Bioorthogonal “click” chemistries are now widely used in chemical biology for a myriad of applications such as activity based protein profiling, monitoring cell proliferation, generation of novel enzyme inhibitors, monitoring the synthesis of newly formed proteins, protein target identification, and studying glycan processing.[1, 2] Arguably, the most fascinating applications involve using these bioorthogonal chemistries to assemble molecules in the presence of living systems such as live cells or even whole organisms.[3, 4] These latter applications require that the chemistry does not employ toxic metal catalysts and maintains kinetics that allow fast reaction to occur with micromolar concentrations of reagents in a time span of minutes to hours. To fulfill these criteria, various “copper-free” click chemistries have been reported, such as the strain-promoted azide-alkyne cycloaddition and the Staudinger ligation, to react with azides on the surface of live cells both in culture and in in vivo systems such as mice and zebrafish.[4] However, to date, the application of “click” chemistries in living systems, has been largely limited to extracellular targets. [5] The reasons for this are likely several. In addition to fulfilling the stability, toxicity, and chemoselectivity requirements of “click” chemistry, intracellular live cell labeling requires reagents that can pass easily through biological membranes and kinetics that enable rapid labeling even with the low concentrations of agent that make it across the cell membrane. Additionally, a practical intracellular bioorthogonal coupling scheme would need to incorporate a mechanism by which the fluorescent tag increases in fluorescence upon covalent reaction to avoid visualizing accumulated but unreacted imaging agent (i.e. "background"). Such activatable “turn-on” probes would significantly increase the signal-to-background ratio, which is particularly relevant to imaging targets inside living cells since a stringent washout of unreacted probe is not possible.
In previous years a number of elegant probes have been introduced whose fluorescence increases after azide-alkyne cycloaddition or staudinger ligation coupling reactions. [6] Most of these strategies utilize a reactive group intimately attached to the fluorophore thus necessitating synthesis of new fluorophore scaffolds or take advantage of a FRET based activation requiring appending of an additional molecule that can act as an energy transfer agent. Furthermore, most probes employing these popular coupling schemes have not been used to label intracellular targets in live cells. Here we report a series of activatable “turn-on” tetrazine-linked fluorescent probes, which react rapidly via an inverse electron demand cycloaddition with strained dienophiles such as trans-cyclooctene. Upon cycloaddition, the fluorescence intensity increases dramatically, in some cases by ~20 fold. This fluorescence “turn-on” significantly lowers background signal. We have used these novel probes for live cell imaging of a trans-cyclooctene modified taxol analog bound to intracellular tubules. The rapid reaction rate coupled with fluorescence activation makes this a nearly ideal method for revealing target molecules inside living cells.
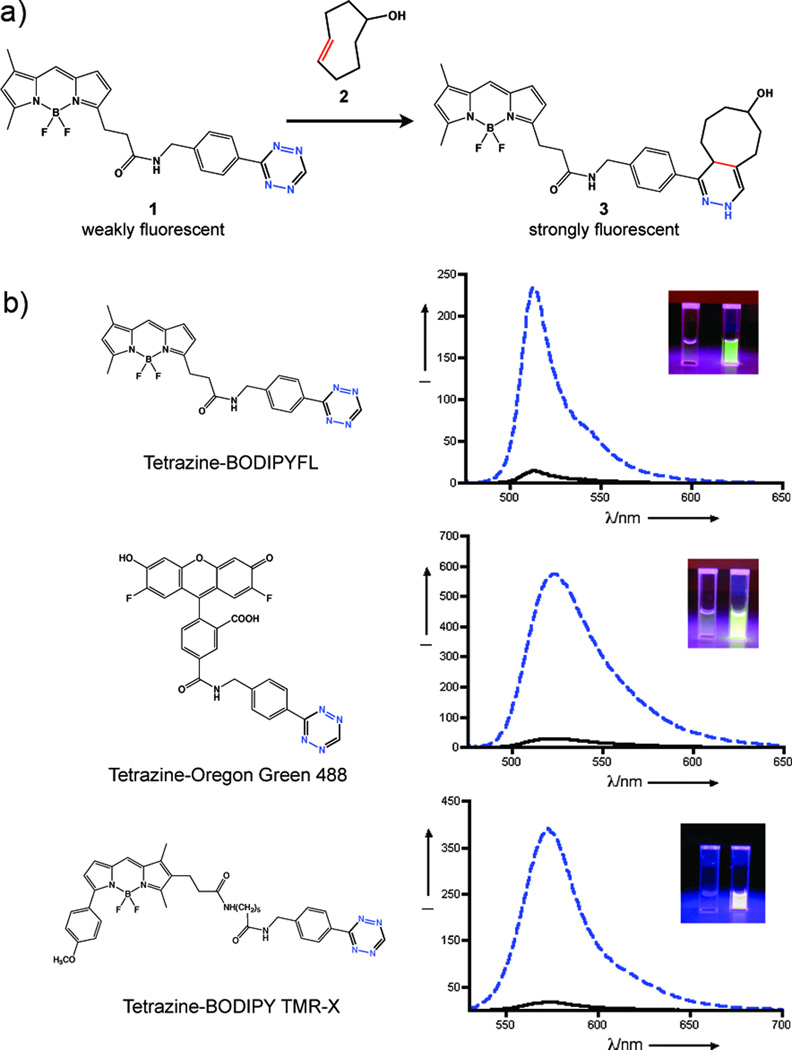
Recently, we and others have explored conjugation reactions using inverse electron demand Diels-Alder cycloadditions between tetrazines and highly strained dienophiles such as norbornene and trans-cyclooctene. [7–9] We have shown that a novel asymmetric tetrazine is quite stable in water and serum and can react with trans-cyclooctene at rates of approximately 103 M−1sec−1 at 37°C.[9] This extremely fast rate constant allowed the labeling of extracellular targets at low nanomolar concentrations of tetrazine labeling agent with concentrations that are sufficiently low to enable real-time imaging of probe accummulation. Previous work from our group relied on tetrazines conjugated to highly charged carbocyanine-based near-IR emitting fluorophores. In our efforts to explore the utility of this reaction for intracellular labeling, we conjugated 3-(4-benzylamino)-1,2,4,5-tetrazine to the succinimidyl esters of visible light emitting boron-dipyrromethene (BODIPY) dyes. BODIPY dyes are uncharged and lipophilic and for these reasons have seen use in intracellular applications. [10] We also wondered whether or not visible fluorophores would show electronic interactions with the tetrazine chromophores, which have absorption maxima at 500–525 nm. In fact, the tetrazine BODIPY conjugates exhibited strongly reduced fluorescence compared to the parent succinimidyl esters of the fluorophores. Upon reaction with a strained dienophile such as trans-cyclooctenol or norbornene, the fluorescence was “switched” back on.
To explore the generality of this phenomenon we reacted the benzylamino tetrazine with commercially available succinimidyl esters of 7-diethylaminocoumarin-3-carboxylic acid, BODIPY FL, BODIPY TMR-X, Oregon Green 488 (Invitrogen) and Vivotag-680 (VT680, Visen Medical). Figure 1b shows the fluorescence emission spectra of selected dye-tetrazine conjugates before and after cycloaddition to trans-cyclooctenol. Table 1 lists the photophysical properties of the dyes before and after reaction. For all dyes with emission between 400–600 nm conjugation to the tetrazine caused fluorescence quenching, which was restored after reaction with dienophiles. Quenching of the fluorophore by the tetrazine is wavelength dependent. Green and red emitting tetrazine dyes showed fluorescent enhancements upon cycloaddition of approximately 15–20 fold in PBS. In contrast, the shorter wavelength emitting tetrazine-coumarin showed only a three fold enhancement. The green and red emitting tetrazine dyes also show strong fluorgenic responses in 100% fetal bovine serum (table S2, supporting information). Near-IR emitting dyes such as our previously used carbocyanine tetrazine-VT680 as well as tetrazine-BODIPY 650–665 (data not shown) were not quenched, explaining why this phenomenon was not observed in previous studies.
Figure 1.
a) Tetrazine-BODIPY FL (1) reacts rapidly with trans-cyclooctenol (2) via an inverse electron demand Diels-Alder cycloaddition to form isomeric dihydropyrazine products (3). b) Emission spectra of various tetrazine probes (black lines) and the corresponding dihydropyrazine products (dashed blue lines). Inset images compare the visible fluorescence emission of the tetrazine probes (left cuvettes) to their corresponding dihydropyrazine products (right cuvettes) under excitation from a handheld UV lamp.
Table 1.
All measurements are in PBS, pH 7.4 (dye concentration 1 µM) Quantum yield measurements are in triplicate with fluorescein (in water, pH 10), Rhodamine 6G (in EtOH), or Cy 5.5 in PBS as standards.
| Dye | Abs(nm)a | Em(nm)a | Quantum yield w/o octeneb | Quantum yield w/ octenec | Fold increase in fluorescence |
|---|---|---|---|---|---|
| Tetrazine-Coumarin | 430 | 480 | 0.01 | 0.03 | 3.3 |
| Tetrazine-BODIPY FL | 505 | 512 | 0.02 | 0.24 | 15.0 |
| Tetrazine-Oregon Green 488 | 495 | 523 | 0.04 | 0.82 | 18.5 |
| Tetrazine-BODIPY TMR-X | 543 | 573 | 0.02 | 0.40 | 20.6 |
| Tetrazine-VT680 | 669 | 687 | 0.16 | 0.16 | 1.0 |
Notes:
abs/em values are before addition of trans-cyclooctene (10 µM), but there are no significant changes in these values after trans-cyclooctene addition.
Quantum yields for the tetrazine-fluorophore conjugates,
Quantum yields for the dihydropyrazine fluorophore products.
We speculate that the mechanism of fluorescence quenching may be due to resonance energy transfer between the fluorescent chromophore and the tetrazine, which has a visible absorbance maximum at 515 nm.[8] This would explain the wavelength dependence of the quenching. Another possibility could be that the quenching is the result of photoinduced electron transfer (PET) between the excited fluorophore and a potential tetrazine acceptor. Tetrazines are well known to be an electron-poor class of heterocycles, hence their utility in inverse-electron demand cycloadditions. The PET based mechanism would be reminiscent of the well known quenching of fluorophores by electron poor nitroaromatic compounds.[11] The fluorescence signals from the quenched tetrazine-BODIPY FL and tetrazine-BODIPY TMR-X conjugates vary by less than 5% between pH 9 and 3, indicating the quenching mechanism is not pH dependent. It is important to note that these fluorogenic compounds can be formed from commercially available fluorophores and that the tetrazine appears to be a sufficiently strong quencher that does not require intimate connection to the fluorophore and can achieve a quenching effect even when separated by aliphatic spacers. We are currently investigating the mechanism of quenching and designing next generation tetrazine-fluorophores that show further enhancements of fluorescence upon cycloaddition.
Although we envision that the featured fluorogenic probes could have a myriad of applications one use that would immediately benefit from a fluorogenic probe is the detection of target molecules inside live cells. This will allow application in determining the subcellular distribution of pharmaceutical and metabolic analogs containing a dienophile tag. To test if the fluorogenic tetrazines reported here are relevant for imaging intracellular molecular targets, we chose dienophile-modified paclitaxel (Taxol®) as a model system. Taxol was selected because of its tremendous clinical impact, the large body of prior work that serves as reference, and based on its well studied ability to stabilize microtubules, providing us a well-defined intracellular structure to image.[12–15] The trans-cyclooctene taxol derivative (Figure 2a) was synthesized by coupling trans-cyclooctene succinimidyl carbonate to 7-β-alanyl taxol via reported procedures.[13] The dienophile was introduced in the C7 position since prior structure activity relationship studies have established that modifications at the C7 position do not significantly affect the biological activity of taxol.[13, 15, 16] Trans-cyclooctene taxol rapidly reacts with our tetrazine probes forming isomeric dihydropyrazine products which can be detected by LC-MS (see supplementary information).
Figure 2.
a) Structure of taxol (1) and Trans-cyclooctene modified taxol (2) (TCO-taxol). b) Comparison of the ability of 10 µM taxol, trans-cyclooctene taxol, and a DMSO control to polymerize tubulin in the absence of GTP (polymerization assayed via absorbance at 350nm). Note that trans-cyclooctene taxol promotes polymerization similar to taxol and significantly better than a DMSO control. c) microtubule bundles formed in the presence of trans-cyclooctene taxol treated with tetrazine-BODIPY FL and visualized by fluorescence microscopy.
To test the activity of the trans-cyclooctene taxol analog, we focused on the well established ability of taxol to polymerize tubulin in the absence of GTP.[17] Optical density measurements at 350 nm (Figure 2b) were used to determine the degree of tubulin polymerization after exposure of tubulin monomer to taxol, trans-cyclooctene taxol, and a DMSO control. Both native taxol and trans-cyclooctene taxol induce polymerization compared to a DMSO control. Trans-cyclooctene taxol induced tubule bundles were visualized by subsequent staining with tetrazine fluorophore probes such as tetrazine-BODIPY FL, which covalently couples to the microtubule-bound trans-cyclooctene taxol molecules, yielding brightly fluorescent tubule structures that can be imaged by fluorescence microscopy (Figure 2c).
For live cell studies, PtK2 kangaroo rat kidney cells were incubated in cell media containing 1 µM trans-cyclooctene taxol for 1 hour at 37°C. PtK2 are commonly used in microtubule studies due to their flattened morphology.[15] After washing with media three times, the cells were exposed to media containing 1 µM tetrazine-BODIPY FL for 20 minutes at room temperature. The cells were then washed and imaged on a confocal microscope (Figure 3). Intracellular structures reflecting tubule networks become readily apparent. This staining pattern corresponds to immunostaining using anti-α-tubulin (Figure S2). Taxol is known to bind the microtubular networks of cells and there are several reports of fluorescent taxol derivatives for imaging microtubular networks.[12, 13, 15] Control experiments employing tetrazine-BODIPY FL alone or with unmodified taxol yielded minimal fluorescence background signal and demonstrate that there is little non-specific or background turn-on and that the images result from the specific tetrazine trans-cyclooctene cycloaddition reaction (Figure 3c). Furthermore, cells treated with trans-cyclooctene modified taxol followed by highly charged non-membrane permeable tetrazine probes such as the sulfonated tetrazine-VT680 showed very little staining and an absence of tubular structures, giving further evidence that tetrazine-BODIPY FL is able to penetrate the cell membrane and label trans-cyclooctene located within the cell (Figure 3d).[8, 9] Attempts to label cells with fluorescent tetrazine taxol conjugates led to nonspecific weak staining signals (Figure S3).
Figure 3.
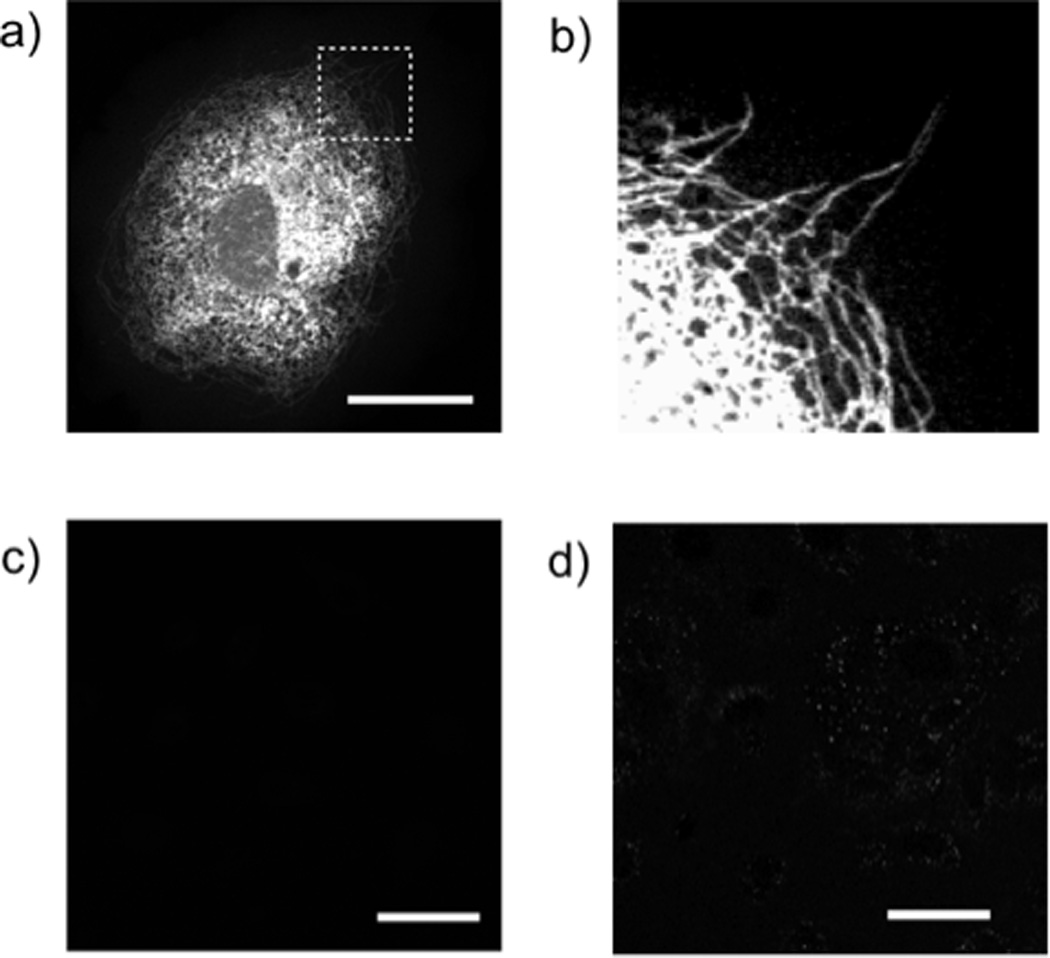
a) Confocal microscopy of a PtK2 cells after treatment with 1µM trans-cyclooctene-taxol followed by 1µM tetrazine-BODIPY FL (green). Scale bar: 30 µm. Expansion of the section indicated by the dashed white line (b) reveals that tubular structures are clearly stained. c) Confocal microscopy of PTK2 cells after treatment with 1µM taxol followed by 1µM tetrazine-BODIPY FL. Scale bar: 50 µm. d) Confocal microscopy of PTK2 cells after treatment with 1µM trans-cyclooctene-taxol followed by 1µM tetrazine-VT680. Scale bar: 50 µm.
In conclusion we have developed a robust method for the bioorthogonal tagging and imaging of targets inside living cells. Fluorogenic tetrazine probes react specifically and rapidly with strained dienophiles such as trans-cyclooctene. The ability of these probes to show fluorescence “turn-on” upon cycloaddition is a major advantage especially for applications where probe washout is not desired or possible. We imagine that this method will be useful for applications requiring the imaging of the intracellular distribution of tagged small molecules. We are currently exploring imaging other dienophile containing small molecules that target therapeutic targets both in vitro with live cells and in vivo in relevant animal models.
Supplementary Material
Footnotes
We thank Dr. Justin Ragains for helpful advice and Alex Chudnovsky for assistance with tissue culture. This research was supported in part by NIH grants U01-HL080731 and T32-CA79443
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; Rostovtsev VV, Green LG, Fokin VV, B SK. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Speers AE, Adam GC, Cravatt BF. J Am Chem Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]; Salic A, Mitchison TJ. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lewis WG, Green LG, Grynszpan F, Radic Z, Carlier PR, Taylor P, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:1053–1057. doi: 10.1002/1521-3773(20020315)41:6<1053::aid-anie1053>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]; Beatty KE, Xie F, Wang Q, Tirrell DA. J Am Chem Soc. 2005;127:14150–14151. doi: 10.1021/ja054643w. [DOI] [PubMed] [Google Scholar]; Gubbens J, Ruijter E, de Fays LE, Damen JM, de Kruijff B, Slijper M, Rijkers DT, Liskamp RM, de Kroon AI. Chem Biol. 2009;16:3–14. doi: 10.1016/j.chembiol.2008.11.009. [DOI] [PubMed] [Google Scholar]; Hsu TL, Hanson SR, Kishikawa K, Wang SK, Sawa M, Wong CH. Proc Natl Acad Sci U S A. 2007;104:2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Neef AB, Schultz C. Angew. Chem. Int. Ed. 2009;48:1498–1500. doi: 10.1002/anie.200805507. [DOI] [PubMed] [Google Scholar]; Ning XH, Guo J, Wolfert MA, Boons GJ. Angew. Chem. Int. Ed. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]; Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prescher JA, Bertozzi CR. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 5.Baskin JM, Bertozzi CR. QSAR Comb. Sci. 2007;26:1211–1219. [Google Scholar]
- 6.Sivakumar K, Xie F, Cash BM, Long S, Barnhill HN, Wang Q. Org Lett. 2004;6:4603–4606. doi: 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]; Zhou Z, Fahrni CJ. J Am Chem Soc. 2004;126:8862–8863. doi: 10.1021/ja049684r. [DOI] [PubMed] [Google Scholar]; Hangauer MJ, Bertozzi CR. Angew. Chem. Int. Ed. 2008;47:2394–2397. doi: 10.1002/anie.200704847. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lemieux GA, De Graffenried CL, Bertozzi CR. J Am Chem Soc. 2003;125:4708–4709. doi: 10.1021/ja029013y. [DOI] [PubMed] [Google Scholar]
- 7.Blackman ML, Royzen M, Fox JM. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pipkorn R, Waldeck W, Didinger B, Koch M, Mueller G, Wiessler M, Braun K. J Pept Sci. 2009;15:235–241. doi: 10.1002/psc.1108. [DOI] [PubMed] [Google Scholar]
- 8.Devaraj NK, Weissleder R, Hilderbrand SA. Bioconjug Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew. Chem. Int. Ed. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole L, Davies D, Hyde GJ, Ashford AE. J Microsc. 2000;197:239–249. doi: 10.1046/j.1365-2818.2000.00664.x. [DOI] [PubMed] [Google Scholar]; Farinas J, Verkman AS. J Biol Chem. 1999;274:7603–7606. doi: 10.1074/jbc.274.12.7603. [DOI] [PubMed] [Google Scholar]; Miller EW, Zeng L, Domaille DW, Chang CJ. Nat Protoc. 2006;1:824–827. doi: 10.1038/nprot.2006.140. [DOI] [PubMed] [Google Scholar]; Takahashi N, Nemoto T, Kimura R, Tachikawa A, Miwa A, Okado H, Miyashita Y, Iino M, Kadowaki T, Kasai H. Diabetes. 2002;51(Suppl 1):S25–S28. doi: 10.2337/diabetes.51.2007.s25. [DOI] [PubMed] [Google Scholar]; Viht K, Padari K, Raidaru G, Subbi J, Tammiste I, Pooga M, Uri A. Bioorg Med Chem Lett. 2003;13:3035–3039. doi: 10.1016/s0960-894x(03)00641-3. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster JV, McGuffin VL. Anal Chem. 2001;73:2004–2011. doi: 10.1021/ac001347n. [DOI] [PubMed] [Google Scholar]; Kim Y, Zhu Z, Swager TM. J Am Chem Soc. 2004;126:452–453. doi: 10.1021/ja038472b. [DOI] [PubMed] [Google Scholar]
- 12.Evangelio JA, Abal M, Barasoain I, Souto AA, Lillo MP, Acuna AU, Amat-Guerri F, Andreu JM. Cell Motil Cytoskeleton. 1998;39:73–90. doi: 10.1002/(SICI)1097-0169(1998)39:1<73::AID-CM7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 13.Guy R, Scott Z, Sloboda R, Nicolaou K. Chem Biol. 1996;3:1021–1031. doi: 10.1016/s1074-5521(96)90168-4. [DOI] [PubMed] [Google Scholar]
- 14.Manfredi JJ, Parness J, Horwitz SB. J Cell Biol. 1982;94:688–696. doi: 10.1083/jcb.94.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nicolaou KC, Dai WM, Guy RK. Angew. Chem. Int. Ed. 1994;33:15–44. [Google Scholar]; Rowinsky EK, Cazenave LA, Donehower RC. J. Natl. Cancer Inst. 1990;82:1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 15.Souto AA, Acuna AU, Andreu JM, Barasoain I, Abal M, AmatGuerri F. Angew. Chem. Int. Ed. 1995;34:2710–2712. [Google Scholar]
- 16.Chen SH, Kant J, Mamber SW, Roth GP, Wei JM, Marshall D, Vyas DM, Farina V, Casazza A, Long BH, Rose WC, Johnston K, Fairchild C. Bioorg. Med. Chem. Lett. 1994;4:2223–2228. [Google Scholar]; Mellado W, Magri NF, Kingston DG, Garcia-Arenas R, Orr GA, Horwitz SB. Biochem Biophys Res Commun. 1984;124:329–336. doi: 10.1016/0006-291x(84)91557-2. [DOI] [PubMed] [Google Scholar]
- 17.Shelanski ML, Gaskin F, Cantor CR. Proc Natl Acad Sci U S A. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schiff PB, Horwitz SB. Biochemistry. 1981;20:3247–3252. doi: 10.1021/bi00514a041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.