Abstract
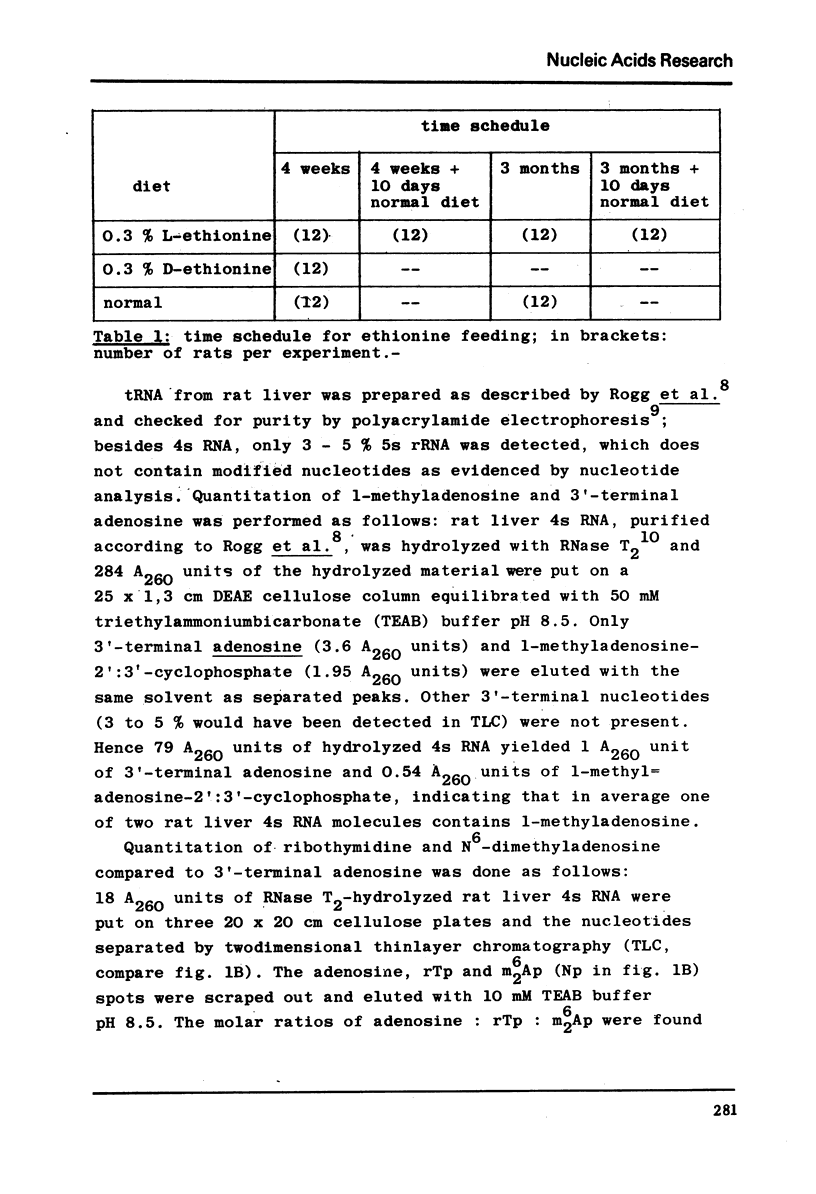
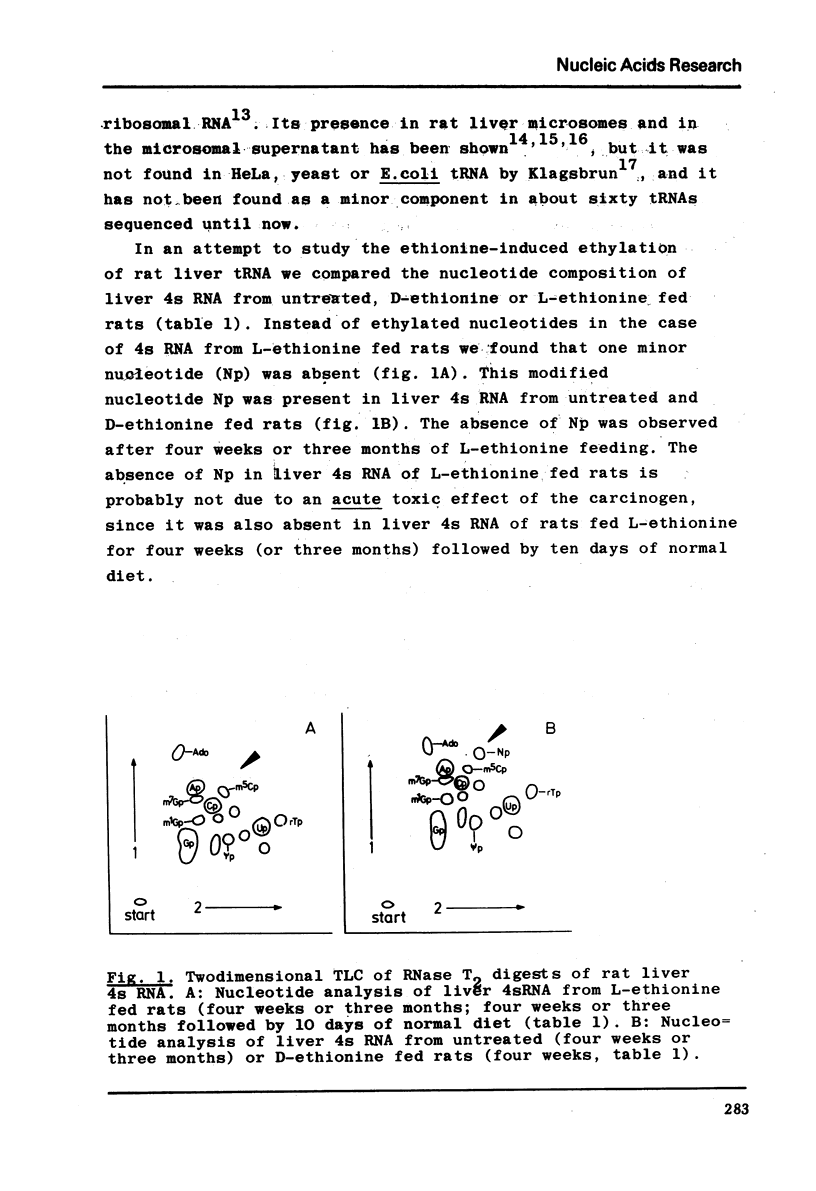
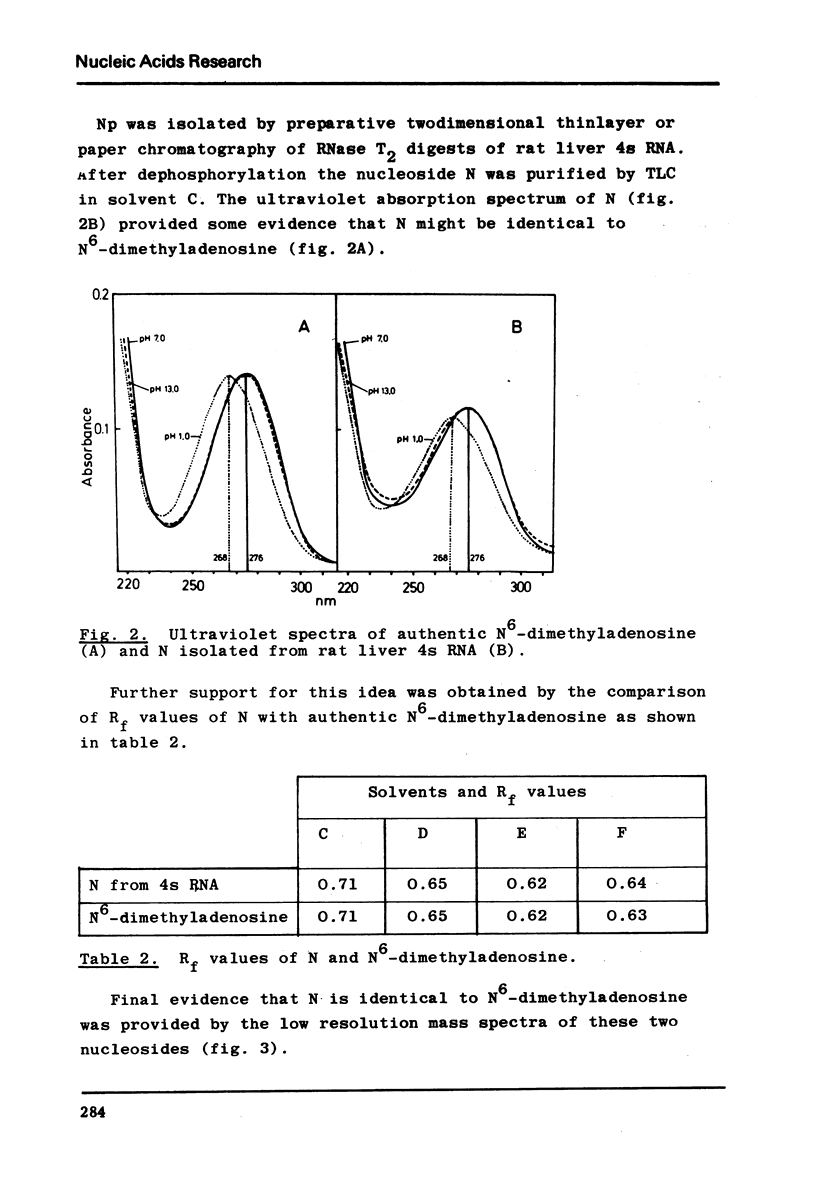
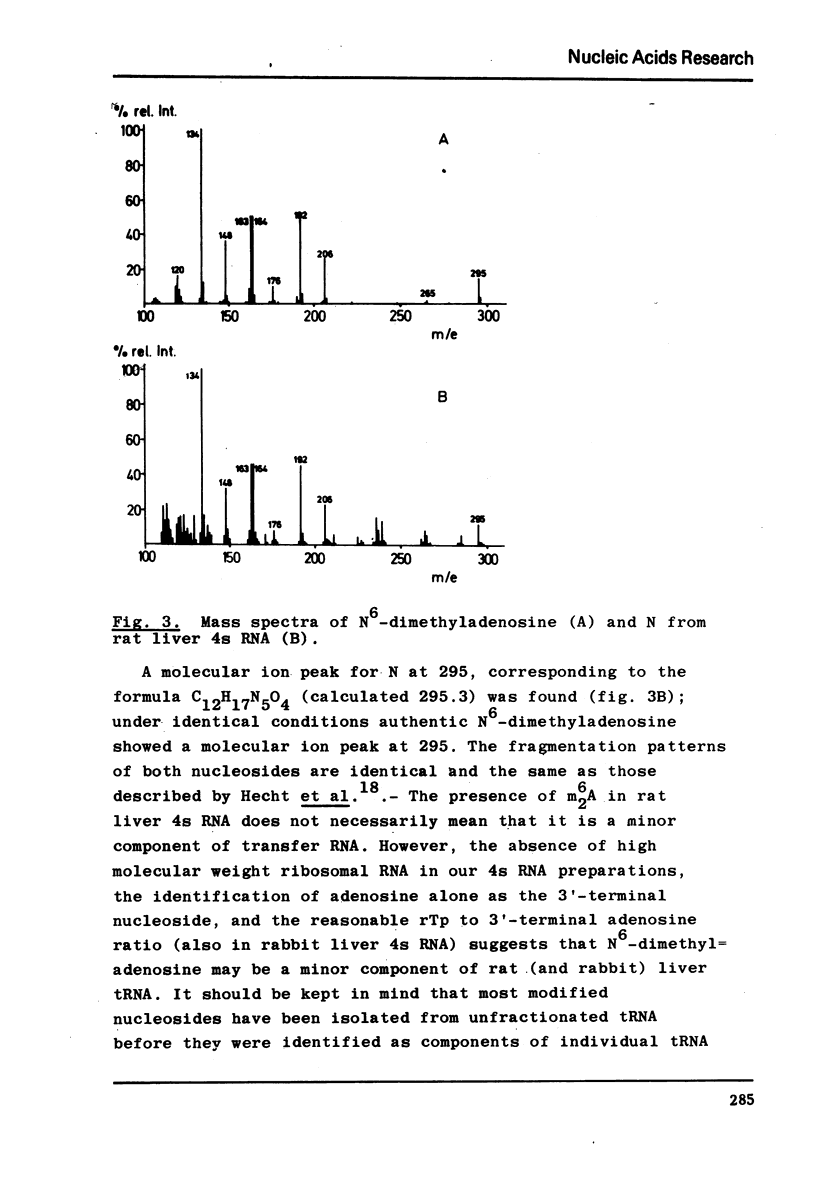
The nucleotide composition of 4s RNA from livers of rats fed with a diet containing 0.3% D-ethionine was found to be identical with that from untreated animals. In contrast, one single modified nucleotide was absent in 4s RNA from livers of rats fed with a 0.3% L-ethionine diet. The minor nucleo=tide was also absent in liver 4s RNA from rats fed with a 0.3% L-ethionine diet followed by ten days of normal food. It was identified after dephosphorylation by ultraviolet absorption spectra, cochromatography with authentic material and mass spectra as N6-dimethyladenosine. It is concluded that S-adenosylethionine, the primary product of L-ethionine in the liver, causes strong and selective inhibition of the specific RNA-methylase responsible for adenosine to N6-dimethyl=adenosine methylation in rat liver 4s RNA. Compared to the strong inhibition of N6-dimethyladenosine formation described here, L-ethionine-dependent ethylation of liver 4s RNA is far less efficient. The quantitation of l-methyladenosine, ribothymidine and 3′-terminal adenosine in this 4s RNA as well as its aminoacid acceptor activity is typical for tRNA; hence it may be concluded that N6-dimethyladenosine is a component of rat liver tRNA. This may demonstrate the first evidence for the existence of specifically methyl-deficient mammalian tRNA. A possible correlation between the activity of L-ethionine as a liver carcinogen and its ability to induce the formation of methyl-deficient tRNA by selectively inhibiting the synthesis of N6-dimethyladenosine on the tRNA level in the same organ is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Weinstein B., Farber E. Patterns of transfer RNA in normal rat liver and during hepatic carcinogenesis. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1255–1260. doi: 10.1073/pnas.58.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGQUIST P. L., MATTHEWS R. E. Occurrence and distribution of methylated purines in the ribonucleic acids of subcellular fractions. Biochem J. 1962 Nov;85:305–313. doi: 10.1042/bj0850305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN D. B. Additional components in ribonucleic acid of rat-liver fractions. Biochim Biophys Acta. 1959 Jul;34:286–288. doi: 10.1016/0006-3002(59)90274-4. [DOI] [PubMed] [Google Scholar]
- FARBER E. Carcinoma of the liver in rats fed ethionine. AMA Arch Pathol. 1956 Dec;62(6):445–453. [PubMed] [Google Scholar]
- FARBER E. ETHIONINE CARCINOGENESIS. Adv Cancer Res. 1963;7:383–474. doi: 10.1016/s0065-230x(08)60986-0. [DOI] [PubMed] [Google Scholar]
- FARBER E., SHULL K. H., VILLA-TREVINO S., LOMBARDI B., THOMAS M. BIOCHEMICAL PATHOLOGY OF ACUTE HEPATIC ADENOSINETRIPHOSPHATE DEFICIENCY. Nature. 1964 Jul 4;203:34–40. doi: 10.1038/203034a0. [DOI] [PubMed] [Google Scholar]
- Fellner P., Sanger F. Sequence analysis of specific areas of the 16S and 23S ribosomal RNAs. Nature. 1968 Jul 20;219(5151):236–238. doi: 10.1038/219236a0. [DOI] [PubMed] [Google Scholar]
- Friedman M., Shull K. H., Farber E. Highly selective in vivo ethylation of rat liver nuclear protein by ethionine. Biochem Biophys Res Commun. 1969 Mar 31;34(6):857–864. doi: 10.1016/0006-291x(69)90259-9. [DOI] [PubMed] [Google Scholar]
- Gross H. J. Transfer RNA: evidence for decreasing size variation during evolution. J Mol Evol. 1973 Nov 27;2(4):339–342. doi: 10.1007/BF01654102. [DOI] [PubMed] [Google Scholar]
- Hancock R. L., Forrester P. I. Increase of soluble RNA methylase activities by chemical carcinogens. Cancer Res. 1973 Jul;33(7):1747–1753. [PubMed] [Google Scholar]
- Hancock R. L. S-adenosylmethionine-synthesizing activity of normal and neoplastic mouse tissues. Cancer Res. 1966 Dec;26(12):2425–2430. [PubMed] [Google Scholar]
- Hecht S. M., Gupta A. S., Leonard N. J. Mass spectra of nucleoside components of tRNA. Anal Biochem. 1969 Aug;30(2):249–270. doi: 10.1016/0003-2697(69)90396-0. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. An evolutionary study of the methylation of transfer and ribosomal ribonucleic acid in prokaryote and eukaryote organisms. J Biol Chem. 1973 Apr 10;248(7):2612–2620. [PubMed] [Google Scholar]
- Klagsbrun M. The contrast between the methylation of transfer ribonucleic acid in vivo and in vitro by normal and SV40 transformed 3T3 cells. J Biol Chem. 1972 Dec 10;247(23):7443–7451. [PubMed] [Google Scholar]
- Klootwijk J., Planta R. J. Analysis of the methylation sites in yeast ribosomal RNA. Eur J Biochem. 1973 Nov 15;39(2):325–333. doi: 10.1111/j.1432-1033.1973.tb03130.x. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., DUNN D. B. The occurrence and distribution of thymine and three methylated-adenine bases in ribonucleic acids from several sources. Biochem J. 1958 Dec;70(4):642–651. doi: 10.1042/bj0700642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. G., Smith R. C. S-Adenosylethionine as an inhibitor of tRNA methylation. Can J Biochem. 1969 May;47(5):561–565. doi: 10.1139/o69-088. [DOI] [PubMed] [Google Scholar]
- Ortwerth B. J., Novelli G. D. Studies on the incorporation of L-ethionine-ethyl-l-14C into the transfer RNA of rat liver. Cancer Res. 1969 Feb;29(2):380–390. [PubMed] [Google Scholar]
- Pegg A. E. Studies of the ethylation of rat liver transfer ribonucleic acid after administration of L-ethionine. Biochem J. 1972 Jun;128(1):59–68. doi: 10.1042/bj1280059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Studies on inhibitors of mammalian tRNA methylases. FEBS Lett. 1971 Jul 15;16(1):13–16. doi: 10.1016/0014-5793(71)80672-5. [DOI] [PubMed] [Google Scholar]
- Rogg H., Wehrli W., Staehelin M. Isolation of mammalian transfer RNA. Biochim Biophys Acta. 1969 Nov 19;195(1):13–15. doi: 10.1016/0005-2787(69)90597-8. [DOI] [PubMed] [Google Scholar]
- Rosen L. Ethylation in vivo of purines in rat-liver RNA by L-ethionine. Biochem Biophys Res Commun. 1968 Nov 25;33(4):546–550. doi: 10.1016/0006-291x(68)90329-x. [DOI] [PubMed] [Google Scholar]
- Shull K. H., McConomy J., Vogt M., Castillo A., Farber E. On the mechanism of induction of hepatic adenosine triphosphate deficiency by ethionine. J Biol Chem. 1966 Nov 10;241(21):5060–5070. [PubMed] [Google Scholar]
- Swann P. F., Pegg A. E., Hawks A., Farber E., Magee P. N. Evidence for ethylation of rat liver deoxyribonucleic acid after administration of ethionine. Biochem J. 1971 Jun;123(2):175–181. doi: 10.1042/bj1230175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T. Purification and properties of RNase T2. J Biochem. 1966 Aug;60(2):115–132. doi: 10.1093/oxfordjournals.jbchem.a128410. [DOI] [PubMed] [Google Scholar]



