Abstract
In heterozygous mice, attenuation of G-protein-coupled receptor kinase 2 (GRK2) level in nociceptors is associated with enhanced and prolonged inflammatory hyperalgesia. To further elucidate the role of GRK2 in nociceptor function we reversibly decreased GRK2 expression using intrathecal antisense oligodeoxynucleotide (AS-ODN). GRK2 AS-ODN administration led to an enhanced and prolonged hyperalgesia induced by prostaglandin E 2, epinephrine and carrageenan. Morover, this effect persisted unattenuated 2 weeks after the last dose of antisense, well after GRK2 protein recovered, suggesting that transient attenuation of GRK2 produced neuroplastic changes in nociceptor function. Unlike hyperalgesic priming induced by transient attenuation of GRK2 produced neuroplastic changes in nociceptor function. Unlike hyperalgesic priming induced by transient activation of protein kinase C epsilon (PKCε), (Aley et al., 2000, Parada et al., 2003b), the enhanced and prolonged hyperalgesia following attenuation of GRK2 is PKCε- and cytoplasmic polyadenylation element binding protein (CPEB)-independent and is protein kinase A (PKA)- and Src tyrosine kinase (Src)-dependent. Finally, rats treated with GRK2 AS-ODN exhibited enhanced and prolonged hyperalgesia induced by direct activation of second messengers, adenyl cyclase, Epac or PKA, suggesting changes downstream of G-protein-coupled receptors. Because inflammation can produce a decrease in GRK2, such a mechanism could help explain a predilection to develop chronic pain, after resolution of acute inflammation.
Keywords: hyperalgesia, nociceptor, G-protein-coupled receptor, G-protein-coupled receptor kinase 2, chronic pain
We have previously provided evidence that transient activation of protein kinase C epsilon (PKCε) produces a very long lasting neuroplastic change in primary afferent nociceptor function, referred to as hyperalgesic priming, such that subsequent exposure to proinflammatory cytokines produces enhanced and markedly prolonged mechanical hyperalgesia (Aley et al., 2000, Parada et al., 2005). Recently, G-protein-coupled receptor kinase 2 (GRK2), which phosphorylates agonist-activated G-protein-coupled receptors (Zhang et al., 1997, Luttrell and Lefkowitz, 2002, Ribas et al., 2007), was also shown to play a role in chronic pain (Kleibeuker et al., 2007, Kavelaars et al., 2011). The attenuation of GRK2 in nociceptors in knockout mice leads to enhanced and prolonged inflammatory mediator-induced mechanical hyperalgesia (Kleibeuker et al., 2007, Eijkelkamp et al., 2010a, Eijkelkamp et al., 2010b, Wang et al., 2011). Since inflammation produces a decrease in GRK2, in leukocytes and nociceptors, these authors suggested that GRK2 attenuation contributes to the development of enhanced and prolonged pain. In the present study we determined whether GRK2 level or, analogous to hyperalgesic priming, a downstream effect produced by a transient decrease in GRK2, is responsible for the exacerbation of inflammatory hyperalgesia. Spinal intrathecal administration of antisense was used to attenuate protein expression in sensory neurons (Parada et al., 2003a, Parada et al., 2003b) innervating the skin. Our findings indicate that: 1) transient attenuation of GRK2 produces very long-lasting enhancement of inflammatory mediator-induced nociceptor sensitization, 2) these differences in nociceptor function are due to changes downstream from the G-protein-coupled receptors at which inflammatory mediators act to produce hyperalgesia, and 3) the neuroplastic change induced by transient decrease in GRK2, a phenomenon we refer to as GRKing, can be mechanistically distinguished from hyperalgesic priming (Aley et al., 2000, Parada et al., 2003b, Parada et al., 2005, Bogen et al., 2012). The elucidation of the mechanism underlying this long-term neuroplastic changes may provide insight into PKCε-independent mechanisms of chronic pain.
2. Experimental Procedures
2.1. Animals
The experiments were performed on 200–220 g, adult male Sprague–Dawley rats (Charles River, Hollister, CA, USA). Animals were housed in the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12-h light/dark cycle. All experimental protocols were approved by the UCSF Institutional Animal Care and Use Committee (IACUC), and conformed to NIH guidelines for the care and use of experimental animals. Effort was made to limit the numbers of animals used and their discomfort.
2.2. Measurement of mechanical nociceptive threshold
Mechanical nociceptive threshold was quantified using the Randall–Selitto paw pressure test in which a force that increases linearly over time is applied to the dorsum of the rat’s hind paw (Randall and Selitto, 1957, Taiwo et al., 1989b, Taiwo and Levine, 1989), using an Ugo Basile Algesymeter® (Stoelting, Chicago, IL, USA). Rats were placed in cylindrical acrylic restrainers designed to provide adequate comfort and ventilation, allow extension of the hind leg from the cylinder, and minimize restraint stress. All rats were acclimatized to the testing procedure, and testing was performed in parallel across groups. Rats were adapted to the restrainer for 1 h prior to starting each study and for 30 min prior to experimental manipulations. Nociceptive threshold was defined as the force in grams (g) at which the rat withdrew its paw. The baseline paw-withdrawal threshold was defined as the mean of three readings. Each paw was treated as an independent measure and each experiment performed on a separate group of rats. All behavioral testing was done between 10:00 and 17:00 h.
2.3. Drugs
The chemicals used in this study were: epinephrine (EPI), prostaglandin E2 (PGE2), carrageenan, adenyl cyclase activator forskolin (all from Sigma, St. Louis, MO), 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′5′-cyclic monophosphate (CPToMe, Epac activator, TOCRIS Bioscience, Ellisville), cell-permeable cAMP analog 8-bromo-cAMP (TOCRIS Bioscience, Ellisville), intracellular messengers inhibitors bisindolylmaleimide (BIM, PKC inhibitor, Calbiochem - EMD Biosciences, La Jolla, CA), U73122 (PLCγ inhibitor, Sigma, St. Louis, MO), wortmannin (PI-3K inhibitor, Sigma, St. Louis, MO), WIPTIDE (PKA inhibitor, AnaSpec Inc, San Jose, CA), SU6656 (Src inhibitor, Sigma), UO126 (ERK/MEK inhibitor, Biomol, Plymouth Meeting, PA, USA) and roscovitine (CDK5 inhibitor, Calbiochem - EMD Biosciences Inc., La Jolla, CA).
The selection of the doses used was based on dose–response curves determined in this or previous studies (Taiwo et al., 1989a, Taiwo and Levine, 1991, Bogen et al., 2008). The stock solution of PGE2 (1 μg/μl) was prepared in 10% ethanol, and additional dilutions made with physiological saline (0.9% NaCl), yielding a final concentration of ethanol less than 1%. Epinephrine was dissolved in distilled water with an equivalent amount of ascorbic acid, immediately before injection. Carrageenan (0.1 or 1%, w/v) and WIPTIDE were dissolved in saline. CPToMe and 8-bromo-cAMP were dissolved in distilled water. Forskolin, BIM, U73122, wortmannin, SU6656, UO126 and roscovitine were dissolved in 10% DMSO and diluted in saline before the injections. All drugs were administered intradermally in a volume of 5 μl using a 30-gauge hypodermic needle adapted to a Hamilton (Reno, NV, USA) syringe, except carrageenan, which was injected using a 27-gauge hypodermic needle, because of its high viscosity. For test agents with low cell membrane permeability (WIPTIDE, forskolin, CPToMe, BIM, U73122, wortmannin, SU6656, UO126 and roscovitine), co-injection of 2 μl of distilled water, to produce hypo-osmotic shock and facilitate cell permeability to these agents, was used (Borle and Snowdowne, 1982, Burch and Axelrod, 1987). When combinations of agents were used, they were administered from the same syringe in such a way that the agent mentioned first reached the intradermal site first; similarly, the distilled water preceded agents with low cell membrane permeability. The agents were separated in the syringe by a small air bubble to prevent their mixing in the syringe. Paw withdrawal threshold was determined before and 30 minutes after inhibitor administration. The effect of each chemical was determined on different groups of rats.
2.4. Antisense and mismatch oligodeoxynucleotides (ODNs)
ODNs were reconstituted in nuclease-free 0.9% NaCl to a concentration of 10 μg/μl and stored at -20oC until use. As described previously (Alessandri-Haber et al., 2003), for administration of the ODNs rats were anesthetized with 2.5% isoflurane (97.5% O2) and an insulin syringe with a 29-gauge needle (Becton Dickinson, Franklin Lakes, NJ) was inserted intrathecally, on the midline between the fifth and sixth lumbar vertebrae, at the level of the cauda equina; intrathecal location of the injection needle was confirmed by a flicking of the rat’s tail (Papir-Kricheli et al., 1987). A dose of 40 μg (injection volume 20 μl) of the ASODN or MM-ODN was administered, once daily for 3 or more consecutive days, depending on the specific protocol for an experiment.
2.5. Protein extraction and Western blot analysis
To confirm that the AS-ODN to GRK2 mRNA produced a decrease in the protein expression level of GRK2 in primary afferent fibers, and that GRK2 content recovered after the last administration of GRK2 antisense, Western blot analysis was performed.
Saphenous nerves from anesthetized rats were ligated with silk surgical suture (4-0) 1 cm above the knee-level bifurcation of the nerve on the day of the first injection (knockdown) or one week after the last injection (recovery). Three days post-ligation, a 5-mm section of saphenous nerve proximal to the ligation was harvested and protein extraction and Western blotting was performed as previously described (Joseph et al., 2007, Bogen et al., 2008). All samples (12 saphenous nerves from 3 mismatch and 3 antisense treated rats) of each treatment group (knockdown and recovery) were processed and analyzed on the same day, and all twelve samples were run at the same time on the same gel.
GRK2 immunoreactivity was detected with affinity-purified polyclonal anti-GRK2 antibody (SC-562, dilution: 1/500, Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with horseradish peroxidase-conjugated (HRP) goat anti-rabbit antibody (1/5000, Pierce Biotechnology, Rockford, IL). To normalize the sample loaded, affinity purified mouse monoclonal anti-GAPDH antibody (ab9424, 1/5000, Abcam) was used followed by incubation with HRP-conjugated goat anti-mouse antibody (1/10,000, Pierce Biotechnology). Immunoreactivities were visualized with enhanced chemiluminescence reagents (Pierce Biotechnology) and images were acquired with CHEMILMAGER™ chemiluminescence imaging system and analyzed with AlphaEaseFC software (Alpha Innotech Corporation, San Leandro, CA).
2.6. Statistics
In all experiments, the dependent variable was change in mechanical threshold for paw withdrawal as a percent of baseline threshold. Importantly, there was no significant difference between paw withdrawal threshold values before and after treatment with GRK2 AS- or MM-ODN: average paw withdrawal threshold before and after GRK2 AS treatment was 111.1 ± 0.4 g and 110.9 ± 0.4 g, respectively (p=0.7695); for the MM group, average paw withdrawal thresholds before and after ODN treatments were 111.2 ± 0.7 g and 109.9 ± 0.7 g, respectively (p=0.0776), (paired Student’s t-test, N=138 in the AS-ODN group; N=60 in the MM-ODN group, data not shown). Other AS-/MM-ODNs used in this study (PKCε, CPEB and PLC-β3) also did not induce significant changes in the mechanical nociceptive threshold, as shown previously (Parada et al., 2003b, Joseph et al., 2007, Bogen et al., 2012).
In Figures 1A and 3A, an unpaired Student’s t-test was used to compare the levels of GRK2 expression in the sensory neuron after treatment with AS- or MM-ODNs. In Figures 1B, 2, 3B, 4 and 5, to test for significant differences in the effect of experimental interventions on mechanical paw withdrawal threshold, over time, two-way repeated ANOVA was performed and, when the interaction was significant, one-way repeated measures ANOVA with simple contrasts was performed for each group, to compare the responses at each time point to the initial time point. In Figure 6, to evaluate the effect of PLC-β3 AS-ODN or of each inhibitor of signaling pathway on the prolongation of PGE2-induced hyperalgesia, paired Student’s t-test was performed for each experiment, considering statistically significant different p values <0.05. To compare the magnitude of hyperalgesia induced by second messenger activators in animals previously treated with GRK2 AS- or MM-ODN in Figure 7, unpaired Student’s t-test was used. All data are presented in figures as mean standard error of the mean (S.E.M.).
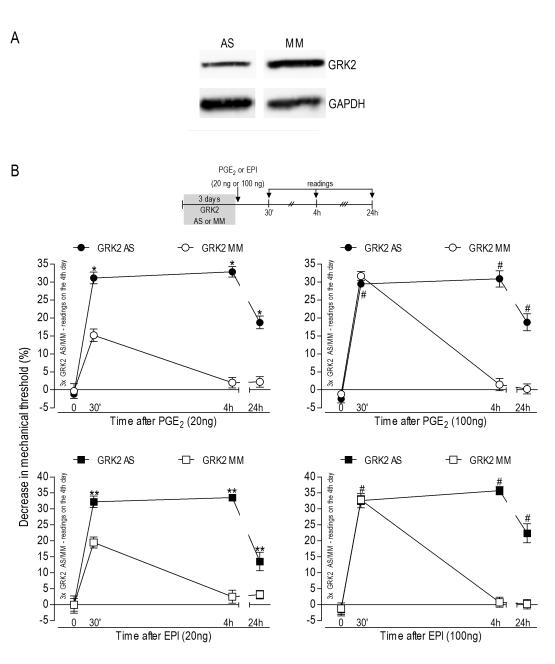
Figure 1. Inflammatory hyperalgesia during GRK2 knockdown.
A Western blot analysis. Analysis by Western blotting demonstrates a significant decrease in GRK2 protein levels in the saphenous nerve of rats treated for 3 consecutive days with antisense (AS)-ODN for GRK2 mRNA (39±9% when compared to the MM-treated rats; p=0.012, N=6 per group); B Potentiation of mechanical PGE2or epinephrine (EPI)induced hyperalgesia during GRK2 knockdown. Groups of rats were treated with antisense (AS)- or mismatch (MM)-ODN for GRK2 mRNA, for 3 consecutive days. On the 4th day, PGE2 (20 ng or 100 ng) or EPI (20 ng or 100 ng) was injected into the hind paw, in separate groups of rats. Mechanical nociceptive thresholds were evaluated 30 min, 4 h and 24 h later, using the Randall-Selitto paw-withdrawal test (N=6 for all groups). Average paw withdrawal threshold immediately before injection of PGE2 or EPI was 110.6 ± 1.0 g for the AS- and 110.8 ± 0.9 g for the MM-ODN groups. Paired Student’s t-test showed no difference in the mechanical threshold before and after the ODN treatments: p=0.5360 for the AS and p=0.3138 for the MM groups (N=24, data not shown). Left panels, low dose (20 ng) of PGE2 or EPI: Two-way repeated measures ANOVA showed a significant group time interaction (PGE2: F3.30=30.743; p<0.001; EPI: F3.30=17.254; p<0.001) and a significant main effect of group (PGE2: F1,10=300.167; p<0.001; EPI: F1,10=193.704; p<0.001). Based on the significant interaction, one-way repeated measures ANOVA with simple contrasts were performed for the AS and MM groups, showing that, for both groups, there was a significant main effect of time (PGE2 MM: F3,15=14.226; p=0.001; PGE2 AS: F3,15=124.234; p<0.001; EPI MM: F3,15=21.104; p=0.001; EPI AS: F3,15=43.756; p<0.001). However, while in the MM groups only the 30 min time point differed significantly from baseline (PGE2: p<0.001; EPI: p=0.002), in the AS groups the 30 min, 4 h, and 24 h time points were all significantly different from baseline (PGE2: *p<0.001 in each case; EPI: **p≤0.001 in each case); Right panels, high dose (100 ng) of PGE2 or EPI: Two-way repeated measures ANOVA showed a significant group time interaction (PGE2: F3,30=60.135; p<0.001; EPI: F3.30=35.221; p<0.001) and a significant main effect of group (PGE2: F1,10=68.594; p<0.001; EPI: F1,10=209.275; p<0.001). Based on the significant interaction, one-way repeated measures ANOVA with simple contrasts were performed for the AS and MM groups, showing that, for both groups, there was a significant main effect of time (PGE2 MM: F3,15=94.239; p<0.001; PGE2 AS: F3,15=49.454; p<0.001; EPI MM: F3,15=94.239; p<0.001; EPI AS: F3,15=49.454; p<0.001). However, in the MM groups, only the 30 min time point differed significantly from baseline (p<0.001 in both cases), while, in the AS groups, the 30 min, 4 h, and 24 h time points were all significantly different from baseline (#p≤0.004 in both cases).
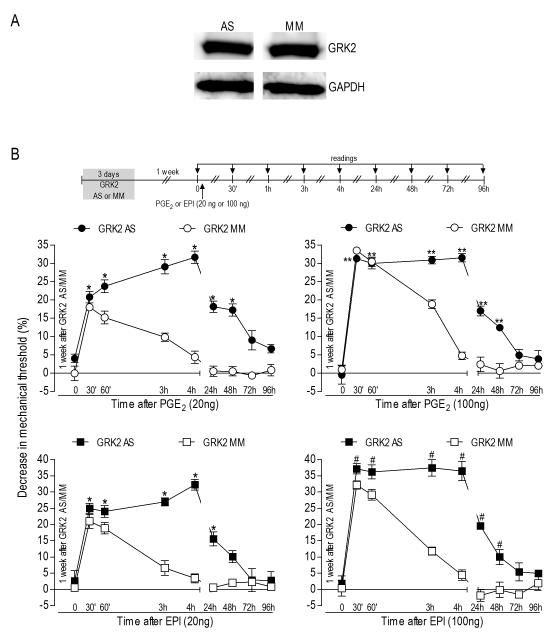
Figure 3.
A Hyperalgesia after recovery of GRK2 levels. Rats were treated with antisense (AS)- or mismatch (MM)-ODN for GRK2 mRNA for 3 consecutive days. 7 days after the last dose of the AS or MM, the saphenous nerves were harvested and Western blot analysis was performed. No differences in the GRK2 protein levels were observed between these two groups (p=0.3309, unpaired Student’s t-test, N=6 per group); B - Potentiation of PGE2- or epinephrine (EPI)induced hyperalgesia. Rats were treated with GRK2 antisense (AS)- or mismatch (MM)-ODN for 3 consecutive days. One week after the last dose of AS- or MM-ODN, when levels of GRK2 had recovered to pretreatment levels, PGE2 (20 ng or 100 ng) or EPI (20 ng or 100 ng) was injected into the hind paw. Mechanical thresholds were evaluated 30 min later and at further time points, until nociceptive threshold had returned to the baseline values (N=6 per group). Average paw withdrawal threshold right before injection of PGE2 or EPI was 110.6 ± 1.0 g in the AS group and 110.8 ± 0.9 g in the MM group. Comparison of thresholds before and after ODN treatments showed no significant changes after 3 injections of AS or MM: p=0.5360 for the AS and p=0.3138 for the MM group (paired Student’s t-test, data not shown). Left panels, low dose (20 ng) of PGE2 or EPI: Two-way repeated measures ANOVA showed a significant group time interaction (PGE2: F8,80=22.499; EPI: F8,80=21.874; p<0.001 for both cases) and a significant main effect of group (PGE2: F1,10=65.755; EPI: F1,10=25.784; p<0.001 for both cases). Based on the significant interaction, one-way repeated measures ANOVA with simple contrasts were performed for each of the two groups (AS and MM), showing that, in the MM groups, there was a significant main effect of time (PGE2: F8,40=39.788; EPI: F8,40=47.896; p<0.001 for both cases), with significant difference from baseline (p<0.001 in all cases) only in the 30 min and 1 h time points. For the AS groups there was also a significant main effect of time (PGE2: F8,40=54.597; EPI: F8,40=44.886; p< 0.001 for both cases), with significant difference from baseline in the first five time points (*p≤0.003); Right panels, high dose (100 ng) of PGE2 or EPI: The two-way repeated measures ANOVA showed a significant group time interaction (PGE2: F8,80=28.320; EPI: F8,80=23.774; p<0.001 for both cases) and a significant main effect of group (PGE2: F1,10=54.643; EPI: F1,10=46.212; p<0.001 for both cases). Based on the significant interaction, one-way repeated measures ANOVA with simple contrasts were performed for each of the two groups (AS and MM). For the MM groups, a significant main effect of time (PGE2: F8,40=120.213; EPI: F8,40=65.319; p<0.001 for both cases) was observed, and simple contrasts showed that, for PGE2, the first three time points, 30 min, 60 min and 3 h, and for EPI, the first two time points, 30 min and 60 min, differed significantly from baseline (p<0.001 in each case). For the AS groups there was also a significant main effect of time (PGE2: F8,40=95.097; EPI: F8,40=105.009; p<0.001), and simple contrasts showed that the first six time points were significantly different from baseline (PGE2: **p≤0.004, EPI: #p≤0.001 in all cases).
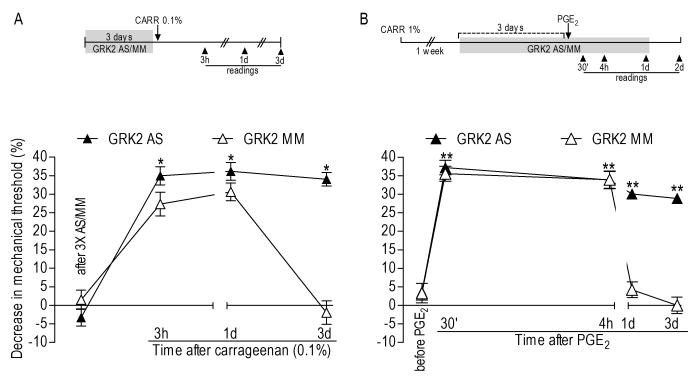
Figure 2.
A Carrageenan hyperalgesia during GRK2 knockdown. Rats were treated with antisense (AS)- or mismatch (MM)-ODN to GRK2 mRNA for 3 consecutive days. On the 4th day carrageenan (0.1%) was injected into the hind paw. Mechanical thresholds in the AS- and MM-treated groups right before carrageenan injection were 112.3 ± 1.5 g and 107.3 ± 2.2 g, respectively. No significant differences were observed on the mechanical threshold in both groups before and after ODN treatments: AS-treated group: p=0.2666; MM-treated group: p= 0.4818, paired Student’s t-test, N=6 per group, data not shown). Two-way repeated measures ANOVA showed a significant group time interaction (F3,30=36.847; p<0.001) and a significant main effect of group (F1,10=17.174; p=0.002). Based on the significant interaction, one-way repeated measures ANOVA with simple contrasts were performed for each of the two groups, showing that, for the MM group, there was a significant main effect of time (F3,15=65.668; p=0.001) but only the 3 h and 1 d time points differed significantly from baseline (p<0.001 in both cases). However, for the AS group, although there was also a significant main effect of time (F3,15=94.568; p<0.001), the 3 d time point also was significantly different from baseline (*p<0.001); B - Prolonged PGE2-induced hyperalgesia in primed rats during GRK2 knockdown. Rats received intradermal injection of carrageenan (1%) in the hind paw. One week later (when the mechanical threshold returned to the baseline), GRK2 AS- or MM-ODN was injected for 3 consecutive days. Test with PGE2 was performed on the 4th day and the AS/MM treatment continued until the MM group thresholds returned to baseline (N=6 per group). Mechanical thresholds on the 3rd day of the AS-/MM-ODN treatment (right before PGE2 injection) were 106.0 ± 1.8 g in the AS- and 108.0 ± 2.8 g in the MM-treated group. ODN treatments did not induce significant changes in the mechanical threshold (AS-treated group: p= 0.1464; MMtreated group: p= 0.9067, paired Student’s t-test, data not shown). Two-way repeated measures ANOVA showed a significant group time interaction (F4,40=82.270; p<0.001) and a significant main effect of group (F1,10=102.059; p<0.001). Based on the significant interaction, one-way repeated measures ANOVA with simple contrasts were performed for each of the two groups, showing that, for the MM group, there was a significant main effect of time (F4,20=55.933; p<0.001) with the 30 min and 4 h time points significantly different from the baseline (p<0.001); for the AS group there was also a significant main effect of time (F4,20=47.785; p<0.001). However, all time points were significantly different from baseline (**p≤0.001).
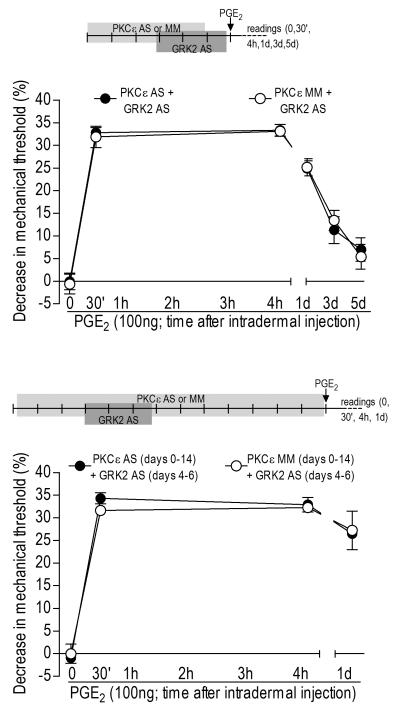
Figure 4. Role of PKCε in prolongation of PGE2 hyperalgesia induced by knockdown of GRK2.
Rats were treated with antisense (AS)- or mismatch (MM)-ODN for PKC? for 6 (upper panel) or 13 (lower panel) days; GRK2 AS-ODN was administered from day 4 to 6 and did not induce significant changes in the mechanical threshold (upper panel: before and after AS: p=0.8771; lower panel: before and after AS: p=0.8771, paired Student’s t-test, data not shown). PGE2 (100 ng, i.d.) was injected into the hind paw on the 7th or 14th day. Mechanical thresholds were evaluated before PGE2 injections and 30 min, 4 h and 24 h later – (upper panel shows the readings until the 5th day) (N=6 per group). Average baseline mechanical thresholds in each group immediately before PGE2 injection were: upper panel - GRK2 AS/PKCε AS: 110.6 ± 2.2 g; GRK2 AS/ PKCε MM: 108.0 ± 2.0 g; lower panel - GRK2 AS/PKCε AS: 111.3 ± 0.9 g; GRK2 AS/ PKCε MM: 101.3 ± 0.6 g. Although the two-way repeated measures ANOVA showed a significant hyperalgesia for both groups (significant main effect of time: upper panel: F5,35=104.906; lower panel: F3,21=273.564; p<0.001 in both cases), there was no effect of the PKCε AS-ODN on the prolongation of PGE2 hyperalgesia, neither during GRK2 knockdown (group time interaction: F5,35=0.220; p=0.857; main effect of group: F1,7=0.006; p=0.941) nor after GRK2 recovery (group time interaction: F3,21=0.737; p=0.495; main effect of group: F1,7=0.061; p=0.812).
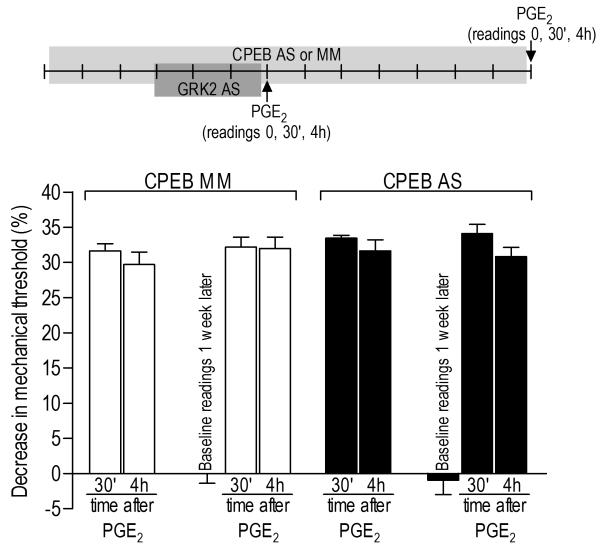
Figure 5. Prolonged hyperalgesia induced by previous knockdown of GRK2 is not dependent on CPEB.
Rats were treated with mismatch (MM, clear bars)- or antisense (AS, black bars)-ODN for CPEB, for 6 or 14 days. In both cases GRK2 AS-ODN was administered from days 4 to 6. No significant changes in the mechanical threshold were observed after GRK2 AS-ODN treatment (7th day: before and after AS: p=0.7227; 14th day: before and after AS: p= 0.6639, paired Student’s t-test, data not shown). PGE2 (100 ng, i.d.) was injected into the hind paw on the 7th or 14th day. Mechanical threshold was evaluated before PGE2 injections and 30 min and 4 h later (N=6 per group). Average mechanical thresholds immediately before PGE2 injection were 111.3 ± 1.9 g (GRK2 AS/CPEB MM, 7th day), 114.0 ± 1.9 g (GRK2 AS/CPEB AS, 7th day), 110.0 ± 1.5 g (GRK2 AS/CPEB MM, 14th day) and 113.0 ± 2.3 4g (GRK2 AS/CPEB AS, 14th day). The two-way repeated measures ANOVA showed a significant main effect of time (F4,40=207.197; p<0.001), indicating an overall significant mechanical hyperalgesia in the two groups, but neither the group time interaction (F4,40=0.636; p=0.566) nor the main effect of group (F1,10=0.562; p=0.471) were significant, indicating no difference between the groups treated with CPEB AS or MM.
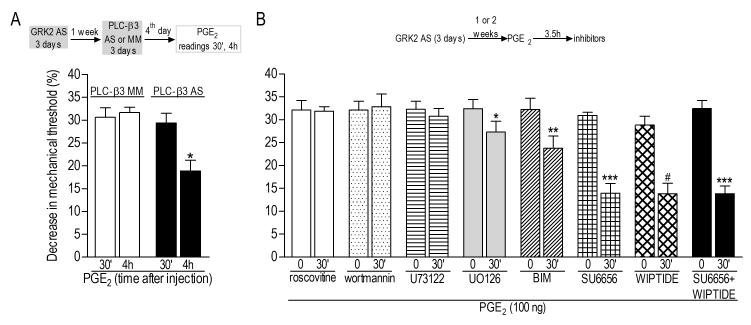
Figure 6. Second messengers involved in the prolongation of PGE2 hyperalgesia induced by previous knockdown of GRK2.
Rats were treated with antisense (AS) for GRK2 for 3 consecutive days. At least one week after AS treatment, PGE2 (100 ng, i.d.) was injected into the hind paw. A. Rats previously (1 week before) treated with GRK2 AS received AS- or MM-ODN for PLC-β3 for 3 consecutive days. Test with PGE2 was performed on the 4th day. Average baseline mechanical thresholds immediately before PGE2 injection were 107.0 ± 2.4 g (GRK2 AS/PLC-β3 MM) and 110.3 ± 2.3 g (GRK2 AS/ PLC-β3 AS). Importantly, treatment with ODNs did not induce significant changes on the baseline thresholds (data not shown, see Section 2.6). Although paired Student’s t-test showed no difference in the PGE2-induced hyperalgesia 30 min and 4 h after injection in the PLC-β3 MM group (p=0.6392, N=6 per group), in the AS group there was significant decrease in the magnitude of mechanical hyperalgesia on the 4th h after the injection (*p=0.0046, N=6 per group); B. Inhibitors of signaling pathways were injected, in different groups of rats, 3.5 h after PGE2, when the mechanical threshold had decreased, in average, 31.6 ± 0.6 % (comparison of the mechanical thresholds before (110.8 ± 0.6 g) and 3.5 h after PGE2 injection (75.7 ± 0.7 g, immediately before inhibitors injection) showed significant hyperalgesia: p<0.0001, paired Student’s t-test, N=48, data not shown). Readings were taken before and after the inhibitors (roscovitine: Cdk5 inhibitor / wortmannin: PI-3K inhibitor / U73122: PLCγ inhibitor / UO126: ERK/MEK inhibitor / BIM: non-selective PKC inhibitor / SU6656: Src inhibitor / WIPTIDE: PKA inhibitor) (N=6 per group; each group of bars represents a different group of rats/inhibitor test). Paired Student’s t-test for each inhibitor showed lack of effect of roscovitine (p=0.8945), wortmannin (p=0.6109) and U73122 (p=0.1852). However, significant inhibition of prolonged PGE2 hyperalgesia was observed after injection of U0126 (*p=0.0074), BIM (**p=0.0026), SU6656 (***p=0.0002), WIPTIDE (#p=0.0010), and SU6656+WIPTIDE (***p=0.0002).
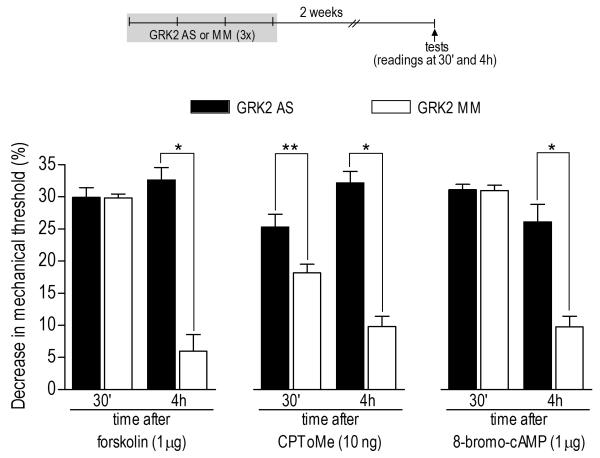
Figure 7. Prolongation of hyperalgesia induced by direct activation of intracellular second messengers by previous knockdown of GRK2.
Rats were treated with antisense (AS, black bars) or mismatch (MM, clear bars) for GRK2 for 3 consecutive days. Two weeks after ODN treatments, activators of adenyl cyclase (forskolin, 1 μg) or Epac (CPToMe, 10 ng), or the cAMP analog 8-bromo-cAMP (1 μg) were injected into the hind paw of different groups of rats. Mechanical thresholds (not significantly affected by AS- and MM-ODN treatment, data not shown), just before the injection of the second messenger activators were: forskolin/GRK2 AS: 112.3 ± 2.0 g; forskolin/GRK2 MM: 115.0 ± 2.2 g; CPToMe/GRK2 AS: 113.3 ± 3.3 g; CPToMe/GRK2 MM: 103.3 ± 2.1 g; 8-bromo-cAMP/GRK2 AS: 115.0 ± 2.2 g; 8-bromo-cAMP/GRK2 MM: 112.0 ±1.6 g. Measurements of nociceptive thresholds were performed 30 min and 4 h after the injections. Comparison between GRK2 AS- and MMODN groups at the 4th h post injections showed prolongation of hyperalgesia in all cases (GRK2 AS/forskolin at 4th h X GRK2 MM/forskolin at 4th h: *p< 0.0001; GRK2 AS/8-bromocAMP at 4th h X GRK2 MM/8-bromo-cAMP at 4th h: *p< 0.0001; GRK2 AS/CPToMe at 4th h X GRK2 MM/ CPToMe at 4th h: *p< 0.0001, unpaired Student’s t-test, N=6 per group). In addition, contrary to forskolin (p=0.9523) and 8-bromo-cAMP (p=0.8912), for CPToMe, unpaired Student’s t-test showed a significant difference, between GRK2 AS and MM groups, in the hyperalgesia 30 min after injection of lower dose of CPToMe (**p=0.0137, N=6 per group), suggesting potentiation of the effect in the GRK2 AS-treated group.
3. Results
3.1. Enhanced inflammatory hyperalgesia during GRK2 knockdown
Decreased GRK2 levels in sensory neurons from GRK+/− mice, ~50%, is associated with increased inflammatory hyperalgesia (Eijkelkamp et al., 2010b, Wang et al., 2011). In this study, we employed AS-ODN to GRK2 mRNA to evaluate the impact of an acute change in its protein expression level in a model of inflammatory hyperalgesia. Western blot analysis was performed to confirm that intrathecal administration of AS-ODN decreased GRK2 expression in the sensory neuron. As shown in the Figure 1A, daily injections of AS-ODN (40 μg/day) for 3 consecutive days produced a 39±9% decrease in GRK2 protein expression in the saphenous nerve (p=0.012, unpaired Student’s t-test, N=6 for both antisense and mismatch treated groups of rats). Of note, the Western blot was performed using the whole nerve tissue, which, in addition to primary afferent nerve fibers, includes many other non-neuronal cells that were not exposed to the intrathecally-administered AS-ODN. Thus, this might cause an underestimation of the effectiveness of the AS-ODN treatment on GRK2 expression in the cells of interest, i.e., the sensory neurons. After 3 injections of AS-ODN, the mechanical hyperalgesia induced by low dose (20 ng) of PGE2 or epinephrine (EPI) was increased both in magnitude and duration: 30 min after PGE2 injection, the MM-ODN group showed ~15% decrease in the mechanical threshold, while in the AS-ODN group this decrease was ~31%; for EPI, the decrease in mechanical threshold was ~19% in the MM group and ~32% in the AS group (Figure 1B, left panels, N=6, p<0.001 in all cases). Four hours after injection of PGE2 or EPI, the mechanical threshold had returned to baseline values in GRK2 MM-ODN treated rats. However, the mechanical threshold in PGE2 - and ~33% in EPI-treated rats (p<0.001 in both cases, when compared to the MM groups). Even after 24 h PGE2 and EPI hyperalgesia were still significant in the AS-ODN groups: ~18% and ~13% decrease in the mechanical threshold for PGE2 and EPI, respectively (p<0.001 in both cases).
The hyperalgesia induced by a higher dose of PGE2 or EPI (100 ng) was also prolonged in the rats treated with GRK2 AS-ODN. When evaluated at the 4th hour after injection, the decrease in the mechanical threshold induced by 100 ng of PGE2 or EPI was ~30% and ~35%, respectively, while in the MM-ODN groups it was no longer significantly different from baseline (Figure 1B, right panels, N=6, p <0.001 in all cases).
We also evaluated the effect of GRK2 knockdown on the mechanical hyperalgesia induced by carrageenan (Figure 2A). Intradermal injection of carrageenan induces a decrease in the mechanical threshold in 30-60 min, reaching a maximum between 2 and 6 h, returning to baseline values within 72 h (Aley et al., 2000). When we injected a low dose of carrageenan (5 μl of a 0.1% solution) into the hind paw of rats treated with GRK2 AS-ODN for 3 days, we observed increased (~34% of decrease in the mechanical threshold in the 3rd h after injection in the AS group, compared to ~27% in the MM group - p<0.001) and prolonged (~34% of decrease in the mechanical threshold 72 h after injection in the AS group; MM group returned to baseline threshold - p=0.001) mechanical hyperalgesia (Figure 2A). The intradermal injection of carrageenan induces hyperalgesic priming (Aley et al., 2000, Parada et al., 2003b); a subsequent injection of PGE2 after recovery from carrageenan hyperalgesia, which in normal tissue induces mechanical hyperalgesia no longer than 4 hours, now induces prolonged hyperalgesia decreasing but still significant 24 h after injection. In primed rats treated with GRK2 AS- or MM-ODN, although the magnitude of PGE2-induced hyperalgesia was similar at 30 min and 4 h in both groups, by 24 h hyperalgesia continued to be present in the AS-ODN, but not in the MM-ODN, group; when the levels of GRK2 were kept attenuated by AS-ODN treatment the significant hyperalgesia remained (decrease in the mechanical threshold: ~30% in the 24th h and 28% in the 48th h - Figure 2B, N=6, p≤0.001 when compared to the MM group).
3.2. Transient knockdown of GRK2 in peripheral neurons produces long–term increased sensitivity to hyperalgesic mediators
To determine if the effect of GRK2 AS-ODN treatment on the PGE2-induced hyperalgesia was reversible, we waited for recovery of GRK2 protein level and evaluated the hyperalgesia induced by intradermal injection of PGE2. However, one week after the last injection of GRK2 AS-ODN, we still observed prolonged hyperalgesia induced by PGE2 (20 ng) or EPI (20 ng) (Figure 3B, left panels) similar to that observed during antisense administration. Although the AS-ODN groups showed magnitude of hyperalgesia similar to the MM-ODN groups in the first 30 min (PGE2: ~20% and ~18% in AS- and MM-ODN groups respectively; EPI: ~24% and ~20% decrease in mechanical threshold in AS- and MM-ODN groups respectively), in the AS groups its intensity actually increased with time, reaching the peak at the 4th h after the injections (~31% for PGE2 and ~32% for EPI), while in the MM groups the mechanical threshold was almost at baseline (N=6, p <0.001 when AS and MM groups were compared).
The hyperalgesia induced by higher doses of PGE2 or EPI (100 ng) was also prolonged in the groups pretreated with AS-ODN. In the 4th h the values were similar (~31% for PGE2 for PGE2 and ~37% for EPI). However, in the MM-ODN groups, at the same time point, the hyperalgesia was almost back to baseline (Figure 3B, right panels; N=6, p<0.001 when AS and MM groups were compared).
To confirm that these changes in nociceptor function were present at a time when GRK2 levels had recovered, we treated animals with GRK2 AS- or MM-ODN for 3 days and harvested the nerves a week later for Western blot analysis. As shown in the figure 3A, the levels of GRK2 protein in the peripheral nerve had already returned to the values not significantly different from that in mismatch-treated animals (Figure 3A, p=0.3309, unpaired Student’s t-test, N=6 each for AS and MM).
3.3. Second messengers involved in the long–lasting changes in nociceptor function induced by GRK2 knockdown
After demonstrating long-term changes in nociceptor function induced by transient attenuation of GRK2 levels, we next investigated the involvement of intracellular second messengers, especially ones known to have a role in hyperalgesic priming, PKCε, PLC-β3 and CPEB (Khasar et al., 1999, Aley et al., 2000, Parada et al., 2003b, Hucho et al., 2005, Joseph et al., 2007, Bogen et al., 2008, Reichling and Levine, 2009, Bogen et al., 2012), in our model of GRKing. We used AS-ODN to decrease the expression of PKCε or CPEB (Parada et al., 2003b, Joseph et al., 2007, Bogen et al., 2012) in sensory neurons, prior to transiently downregulating GRK2 expression and evaluated the PGE2 hyperalgesia either during attenuation or after the recovery of GRK2 protein expression levels. Intrathecal injections of AS- or MM-ODNs for PKCε or CPEB were performed for 3 days before treatment with GRK2 AS-ODN, to avoid the activation of PKCε or CPEB during the transient attenuation of GRK2. From days 4 to 6, animals received both PKCε or CPEB and GRK2 AS-ODN. Control groups received PKCε or CPEB MM-ODN for 6 days and GRK2 AS-ODN from days 4 to 6, until testing on the 7th day with PGE2. As seen in the Figure 4, upper panel, and in Figure 5, p PGE2 hyperalgesia was prolonged in all cases, i.e., neither PKCε (Figure 4, upper panel, N=6, =0.941) nor CPEB (Figure 5, N=6, p=0.471) AS-ODN had effect in the prolonged PGE2 hyperalgesia during the knockdown of GRK2.
Similarly, we tested the possibility that these mediators play a role in the plasticity observed after the recovery of GRK2 to control level. In this case, we treated separate groups of animals with PKCε or CPEB AS- or MM-ODN for 13 days, which prevents hyperalgesic priming. These animals received GRK2 AS-ODN from days 4 to 6, allowing 1 week until injection of PGE2, on the 14th day. PGE2 hyperalgesia was prolonged in all groups (Figure 4, lower panel, N=6, p=0.812, and Figure 5, N=6 for each group, p=0.471), showing that, unlike hyperalgesic priming, PKCε and CPEB do not play a role in GRKing. The role of PLC-β3, also shown to be involved in hyperalgesic priming (Joseph et al., 2007), was also tested in the prolonged PGE2 hyperalgesia induced by previous GRK2 knockdown. In contrast to PKCε and CPEB, treatment with AS-ODN against PLC-β3 for 3 consecutive days produced an attenuation of ~36% in the PGE2-induced hyperalgesia on the 4th h when compared to the MM-ODN group (Figure 6, panel A, N=6, p=0.0007).
Inhibitors of other intracellular second messengers implicated in nociceptor function were also tested in our model, GRKing, up to two weeks after the last treatment with GRK2 ASODN. Inhibitors for Cdk5 (roscovitine), PI-3K (wortmannin), PLCγ (U73122), ERK/MEK (UO126), nonselective PKC (BIM), Src (SU6656) and PKA (WIPTIDE) were injected 3.5 h after PGE2 in the same site. Treatment with UO126, BIM, SU6656 or WIPTIDE partially inhibited the prolonged PGE2 hyperalgesia (UO126: ~15%, p=0.0074; BIM: ~26%, p=0.0026; SU6656: ~55%, p=0.0002; WIPTIDE: ~52%, p=0.0010, of inhibition of PGE2 hyperalgesia in the 4th h). Moreover, there was no difference in the inhibition (compared to each inhibitor alone) when SU6656 and WIPTIDE were combined (~57%, p=0.0002), suggesting that these two mediators may be part of the same pathway involved in GRKing (Figure 6, panel B).
3.4. GRK2 knockdown induces changes in nociceptor function downstream of G-protein-coupled receptors
The plasticity in nociceptor function mediating the enhancement and prolongation of PGE2 hyperalgesia induced by GRK2 knockdown involved changes in the intracellular pathways activated by PGE2. However, considering that we also observed an increase with time in the hyperalgesia induced by low doses of PGE2 or EPI (Figure 3B) we hypothesized that changes downstream of their respective receptors could be responsible by this amplification of the receptor signaling. To investigate this possibility, we used specific agonists to directly activate adenyl cyclase (forskolin), Epac (CPToMe) or cAMP-dependent pathways (by injecting the cAMP analog 8-bromo-cAMP) in animals previously (2 weeks before) treated with GRK2 AS-ODN. The hyperalgesia induced by all three agonists was prolonged when compared to the control group (GRK2 MM-ODN) (Figure 7). Interestingly, the low dose of CPToMe induced hyperalgesia that increased with time, again suggesting that the changes induced by previous GRK2 knockdown may allow the activation of a pathway that would amplify the signaling triggered by activation of a pronociceptive G-protein-coupled receptor.
4. Discussion
It is possible to envision two, not necessarily mutually exclusive, mechanisms for chronic pain. In what we will refer to here as Type I chronic pain, ongoing processes such as inflammation produce continuous sensitization in primary afferent nociceptors. Unique amongst sensory modalities, such sensitization can persist for a long period of time. An example of this would be the patient with previously untreated, longstanding rheumatoid arthritis, whose pain markedly improves when treated with an antagonist of the pronociceptive inflammatory mediator, tumor necrosis alpha (TNFα), and whose pain recurs if for any reason this treatment needs to be discontinued (Feldmann and Maini, 2001, El Bahri et al., 2007). In contrast, nociceptors may undergo neuroplastic changes such that even after resolution of an initiating insult, such as an ongoing inflammatory process, their function remains altered in such a way as to predispose them to enhanced and prolonged sensitization by exposure to even low levels of pronociceptive inflammatory mediators, as might be encountered in the systemic circulation (Dina et al., 2011). An example of the latter condition, which we will refer to here as Type II chronic pain, is the individual with a work-related ergonomic musculoskeletal pain syndrome (e.g., repetitive motion disorders (Ortiz-Hernandez et al., 2003, Schlossberg et al., 2004, Lassen et al., 2005, Grooten et al., 2007, van Tulder et al., 2007)), in which the pain may disappear, for months or years, only to recur after a minor insult, such as attempting to resume the work exposure that had produced the initial acute pain syndrome. Such an ergonomic exposure now produces an intense long-lasting recurrence of pain at the same site (Harris-Hayes et al., 2005, Manchikanti et al., 2009, Bron et al., 2011). We recently developed a model of Type II chronic pain, that we have referred to as hyperalgesic priming, in which after recovery from an initiating insult, such as work-related ergonomic insults (Dina et al., 2010, Alvarez et al., 2012), inflammation (Aley et al., 2000, Parada et al., 2005, Dina et al., 2008, Dina et al., 2009) or psychological stress (Khasar et al., 2005, Dina et al., 2009, Khasar et al., 2009, Green et al., 2011), administration of a low dose of an inflammatory mediator produces enhanced and markedly prolonged hyperalgesia. Of note, following induction of hyperalgesic priming, there is a change in the second messenger signaling pathway that mediates nociceptor sensitization induced by direct-acting inflammatory mediators (i.e., pronociceptive mediators that act at their cognate receptor, on the peripheral terminal of the nociceptor, to induce sensitization) (Aley et al., 2000, Hucho et al., 2005, Khasar et al., 2008). For example, after recovery from an inflammatory insult, the signaling pathway mediating PGE2 hyperalgesia shifts from being dependent on cAMP and PKA to now also being dependent on PLC-β3 and PKCε (Parada et al., 2005, Joseph et al., 2007).
In a more recently developed model, GRK2-related chronic pain, Kavelaars and colleagues have shown that GRK2+/− mice which have an approximately 50% decrease in GRK2 in their nociceptors, similarly demonstrate enhanced and prolonged PGE2 and EPI hyperalgesia (Eijkelkamp et al., 2010b, Wang et al., 2011). Since inflammation produces attenuation of GRK2 in nociceptors (Kleibeuker et al., 2007, Eijkelkamp et al., 2010b), these investigators suggested that enhanced and prolonged PGE2 and EPI hyperalgesia induced by a decrease in GRK2 contributes to the production of chronic inflammatory pain (Eijkelkamp et al., 2010b, Wang et al., 2011). To determine if the enhanced and prolonged PGE2 and EPI hyperalgesia observed in association with a decrease in GRK2 should be classified as Type I or Type II chronic pain, we assessed the hyperalgesia induced by PGE2 and EPI in rats in which GRK2 in nociceptors was attenuated by intrathecal administration of antisense against GRK2 mRNA, after antisense was stopped and GRK2 levels allowed to recover. Regarding the use of antisense ODN injected via the intrathecal route, to produce a decrease in protein expression, Western blot analysis confirmed an effect on peripheral neurons (for GRK2 in this study and for CPEB, PLC-β3 and PKCε in previous works (Parada et al., 2003b, Joseph et al., 2007, Bogen et al., 2012)). While the focus of this study is to show the consequences of a transitory decrease of GRK2 in the sensory neuron on peripherally-induced hyperalgesia, this does not exclude an additional role of central mechanisms. Importantly, the GRK2 AS treatment did not affect baseline nociceptive threshold, only the duration of the hyperalgesia induced by peripheral administration of PGE2, EPI or second messengers in the peripheral terminal of the nociceptor mediating sensitization.
Based on our finding that enhancement and prolongation of PGE2 and EPI hyperalgesia, and the change in second messenger signaling mediating the enhanced and prolonged hyperalgesia, persists after recovery of GRK2 to pre antisense control levels, we suggest that attenuation of GRK2, in the nociceptor, produces Type II rather than Type I chronic pain; that is, the nociceptor has undergone a neuroplastic change that can contribute to chronic pain.
To determine if the second messenger signaling mechanisms underlying the prolonged and enhanced PGE2 and EPI hyperalgesia are the same in primed rats and those experiencing transient attenuation of GRK2 in the nociceptor, we next compared the mechanism of enhanced and prolonged hyperalgesia induced by the same direct-acting pronociceptive mediators in primed rats and in rats during or after attenuation of GRK2. While both interventions produced similarly enhanced and prolonged PGE2 and EPI hyperalgesia, the hyperalgesia induced by prior inflammation was CPEB and PKCε dependent (Aley et al., 2000, Parada et al., 2003b, Bogen et al., 2012), while that induced by transient decrease in GRK2 was CPEB and PKCε independent, rather being inhibited by antagonists of PKA and Src signaling. In addition, second messengers involved in other models of hyperalgesia such as PLCγ, PI-3K and ERK/MEK (involved in the NGF and GDNF-induced hyperalgesia (Malik-Hall et al., 2005, Bogen et al., 2008)), Cdk5 (GDNF (Bogen et al., 2008)), and other types of PKC (Schepelmann et al., 1993, Ahlgren and Levine, 1994, Velazquez et al., 2007) also showed no role in the prolongation of PGE2 hyperalgesia, pointing out a specific signaling pathway activated by PGE2 in this model, which involves both PKA and Src. However, to determine the detailed mechanism downstream the GPCR initiating the prolonged inflammatory pain in GRKing further investigation is needed.
We have also recently reported another form of enhancement and prolongation of inflammatory mediator hyperalgesia, induced by exposure of nociceptors to an opioid followed by induction of opioid withdrawal (Joseph et al., 2010). Repeated intradermal administration of the potent and highly selective mu-opioid agonist, DAMGO, to produce tolerance for its inhibition of PGE2 hyperalgesia, produced PKCε-dependent enhancement and prolongation of PGE2 hyperalgesia thus resembling hyperalgesic priming, rather than the enhanced and prolonged hyperalgesia induced by attenuation of GRK2.
In contrast to our finding that the enhanced and prolonged PGE2 and EPI hyperalgesia induced by decrease in GRK2 is PKCε and ERK/MEK independent, Kavelaars and colleagues found that the enhancement and prolongation of PGE2 hyperalgesia in GRK2+/− mice was completely attenuated by inhibition of PKCε or ERK/MEK second messengers. Several differences between the present and previous studies of GRK2 function in nociceptors may contribute to observed differences in underlying mechanisms of enhanced and prolonged PGE2 and EPI hyperalgesia. First, the studies by Kavelaars and colleagues used GRK2 knockout mice (Kleibeuker et al., 2007, Eijkelkamp et al., 2010a, Eijkelkamp et al., 2010b, Willemen et al., 2010, Wang et al., 2011), which might have compensatory changes in mechanisms underlying relevant nociceptor functions. Second, most of their studies were performed in mice, while the present experiments were performed in the rat. And, third, most of their experiments were done in female animals, while all our studies were done in males, because hyperalgesic priming does not occur in gonad intact female rats (Joseph et al., 2003). In fact, we have previously shown that hyperalgesic priming, initially described in male rats, occurs in ovariectomized but not gonad intact females and that the second messenger signaling for EPI hyperalgesia differs in the male and female rat; while in male rats it is mediated by PKCε, PKA and MEK, in females it is only mediated by MEK (Dina et al., 2001). The impact of these differences in experimental protocols on the role of GRK2 in chronic pain in the female mouse including whether this form of chronic pain should be classified as Type I or Type II, will require studies in which protocols are developed that allow reversal of the attenuation of GRK2, in the mouse. Furthermore, another point that must be emphasized is that while we observed changes in the nociceptor function in a moment where there is no ongoing inflammation and the levels of GRK2 are recovered, the results reported by Kavelaars and colleagues were observed during inflammation (chronic constriction injury or interleukin-1β administration (Kleibeuker et al., 2007) and carrageenan injection (Eijkelkamp et al., 2010b)), or in mice with reduced GRK2 levels (Eijkelkamp et al., 2010b, Wang et al., 2011), a condition that would mimic the effects of ongoing inflammation. Thus, considering the differences between the experimental approaches used by other authors and our protocols, we suggest that those are different conditions with distinct mechanisms involved.
In conclusion, Kavelaars and colleagues have shown, using GRK+/- mice, that attenuation of GRK2 level in nociceptors is associated with enhanced and prolonged inflammatory hyperalgesia. To further elucidate the role of GRK2 in nociceptor function we used antisense to produce a reversible decrease in GRK2. GRK2 AS-ODN also produced enhanced and prolonged PGE2 and EPI hyperalgesia, an effect that persisted after recovery from antisense treatment, indicating that transient attenuation of GRK2 could produce a longlasting neuroplastic change in nociceptor function. While similar in magnitude and duration to a chronic effect induced by transient activation of PKCε, hyperalgesic priming (Aley et al., 2000, Parada et al., 2003b), the enhanced and prolonged hyperalgesia following transient attenuation of GRK2, in the rat, is PKCε-, MEK- and CPEB-independent. Consistent with the observations of Kavelaars and colleagues in GRK2+/- mice, rats treated with GRK2 AS-ODN exhibit enhanced and prolonged hyperalgesia induced by direct activation of adenyl cyclase, Epac, or PKA, suggesting that the changes in the nociceptor underlying neuroplastic changes occur downstream of G-protein-coupled receptors for pronociceptive inflammatory mediators. Because inflammation can produce a decrease in GRK2, such a mechanism could help explain a predilection to develop chronic pain, after resolution of acute inflammation, a major health problem, especially in work-related musculoskeletal diseases.
Acknowledgements
This work was funded by the NIH.
List of abbreviations
- ASODN
antisense oligodeoxynucleotide
- BIM
bisindolylmaleimide
- cAMP
cyclic AMP
- CPEB
cytoplasmic polyadenylation element binding protein
- CPToMe
8-(4-chlorophenylthio)-2′-O-methyladenosine-3′5′-cyclic monophosphate
- EPI
epinephrine
- GPCRs
G-protein-coupled receptors
- GRK2
G-protein-coupled receptor kinase 2
- MMODN
mismatch oligodeoxynucleotide
- PGE2
prostaglandin E2
- PKA
protein kinase A
- PKCε
protein kinase C epsilon
- PLCβ3
phospholipase C-beta 3
- PLCγ
phospholipase C gamma
- S.E.M.
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahlgren SC, Levine JD. Protein kinase C inhibitors decrease hyperalgesia and C-fiber hyperexcitability in the streptozotocin-diabetic rat. J Neurophysiol. 1994;72:684–692. doi: 10.1152/jn.1994.72.2.684. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Exp Neurol. 2012;233:859–865. doi: 10.1016/j.expneurol.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci. 2012;32:2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982;217:252–254. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- Bron C, de Gast A, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. Treatment of myofascial trigger points in patients with chronic shoulder pain: a randomized, controlled trial. BMC Med. 2011;9:8. doi: 10.1186/1741-7015-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987;84:6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. J Pain. 2010;11:369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160:501–507. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011;15:796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Heijnen CJ, Willemen HL, Deumens R, Joosten EA, Kleibeuker W, den Hartog IJ, van Velthoven CT, Nijboer C, Nassar MA, Dorn GW, 2nd, Wood JN, Kavelaars A. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci. 2010a;30:2138–2149. doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci. 2010b;30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bahri DM, Meddeb N, Sellami S. [Rheumatoid arthritis: current status of therapy] Tunis Med. 2007;85:1–8. [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152:2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooten WJ, Mulder M, Josephson M, Alfredsson L, Wiktorin C. The influence of work-related exposures on the prognosis of neck/shoulder pain. Eur Spine J. 2007;16:2083–2091. doi: 10.1007/s00586-007-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Hayes M, Van Dillen LR, Sahrmann SA. Classification, treatment and outcomes of a patient with lumbar extension syndrome. Physiother Theory Pract. 2005;21:181–196. doi: 10.1080/09593980500212987. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105:143–150. doi: 10.1016/s0304-3959(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Reichling DB, Levine JD. Shared mechanisms for opioid tolerance and a transition to chronic pain. J Neurosci. 2010;30:4660–4666. doi: 10.1523/JNEUROSCI.5530-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavelaars A, Eijkelkamp N, Willemen HL, Wang H, Carbajal AG, Heijnen CJ. Microglial GRK2: a novel regulator of transition from acute to chronic pain. Brain Behav Immun. 2011;25:1055–1060. doi: 10.1016/j.bbi.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleibeuker W, Ledeboer A, Eijkelkamp N, Watkins LR, Maier SF, Zijlstra J, Heijnen CJ, Kavelaars A. A role for G protein-coupled receptor kinase 2 in mechanical allodynia. Eur J Neurosci. 2007;25:1696–1704. doi: 10.1111/j.1460-9568.2007.05423.x. [DOI] [PubMed] [Google Scholar]
- Lassen CF, Mikkelsen S, Kryger AI, Andersen JH. Risk factors for persistent elbow, forearm and hand pain among computer workers. Scand J Work Environ Health. 2005;31:122–131. doi: 10.5271/sjweh.859. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician. 2009;12:E35–70. [PubMed] [Google Scholar]
- Ortiz-Hernandez L, Tamez-Gonzalez S, Martinez-Alcantara S, Mendez-Ramirez I. Computer use increases the risk of musculoskeletal disorders among newspaper office workers. Arch Med Res. 2003;34:331–342. doi: 10.1016/S0188-4409(03)00053-5. [DOI] [PubMed] [Google Scholar]
- Papir-Kricheli D, Frey J, Laufer R, Gilon C, Chorev M, Selinger Z, Devor M. Behavioural effects of receptor-specific substance P agonists. Pain. 1987;31:263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–190. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003a;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003b;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Schepelmann K, Messlinger K, Schmidt RF. The effects of phorbol ester on slowly conducting afferents of the cat’s knee joint. Exp Brain Res. 1993;92:391–398. doi: 10.1007/BF00229027. [DOI] [PubMed] [Google Scholar]
- Schlossberg EB, Morrow S, Llosa AE, Mamary E, Dietrich P, Rempel DM. Upper extremity pain and computer use among engineering graduate students. Am J Ind Med. 2004;46:297–303. doi: 10.1002/ajim.20071. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989a;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res. 1989b;487:148–151. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res. 1989;492:397–399. doi: 10.1016/0006-8993(89)90928-1. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- van Tulder M, Malmivaara A, Koes B. Repetitive strain injury. Lancet. 2007;369:1815–1822. doi: 10.1016/S0140-6736(07)60820-4. [DOI] [PubMed] [Google Scholar]
- Velazquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: involvement of multiple isoforms. Pharmacol Res. 2007;55:578–589. doi: 10.1016/j.phrs.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Heijnen CJ, Eijkelkamp N, Carbajal A Garza, Schedlowski M, Kelley KW, Dantzer R, Kavelaars A. GRK2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain. 2011;152:1649–1658. doi: 10.1016/j.pain.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz MC, Bender K, Brandts B, Meyer T, Pott L. Antisense oligonucleotides against receptor kinase GRK2 disrupt target selectivity of beta- adrenergic receptors in atrial myocytes. FEBS Lett. 1999;451:279–283. doi: 10.1016/s0014-5793(99)00594-3. [DOI] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Wang H, Dantzer R, Dorn GW, 2nd, Kelley KW, Heijnen CJ, Kavelaars A. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain. 2010;150:550–560. doi: 10.1016/j.pain.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels. 1997;5:193–199. [PubMed] [Google Scholar]