Abstract
tRNase Z is the endonuclease responsible for removing the 3'-trailer sequences from precursor tRNAs, a prerequisite for the addition of the CCA sequence. It occurs in the short (tRNase ZS) and long (tRNase ZL) forms. Here we report the identification and sequence analysis of candidate tRNase Zs from 81 metazoan species. We found that the vast majority of deuterostomes, lophotrochozoans and lower metazoans have one tRNase ZS and one tRNase ZL genes, whereas ecdysozoans possess only a single tRNase ZL gene. Sequence analysis revealed that in metazoans, a single nuclear tRNase ZL gene is likely to encode both the nuclear and mitochondrial forms of tRNA 3′-end processing enzyme through mechanisms that include alternative translation initiation from two in-frame start codons and alternative splicing. Sequence conservation analysis revealed a variant PxKxRN motif, PxPxRG, which is located in the N-terminal region of tRNase ZSs. We also identified a previously unappreciated motif, AxDx, present in the C-terminal region of both tRNase ZSs and tRNase ZLs. The AxDx motif consisting mainly of a very short loop is potentially close enough to form hydrogen bonds with the loop containing the PxKxRN or PxPxRG motif. Through complementation analysis, we demonstrated the likely functional importance of the AxDx motif. In conclusion, our analysis supports the notion that in metazoans a single tRNase ZL has evolved to participate in both nuclear and mitochondrial tRNA 3′-end processing, whereas tRNase ZS may have evolved new functions. Our analysis also unveils new evolutionarily conserved motifs in tRNase Zs, including the C-terminal AxDx motif, which may have functional significance.
Introduction
tRNA 3′-processing enzyme tRNase Z (also termed RNase Z or 3′-tRNase) is an endonuclease involved in tRNA 3′-end maturation (for reviews see [1], [2], [3], [4]). It cleaves tRNA precursors (pre-tRNAs) after the discriminator nucleotide (the first nucleotide after the acceptor stem) to generate the tRNA 3′-end suitable for CCA addition, which is essential for tRNA aminoacylation.
There are two forms of tRNase Z encoded by distinct genes. The short forms (tRNase ZS) and long forms (tRNase ZL) vary in length from 279–554 aa and 648–997 aa, respectively. Bioinformatics analyses suggest that tRNase ZL may evolve through tandem gene duplication of tRNase ZS followed by sequence divergence [5], [6], [7].
The species distribution of tRNase Z is complex. tRNase ZS is observed in all archaea sequenced to date and in many bacteria [3], [5], [7]. Prokaryotes only possess tRNase ZS [3]. Usually there is one tRNase ZS gene per genome. In contrast, eukaryotes can have both forms of tRNase Z. In fungi, tRNase ZL is universally distributed, whereas tRNase ZS exists only in certain taxonomic groups [7]. The vast majority of fungi examined have one gene for tRNase ZL, which appears to be targeted to both the nucleus and mitochondria [7]. Unlike fungi, plants are represented by multiple genes for tRNase Zs including two tRNase ZSs and one to two tRNase ZLs [5]. These plant proteins seem to have different subcellular localization. In A. thaliana, the two tRNase ZSs are localized in the cytosol and chloroplast, respectively, whereas the two tRNase ZLs are targeted to both the nucleus and mitochondria and to the mitochondria, respectively [8].
tRNase Z has been reported in a few metazoan species. Humans contain one tRNase ZS (also called ELAC1) and one tRNase ZL (also called ELAC2). Human tRNase ZS is found primarily in the cytosol [9], [10], [11], whereas human tRNase ZL is localized in both the nucleus and mitochondria [9], [10]. Unlike humans, the fruit fly Drosophila melanogaster and nematode Caenorhabditis elegans contain just one tRNase ZL. However, the phylogenetic diversity of tRNase Z in the metazoan kingdom has yet to be comprehensively studied.
tRNase Z belongs to the superfamily of metallo-β-lactamase (MBL) which is a large group of proteins with diverse biological functions including antibiotic degradation, stress response, DNA repair and RNA maturation [6], [12], [13], [14], [15]. The MBL superfamily members have very low sequence homology but share five conserved sequence motifs termed Motifs I-V, among which the most characteristic is Motif II (HxHxDH, also called the His motif). These motifs contain invariant histidine and/or aspartic acid residues essential for zinc binding and catalysis. Besides these catalytic motifs, both tRNase ZS and tRNase ZL contain the GP, PxKxRN, HEAT and HST motifs, which adopt loop structures [5]. The GP and PxKxRN motifs may play a role in cleavage specificity [16], [17], whereas the conserved Glu-His pair from the HEAT and HST motifs may facilitate proton transfer in catalysis [18], [19], [20].
The three-dimensional structures of bacterial tRNase ZSs from Bacillus subtilis, Escherichia coli and T. maritima [18], [21], [22], [23], [24] and the only crystal structure of eukaryotic tRNase ZS from humans (http://www.rcsb.org/pdb/explore.do?structureId=3ZWF) available so far reveal that the enzymes form a dimer. Each monomer adopts a typical MBL fold, which comprises external α-helices and internal β-sheets forming a four-layered αβ/αβ sandwich. The zinc ligands at the active sites of these enzymes are located at loops and turns that connect the β-sheets, and are composed of conserved residues mainly from Motifs I-V. More importantly, the crystal structures reveal the tRNase Z-specific domain termed flexible arm (also called exosite) consisting of a compact globular domain and an extended two-stranded stalk. This flexible arm is located on the opposite side of the active site and protrudes from the protein core, and is involved in the substrate binding primarily through binding the D and T loops of the pre-tRNA [24], [25], [26]. However, no high-resolution structures have been solved for any tRNase ZL.
We have recently undertaken comprehensive surveys of tRNase Zs in fungi [7] and plants [5]. Here, we extend the analysis to metazoans. Our results provide insights into the origin and evolution of metazoan tRNase Zs and should facilitate further molecular evolutionary and structure-function relationship analyses of tRNase Z.
Results
Identification of Candidate Metazoan tRNase Z Genes
As part of our effort to explore genomic distribution and sequence conservation of metazoan tRNase Zs, we performed BLAST similarity searches of metazoan genomic and EST databases. We obtained a total of 45 candidate tRNase ZS genes and 71 tRNase ZL genes from 82 species, including 77 bilaterians, 4 nonbilaterian metazoans, and 1 choanoflagellate (Table S1). It should be noted that tRNase ZL was identified in all metazoan species examined, although the protein sequences of some tRNase ZLs could not be accurately predicted due to gaps in their nucleotide sequences. Although the number of basal metazoan and protozoan species that have had their complete genome sequences reported is very limited, the inclusion of these species in our analysis would enable us to have a better understanding of the origin and evolution of metazoan tRNase Zs. Among these candidate metazoan tRNase Z genes identified, only those from D. melanogaster [27], [28], C. elegans [29] and humans have been experimentally characterized. To present the data in a concise manner, we have selected a list of tRNase Zs of representative species spanning a diverse range of taxonomic groups (Table 1).
Table 1. Distribution of candidate tRNase Zs from representative metazoans.
| Species | Protein name | Form | Accession number | Database | No. aa# | EST |
| Deuterostomes | ||||||
| Branchiostoma floridae | BflTRZ1 | tRNase ZS | XP_002598072.1 | NCBI | 367* | + |
| Branchiostoma floridae | BflTRZ2 | tRNase ZL | XP_002603583.1 | NCBI | 785* | |
| Canis familiaris | CfaTRZ1 | tRNase ZS | XP_849493.1 | NCBI | 374 | + |
| Canis familiaris | CfaTRZ2 | tRNase ZL | XP_546630.2 | NCBI | 821 | + |
| Ciona intestinalis | CinTRZ1 | tRNase ZS | 299192 | Metazome | 383 | + |
| Ciona intestinalis | CinTRZ2 | tRNase ZL | 295609 | Metazome | 727 | + |
| Danio rerio | DreTRZ1 | tRNase ZS | NP_001003503.1 | NCBI | 372 | + |
| Danio rerio | DreTRZ2 | tRNase ZL | XP_003198113.1 | NCBI | 895 | + |
| Homo sapiens | HsaTRZ1 | tRNase ZS | NP_061166.1 | NCBI | 363 | + |
| Homo sapiens | HsaTRZ2 | tRNase ZL | NP_060597.4 | NCBI | 826 | + |
| Loxodonta africana | LafTRZ1 | tRNase ZS | ENSLAFP00000001283 | Ensembl | 364 | |
| Loxodonta africana | LafTRZ2 | tRNase ZL | ENSLAFP00000025345 | Ensembl | 819 | |
| Mus musculus | MmuTRZ1 | tRNase ZS | NP_444485.2 | NCBI | 362 | + |
| Mus musculus | MmuTRZ2 | tRNase ZL | CAI24609 | NCBI | 824 | + |
| Salmo salar | SsaTRZ1 | tRNase ZS | NCBI | 370 | + | |
| Salmo salar | SsaTRZ2 | tRNase ZL | C0H8T6 | Uniprot | 875 | + |
| Strongylocentrotus purpuratus | SpuTRZ1 | tRNase ZS | XP_001189573.1 | NCBI | 362 | |
| Strongylocentrotus purpuratus | SpuTRZ2 | tRNase ZL | XP_783904.2 | NCBI | 931* | + |
| Xenopus tropicalis | XtrTRZ1 | tRNase ZS | 476074 | Metazome | 363 | + |
| Xenopus tropicalis | XtrTRZ2 | tRNase ZL | 157533 | Metazome | 835* | + |
| Protostomes | ||||||
| Bombyx mori | BmoTRZ1 | tRNase ZL | Bmb008309 | Metazome | 889* | + |
| Caenorhabditis elegans | CelTRZ1 | tRNase ZL | NP_001023109.1 | NCBI | 833 | + |
| Capitella teleta | CteTRZ1 | tRNase ZL | 228344 | JGI | 758 | + |
| Capitella teleta | CteTRZ2 | tRNase ZL-Like | 218896 | JGI | 738 | + |
| Drosophila melanogaster | DmeTRZ1 | tRNase ZL | NP_724916.1 | NCBI | 766 | + |
| Lottia gigantea | LgiTRZ1 | tRNase ZS | 218281 | JGI | 356 | + |
| Lottia gigantea | LgiTRZ2 | tRNase ZL | 156489 | JGI | 738* | + |
| Lottia gigantea | LgiTRZ3 | tRNase ZL | 230203 | JGI | 780 | + |
| Tribolium castaneum | TcaTRZ1 | tRNase ZL | XP_968692.2 | NCBI | 827 | + |
| Basal Metazoans | ||||||
| Amphimedon queenslandica | AquTZ1 | tRNase ZS | Aqu1.217211 | JGI | 373* | |
| Amphimedon queenslandica | AquTZ2 | tRNase ZL | XP_003385508.1 | NCBI | 712* | |
| Hydra magnipapillata | HmaTRZ1 | tRNase ZS | Hma2.228140 | JGI | 359 | + |
| Hydra magnipapillata | HmaTRZ2 | tRNase ZL | Hma2.206546 | JGI | 723* | |
| Nematostella vectensis | NveTRZ1 | tRNase ZS | 186754 | JGI | 342 | + |
| Nematostella vectensis | NveTRZ2 | tRNase ZL | 11980 | JGI | 827* | |
| Nematostella vectensis | NveTRZ3 | tRNase ZL | 108544 | JGI | 769* | + |
| Trichoplax adhaerens | TadTRZ1 | tRNase ZL | B3RMG1 | Uniprot | 728* | |
| Protozoans | ||||||
| Monosiga brevicollis | MbrTRZ1 | tRNase ZS | 25108 | JGI | 380* | |
| Monosiga brevicollis | MbrTRZ2 | tRNase ZL | 23750 | JGI | 812* |
The number of amino acids in metazoan tRNase Z proteins.
Indicates that mispredicted sequences obtained from the databases have been corrected.
A plus sign indicates that gene expression is confirmed by the presence of ESTs.
It appears that the tRNase Z gene is variably distributed within the three major divisions of Bilateria (the Deuterostomia, the Ecdysozoa, and the Lophotrochozoa). All of the vertebrate deuterostomes examined to date have one tRNase ZS and one tRNase ZL genes. Similarly, four invertebrate deuterostomes, the amphioxus Branchiostoma floridae (cephalochordate), two sea squirts Ciona intestinalis and Ciona savignyi (urochordate), and the sea urchins Strongylocentrotus purpuratus (echinoderm) have one tRNase ZS and one tRNase ZL genes.
Unlike deuterostomes, protostomes contain variable numbers of tRNase Zs. Ecdysozoans, one of two major groups of protostomes and including animals such as arthropods and nematodes, appear to possess a single tRNase ZL gene but lack the tRNase ZS gene. Lophotrochozoa, which is another major group of protostomes and comprises annelids and molluscs, is significantly underrepresented in terms of whole genome data. Among sequenced lophotrochozoans, the marine mollusk Lottia gigantea appears to have the largest number of tRNase Zs: one tRNase ZS and two tRNase ZL. Like deuterostomes, the marine mollusk Aplysia californica and the two parasitic flatworms (Schistosoma mansoni and Schistosoma japonicum) harbor one tRNase ZS and one tRNase ZL genes. Like ecdysozoans, the two marine annelids Helobdella robusta and Capitella teleta appear to have one tRNase ZL gene, but have no tRNase ZS gene. In addition, C. teleta has one tRNase ZL-like protein, which lacks the GP-motif.
The number of tRNase Z genes in basal metazoans examined so far seems highly variable. The freshwater hydra Hydra magnipapillata, a representative cnidarian, and the marine demosponge Amphimedon queenslandica (phylum Porifera) each contain one tRNase ZS and one tRNase ZL genes. In the starlet sea anemone Nematostella vectensis, another representative cnidarian, one tRNase ZS and two tRNase ZL genes were identified. Trichoplax adhaerens, the sole representative of the phylum Placozoa, represents the simplest known animal with the smallest known animal genome. In this species, only one tRNase ZL gene was found, but no tRNase ZS genes were identified. In the choanoflagellate Monosiga brevicollis, which is the closest known unicellular relative to metazoans, two tRNase Z genes (one tRNase ZS gene and one tRNase ZL gene) were identified.
Prediction of the Number of Introns in Metazoan tRNase ZS and tRNase ZL Genes
To understand the structure of metazoan tRNase Z genes, we predicted their introns. Metazoan tRNase ZS genes appear to have few introns. For example, all mammalian tRNase ZS genes examined have just two introns (Table S2). Mammals and inverterbrate tRNase ZLs seem to have different numbers of introns. All mammalian tRNase ZL genes examined contain 23 introns. In contrast, tRNase ZL genes of 3 Caenorhabditis species including C. elegant and 9 Drosophila species including D. melanogaster contain 6 and 1 introns, respectively. The difference in intron numbers between mammalian and invertebrate tRNase ZL genes can be ascribed to massive, lineage-specific intron loss in worms and insects [30]. Interestingly, the intron found in Drosophila tRNase ZL genes splits the gene into two fragments that encode roughly the N- and C-terminal halves of the protein. This is consistent with the notion that proteins are often made from independently-folded functional domains encoded by separate exons, which can be separated by introns [31], [32]. Unexpectedly, we found that the ascidian C. intestinalis tRNase ZL gene encoding a protein of 727-aa apparently does not have any intron by comparing the gene sequence with corresponding EST sequences in NCBI databases.
Alternative Splicing of tRNase ZL Transcripts
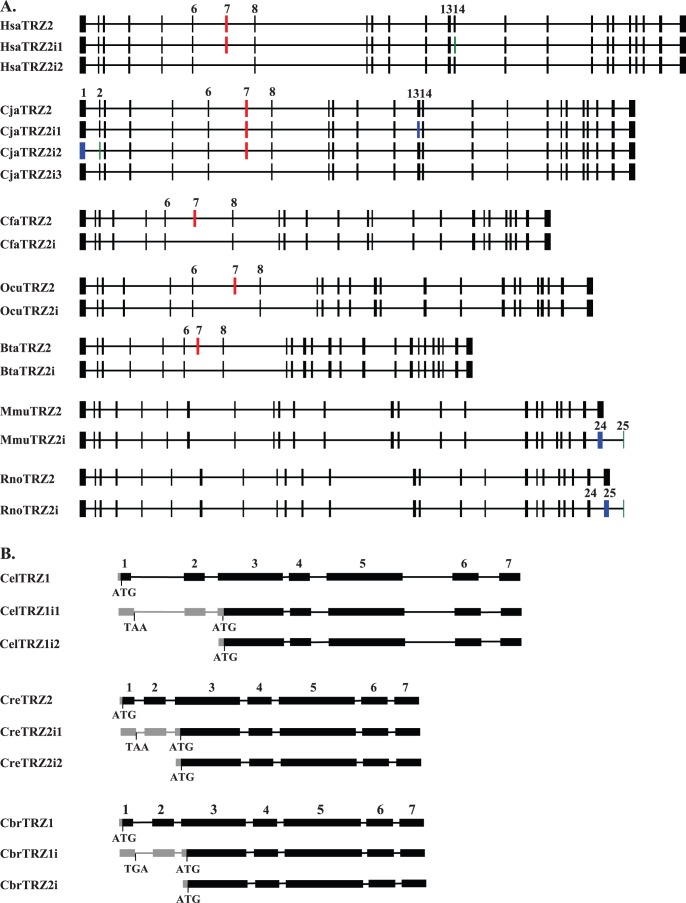
Alternative splicing can play a major role in modulating gene structure and function. To identify potential alternative spliced isoforms of tRNase ZL, we performed BLAST searches against the NCBI EST database. Candidate splice variants of tRNase ZL were largely found in vertebrates, consistent with the observation that vertebrates have substantially higher rates of alternative splicing compared to other species. Figure 1 shows a schematic representation of predicted alternatively spliced variants of metazoan tRNase ZL. The common marmoset (Callithrix jacchus) appears to have three alternatively spliced isoforms of tRNase ZL, designated CjaTRZ2i1, CjaTRZ2i2 and CjaTRZ2i3. CjaTRZ2i1 (mRNAs) is created by using an alternative splice donor within exon 13. The use of this alternative acceptor site (within tRNase ZL exon) causes a deletion of V703. CjaTRZ2i2 uses an alternative donor site within exon 1 and an acceptor site within exon 2 that results in a deletion of 57 nt. CjaTRZ2i3 is generated by skipping of exon 7 that causes an internal 120-nt deletion.
Figure 1. Schematic representation of the predicted alternative splice variants of mammalian and nematode tRNase ZL genes (drawn to scale).
(A) Mammalian tRNase ZLs are from H. sapiens (Hsa), C. jacchus (Cja), C. familiaris (Cfa), O. cuniculus (Ocu), B. taurus (Bta), M. musculus (Mmu) and R. norvegicus (Rno). HsaTRZ2i1, HsaTRZ2i2, CjaTRZ2i1, CjaTRZ2i2, CjaTRZ2i3, CfaTRZ2i, OcuTRZ2i, BtaTRZ2i, MmuTRZ2i, and RnoTRZ2i correspond to alternatively spliced variants. Constitutive exons and introns are represented by the filled boxes and intervening horizontal lines respectively. Alternative exons are colored as follows: exon skipping in red, exon with alternative donors in blue and exon with alternative acceptors in green. The locations of the alternative exons in the tRNase ZL genes and its splice variants are shown on the top of the exon. (B) Nematode tRNase Zs are from C. elegans (Cel), C. remanei (Cre) and C. briggsae (Cbr). CelTRZ1i1, CelTRZ1i2, CreTRZ1i1, CreTRZ1i2, CbrTRZ1i1 and CbrTRZ1i2 are splice variants. Exons are shaded black, introns are indicated by lines and the 5′-untranslated region (5′-UTR) sequences are indicated by gray boxes. The exon number indicated on top. The ATG start codon and TAA stop codon are indicated.
Alternative splicing of tRNase ZL may also occur in humans and may result in the expression of two splicing variants, designated HsaTRZ2i1 and HsaTRZ2i2. Whereas HsaTRZ2i1 is produced by using an alternative in-frame acceptor site within exon 14 that leads to loss of one amino acid (aa) residue Lys407 compared to the human tRNase ZL, HsaTRZ2i2 is generated as a result of exon 7 skipping that deletes 120 nt. Evidence of skipping of exon 7 is provided by annotated GenBank cDNAs isolated from the cDNA libraries of human brain, spleen and lung (GenBank accession numbers: DA954223.1, DA178234.1 and AW206103.1).
Alternative splicing appears to generate two tRNase ZL isoforms in the domestic dog (Canis familiaris) and European rabbit (Oryctolagus cuniculus). Similar to CjaTRZ2i3 and HsaTRZ2i1, both these two alternatively spliced tRNase ZLs are produced by skipping exon 7.
The mouse tRNase ZL has two alternative spliced transcripts (MmuTrz2 and MmuTrz2i), which has similar organization of the exons and introns except for the alternatively spliced form (MmuTrz2i), which contains a truncated (24th) exon 24 and an additional new exon of 33 bp (exon 25) at the end of the gene (Figure 1). Similar alternative splicing occurs in the rat tRNase ZL. As a result, the major mouse and rat tRNase ZL transcripts contain 24 exons encoding proteins of 824 and 827 aa respectively, whereas their alternative spliced transcripts contain 25 exons and encode proteins of 831 and 834 aa respectively. The amino acid sequences encoded by the additional exon 25 are poorly conserved between rodents (mouse and rat). The generation of this alternate splice form appears to occur only in rodents, but not in humans, and both splice forms are present in most mouse and rat tissues analyzed [33] (see Discussion).
In addition to vertebrates, splice variants appear to be present in three Caenorhabditis nematodes (C. elegans, Caenorhabditis briggsae and Caenorhabditis remanei). In C. elegans, three different splice transcripts of tRNase ZL (CelTrz1, CelTrz2i1 and CelTrz2i2 corresponding to E04A4.A, E04A4.4B and E04A4.4C described in [29], respectively) were detected by reverse transcriptase (RT)-PCR [29]. CelTrz1, which can be trans-spliced to one of two short leader RNAs, SL1 or SL2, encodes the full length protein containing a putative mitochondrial targeting sequence (MTS). CelTrz2i1 is trans-spliced to either SL1 or SL2. Although CelTrz2i1 begins with the first exon, translation begins from a start codon within the third exon since it contains an in-frame stop codon (TAA) in the 5′-end of its pre-mRNAs (Figure 1). CelTrz2i2 is trans-spliced to SL1 and begins at the start of exon 2. Like CelTrz2i1, the translation of CelTrz2i2 starts from an ATG present in exon 3. Both CelTrz2i1 and CelTrz2i2 transcripts apparently encode the same tRNase ZL isoform that lacks an N-terminal MTS and is likely targeted to the nucleus. Comparison of the tRNase ZL gene structures of three Caenorhabditis nematodes suggested that similar mechanisms to generate splice variants a single tRNase ZL gene may operate in other two nematodes (Figure 1).
Phylogenetic Analysis
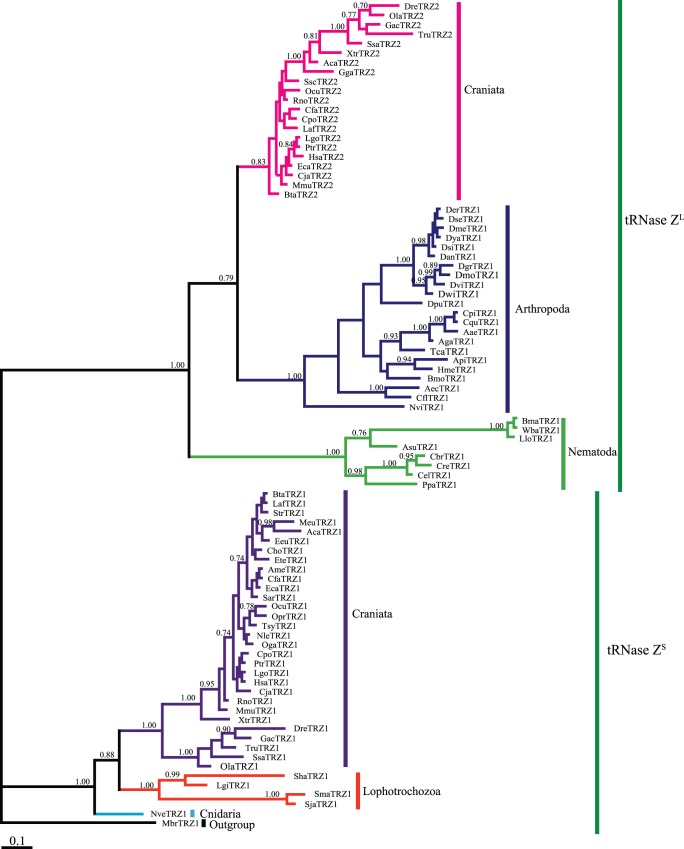
To evaluate the evolutionary relationship among the metazoan tRNase Zs, we performed a Bayesian phylogenetic analysis using a data set composed of 86 representative tRNase Zs from taxonomically diverse metazoans. In addition to the metazoan species, the premetazoan choanoflagellate M. brevicollis tRNase ZS was included as an outgroup. Our phylogenetic analysis showed that candidate sequences cluster into two well-supported sister clades (Figure 2). One clade, termed the tRNase ZL clade, comprises all tRNase ZLs, and the other one, termed the tRNase ZS clade, includes all tRNase ZSs. This phylogenetic tree suggests that the metazoan tRNase ZSs and tRNase ZLs share a common ancestor. Moreover, the phylogenetic relationships of tRNase Zs within each of the clades generally follow the species phylogeny [34] except for nematode tRNase ZLs (Figure 2).
Figure 2. Bayesian phylogenetic tree of predicted metazoan tRNase Zs.
This analysis is based on the sequence alignment of the full-length metazoan tRNase ZSs with the C-terminal half of metazoan tRNase ZLs. The accession number for each candidate tRNase Z is identified in Table S1. Bayesian posterior probabilities are indicated at the nodes. The scale bar indicates 0.1 nucleotide substitutions per site. Taxonomic designations are indicated on the right side of the tree.
Prediction of Subcellular Localization of Metazoan tRNase Zs
To understand the functions of metazoan tRNase Zs, we predicted subcellar localization of candidate metazoan tRNase Zs. All metazoan tRNase ZSs examined do not contain any predictable subcellular localization signal and are expected to be cytoplasmic proteins. In contrast, the vast majority of analyzed candidate tRNase ZLs contain both an N-terminal MTS and a nuclear localization signal (NLS), and are predicted to be targeted to both the nucleus and mitochondria (Table S3). Indeed, subcellular localization studies using fluorescence microscopy have shown that human tRNase ZL localizes to both the nucleus and mitochondria, whereas human tRNase ZS exists in the cytosol [9], [35], [36]. Also consistent with the prediction of subcellular localization, the Drosophila tRNase ZL has been shown to participate in both nuclear and mitochondrial tRNA 3′-end processing [28].
Analysis of Metazoan tRNase Z Protein Sequences
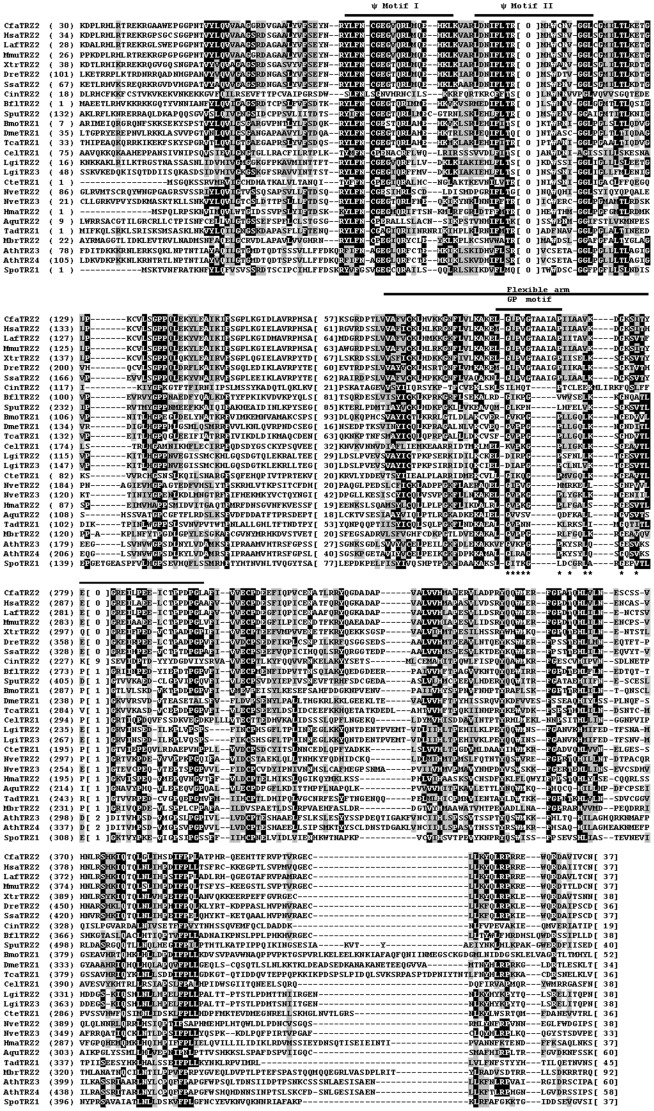
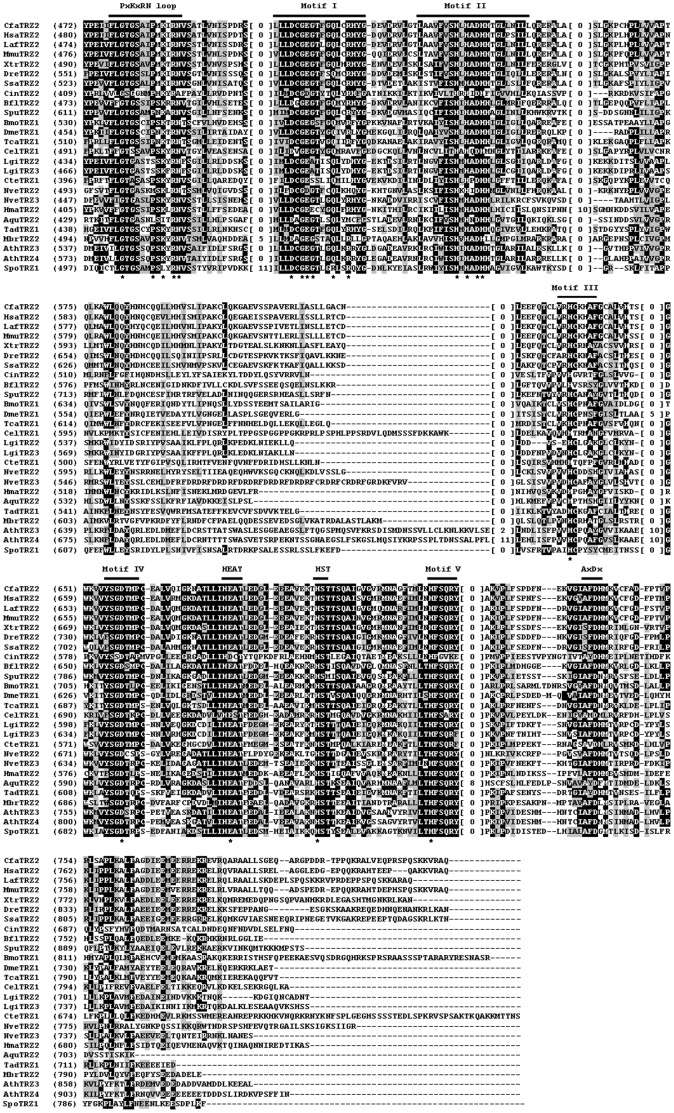
To assess the sequence conservation and structural features of metazoan tRNase Zs, we performed multiple sequence alignments of candidate sequences. We analyzed candidate tRNase ZLs first since they are most likely candidate endonucleases for tRNA 3′-end processing. Since tRNase ZL can be separated into the N- and C-terminal halves with weak sequence similarity to each other, we aligned these two halves separately. Figures 3 and 4 show the alignments of the N- and C-terminal halves of tRNase ZLs from representative metazoan species (see Figure S1 for a complete list). Amino acid sequence comparison shows that the coding sequences of the vertebrate tRNase ZLs are highly conserved. For example, the human tRNase ZL exhibits an overall sequence identity of 79–82% to dog (C. familiaris), bovine (S. scrofa), rabbit (O. cuniculus), mouse (M. musculus), Xenopus (X. tropicalis) and zebrafish (D. rerio) tRNase ZLs (summarized in Table S4).
Figure 3. Multiple sequence alignment of representatives of N-terminal halves of the metazoan and non-metazoan tRNase ZLs.
Representative metazoan tRNase ZLs are from ten deuteromes including C. familiaris (Cfa), H. sapiens (Hsa), L. Africana (Laf), M. musculus (Mmu), X. tropicalis (Xtr), D. rerio (Dre), S. salar (Ssa), C. intestinalis (Cin), B. floridae (Bfl), and S. purpuratus (Spu), six protostomes including B. mori (Bmo), D. melanogaster (Dme), T. castaneum (Tca), C. elegans (Cel), L. gigantean (Lgi), and C. teleta (Cte), and four basal metazoans including N. vectensis (Nve), H. magnipapillata (Hma), A. queenslandica (Aqu), and T. adhaerens (Tad). Non-metazoan tRNase ZLs are from the unicellular choanoflagellate M. brevicollis (Mbr), green plant A. thaliana (Ath) and fungal S. pombe (Spo). See Table 1 for more information. The alignment was constructed using the computer program Clustal W [52]. Identical residues are on a black ground and conserved residues shaded in gray. The conserved motifs of tRNase Zs indicated above the alignment are labeled according to references [17], [19], [58]. The numbers in brackets indicate the length of the region in the protein, which are species-specific and could not be correctly aligned. Hyphens represent gaps introduced into sequences for maximum alignment. Amino acid residues predicted to be critical for activity are indicated by a star.
Figure 4. Multiple sequence alignment of representatives of C-terminal halves of the metazoan tRNase ZLs.
Same legend as in Figure 3.
In contrast, the primary sequences of vertebrate and invertebrate tRNase ZLs are highly divergent. As summarized in Table S4, the sequence identities between the human and invertebrate tRNase ZLs are below ∼32%. Notably, human tRNase ZL shares 19% and 20% sequence identity, respectively, with its orthologs from two ascidian species C. intestinalis and C. savignyi (marine invertebrates of the chordate subphylum Urochordata), suggesting a very distant phylogenetic relationship.
Sequence conservation between the metazoan and plant, yeast or protozoan tRNase ZLs is low (∼24% identity between human tRNase ZL and either plant or yeast, tRNase ZL, ∼22% between human and protozoan tRNase ZLs). Moreover, the conserved amino acid sequences in these tRNase ZLs are primarily located at the sequence motifs common to all eukaryotic tRNase ZLs, suggesting that the overall fold of tRNase ZLs is most likely to be evolutionarily conserved across diverse species from unicellular eukaryotes to metazoans.
Interestingly, all 42 candidate tRNase ZLs from Craniata (hagfishes, lampreys and vertebrates), the largest subphylum of chordates, contain a distinct GP motif, in which a 5-aa peptide is inserted between conserved residues Gly and Pro in the GP motif. These insertion sequences in tRNase ZLs from mammals, birds, reptiles, amphibians and fishes that have been examined are TAAIA, TPAIL, TAAIA, TAAIG and TAAIG, respectively. This 5-aa insertion seems to be unique to craniate tRNase ZLs since this insertion is only found in craniate tRNase ZLs, but not found in any of other eukaryotic tRNase ZLs. It is unknown why craniate tRNase ZLs have a diagnostic 5-aa insertion, which does not seem to be needed for enzyme activity since they are not found in many other tRNase ZLs.
Figure 5 shows alignments of representative metazoan tRNase ZSs (for a complete list, see Figure S2). Sequence alignment revealed that similar to tRNase ZL, tRNase ZS is highly conserved throughout vertebrate evolution (see Figure 5 and Table S5). For example, the human tRNase ZS shares approximately 92% and 57% sequence identity with the mouse and zebrafish tRNase ZSs, respectively. In contrast, vertebrate tRNase ZSs show much lower overall sequence identity to tRNase ZSs from protostome invertebrates and basal metazoans. The amino acid sequence identity of the human tRNase ZS with the tRNase ZSs from the human parasite Schistosoma mansoni and starlet sea anemone Nematostella vectensis is 35% and 45%, respectively. The amino acid sequences of vertebrate tRNase ZSs are also markedly diverged from those of bacterial tRNase ZSs. The sequence of the human tRNase ZS shares 34% and 47% sequence identity to those from B. subtilis and E. coli, respectively.
Figure 5. Multiple sequence alignment of representatives of the metazoan and non-metazoan tRNase ZSs.
Metazoan tRNase ZSs are from ten deuteromes including C. familiaris (Cfa), L. africana (Laf), H. sapiens (Hsa), M. musculus (Mmu), X. tropicalis (Xtr), D. rerio (Dre), S. salar (Ssa), C. intestinalis (Cin), B. floridae (Bfl), and S. purpuratus (Spu), one protostome, L. gigantean (Lgi), and three basal metazoans, N. vectensis (Nve), H. magnipapillata (Hma), and A. queenslandica (Aqu). Non-metazoan tRNase ZSs are from the unicellular choanoflagellate M. brevicollis (Mbr), B. subtilis (Bsu) and E. coli (Eco). The annotation of the alignment is as described in the legend to Figure 3.
Unlike bacterial tRNase ZSs and eukaryotic tRNase ZLs, metazoan tRNase ZSs appear to contain a variant PxKxRN motif (i.e., the PxPxRG motif) that contains Pro and Gly in place of Lys and Asn respectively (Figure 6). The Lys to Pro substitution is expected to increase the rigidity of the PxKxRN loop, whereas the Asn to Gly substitution may decrease the rigidity of the loop.
Figure 6. Sequence logos for the predicted PxRxRN, PxPxRG and AxDx motifs.
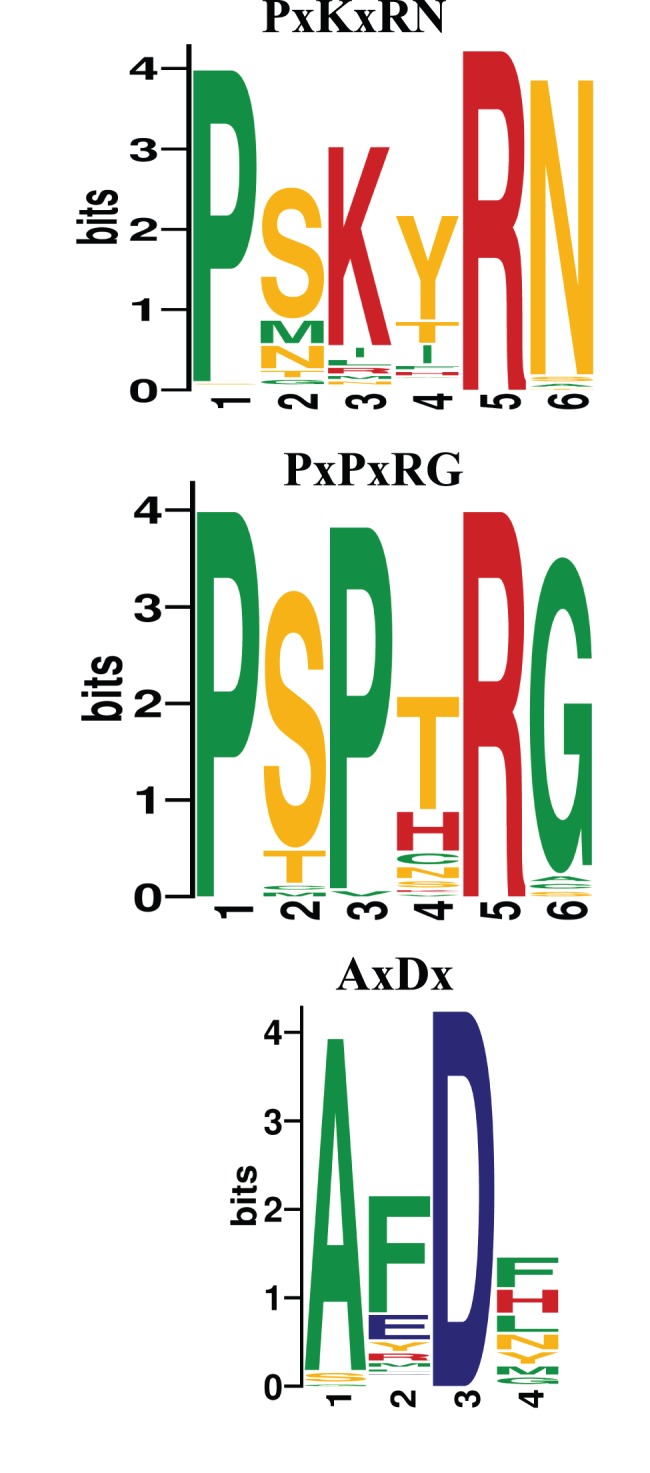
The sequence logo of the PxRxRN motif was derived from 174 tRNase ZLs from 75 fungi, 21 plants and 68 metazoans. The sequence log of the PxPxRG motif is derived from alignment of 44 metazoan tRNase ZSs. The sequence logo of the AxDx motif is derived from 240 alignments including 178 tRNase ZLs from 71 metazoan, 21 green plants, 77 fungi, and 62 tRNase ZSs from 30 metazoans, 9 fungi, 2 bacteria and M. brevicollis. The sequence logos were created using WebLogo (http://weblogo.berkeley.edu). The height of each amino acid indicates the level of conservation at that position. Amino acids are colored as follows: red, basic; blue, acidic; orange, polar; and green, hydrophobic.
Careful examination of the sequences of metazoan tRNase ZSs revealed an AxDx motif located near the C-terminal region of the proteins (Figure 5). A reexamination of all previously identified tRNase Z sequences revealed that this motif is also present in the C-terminal regions of tRNase ZLs and bacterial-type tRNase ZSs but not in Thermotoga maritima (TM)-type tRNase ZSs [5], [7]. The AxDx motif contains an absolutely conserved Asp residue and a highly conserved Ala residue (Figure 6).
The Effect of the Point Mutation in the AxDx Motif on the Complementation Ability of the S. pombe tRNase ZL
To establish the functional significance of the AxDx motif, we employed a Schizosaccharomyces pombe-based functional complementation assay to assess the functional consequence of the point mutation in the conserved AxDx motif of the S. pombe Trz1. As such, we introduced the D772A mutation into the AxDx motif of the S. pombe Trz1 and tested if these mutants could rescue a S. pombe trz1 + temperature-sensitive (ts) mutant strain trz1-1 as described before [37]. As shown in Figure 7, the wild type S. pombe Trz1 could complement the temperature sensitive growth phenotype of trz1-1 when expressed from the pREP4X plasmid. On the other hand, the D772A mutant protein showed an impaired ability to complement the temperature-sensitive growth defect of trz1-1. Based on these results, we concluded that the highly conserved AxDx motif and particularly Asp772 is vital for the S. pombe Trz1 activity and function.
Figure 7. Growth complementation analysis of S. pombe Trz1 with a mutation in the AxDx motif.
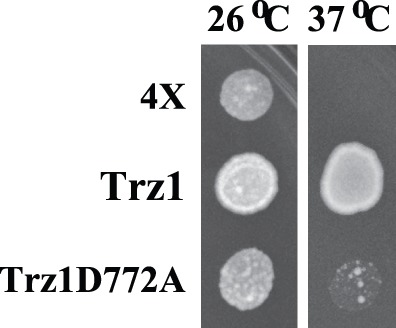
A S. pombe strain bearing a temperature-sensitive allele of trz1 (trz1-1) was transformed with empty vector (4X) or vector expressing the wild-type Trz1 or the Trz1D772A mutant, and equal amounts of cells were spotted on EMM medium supplemented with leucine and adenine and grown at 26°C or 37°C.
Discussion
The Origin and Evolution of tRNase Z in Metazoans
Our survey of available metazoan genomes revealed that the tRNase ZL gene is ubiquitous across the metazoan kingdom, including nonbilaterian metazoans. On the other hand, the tRNase ZS gene does not exist in any of the sequenced ecdysozoan genomes, which is the largest clade of metazoans. The presence of the tRNase ZS and tRNase ZL genes in many higher metazoans such as lophotrochozoans and deuterostomes, and in lower metazoans including poriferans and cnidarians indicates that the tRNase ZS and tRNase ZL genes were already present very early in the evolution of metazoans, and that the absence of tRNase ZS in ecdysozoans is likely due to gene loss. This hypothesis is further reinforced by our finding that both tRNase ZS and tRNase ZL genes already existed in the unicellular choanoflagellate M. brevicollis, which is the closest known relative of metazoans. Our findings are consistent with the observations that ecdysozoans have high rates of gene loss [38], [39].
There are two possible explanations for the loss of the tRNase ZS gene in ecdysozoans. First, the function of the tRNase ZS gene might largely represent the vertebrate-specific feature underlying the biological complexity of vertebrates. However, the role of the vertebrate tRNase ZS gene has not been fully addressed (see below discussion). Second, the tRNase ZS gene could be an animal ancestral gene that has been lost during Ecdysozoan evolution.
The Role of Metazoan tRNase Zs
In our early bioinformatics studies of fungi and plant tRNase Zs, we concluded that tRNase ZL takes over the essential role of tRNase ZS in the nuclear and mitochondrial tRNA 3′-end processing. The present bioinformatics analysis for metazoan tRNase Zs extends and reinforces this conclusion.
The conclusion that tRNase ZL is responsible for the 3′-end processing of nuclear and mitochondrial tRNA precursors in metazoans is further supported by the results of both in vitro and in vivo functional studies. In humans, tRNase ZL exhibits tRNA processing efficiency ∼2000 fold higher than tRNase ZS in vitro [40]. Recent in vivo studies of tRNase ZL from Drosophila and humans provide direct evidence that tRNase ZL is responsible for endonucleolytic processing of the 3′-end of pre-tRNAs in metazoan nucleus and mitochondria [28]. The Drosophila tRNase ZL is encoded by the juvenile hormone (JH) inducible gene, JhI-1 [41]. RNA interference (RNAi)-mediated silencing of the JhI-1 gene expression in D. melanogaster results in the accumulation of 3′-end unprocessed tRNAs in both the nucleus and mitochondria [28], indicating that tRNase ZL is responsible for generating the mature 3′-end of tRNAs in the nucleus and mitochondria of D. melanogaster. Likewise, in humans, tRNase ZL is the primary enzyme responsible for mitochondrial tRNA 3′-end since silencing of the human tRNase ZL gene inhibits the 3′-end processing of mitochondrial tRNAs [9], [11]. However, among some of the mitochondrial tRNAs analyzed, siRNA against the human tRNase ZL gene only affects the mature level of a few mitochondrial tRNAs in humans [9], [11].
In Drosophila, knockdown of the tRNase ZL gene significantly decreases the mature levels of the mitochondrial tRNAs but not the mature levels of all five tested nuclear tRNAs [28]. It is not known why the steady-state levels of many tested mature forms of tRNAs are not visibly affected. One possibility is that additional enzymes may also be involved in 3′-end processing of tRNAs.
Besides its primary role in tRNA maturation, the human tRNase ZL functions in other cellular pathways. For example, the human tRNase ZL has been demonstrated to be involved in the biogenesis of non-coding RNAs (ncRNAs), other than tRNA, such as metastasis associated lung adenocarcinoma transcript 1 (MALAT1) [42], tRNA-derived small RNAs [43], [44] and viral microRNAs (miRNAs) [45], [46]. It would be interesting to see if other metazoan tRNase ZLs also have additional functions apart from tRNA 3′-end processing.
So far, the metazoan tRNase ZS has only been studied in humans and its function is still poorly understood. The human tRNase ZS gene appears to be nonessential since this gene was found to be deleted in a lung cancer cell line, Ma29 [47]. The human tRNase ZS is localized in the cytoplasm and has been proposed to play a role in fine-tuning of gene expression by processing and degradation of unstructured RNAs [11]. It will be important to determine the RNA substrates of the metazoan tRNase ZSs by various methods including coimmunoprecipitation with an antibody against tRNase ZS, followed by sequencing of associated RNAs.
Mechanisms for Dual Localization of Metazoan tRNase ZLs
How does the metazoan tRNase ZL participate in both the mitochondrial and nuclear tRNA 3′-end processing? A recent study on the subcellular localization of tRNase ZL suggests that mammalian tRNase ZL genes encode both nuclear and mitochondrial forms of tRNase ZL by using alternative translation initiation sites [36]. Our results extend this conclusion to include other metazoan tRNase ZLs. We found that like mammalian tRNase ZLs, most non-mammalian metazoan tRNase ZLs also contain a putative downstream AUG start codon in-frame with the first one (Figure S3). In additional, the nucleotide context around the first start codon is apparently suboptimal for translational initiation as defined by the consensus sequences [48], [49]. We also found that most metazoan tRNase ZLs analyzed so far are characterized by the presence of the N-terminal MTS. Thus, it is likely that a single metazoan tRNase ZL mRNA is translated via leaky scanning to make proteins with or without an MTS by initiating translation at two in-frame AUG codons that should produce essentially the same proteins, one mitochondrial and the other nuclear. Indeed, changing the nucleotide sequences surrounding the first AUG start codon of the human tRNase ZL to conform to the eukaryotic consensus sequence results in exclusive mitochondrial localization of the protein, whereas removing the N-terminal region of the human tRNase ZL encoding most of the predicted MTS causes the protein to be targeted exclusively to the nucleus [36].
Besides leaky scanning, other mechanisms including alternative splicing [50] may also be used to produce the nuclear and mitochondrial forms of the metazoan tRNase ZL from the same gene. Examination of the tRNase ZL genes from three Caenorhabditis nematodes revealed that all of the candidate genes contain a second putative in-frame start codon located in exon 3 (Figure 1). The two AUG start codons are separated by 406 nt (C. remanei tRNase ZL), 1248 nt (C. elegans) and 413 nt. (C. briggsae), respectively. These distances seem too long for efficient leaky scanning [51]. In C. elegans, RT-PCR analysis showed that the full length tRNase ZL and its splice variants that differ by the presence or absence of a an N-terminal putative MTS are likely generated by alternative splicing of 5' exons [29]. Thus, it is possible that both the nuclear and mitochondrial forms of the Caenorhabditis tRNase ZL genes could be translated from a single mRNA species through an alternative splicing mechanism.
The Role of the AxDx Motif
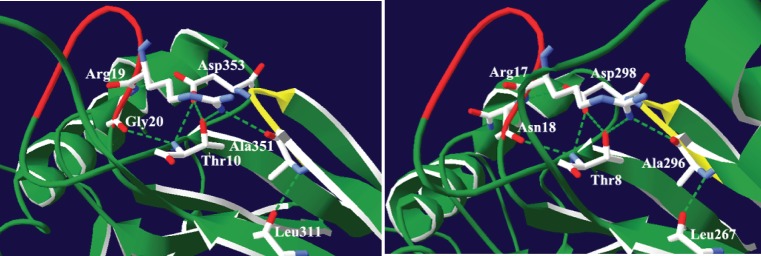
Our sequence analysis revealed an AxDx sequence motif, which is conserved in the eukaryotic tRNase ZLs and bacterial tRNase ZSs, but not in the TM-type tRNase ZSs. In the crystal structures of human and B. subtilus tRNase ZSs, the two AxDx motifs have entirely the same folding topology (http://www.rcsb.org/pdb/explore.do?structureId=3ZWF and [18]). The first residue of the motif (Ala) is located at the end of β15 while other residues are found in a short loop connecting β15 and β16. We noted that the AxDx motifs of human and Bacillus tRNase ZSs and the PxKxRN motif or its variant PxPxRG are in close enough proximity to make hydrogen bonding interactions (Figure 8). The PxKxRN motif or its variant PxPxRG forms part of a loop which acts like a flap covering the entrance to the active site. Strikingly, the hydrogen bonding networks formed by the AxDx motif and the PxPxRG or PxKxRN motif are very similar with one notable difference: the OD2 atom of Asp353 and the main-chain amino group of Thr10 in the human tRNase ZS make hydrogen bonds with the main-chain amino and carbonyl groups of Gly20 of the PxPxRG motif, respectively, whereas the corresponding residues in the Bacillus tRNase ZS form hydrogen bonds with the main-chain amino and carbonyl groups of Asn18 in the PxKxRN motifs (Figure 8). Interestingly, our homology modeling of the human tRNase ZL based on the crystal structure of the human tRNase ZS suggests that the potential hydrogen bonding network involving the AxDx motif is likely to be conserved in the structure of the human tRNase ZL (Figure S4).
Figure 8. Depiction of the predicted hydrogen bonding network in the AxDx strand/loop region of human and B. subtilus tRNase ZSs.
The atomic coordinates of human and B. subtilus tRNase ZSs were obtained from the Protein Data Bank, www.rcsb.org (PDB accession codes 3ZWF and 1Y44, respectively). Images were made with Swiss-PdbViewer [59]. Potential hydrogen bonds were determined using the Swiss-PdbViewer and Insight II. Secondary structure elements are colored green. The AxDx strand/loop is colored yellow and the PxPxRG or PxKxRN loop is colored red. Hydrogen bonds are represented by a dashed green line. (A) In the human tRNase ZS, the AxDx strand/loop-centered hydrogen bond network involves Ala351 (O) and Arg19 (NH1), Asp353 (OD2) and Gly20 (HN), Ala351 (HN) and Leu311 (O), Asp353 (OD1) and Thr10 (HN and OG1), and Gly20 (O) and Thr10 (HN). (B) In B. subtilus, the AxDx strand/loop-centered hydrogen bond network includes Ala296 (O) and Arg17 (NH1), Asp298 (OD2) and Asn18 (HN), Ala 296 (HN) and Leu267 (O), Asp298 (OD2) and Thr8 (HN and OG1), and Asn18 (O) and Thr8 (HN).
The PxKxRN motif, in particular, the two basic residues in the motif, has been proposed to may play a role in cleavage site specificity and, by inference, CCA anti-determination, which prevents removal of mature 3′-end of tRNAs by tRNase Z, and thus avoids the repeated addition and removal of the CCA sequence [17], [18]. In D. melanogaster, alanine substitutions of conserved residues in the PxKxRN motif significantly increased the Km value [17]. Considering its location and the possible role of the PxKxRN motif, we speculate that the AxDx motif may be indirectly involved in cleavage specificity through stabilization of an appropriate conformation of the PxKxRN/PxPxRG loop for tRNA processing.
Using a S. pombe-based functional complementation assay we have demonstrated the importance of the conserved Asp in the AxDx motif for the function of the S. pombe nuclear tRNase ZL. However, its exact role in tRNA processing is unclear and awaits further biochemical characterization.
Alternative Splicing
Our database searches revealed candidate alternatively spliced isoforms of tRNase ZL in mammalian and nematode species. Of the types of alternative splicing of the mammalian tRNase ZL, skipping of exon 7 appears to be the most common type of alternative splicing. This exon is skipped due a downstream splice site of exon 8. The sizes of exon 7 of mammalian tRNase ZLs range from 108 (in dog) to 135 bp (in rat). The region encoded by exon 7 is located upstream from the exon 8 encoding the flexible arm. Further, the region encoded by exon 7 is not found in the fly and nematode tRNase ZLs. Thus, exon 7 apparently does not required for the tRNA 3′-end processing activity of the metazoan tRNase ZL.
So far, alternative splicing of the mammalian tRNase ZL mRNA has only been experimentally verified in mouse and rat tissues [33]. The mouse and rat tRNase ZLs have two mRNA splice forms. Real-time quantitative PCR analysis revealed that both splice forms of tRNase ZL are widely expressed in most mouse and rat tissues. In the mouse, the highest levels of expression of the two splice forms (MmuTRZ2 and MmuTRZ2i) were detected in the testis and spleen, respectively, whereas the lowest levels of MmuTRZ2 and MmuTRZ2i were observed in both the prostate and muscle, and the muscle, respectively. In the prostate and muscle, the MmuTRZ2i mRNA is the predominant transcript, whereas in other tissues, the MmuTRZ2 transcript is predominant. Similarly, in the prostate, the rat tRNase ZL expression is dramatically reduced and the alternative form of tRNase ZL is more abundant than the normally spliced form. However, the spliced variants of mouse and rat tRNase ZLs have been experimentally validated on the mRNA level, but not on the protein level. Furthermore, the function of these spliced variants of tRNase ZL remains to be characterized.
Conclusions
The present study complements our previous work by identifying and analyzing metazoan tRNase Zs. We found that although both tRNase ZS and tRNase ZL arose very early in metazoan evolution, only tRNase ZL has been retained throughout metazoan evolution. Similar to the situation in fungi and plants, in metazoans, a single tRNase ZL seems to be responsible for both nuclear and mitochondrial tRNA 3′-end processing. In contrast, tRNase ZS, which is retained only in deuterostomes, lophotrochozoans and lower metazoans, may have a function other than tRNA 3′-end processing. We also found the existence of the predicted alternative splice variants of the mammalian and nematode tRNase ZL genes in the GenBank database. Using sequence conservation analysis, we identified a conserved AxDx motif in all metazoan tRNase Zs examined. We predicted that potential hydrogen bonding interactions involving the AxDx and PxKxRN/PxPxRG motif are a highly conserved structural feature of tRNase Zs. We showed that the AxDx motif is important to the function of the S. pombe tRNase ZL in a functional complementation assay. Our results should open up interesting new perspectives for future studies of tRNase Z.
Methods
Metazoan Genome Database Searches and Protein Sequence Analyses
Candidate tRNase Zs were identified by blastp and tblastn searches of genomic, protein and EST databases, which include the National Center for Biotechnology Information nonredundant protein sequence database (NCBI; http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi?organism=euk), Metazome (http://www.metazome.net/search.php?show=blast), Ensemble (http://www.ensembl.org/index.html), ExPASy (http://www.expasy.ch/), the Joint Genome Institute (JGI; http://www.jgi.doe.gov/), the Broad Institute (http://www.broadinstitute.org/scientific-community/data), Wormbase (http://www.wormbase.org/), the Universal Protein Resource (UniProt; http://www.uniprot.org/), and SpongeBase (http://spongebase.unimainz.de/), using default parameters. The resulting sequences were subject to validation as described [5], [7]. Briefly, each prediction was first subjected to reciprocal BLAST searches against the GenBank database. In back-searches, a candidate was confirmed if the best hits from reciprocal BLAST are tRNase Zs. Accuracy of prediction was further evaluated by multiple sequence alignment. All discordant candidate sequences were checked manually for possible errors including sequencing errors, intron mispredictions and existence of gaps in the genome sequences. When possible, we also used mRNA/EST sequences to check the predicted intron positions. We kept the candidate if it could be accurately predicted, otherwise we discarded it. However, despite our best efforts, we could not conclusively rule out the possibility that some of candidates might contain errors. The splicing pattern was verified using the Fgenesh and Fgenesh_GC programs provided at the Softberry website (http://linux1.softberry.com/berry.phtml?topic=fgenesh). Prediction of subcellular localization of proteins was made using web-based prediction programs such as MITOPROT (http://ihg2.helmholtz-muenchen.de/ihg/mitoprot.html) and PSORT (http://psort.hgc.jp/form.html). Multiple sequence alignments were done by Clustal W [52].
Phylogenetic Analysis
The phylogenetic tree for candidate metazoan tRNase Zs was constructed as described [5], [7]. Accession numbers for the sequences used in the analysis are summarized in Table S1. Briefly, a multiple amino acid sequence alignment of metazoan tRNase ZSs and C-terminal halves of metazoan tRNase ZLs was generated with Clustal W implemented in MEGA5.0 [53] were manually adjusted. Model selection was performed using ProtTest 2.4 [54]. The Bayesian phylogenetic tree was inferred by using MrBayes version 3.1.2 [55] and a mixture of the fixed amino acid models and I + G distribution. Statistical confidence was assessed by using Markov Chain Monte Carlo (MCMC) sampling approaches. Two separate runs including a total of four independent tree searches were conducted. All searches consisted of one ‘cold’ and three ‘heated’ Markov chains estimated for 107 generations, and every 100 generations were sampled. The burn-in parameter was estimated by plotting -lnL against the generation number using Tracer (v1.4.1, http://beast.bio.ed.ac.uk/Tracer), and the retained trees were used to estimate the consensus tree and the Bayesian posterior probabilities.
PCR Mutagenesis
The aspartate to alanine mutation in the 770AxDx773 motif of SpTrz1 was generated by the overlapping PCR method [56]. Briefly, the plasmid expressing wild type Sptrz1 [57] was used as template for PCR. The upstream region of the mutant was generated using the following pairs of primers: 5'-GACTGAGTCGACATGGATTACAAAGACGATGACGAC (designated SalI) and 5'-GACTTACATATAGCTTTGGCGTTTGCTGGAATGACTCTTAAAATATCGG (designated spTrz1-D-fo). The downstream region of the mutant was created using the following primer pairs : 5'-CCGATATTTTAAGAGTCATTCCAgCAAACGCCAAAGCTATATGTAAGTC (designated spTrz1-D-re) and 5'-ACGTCGTCCCGGGTTAGAACTTTAAAGGATCCGACTCCTC (designated spTrz1-re). The up and downstream fragments of the mutant from the first round of PCR were mixed in a 1∶1 molar ratio, diluted and used as template for the second round of PCR. The primer pair SalI and spTrz1-re was used in the second round of PCR. The resulting PCR product containing mutated sptrz1 (sptrz1-D772A) was digested and cloned into the Sal I/Sma I sites of pREP4X. The point mutation was verified by DNA sequencing.
Supporting Information
Alignment of candidate tRNase ZLs identified in metazoans. The accession numbers for the candidates are listed in Table S1. The annotation of the alignment is described in the legend to Figure 3.
(DOC)
Alignment of candidate tRNase ZSs identified in metazoans. The accession numbers for the candidates are listed in Table S1. The annotation of the alignment is described in the legend to Figure 3.
(DOC)
Comparison of the sequences flanking the two in-frame AUG start codons in 58 metazoan tRNase ZL mRNAs. Sequences were aligned by the start codon. The AUG start codons are indicated in red. In each sequence, nucleotides at positions −3 and +4, which are the most important determinants of context are shown in blue and green, respectively. Although the consensus sequences surrounding AUG start codons vary considerably between eukaryotic groups, they share a strong preference for purines at the position −3 and G at the position +4 for translational initiation.
(DOC)
The predicted hydrogen bonding network in the AxDx strand/loop region of human tRNase ZL. The published structure of human tRNase ZS (PDB code 3ZWF) was used as a template to build a modeled structure of C-terminal region of human tRNase ZL (residues 481–754) using the homology modeling server SWISSMODEL (http://swissmodel.expasy.org/). The picture is labeled as described in the legend to Figure 8. The potential hydrogen bond network is formed by the O atom of Ala746 and the NH1 atom of Arg497 (3.07Å), the OD2 atom of Asp748 and the HN atom of Asn498 (2.78Å), the HN atom of Ala 746 and the O atom of Leu722 (2.86Å), the OD2 atom of Asp748 and the HN atom of Thr488 (3.06Å), and the OD2 atom of Asp748 and the OG1 atom of Thr488 (2.71Å), the OG1 atom of Thr488 and the NH1 atom of Arg497 (2.81Å) and the O atom of Asn498 and the HN atom of Thr488 (3.01Å). The figure was prepared using Swiss-PdbViewer [59].
(TIF)
Distribution of candidate tRNase Zs identified in metazoans. Abbreviations for species names are indicated in the parentheses. +The number of amino acids in metazoan tRNase Z and tRNase Z-like proteins. *Indicates that mispredicted sequences obtained from the databases have been corrected. ?Indicates the sequence could not be correctly predicted.
(DOC)
Prediction of the number of introns in metazoan tRNase Z genes.
(DOC)
Subcellular localization prediction of metazoan tRNase ZLs. The putative NLSs were predicted using PSORT (http://psort.hgc.jp/form.html), while the putative MTSs were predicted using MITOPROT (http://ihg2.helmholtz-muenchen.de/ihg/mitoprot.html). Nuc: stand for the nucleus; Mito: mitochondria. N indicates nuclear localization and M denotes mitochondrial localization. “-”, the localization or targeting sequence could not be predicted. The numbers refer to amino acid positions starting from the N-terminus.
(DOC)
Percentage amino acid identity among tRNase ZLs from selected metazoans. The pairwise percent identity scores were generated with Clustal W [52]. tRNase ZLs are from H. sapiens (Hsa), C. familiaris (Cfa), M. musculus (Mmu), R. norvegicus (Rno), O. cuniculus (Ocu), S. scrofa (Ssc), A. carolinensis (Aca), X. tropicalis (Xtr), D. rerio (Dre), G. aculeatus (Gac), C. intestinalis (Cin), C. savignyi (Csa), B. mori (Bmo), D. melanogaster (Dme), C. elegans (Cel), H. robusta (Hro), S. mansoni (Sma), T. adhaerens (Tad), M. brevicollis, (Mbr), A. thaliana (Ath), and S. pome (Spo).
(DOC)
Percentage amino acid identity among tRNase ZSs from selected metazoans. The pairwise percent identity scores were generated with Clustal W [52]. H. sapiens, Hsa; M. musculus, Mmu; R. norvegicus, Rno; O. cuniculus, Ocu; S. araneus, Sar; T. syrichta, Tsy; A. carolinensis, Aca; X. tropicalis, Xtr; D. rerio, Dre; G. aculeatus, Gac; C. intestinalis, Cin; C. savignyi, Csa; B. floridae, Bfl, L. gigantean, Lgi; S. mansoni, Sma; A. queenslandica, Aqu; N. vectensis, Nve; M. brevicollis, Mbr; B. subtilis, Bsu; E. coli, Eco.
(DOC)
Acknowledgments
We thank Lijuan Fan for initial efforts in this work, Hongmei Dai and Haiyan Yu for technical help, and Jie Yan for help in construction of phylogenetic trees and for valuable discussion.
Funding Statement
This work was supported in part by grants from the National Science Foundation of China (31070703) (http://www.nsfc.gov.cn/Portal0/default152.htm) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (http://jsycw.ec.js.edu.cn/) and Nanjing Normal University (2007104XGQ0148). The funders had no role in study design, data colletion and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ceballos M, Vioque A (2007) tRNase Z. Protein Pept Lett. 14: 137–145. [DOI] [PubMed] [Google Scholar]
- 2. Hartmann RK, Gossringer M, Spath B, Fischer S, Marchfelder A (2009) Chapter 8 The Making of tRNAs and More - RNase P and tRNase Z. Prog Nucleic Acid Res Mol Biol. 85C: 319–368. [DOI] [PubMed] [Google Scholar]
- 3. Redko Y, Li de Lasierra-Gallay I, Condon C (2007) When all's zed and done: the structure and function of RNase Z in prokaryotes. Nat Rev Microbiol 5: 278–286. [DOI] [PubMed] [Google Scholar]
- 4. Spath B, Canino G, Marchfelder A (2007) tRNase Z: the end is not in sight. Cell Mol Life Sci 64: 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan L, Wang Z, Liu J, Guo W, Yan J, et al. (2011) A survey of green plant tRNA 3′-end processing enzyme tRNase Zs, homologs of the candidate prostate cancer susceptibility protein ELAC2. BMC Evol Biol 11: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tavtigian SV, Simard J, Teng DH, Abtin V, Baumgard M, et al. (2001) A candidate prostate cancer susceptibility gene at chromosome 17p. Nat Genet 27: 172–180. [DOI] [PubMed] [Google Scholar]
- 7. Zhao W, Yu H, Li S, Huang Y (2010) Identification and analysis of candidate fungal tRNA 3′-end processing endonucleases tRNase Zs, homologs of the putative prostate cancer susceptibility protein ELAC2. BMC Evol Biol 10: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canino G, Bocian E, Barbezier N, Echeverria M, Forner J, et al.. (2009) Arabidopsis encodes four tRNase Z enzymes. Plant Physiol. [DOI] [PMC free article] [PubMed]
- 9.Brzezniak LK, Bijata M, Szczesny RJ, Stepien PP (2011) Involvement of human ELAC2 gene product in 3′ end processing of mitochondrial tRNAs. RNA Biol 8. [DOI] [PubMed]
- 10.Rossmanith W (2011) Of P and Z: Mitochondrial tRNA processing enzymes. Biochim Biophys Acta. [DOI] [PMC free article] [PubMed]
- 11. Takahashi M, Takaku H, Nashimoto M (2008) Regulation of the human tRNase ZS gene expression. FEBS Lett 582: 2532–2536. [DOI] [PubMed] [Google Scholar]
- 12. Aravind L (1999) An evolutionary classification of the metallo-beta-lactamase fold proteins. In Silico Biol 1: 69–91. [PubMed] [Google Scholar]
- 13. Daiyasu H, Osaka K, Ishino Y, Toh H (2001) Expansion of the zinc metallo-hydrolase family of the beta-lactamase fold. FEBS Lett 503: 1–6. [DOI] [PubMed] [Google Scholar]
- 14. Dominski Z (2007) Nucleases of the metallo-beta-lactamase family and their role in DNA and RNA metabolism. Crit Rev Biochem Mol Biol 42: 67–93. [DOI] [PubMed] [Google Scholar]
- 15. Schiffer S, Rosch S, Marchfelder A (2002) Assigning a function to a conserved group of proteins: the tRNA 3'-processing enzymes. EMBO J 21: 2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minagawa A, Ishii R, Takaku H, Yokoyama S, Nashimoto M (2008) The flexible arm of tRNase Z is not essential for pre-tRNA binding but affects cleavage site selection. J Mol Biol 381: 289–299. [DOI] [PubMed] [Google Scholar]
- 17. Zareen N, Hopkinson A, Levinger L (2006) Residues in two homology blocks on the amino side of the tRNase Z His domain contribute unexpectedly to pre-tRNA 3' end processing. RNA 12: 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de la Sierra-Gallay IL, Pellegrini O, Condon C (2005) Structural basis for substrate binding, cleavage and allostery in the tRNA maturase RNase Z. Nature. 433: 657–661. [DOI] [PubMed] [Google Scholar]
- 19. Karkashon S, Hopkinson A, Levinger L (2007) tRNase Z catalysis and conserved residues on the carboxy side of the His cluster. Biochemistry 46: 9380–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minagawa A, Takaku H, Ishii R, Takagi M, Yokoyama S, et al. (2006) Identification by Mn2+ rescue of two residues essential for the proton transfer of tRNase Z catalysis. Nucleic Acids Res 34: 3811–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishii R, Minagawa A, Takaku H, Takagi M, Nashimoto M, et al. (2007) The structure of the flexible arm of Thermotoga maritima tRNase Z differs from those of homologous enzymes. Acta Crystallogr Sect F Struct Biol Cryst Commun 63: 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishii R, Minagawa A, Takaku H, Takagi M, Nashimoto M, et al. (2005) Crystal structure of the tRNA 3' processing endoribonuclease tRNase Z from Thermotoga maritima. J Biol Chem 280: 14138–14144. [DOI] [PubMed] [Google Scholar]
- 23. Kostelecky B, Pohl E, Vogel A, Schilling O, Meyer-Klaucke W (2006) The crystal structure of the zinc phosphodiesterase from Escherichia coli provides insight into function and cooperativity of tRNase Z-family proteins. J Bacteriol 188: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li de la Sierra-Gallay I, Mathy N, Pellegrini O, Condon C (2006) Structure of the ubiquitous 3' processing enzyme RNase Z bound to transfer RNA. Nat Struct Mol Biol 13: 376–377. [DOI] [PubMed] [Google Scholar]
- 25.Hopkinson A, Levinger L (2008) Effects of conserved D/T loop substitutions in the pre-tRNA substrate on tRNase Z catalysis. RNA Biol 5. [DOI] [PubMed]
- 26. Schilling O, Spath B, Kostelecky B, Marchfelder A, Meyer-Klaucke W, et al. (2005) Exosite modules guide substrate recognition in the ZiPD/ElaC protein family. J Biol Chem 280: 17857–17862. [DOI] [PubMed] [Google Scholar]
- 27. Dubrovsky EB, Dubrovskaya VA, Levinger L, Schiffer S, Marchfelder A (2004) Drosophila RNase Z processes mitochondrial and nuclear pre-tRNA 3' ends in vivo. Nucleic Acids Res 32: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie X, Dubrovskaya VA, Dubrovsky EB (2010) RNAi knockdown of dRNaseZ, the Drosophila homolog of ELAC2, impairs growth of mitotic and endoreplicating tissues. Insect Biochem Mol Biol. [DOI] [PubMed]
- 29. Smith MM, Levitan DJ (2004) The Caenorhabditis elegans homolog of the putative prostate cancer susceptibility gene ELAC2, hoe-1, plays a role in germline proliferation. Dev Biol 266: 151–160. [DOI] [PubMed] [Google Scholar]
- 30. Roy SW, Gilbert W (2005) Complex early genes. Proc Natl Acad Sci U S A 102: 1986–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chothia C, Gough J, Vogel C, Teichmann SA (2003) Evolution of the protein repertoire. Science 300: 1701–1703. [DOI] [PubMed] [Google Scholar]
- 32. Rogozin IB, Sverdlov AV, Babenko VN, Koonin EV (2005) Analysis of evolution of exon-intron structure of eukaryotic genes. Brief Bioinform 6: 118–134. [DOI] [PubMed] [Google Scholar]
- 33. Dumont M, Frank D, Moisan AM, Tranchant M, Soucy P, et al. (2004) Structure of primate and rodent orthologs of the prostate cancer susceptibility gene ELAC2. Biochim Biophys Acta 1679: 230–247. [DOI] [PubMed] [Google Scholar]
- 34. Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, et al. (2008) Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452: 745–749. [DOI] [PubMed] [Google Scholar]
- 35.Lopez Sanchez MI, Mercer TR, Davies SM, Shearwood AM, Nygard KK, et al.. (2011) RNA processing in human mitochondria. Cell Cycle 10. [DOI] [PubMed]
- 36. Rossmanith W (2011) Localization of human RNase Z isoforms: dual nuclear/mitochondrial targeting of the ELAC2 gene product by alternative translation initiation. PLoS One 6: e19152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao Z, Su W, Yuan S, Huang Y (2009) Functional conservation of tRNase ZL among Saccharomyces cerevisiae, Schizosaccharomyces pombe and humans. Biochem J 422: 483–492. [DOI] [PubMed] [Google Scholar]
- 38. Kortschak RD, Samuel G, Saint R, Miller DJ (2003) EST analysis of the cnidarian Acropora millepora reveals extensive gene loss and rapid sequence divergence in the model invertebrates. Curr Biol 13: 2190–2195. [DOI] [PubMed] [Google Scholar]
- 39. Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, et al. (2005) Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433: 156–160. [DOI] [PubMed] [Google Scholar]
- 40. Yan H, Zareen N, Levinger L (2006) Naturally occurring mutations in human mitochondrial pre-tRNASer(UCN) can affect the transfer ribonuclease Z cleavage site, processing kinetics, and substrate secondary structure. J Biol Chem 281: 3926–3935. [DOI] [PubMed] [Google Scholar]
- 41. Dubrovsky EB, Dubrovskaya VA, Bilderback AL, Berger EM (2000) The isolation of two juvenile hormone-inducible genes in Drosophila melanogaster. Dev Biol 224: 486–495. [DOI] [PubMed] [Google Scholar]
- 42. Wilusz JE, Freier SM, Spector DL (2008) 3' end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 135: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, et al. (2010) Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA 16: 673–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee YS, Shibata Y, Malhotra A, Dutta A (2009) A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev 23: 2639–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bogerd HP, Karnowski HW, Cai X, Shin J, Pohlers M, et al. (2010) A mammalian herpesvirus uses noncanonical expression and processing mechanisms to generate viral MicroRNAs. Mol Cell 37: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diebel KW, Smith AL, van Dyk LF (2010) Mature and functional viral miRNAs transcribed from novel RNA polymerase III promoters. Rna 16: 170–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yanaihara N, Kohno T, Takakura S, Takei K, Otsuka A, et al. (2001) Physical and transcriptional map of a 311-kb segment of chromosome 18q21, a candidate lung tumor suppressor locus. Genomics 72: 169–179. [DOI] [PubMed] [Google Scholar]
- 48. Cavener DR (1987) Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res 15: 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kozak M (2002) Pushing the limits of the scanning mechanism for initiation of translation. Gene 299: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith CW, Patton JG, Nadal-Ginard B (1989) Alternative splicing in the control of gene expression. Annu Rev Genet 23: 527–577. [DOI] [PubMed] [Google Scholar]
- 51. Kozak M (1995) Adherence to the first-AUG rule when a second AUG codon follows closely upon the first. Proc Natl Acad Sci U S A 92: 2662–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 53.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al.. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed]
- 54. Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105. [DOI] [PubMed] [Google Scholar]
- 55. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 56. Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59. [DOI] [PubMed] [Google Scholar]
- 57.Gan X, Yang J, Li J, Yu H, Dai H, et al.. (2011) The fission yeast Schizosaccharomyces pombe has two distinct tRNase ZLs encoded by two different genes and differentially targeted to the nucleus and mitochondria. Biochem J. [DOI] [PubMed]
- 58. Levinger L, Hopkinson A, Desetty R, Wilson C (2009) Effect of changes in the flexible arm on tRNase Z processing kinetics. J Biol Chem 284: 15685–15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18: 2714–2723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of candidate tRNase ZLs identified in metazoans. The accession numbers for the candidates are listed in Table S1. The annotation of the alignment is described in the legend to Figure 3.
(DOC)
Alignment of candidate tRNase ZSs identified in metazoans. The accession numbers for the candidates are listed in Table S1. The annotation of the alignment is described in the legend to Figure 3.
(DOC)
Comparison of the sequences flanking the two in-frame AUG start codons in 58 metazoan tRNase ZL mRNAs. Sequences were aligned by the start codon. The AUG start codons are indicated in red. In each sequence, nucleotides at positions −3 and +4, which are the most important determinants of context are shown in blue and green, respectively. Although the consensus sequences surrounding AUG start codons vary considerably between eukaryotic groups, they share a strong preference for purines at the position −3 and G at the position +4 for translational initiation.
(DOC)
The predicted hydrogen bonding network in the AxDx strand/loop region of human tRNase ZL. The published structure of human tRNase ZS (PDB code 3ZWF) was used as a template to build a modeled structure of C-terminal region of human tRNase ZL (residues 481–754) using the homology modeling server SWISSMODEL (http://swissmodel.expasy.org/). The picture is labeled as described in the legend to Figure 8. The potential hydrogen bond network is formed by the O atom of Ala746 and the NH1 atom of Arg497 (3.07Å), the OD2 atom of Asp748 and the HN atom of Asn498 (2.78Å), the HN atom of Ala 746 and the O atom of Leu722 (2.86Å), the OD2 atom of Asp748 and the HN atom of Thr488 (3.06Å), and the OD2 atom of Asp748 and the OG1 atom of Thr488 (2.71Å), the OG1 atom of Thr488 and the NH1 atom of Arg497 (2.81Å) and the O atom of Asn498 and the HN atom of Thr488 (3.01Å). The figure was prepared using Swiss-PdbViewer [59].
(TIF)
Distribution of candidate tRNase Zs identified in metazoans. Abbreviations for species names are indicated in the parentheses. +The number of amino acids in metazoan tRNase Z and tRNase Z-like proteins. *Indicates that mispredicted sequences obtained from the databases have been corrected. ?Indicates the sequence could not be correctly predicted.
(DOC)
Prediction of the number of introns in metazoan tRNase Z genes.
(DOC)
Subcellular localization prediction of metazoan tRNase ZLs. The putative NLSs were predicted using PSORT (http://psort.hgc.jp/form.html), while the putative MTSs were predicted using MITOPROT (http://ihg2.helmholtz-muenchen.de/ihg/mitoprot.html). Nuc: stand for the nucleus; Mito: mitochondria. N indicates nuclear localization and M denotes mitochondrial localization. “-”, the localization or targeting sequence could not be predicted. The numbers refer to amino acid positions starting from the N-terminus.
(DOC)
Percentage amino acid identity among tRNase ZLs from selected metazoans. The pairwise percent identity scores were generated with Clustal W [52]. tRNase ZLs are from H. sapiens (Hsa), C. familiaris (Cfa), M. musculus (Mmu), R. norvegicus (Rno), O. cuniculus (Ocu), S. scrofa (Ssc), A. carolinensis (Aca), X. tropicalis (Xtr), D. rerio (Dre), G. aculeatus (Gac), C. intestinalis (Cin), C. savignyi (Csa), B. mori (Bmo), D. melanogaster (Dme), C. elegans (Cel), H. robusta (Hro), S. mansoni (Sma), T. adhaerens (Tad), M. brevicollis, (Mbr), A. thaliana (Ath), and S. pome (Spo).
(DOC)
Percentage amino acid identity among tRNase ZSs from selected metazoans. The pairwise percent identity scores were generated with Clustal W [52]. H. sapiens, Hsa; M. musculus, Mmu; R. norvegicus, Rno; O. cuniculus, Ocu; S. araneus, Sar; T. syrichta, Tsy; A. carolinensis, Aca; X. tropicalis, Xtr; D. rerio, Dre; G. aculeatus, Gac; C. intestinalis, Cin; C. savignyi, Csa; B. floridae, Bfl, L. gigantean, Lgi; S. mansoni, Sma; A. queenslandica, Aqu; N. vectensis, Nve; M. brevicollis, Mbr; B. subtilis, Bsu; E. coli, Eco.
(DOC)