Abstract
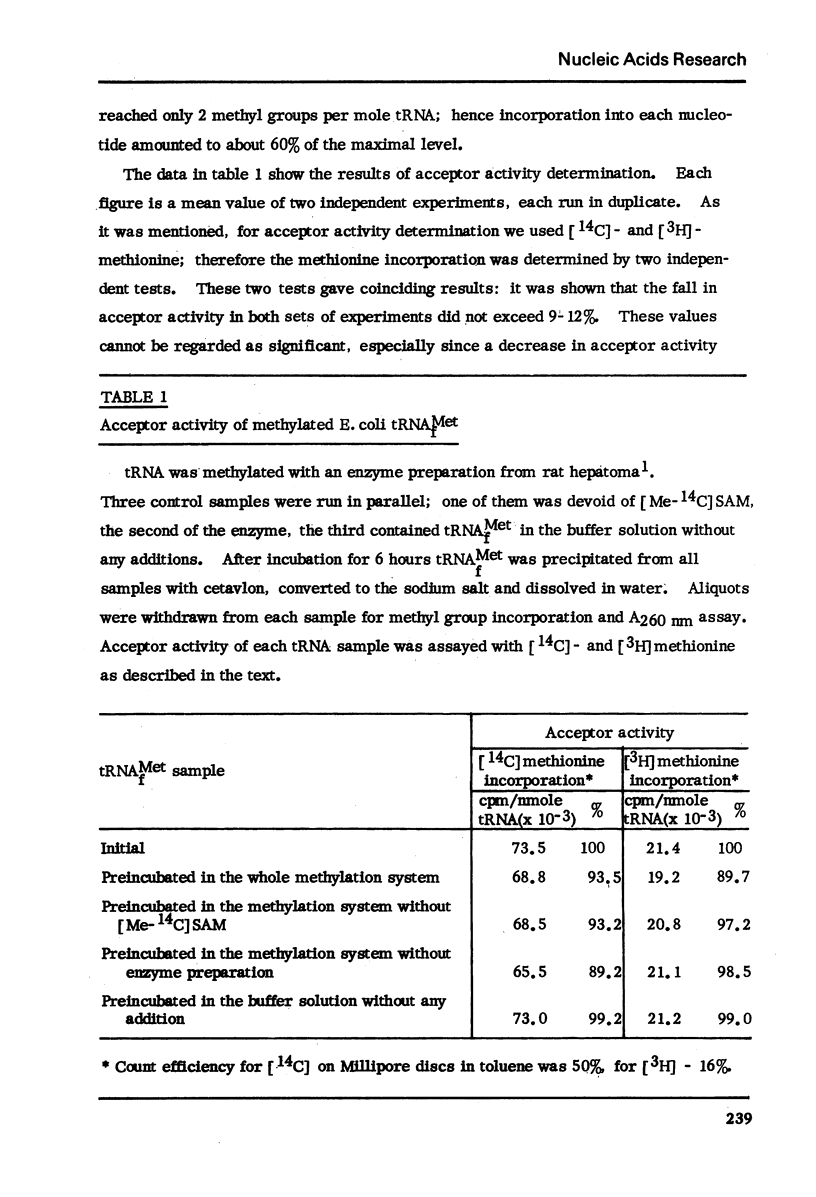
The acceptor activity of normal E. coli tRNAMetf was compared with that of a preparation with surplus methyl groups introduced by a crude methylase preparation from rat hepatoma. No changes in charging were detected when the aminoacylation was carried out in a homologous system. The data indicate that neither the surplus methyl groups by themselves, nor the eventual changes in spatial arrangement are essential for charging of E. coli tRNAMetf.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dube S. K. Evidence of "three point" attachment of tRNA to methionyl tRNA synthetase. Nat New Biol. 1973 May 23;243(125):103–105. [PubMed] [Google Scholar]
- Dube S. K., Marcker K. A., Clark B. F., Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968 Apr 20;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- Kelmers A. D., Novelli G. D., Stulberg M. P. Separation of transfer ribonucleic acids by reverse phase chromatography. J Biol Chem. 1965 Oct;240(10):3979–3983. [PubMed] [Google Scholar]
- Michelson A. M., Pochon F. Polynucleotide analogues. VII. Methylation of polynucleotides. Biochim Biophys Acta. 1966 Mar 21;114(3):469–480. [PubMed] [Google Scholar]
- Pochon F., Michelson A. M. Polynucleotide analogues. XI. Poly N-1-methylguanylic acid and other methylated polynucleotides. Biochim Biophys Acta. 1967 Sep 26;145(2):321–327. [PubMed] [Google Scholar]
- Pochon F., Michelson A. M. Polynucleotide analogues. XIV. Poly N2-dimethylguanylate. Biochim Biophys Acta. 1969 May 20;182(1):17–23. doi: 10.1016/0005-2787(69)90515-2. [DOI] [PubMed] [Google Scholar]
- Schulman L. H. Structure and function of E. coli formylmethionyl tRNA. I. Effect of modification of pyrimidine residues on aminoacyl synthetase recognition. Proc Natl Acad Sci U S A. 1970 Jun;66(2):507–514. doi: 10.1073/pnas.66.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H. Structure and function of Escherichia coli formylmethionine transfer RNA. II. Effect of modification of guanosine residues on aminoacyl synthetase recognition. J Mol Biol. 1971 May 28;58(1):117–131. doi: 10.1016/0022-2836(71)90236-1. [DOI] [PubMed] [Google Scholar]
- Schulman L. H. Structure and function of Escherichia coli formylmethionine transfer RNA: loss of methionine acceptor activity by modification of a specific guanosine residue in the acceptor stem of formylmethionine transfer RNA from Escherichia coli. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3594–3597. doi: 10.1073/pnas.69.12.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shershneva L. P., Venkstern T. V., Bayev A. A. A study of tRNA methylase action. FEBS Lett. 1973 Jan 15;29(2):132–134. doi: 10.1016/0014-5793(73)80543-5. [DOI] [PubMed] [Google Scholar]
- Shershneva L. P., Venkstern T. V., Bayev A. A. A study of transfer RNA methylation. Biochim Biophys Acta. 1973 Jan 19;294(2):250–262. [PubMed] [Google Scholar]
- Thiebe R., Zachau H. G. Further studies on amino acid acceptance and physical properties of tRNA-phe-yeast. Biochim Biophys Acta. 1970 Oct 15;217(2):294–304. [PubMed] [Google Scholar]