 1 (ANGPT1; also known as TIE2) on monocytes. Therefore, the determination of antigenic targets that are recognized by the immune system after the administration of immunomodulatory therapies can reveal how tumours evade immunosurveillance, such as MICA shedding, and can also identify important functional factors that are involved in tumour growth, such as MIF.
In order to identify endogenous prostate tumour-specific antigens recognized on anti-CTLA4 blockade that correlate with anti-tumour effects, specifically without the presence of a vaccine for prostate cancer, we have used sera from responding and non-responding patients with mAIPC, who have been treated with only CTLA4 blockade and GM-CSF, to screen protein arrays86. Our preliminary observations indicate that there is an increased antibody response in the responders compared with the non-responders. The antibodies analysed so far have only a few antigens in common, suggesting that CTLA4 blockade induces endogenous tumour-specific responses that are mainly patient-specific. As irAEs are also observed in these patients, further studies should be carried out to distinguish tumour-specific antigens from self-antigens.
Future directions
In clinical studies, several issues require further investigation. First, what is the appropriate dose level for ipilimumab, either alone or in combination? Clinical responses (and immune-related side effects) are seen consistently at levels of ≥3 mg per kg. Data from melanoma trials suggest that tumour responses improve from 3 to 10 mg per kg68, but it is unclear whether the incidence of immune-related adverse events also increases. The optimal dose that strikes a suitable balance between safety and efficacy has not been determined, and both future and current Phase III trials in mAIPC will only test high-dose ipilimumab.
Second, if ipilimumab triggers long-lasting anti-tumour immunity, but at best clinically stabilizes rather than eradicates disease, treating earlier during the course of prostate cancer may provide a more meaningful benefit. If generalizations can be made from prior studies of immunotherapy in humans, ipilimumab should not be expected to induce immediate tumour regression. The tumour microenvironment may suppress immune responses that would otherwise reject tumours, and, hypothetically, a lower tumour burden, earlier in the course of disease, may induce less immune suppression. It is intriguing to ask whether CTLA4 blockade may be more effective in earlier states, such as in hormone-sensitive prostate cancer; although an argument can be made that each stage during the course of prostate cancer may have its own antigenic profile. Regardless, anti-CTLA4 therapy has the advantage of not being dependent on any one specific tumour antigen.
The treatment of advanced prostate cancer is substantially evolving with the recent FDA approvals of sipuleucel-T, abiraterone acetate and cabazitaxel. Other treatments are also in Phase III trials, including PROSTVAC. The appropriate sequence of use of CTLA4 blockade with all of these agents will need to be addressed by clinical trials, but these issues underscore the need for biomarkers that can help to predict response to CTLA4 blockade and, therefore, allow improved patient (and drug) selection. Retrospective analysis from the combination trial of GVAX and ipilimumab suggests that CTLA4 expression on circulating CD4+ T cells before treatment may be associated with significantly prolonged survival after ipilimumab therapy59. Such an observation would need to be validated prospectively to establish this as a predictive biomarker of clinical response.
By accessing immune-specific responses, such as dissecting the antigens that are recognized by tumour-specific T cells and antibodies in patients treated with CTLA4 blockade, novel cancer pathways are being revealed that are not obvious when examining the genetic, epi-genetic and expression profiles of prostate tumours compared with normal cells. These antigens become sufficiently immunogenic with CTLA4 blockade. Moreover, some of these antigens may also participate in tumorigenesis, especially if the immune-specific responses to these antigens correlate with objective responses. In addition, conventional therapies such as radiation or chemotherapy have also been shown to increase the tumour antigen pool, possibly owing to an increase in apoptosis as a result of these treatments. Antibody responses induced by conventional treatments have been detected using serological identification of antigens by recombinant cDNA expression cloning (SEREX)87. Androgen ablation has also been shown to be pro-immunogenic, and induction of antibodies has been observed88. Correlating the immune responses with objective responses in the current clinical trials that combine conventional therapies with CTLA4 blockade could potentially be useful in delineating responders from non-responders. In addition, as tumour response patterns are variable and clinical effects late in onset, identifying potential markers that could predict treatment efficacy could improve understanding of who would need early transition to other second-line agents.
Further studies are required to determine whether the antigens identified on CTLA4 blockade could be better and more relevant vaccine candidates, especially if targeting these antigens could uncouple anti-tumour responses from on-target side effects. If so, the challenges of developing customized vaccines for personalized medicine, including the time and cost required to identify relevant antigens and to synthesize working vaccines for each individual patient, would have to be addressed. Conversely, it could be that immunomodulatory therapy with CTLA4 blockade is essentially similar to a patient-specific therapy, as it unmasks the individual antigenic pool that can provide an efficacious response and may, therefore, represent a more accessible approach. There is increased recognition that genetic, epigenetic and transcript expression patterns in tumours are patient-specific; antigenic variability now adds an additional layer to the complexity of tumour biology that we need to understand better in order to prolong patient survival with current cancer therapies.
Acknowledgments
S.S.K. is supported by a Peter Michael Foundation Pelican Fellowship. E.C. is supported by an ASCO Young Investivator Award. L.F. is supported by NIH R01 CA136753 and the Prostate Cancer Foundation.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
ClinicalTrials.gov: http://clinicaltrials.gov/NCT00702923|NCT00861614|NCT01057810|NCT01216436|NCT01303705
FURTHER INFORMATION
Lawrence Fong’s homepage: http://medicine.ucsf.edu/hemonc/fonglab/
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Serena S. Kwek, Division of Hematology/Oncology, Department of Medicine, University of California, San Francisco CA 94143-0511, USA
Edward Cha, Division of Hematology/Oncology, Department of Medicine, University of California, San Francisco CA 94143-0511, USA.
Lawrence Fong, Division of Hematology/Oncology, Department of Medicine, University of California, San Francisco CA 94143-0511, USA. UCSF Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco CA 94143, USA.
References
- 1.American Cancer Society. Cancer Facts & Figures. American Cancer Society; 2011. [online], http://www.cancer.org/Research/CancerFactsFigures/index. [Google Scholar]
- 2.Howlader N, et al. SEER Cancer Statistics Review 1975–2008. National Cancer Institute; 2011. [online], http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 3.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laufer M, Denmeade SR, Sinibaldi VJ, Carducci MA, Eisenberger MA. Complete androgen blockade for prostate cancer: what went wrong? J Urol. 2000;164:3–9. [PubMed] [Google Scholar]
- 5.Cha E, Fong L. Immunotherapy for prostate cancer: biology and therapeutic approaches. J Clin Oncol. 2011;29:3677–3685. doi: 10.1200/JCO.2010.34.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Carballido E, Fishman M. Sipuleucel-T for therapy of asymptomatic or minimally symptomatic, castrate-refractory prostate cancer: an update and perspective among other treatments. Onco Targets Ther. 2011;4:79–96. doi: 10.2147/OTT.S14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akhtar NH, Pail O, Saran A, Tyrell L, Tagawa ST. Prostate-specific membrane antigen-based therapeutics. Adv Urol. 2012;2012:973820. doi: 10.1155/2012/973820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clive KS, et al. Use of GM-CSF as an adjuvant with cancer vaccines: beneficial or detrimental? Expert Rev Vaccines. 2010;9:519–525. doi: 10.1586/erv.10.40. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaer DA, Cohen AD, Wolchok JD. Anti-GITR antibodies--potential clinical applications for tumor immunotherapy. Curr Opin Investig Drugs. 2010;11:1378–1386. [PubMed] [Google Scholar]
- 13.Gerritsen W, et al. A dose-escalation trial of GM-CSF-gene transduced allogeneic prostate cancer cellular immunotherapy in combination with a fully human anti-CTLA antibody (MDX-010, ipilimumab) in patients with metastatic hormone-refractory prostate cancer (mHRPC) J Clin Oncol Abstr. 2006;24:2500. [Google Scholar]
- 14.Ledford H. Melanoma drug wins US approval. Nature. 2011;471:561. doi: 10.1038/471561a. [DOI] [PubMed] [Google Scholar]
- 15.Brunet JF, et al. A new member of the immunoglobulin superfamily — CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 16.Peach RJ, et al. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7–1. J Exp Med. 1994;180:2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein PH, Fraser JD, Weiss A. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3′-kinase. Mol Cell Biol. 1994;14:3392–3402. doi: 10.1128/mcb.14.5.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang E, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 19.Dariavach P, Mattei MG, Golstein P, Lefranc MP. Human Ig superfamily CTLA-4 gene: chromosomal localization and identity of protein sequence between murine and human CTLA-4 cytoplasmic domains. Eur J Immunol. 1988;18:1901–1905. doi: 10.1002/eji.1830181206. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 21.Linsley PS, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linsley PS, et al. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linsley PS, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 24.Chuang E, et al. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997;159:144–151. [PubMed] [Google Scholar]
- 25.van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7–1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 27.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider H, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 31.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32:428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kearney ER, et al. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 39.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 40.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 41.Kwon ED, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon ED, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurwitz AA, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 44.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mokyr MB, Kalinichenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 46.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- 47.Matsumura S, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Drake CG, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutherland JS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 51.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 52.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keler T, et al. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J Immunol. 2003;171:6251–6259. doi: 10.4049/jimmunol.171.11.6251. [DOI] [PubMed] [Google Scholar]
- 55.Small EJ, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 56.Fong L, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harzstark AL, et al. Final results of a phase I study of CTLA-4 blockade in combination with GM-CSF for metastatic castration resistant prostate cancer (mCRPC) J Clin Oncol Abstr. 2010;28:4689. [Google Scholar]
- 58.Gerritsen W, et al. Expanded phase I combination trial of GVAX immunotherapy for prostate cancer and ipilimumab in patients with metastatic hormone-refractory prostate cancer (mHPRC) J Clin Oncol Abstr. 2008;26:5146. [Google Scholar]
- 59.Santegoets S, et al. Lymphoid and myeloid biomarkers for clinical outcome of ipilimumab and prostate GVAX treatment: tumor-related CTLA-4 expression by CD4+ T cells as a dominant predictor of survival. J Immunother Abstr. 2011;34:9. [Google Scholar]
- 60.Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohebtash M, et al. Phase I trial of targeted therapy with PSA-TRICOM vaccine (V) and ipilimumab (ipi) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol Abstr. 2009;27:5144. [Google Scholar]
- 62.Beer TM, et al. Phase I trial of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration resistant prostate cancer (mCRPC) J Clin Oncol Abstr. 2008;26:5004. [Google Scholar]
- 63.Slovin SF, et al. Initial phase II experience of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol Abstr. 2009;27:5138. [Google Scholar]
- 64.Small EJ, et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer. J Clin Oncol Abstr. 2006;24:4609. [Google Scholar]
- 65.Tollefson MK, et al. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer. 2010 Genitourinary Cancers Symp. 2010:Abstr. 168. [Google Scholar]
- 66.Lang JM, Staab MJ, Liu G, Wilding G, McNeel DG. Phase I dose-escalation trial of tremelimumab in combination with bicalutamide in patients with recurrent prostate cancer. J Clin Oncol Abstr. 2011;29:174. [Google Scholar]
- 67.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 68.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 69.Madan RA, et al. Overall survival (OS) analysis of a phase I trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab (Ipi) in the treatment of metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol Abstr. 2010;28:2550. [Google Scholar]
- 70.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Comin-Anduix B, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6:22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liakou CI, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kavanagh B, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 77.Fasso M, et al. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci USA. 2008;105:3509–3514. doi: 10.1073/pnas.0712269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan J, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dubovsky JA, Albertini MR, McNeel DG. MAD-CT-2 identified as a novel melanoma cancer-testis antigen using phage immunoblot analysis. J Immunother. 2007;30:675–683. doi: 10.1097/CJI.0b013e3180de4d19. [DOI] [PubMed] [Google Scholar]
- 81.Goff SL, Robbins PF, El-Gamil M, Rosenberg SA. No correlation between clinical response to CTLA-4 blockade and presence of NY-ESO-1 antibody in patients with metastatic melanoma. J Immunother. 2009;32:884–885. doi: 10.1097/CJI.0b013e3181affbf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan J, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108:16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci USA. 2006;103:9190–9195. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schoenfeld J, et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70:10150–10160. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chesney J, et al. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–191. [PMC free article] [PubMed] [Google Scholar]
- 86.Fong L, et al. Identification of novel prostate cancer-associated antigens through antibody profiling of prostate cancer patients treated with CTLA-4 blockade. J Clin Oncol Abstr. 2010;28:2578. [Google Scholar]
- 87.Nesslinger NJ, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 88.Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;71:496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]