Abstract
Purpose
The present study examines the effect of normal aging on respiratory support for speech when utterance length is controlled.
Methods
Fifteen women (mean age 71 years) and 10 men (mean age 73 years) produced two sentences of different lengths in four loudness conditions while respiratory kinematics were measured. Measures included those related to lung volume and chest wall movements.
Results
Data from the older adults were compared to data from 30 young adults, previously published. A significant age by sex effect was demonstrated. Older men produced speech at higher lung volumes than younger men. No significant differences existed between older and younger women. Older adults tended to use more abdominal movement in loud speech than younger adults, especially when talking in noise. Some of the mechanisms used by the older adults to support increased loudness in response to the cues differed from those used by the younger adults. Age-related differences were larger when participants produced the longer utterance as compared to the shorter one.
Conclusions
Reduced chest wall compliance, pulmonary elastic recoil, and laryngeal closure may explain the findings. These data can be used to help distinguish normal age-related changes from disease-related changes.
INTRODUCTION
During speech, the respiratory and laryngeal systems work together to provide a relatively steady pressure to drive vocal fold vibration (Draper, Ladefoged, & Whitteridge, 1959; Hixon, Goldman, & Mead, 1973; Hoit & Hixon, 1987; Huber & Stathopoulos, 2003; Lieberman, 1967; Stathopoulos & Sapienza, 1997). Many studies indicate the presence of normal age-related changes to the respiratory system that may impact the development and maintenance of subglottal pressure for speech. The compliance of the chest wall decreases, and compliance of the lungs may increase with age (Frank, Mead, & Ferris, 1957; Mittman, Edelman, Norris, & Shock, 1965). Increased lung compliance would result in a loss of lung elasticity (and lower elastic recoil forces). The loss of lung elasticity may be more pronounced in men than in women, particularly between ages 45 and 58 years (Bode, Dosman, Martin, Ghezzo, & Macklem, 1976). Inspiratory and expiratory muscle strength are reduced in older adults, with a more prominent effect on inspiratory strength in women (Berry, Vitalo, Larson, Patel, & Kim, 1996; Enright, Kronmal, Manolio, Schenker, & Hyatt, 1994). The overall effect of these age-related mechanistic changes to the respiratory system is a reduction in functional reserve (Tolep & Kelsen, 1993).
Further, studies of older adults have demonstrated anatomical changes in the larynx including calcification or ossification of the laryngeal cartilages, changes to the lamina propria of the vocal folds, vocal fold atrophy, and replacement of intrinsic laryngeal muscle fibers with connective tissue (Hirano, Kurita, & Nakashima, 1983; Honjo & Isshiki, 1980; Segre, 1971; Ximenes Filho, Tsuji, do Nascimento, & Sennes, 2003). These changes may lead to reduced laryngeal muscle force (Baker, Ramig, Sapir, Luschei, & Smith, 2001). As with the respiratory changes, the changes in the larynx begin earlier and occur to a greater extent in men than in women (Hirano et al., 1983; Kahane, 1987; Ximenes Filho et al., 2003). Age-related anatomical changes in the larynx are likely to result in reduced glottal closure during vocal fold vibration (Biever & Bless, 1989; Honjo & Isshiki, 1980) and reduced laryngeal airway resistance (Holmes, Leeper, & Nicholson, 1994; Melcon, Hoit, & Hixon, 1989). However it is not clear if this change in laryngeal airway resistance results in increased air wastage during vocal fold vibration (Sapienza & Dutka, 1996).
Anatomic and physiologic changes to the respiratory and laryngeal systems with aging result in 1) the need for larger subglottal pressures due to changes in laryngeal airway resistance and vocal fold closure (Hoit & Hixon, 1987; Hoit, Hixon, Altman, & Morgan, 1989) and 2) greater difficulty producing adequate pressure for speech due to reduced pulmonary recoil and chest wall compliance. Even in the absence of laryngeal changes, age-related reductions in elastic recoil of the lungs (due to increased lung compliance), compliance of the chest wall, and strength of the respiratory muscles are likely to alter how pressure is generated during speech. Recoil pressure from the lung-thorax unit is a main source of pressure for speech, and maintenance of steady pressure during speech requires fine control of the balance between recoil and muscular pressures across a range of lung volumes (Draper et al., 1959; Hixon et al., 1973). The question of how individuals use the respiratory system to produce a relatively steady and adequate pressure during speech production in spite of decreasing mechanical support is an important one. Pressure generation for speech is important for intelligibility and audibility, both of which impact quality of life from the perspective of communication.
Based on previous studies, there is evidence that older adults initiate speech at a higher lung volume, use a greater percent of their lung volume per speech breath and per syllable, and produce fewer syllables per breath than younger adults (Hoit & Hixon, 1987; Hoit et al., 1989; Sperry & Klich, 1992). However, age-related effects may be different in men than in women. Hoit et al. (1989) compared the data from women in their paper to data from men published in the Hoit et al. (1987) paper. Women used significantly fewer cc per syllable than men, but women did not significantly reduce utterance length as men did. These studies were important in demonstrating that aging does affect respiratory function for speech, potentially to different degrees in women and men; however, there are several significant questions that remain unanswered.
One important remaining question is how older adults handle tasks that challenge or load the respiratory system during speech. For example, previous data have shown that older adults, particularly men, produce shorter breath groups than young adults during connected speech tasks (Hoit & Hixon, 1987). Reducing breath group length may be one mechanism older adults use to reduce the load on their respiratory systems and reduce the effects of changes to the respiratory system. Production of shorter breath groups by older adults suggests a need to experimentally control breath group length in order to fully examine the changes to speech breathing with aging. The current study controls for utterance length by using two sentences of fixed length.
Along the same lines, it is not known how older adults handle the increased load on the respiratory system caused by increasing vocal loudness. Increasing loudness requires the production of higher subglottal pressures (Finnegan, Luschei, & Hoffman, 2000; Holmberg, Hillman, & Perkell, 1988; Stathopoulos & Sapienza, 1997). Changing the lung volume at which speech is produced is a primary mechanism for generating the higher pressures required for louder speech (Finnegan et al., 2000; Stathopoulos & Sapienza, 1997). To examine respiratory changes with louder speech, previous studies have asked participants to speak at twice or four times their comfortable loudness or to speak at 5 or 10 dB above comfortable sound pressure level (SPL) (Dromey & Ramig, 1998; Hixon et al., 1973; Huber, Chandrasekaran, & Wolstencroft, 2005; Russell & Stathopoulos, 1988; Stathopoulos & Sapienza, 1997). These studies have demonstrated that young adults and young children tend to increase the lung volume at which speech is initiated and utilize greater abdominal muscle effort to achieve higher pressures and a louder voice (Dromey & Ramig, 1998; Hixon et al., 1973; Huber et al., 2005; Russell & Stathopoulos, 1988; Stathopoulos & Sapienza, 1997).
However, not all studies have demonstrated such clear trends in respiratory function for loud speech. For example, Winkworth and Davis (1997) reported variable respiratory patterns produced by five young women speaking in noise. Speaking in noise is known to elicit the Lombard Effect, in which speakers naturally speak louder under conditions of background noise (Pick, Siegel, Fox, Garber, & Kearney, 1989). The participants in the Winkworth and Davis study increased SPL as much as in previous studies of respiratory kinematics with increased loudness, but the variability in the respiratory patterns was different from findings of previous studies, which reported a consistent pattern of initiating speech at higher lung volumes. Winkworth and Davis hypothesized that the differences between their findings and earlier findings were due to the methods used to elicit louder speech.
Huber et al. (2005) examined the effects of cueing on respiratory kinematics for loud speech in young men and women utilizing the following cues: asking participants to target an SPL 10 dB above comfortable using an SPL meter for feedback (COMF+10), asking participants to speak at twice their comfortable loudness without feedback (2XCOMF), and asking participants to talk with multi-talker noise playing in the background (NOISE). The cues used were designed to elicit approximately equivalent SPL increases, about 10 dB SPL. Winkworth and Davis (1997) demonstrated that at least a 10 dB SPL increase could be expected when speaking in noise. Based on the psychophysical literature, individuals perceive a sound that is 10 dB louder than a referent as “twice as loud” (Stevens, 1955). Previous studies that used a “twice as loud” cue reported approximately a 9-10 dB SPL increase (Dromey & Ramig, 1998; Kleinow, Smith, & Ramig, 2001). Collectively, these data suggested that the cue to speak at “twice comfortable loudness” would elicit an SPL increase of about 10 dB. Based on the expectations of a 10 dB SPL increase in the two other cues, we chose to ask participants to target 10 dB SPL above comfortable loudness (rather than 5 dB as was used by Stathopoulos & Sapienza (1997)) in Huber et al. (2005) and in the current study.
All three cues to increase loudness resulted in similar increases in SPL, about 10 dB SPL. However, the respiratory mechanisms used to support the increase in loudness differed depending on the cue used to elicit louder speech. In the COMF+10 condition, participants increased the lung volume at which speech was initiated to take advantage of higher recoil pressures. In the 2XCOMF condition, participants increased expiratory force to increase the pressure for speech. However in the NOISE condition, participants combined the two approaches, using both increased recoil pressures and increased expiratory force. In NOISE, participants also slowed speech rate and used larger volume excursions even though the number of syllables produced was the same. Huber et al.’s (2005) results demonstrated that the way young adults are cued to increase loudness affects the respiratory mechanisms used to increase subglottal pressure and loudness, and suggest that different perceptions or expectations of the task were elicited by the different cues.
The aims of the current study were to examine the effects of normal aging on respiratory support for speech when utterance length was controlled and to determine if the results of Huber et al. (2005) could be extended to older adults. In order to examine how older adults use their respiratory systems to handle the challenge of increasing loudness and to examine the effects of cueing, the participants spoke loudly in response to the same three cues used in Huber et al. (2005). Based on the results of Huber et al.’s (2005) study, it was expected that the different cues to increase loudness would affect the respiratory mechanisms used to increase subglottal pressure and loudness by older adults. However, the effects of aging cannot be inferred from data on children or younger adults.
It is important to delineate the effects of cueing in older adults for two reasons. First, age-related changes in respiratory function can be compounded by diseases that affect speech and respiration, such as Parkinson’s disease, cerebrovascular accidents, and Chronic Obstructive Pulmonary Disease, many of which are more prevalent in older adults (Vinters, 2001). Without a valid model for normal respiratory support for speech in older adults, distinguishing normal age-related changes from disease-related changes in respiratory function would prove difficult. Second, some speech disorders that occur in older adults, for example Parkinson’s disease, are known to result in reduced loudness levels. These disorders are often treated using multiple cues to elicit increased loudness (Fox, Morrison, Ramig, & Sapir, 2002; Ramig, Countryman, Thompson, & Horii, 1995; Ramig et al., 2001). Since the respiratory system plays a primary role in increasing loudness, an understanding of how cues affect respiratory function in normal older speakers will assist in treatment planning.
The hypotheses were as follows:
Due to reduced lung recoil pressures, older adults will begin to speak at higher lung volumes than younger adults to take advantage of the greater recoil pressure available at higher lung volumes. This difference will be more prominent in older men than older women, due to the potential for larger age-related changes to recoil in men than in women.
The respiratory mechanisms used by older adults under the three different cueing conditions will be similar to those used by younger adults, although the actual volumes used may differ due to age-related changes to recoil and muscle strength. There is no clear reason to expect that older adults will respond to the different cues to increase loudness in a manner distinct from younger adults.
METHODS
Participants
Twenty-five older adults (15 women and 10 men) participated in the current study. The mean age of the women was 71 years (range = 65 years, 11 months to 76 years, 1 month), and the mean age of the men was 73 years (range = 65 years, 10 months to 88 years). All participants had normal speech and language, as determined by a certified speech-language pathologist, and reported no history of voice or respiratory problems (including asthma), neurological disease, or head or neck surgery and that they had been nonsmokers for the past five years. Older adults had “typical” hearing for their cohort as indicated by a hearing screening, conducted in a quiet room, at 40 dB HL for 500, 1000, and 1500 Hz, bilaterally (Ventry & Weinstein, 1983). Participants had normal vital capacity (VC), forced VC, and forced expiratory volume in one second as defined as ≥80% of expected values based on age, sex, height, weight, and ethnicity (VacuMed Discovery Handheld Spirometer).
The data in the current study were collected as part of a larger study, and the older adults were compared to data from 30 young adults, published previously (Huber et al., 2005). The young adult data were included in the present paper as a comparison for the older adults in order to elucidate the effects of aging on respiratory support for speech. Expected differences between the groups were present for the VC data; men had larger VC than women, and the younger adults had larger VC than the older adults.
Procedures and Speech Stimuli
Purdue University’s Committee on the Use of Human Research Subjects approved the procedures for data collection. Participants were seated during data collection. Participants said two sentences: “Buy Bobby a puppy” (short sentence) and “You buy Bobby a puppy now if he wants one” (long sentence). Each sentence was produced fifteen times, one time per breath, in the following four conditions (Huber et al., 2005):
Comfortable (COMF): at comfortable loudness and pitch
Targeting 10 decibels (dB) above comfortable (COMF+10): Targets were set at 10 dB (± 2 dB) above the participant’s comfortable SPL. An SPL meter was used for feedback. The meter’s output was visible to the participant via its projection on a television screen, placed next to the computer screen displaying the sentence to be produced.
At what the participant felt was twice his/her comfortable level (2XCOMF): External feedback about loudness was not provided in this condition.
While multi-talker noise was presented in the free field in the examination room at 70 dBA (NOISE): External feedback about loudness was not provided in this condition.
The short sentence was produced first in each condition. The COMF condition was completed first. The order of the three loud conditions was counterbalanced across participants.
Equipment
The acoustic signal was transduced via a condenser microphone (B&K) connected to an SPL meter (Quest model 1700). Respiratory inductive plethysmography via the Respitrace system (Ambulatory Monitoring, Inc.) was used to transduce respiratory movements. One elastic band was placed around the rib cage (RC) under the axilla (to track RC movement). A second elastic band was placed around the abdomen (AB) with the top of the band at the level of the umbilicus and below the last rib (to track AB movement). Respiratory kinematic data were digitized at 2,000 Hz. A second microphone signal was collected with the respiratory kinematics to be used to determine speech onsets and offsets for measurement of the kinematic waveform.
Measurements
Sound Pressure Level (SPL)
Sound pressure level was measured using Praat (Boersma & Weenink, 2003). The acoustic signal was digitized from DAT tape into a computer at a sampling rate of 44 kHz. The signal was then downsampled to 18 kHz and low pass filtered at 9 kHz for anti-aliasing. The average SPL across each utterance was measured.
Respiratory Kinematic Measurements
The participants performed VC and AB capacity tasks with the Respitrace bands in place to acquire an estimate of the maximal capacity of the lungs, RC, and AB (Hoit & Hixon, 1987). Since lung volume change reflects combined changes in RC and AB volumes (Konno & Mead, 1967), the sum of the RC and AB signals, corrected for the respective RC and AB contributions to lung volume (LV) change, were computed. Rib cage and AB contributions to LV change were determined using two non-speech tasks, rest breathing and “speech-like” breathing. For the “speech-like” breathing, participants were instructed to read the long sentence silently to themselves, one time per breath. A spirometer (VacuMed Universal Ventilation Meter) with no dead space was used to collect LV data during the rest breathing and “speech-like” breathing tasks. The spirometric (SP) data were digitized along with the RC and AB signals at 2000 Hz.
The solution for the correction factors (k1 and k2) in the following formula was determined for each participant using the sets of RC, AB, and SP data points in the two non-speech tasks:
The Moore-Penrose pseudoinverse function was used in Matlab to calculate the solution for the correction factors with the least error. The correction factors were verified by visually checking the calculated sum signal (k1(RC) + k2(AB)) against the original SP signal for several consecutive “speech-like” breathing cycles. Estimated lung volume was then computed for each point during the speech tasks using the formula:
The least squares method has been established as an acceptable method for estimating lung volume change from the RC and AB signals (Chadha et al., 1982). The exact method described here has been used previously (Huber et al., 2005).
Lung volume, RC, and AB initiations were measured at the onset of the acoustic signal, and terminations were measured at the offset of the acoustic signal. The measurer listened to the audio signal corresponding to the segmented portion to verify that the initiations and terminations were accurately selected and that no part of the speech signal was cut-off. Lung volume, RC, and AB initiations and terminations were measured relative to end expiratory level (EEL), which was measured from the troughs of three steady rest breaths prior to the start of each set of sentence repetitions in each condition, and expressed as a percent of lung, RC, and AB capacity (VC, RCC, and ABC, respectively).
Lung volume, RC, and AB excursions were calculated by subtracting the volume at termination from the volume at initiation and were expressed as a percent of VC, RCC, and ABC. Percent of VC expended per syllable was computed by dividing lung volume excursion by the number of syllables per utterance. Duration was measured as the time between speech onset and speech offset of each utterance.
Statistics
The first two sentence productions of each sentence in each condition were not analyzed to avoid onset effects. The next ten productions of each sentence in each condition, produced without error, were included in the analysis. Means were computed for each participant for each condition and each sentence task. The differences in the means were assessed in four-factor repeated measures analyses of variance (ANOVA). The within factors were cue (condition) and sentence (short vs. long) and the between factors were age and sex. Tukey HSD tests were completed for all factors and interactions that resulted in significance based on the ANOVA. Since multiple ANOVAs were completed, a more stringent alpha level of at p < 0.01 was used for the ANOVAs and the Tukey HSD tests. To further ensure that statistical tests reflected significant differences, Cohen’s effect sizes were computed for all significant pairwise comparisons.
Inter-measurer reliability was completed on eight participants (2 male and 2 female randomly chosen from each age group). Independent t-tests were computed between the first and second measurement for each variable. None of the alpha levels neared significance (p < 0.01), ranging from p=0.061 to p=0.900, indicating good inter-measurer reliability.
RESULTS
Means, standard deviations, and statistical summaries for age, sex, and age by sex effects are presented in Table 1, for sentence and sentence by age interactions in Table 2, and for condition and condition by age, condition by sentence, and condition by age by sentence interactions in Table 3. Statistical summaries for all non-significant interaction effects are presented in Table 4. Effect sizes (d) are presented in Table 5. Results are presented relative to the two main hypotheses of the study.
Table 1.
Means, Standard Deviations, and Statistical Summary for Age and Sex.
| Measures | Statistics | Age | Sex | Age X Sex |
|---|---|---|---|---|
| Sound Pressure Level (dB) |
Mean (SD) | OA: 87.2 (9.3) | W: 86.0 (8.7) | OA W: 85.7 (10.6) |
| YA: 86.8 (6.7) | M: 88.1 (6.8) | OA M: 89.5 (6.2) | ||
| YA W: 86.3 (6.4) | ||||
| YA M: 87.2 (7.1) | ||||
| F (p) | 0.24 (0.625) | 1.87 (0.177) | 0.77 (0.385) | |
|
| ||||
| Duration (seconds) |
Mean (SD) | OA: 2.11 (0.84) | W: 2.03 (0.82) | OA W: 2.17 (0.92) |
| YA: 1.80 (0.66) | M: 1.83 (0.68) | OA M: 2.01 (0.70) | ||
| YA W: 1.88 (0.68) | ||||
| YA M: 1.72 (0.64) | ||||
| F (p) | 10.28 (0.002*) | 3.12 (0.083) | <0.01 (0.967) | |
|
| ||||
| Lung Volume Initiation (%VC) |
Mean (SD) | OA: 36.8 (20.0) | W: 33.6 (14.8) | OA W: 33.5 (14.2) |
| YA: 27.2 (15.2) | M: 29.1 (21.3) | OA M: 41.6 (25.7)a | ||
| YA W: 33.6 (15.3) | ||||
| YA M: 20.7 (12.0)a | ||||
| F (p) | 6.90 (0.011) | 0.36 (0.551) | 6.97 (0.011) | |
|
| ||||
| Lung Volume Termination (%VC) |
Mean (SD) | OA: 23.7 (20.7) | W: 22.3 (14.2) | OA W: 19.8 (13.2) |
| YA: 18.5 (15.4) | M: 19.0 (21.9) | OA M: 24.9 (14.8) | ||
| YA W: 29.5 (27.6) | ||||
| YA M: 12.1 (13.1) | ||||
| F (p) | 2.32 (0.134) | 0.15 (0.697) | 7.81 (0.007*) | |
|
| ||||
| Lung Volume | Mean (SD) | OA: 13.1 (7.3) | W: 11.2 (6.5) | OA W: 13.7 (7.4) |
| Excursion | YA: 8.7 (4.9) | M: 10.1 (6.5) | YA W: 8.7 (4.0) | |
| (%VC) | YA M: 8.7 (5.7) | |||
|
| ||||
| F (p) | 15.15 (<0.001*) | 0.53 (0.469) | 0.53 (0.471) | |
|
| ||||
| %VC per Syllable |
Mean (SD) | OA: 1.46 (0.66) | W: 1.25 (0.59) | OA W: 1.53 (0.66) |
| YA: 0.96 (0.43) | M: 1.12 (0.60) | OA M: 1.37 (0.67) | ||
| YA W: 0.97 (0.34) | ||||
| YA M: 0.96 (0.50) | ||||
| F (p) | 15.31 (<0.001*) | 0.47 (0.494) | 0.39 (0.536) | |
|
| ||||
| Rib Cage Initiation (%RCC) |
Mean (SD) | OA: 38.2 (21.4) | W: 33.8 (19.7) | OA W: 33.5 (22.6) |
| YA: 28.5 (15.9) | M: 31.8 (18.6) | OA M: 45.1 (17.2)a | ||
| YA W: 34 (16.4) | ||||
| YA M: 22.9 (13.4)a | ||||
| F (p) | 6.45 (0.014) | <0.01 (0. 958) | 7.02 (0.011) | |
|
| ||||
| Rib Cage Termination (%RCC) |
Mean (SD) | OA: 27.5 (19.6) | W: 23.8 (17.1) | OA W: 22.4 (18.8) |
| YA: 19.9 (14.8) | M: 22.8 (18.1) | OA M: 35.2 (18.5)a | ||
| YA W: 25.2 (15.2) | ||||
| YA M: 14.5 (12.5)a | ||||
| F (p) | 5.61 (0.022) | 0.08 (0.779) | 9.79 (0.003*) | |
|
| ||||
| Rib Cage | Mean (SD) | OA: 10.7 (8.0) | W: 10.0 (6.4) | OA M: 9.9 (8.4) |
| Excursion | YA: 8.6 (4.5) | YA W: 8.8 (4.3) | ||
| (%RCC) | YA M: 8.4 (4.6) | |||
| F (p) | 2.12 (0.152) | 0.38 (0.541) | 0.10 (0.754) | |
|
| ||||
| Abdominal Volume Initiation (%ABC) | Mean (SD) | OA: 10.6 (10.6) | W: 9.1 (12.7) | OA W: 10.3 (12.3) |
| YA: 8.6 (11.2) | M: 10.1 (8.4) | OA M: 11.2 (7.4) | ||
| YA W: 7.8 (13.1) | ||||
| YA M: 9.4 (9.1) | ||||
| F (p) | 0.83 (0.368) | 0.28 (0.600) | 0.03 (0.874) | |
|
| ||||
| Abdominal Volume Termination (%ABC) |
Mean (SD) | OA: 2.8 (12.4) | W: 3.4 (13.7) | OA W: 1.4 (13.8) |
| YA: 4.9 (11.4) | M: 4.7 (9.2) | OA M: 4.9 (9.4) | ||
| YA W: 5.3 (13.4) | ||||
| YA M: 4.5 (9.0) | ||||
| F (p) | 0.45 (0.504) | 0.28 (0.598) | 0.70 (0.408) | |
|
| ||||
| Abdominal Volume Excursion (%ABC) |
Mean (SD) | OA: 7.8 (7.1) | W: 5.7 (7.2) | OA W: 8.9 (7.6) |
| YA: 3.7 (5.6) | M: 5.4 (6.1) | OA M: 6.2 (6.1) YA W: 2.5 (5.1) | ||
| YA M: 4.9 (5.9) | ||||
| F (p) | 8.25 (0.006*) | 0.01 (0.923) | 3.51 (0.067) | |
Degrees of freedom = [1, 51]. SD = standard deviation, F = F-ratio, p = level of significance. Asterisk indicates significance at the p < 0.01 level; for significant interaction effects
aindicates age effect within sex. OA = older adults, YA = younger adults, W = women, M = men, dB=decibels, %VC, %RCC, %ABC = percent of vital, rib cage, and abdominal capacities.
Table 2.
Means, Standard Deviations, and Statistical Summary for Sentence and Age.
| Measures | Statistics | Sentence | Sentence X Age |
|---|---|---|---|
| Sound Pressure Level (dB) |
Mean (SD) | SH: 87.5 (8.1) | OA SH: 87.6 (9.5) |
| LO: 86.4 (7.8) | OA LO: 86.9 (9.1) | ||
| YA SH: 87.5 (6.9) | |||
| YA LO: 86.1 (6.5) | |||
| F (p) | 27.85 (<0.001*) | 1.63 (0.080) | |
|
| |||
| Duration (seconds) |
Mean (SD) | SH: 1.30 (0.24) | OA SH: 1.40 (0.25) |
| LO: 2.58 (0.54) | OA LO: 2.82 (0.59)a | ||
| YA SH: 1.22 (0.21) | |||
| YA LO: 2.38 (0.41)a | |||
| F (p) | 922.21 (<0.001*) | 8.17 (0.006*) | |
|
| |||
| Lung Volume Initiation (%VC) |
Mean (SD) | SH: 30.2 (17.6) | OA SH: 35.4 (18.6) |
| LO: 32.8 (18.6) | OA LO: 38.1 (21.3) | ||
| YA SH: 25.9 (15.6) | |||
| YA LO: 28.4 (14.7) | |||
| F (p) | 5.03 (0.029) | <0.01 (0.990) | |
|
| |||
| Lung Volume Termination (%VC) |
Mean (SD) | SH: 23.0 (17.3) | OA SH: 26.5 (18.5) |
| LO: 18.7 (18.8) | OA LO: 20.8 (22.4) | ||
| YA SH: 20.1 (15.7) | |||
| YA LO: 16.9 (14.9) | |||
| F (p) | 17.68 (<0.001*) | 1.44 (0.24) | |
|
| |||
| Lung Volume Excursion (%VC) |
Mean (SD) | SH: 7.2 (3.8) | OA SH: 8.9 (4.3) |
| LO: 14.1 (6.8) | OA LO: 17.3 (7.3)a | ||
| YA SH: 5.8 (2.6) | |||
| YA LO: 11.5 (5.1)a | |||
| F (p) | 241.75 (<0.001*) | 7.99 (0.007*) | |
|
| |||
| %VC Expended Per Syllable |
Mean (SD) | SH: 1.21 (0.63) | OA SH: 1.49 (0.72) |
| LO: 1.18 (0.57) | OA LO: 1.44 (0.61) | ||
| YA SH: 0.97 (0.43) | |||
| YA LO: 0.95 (0.43) | |||
| F (p) | 1.03 (0.315) | 0.30 (0.585) | |
|
| |||
| Rib Cage Volume Initiation (%RCC) |
Mean (SD) | SH: 31.5 (19.2) | OA SH: 36.6 (21.2) |
| LO: 34.2 (19.2) | OA LO: 39.7 (21.5) | ||
| YA SH: 27.3 (16.1) | |||
| YA LO: 29.7 (15.7) | |||
| F (p) | 7.90 (0.007*) | 0.08 (0.772) | |
|
| |||
| Rib Cage Volume Termination (%RCC) |
Mean (SD) | SH: 24.8 (17.7) | OA SH: 29.1 (19.5) |
| LO: 21.8 (17.3) | OA LO: 25.9 (19.8) | ||
| YA SH: 21.3 (15.4) | |||
| YA LO: 18.4 (14.2) | |||
| F (p) | 11.24 (0.002*) | 0.03 (0.875) | |
|
| |||
| Rib Cage Volume Excursion (%RCC) |
Mean (SD) | SH: 6.9 (4.5) | OA SH: 7.6 (5.7) |
| LO: 12.4 (6.8) | OA LO: 13.8 (8.8) | ||
| YA SH: 6.0 (2.9) | |||
| YA LO: 11.2 (4.2) | |||
| F (p) | 193.87 (<0.001*) | 1.06 (0.309) | |
|
| |||
| Abdominal Volume Initiation (%ABC) |
Mean (SD) | SH: 9.0 (11.2) | OA SH: 10.2 (10.4) |
| LO: 10.1 (10.8) | OA LO: 11.1 (10.9) | ||
| YA SH: 8.1 (11.7) | |||
| YA LO: 9.2 (10.7) | |||
| F (p) | 1.00 (0.321) | 0.14 (0.714) | |
|
| |||
| Abdominal Volume Termination (%ABC) |
Mean (SD) | SH: 5.5 (11.5) | OA SH: 5.2 (11.2) |
| LO: 2.4 (12.0) | OA LO: 0.5 (13.1) | ||
| YA SH: 5.8 (11.9) | |||
| YA LO: 4.0 (10.9) | |||
| F (p) | 16.42 (<0.001*) | 3.39 (0.071) | |
|
| |||
| Abdominal Volume Excursion (%ABC) |
Mean (SD) | SH: 3.5 (4.2) | OA SH: 5.0 (4.1) |
| LO: 7.7 (8.0) | OA LO: 10.6 (8.3) | ||
| YA SH: 2.2 (3.8) | |||
| YA LO: 5.2 (6.7) | |||
| F (p) | 64.37 (<0.001*) | 5.51 (0.023) | |
Degrees of freedom = [1, 51]. SD = standard deviation, F = F-ratio, p = level of significance. Asterisk indicates significance at the p < 0.01 level; for significant interaction effects,
indicates age effect within sentence. OA = older adults, YA = younger adults, SH = short sentence, LO = long sentence, dB = decibels, %VC, %RCC, %ABC = percent of vital, rib cage, and abdominal capacities.
Table 3.
Means, Standard Deviations, and Statistical Summary for Condition, Sentence, and Age.
| Measures | Effect | F (p) | Group | Mean (SD) | |||
|---|---|---|---|---|---|---|---|
| COMF | COMF+10 | 2XCOMF | NOISE | ||||
| Sound Pressure Level (dB) | Condition | 181.04 | None | 79.6 (6.9) | 89.3 (6.5) | 88.8 (6.8) | 90.1 (6.9) |
| (<0.001*) | |||||||
| Condition X | 0.64 (0.591) | OA | 80.2 (9.1) | 89.3 (8.2) | 89.3 (8.0) | 90.0 (8.3) | |
| Age | YA | 79.1 (4.2) | 89.3 (4.7) | 88.4 (5.6) | 90.2 (5.5) | ||
| Condition X | 3.45 (0.018) | Short | 79.8 (7.2) | 90.1 (6.5) | 89.5 (6.8) | 90.7 (6.9) | |
| Sentence | Long | 79.3 (6.6) | 85.6 (6.4) | 88.2 (6.8) | 89.5 (6.8) | ||
| 1.54 (0.206) | OA Short | 80.3 (9.5) | 90.0 (8.3) | 89.6 (8.0) | 90.5 (8.5) | ||
| Condition X | OA Long | 80.1 (8.9) | 88.7 (8.2) | 89.1 (8.1) | 89.5 (8.2) | ||
| Age X Sentence | YA Short | 79.5 (4.6) | 90.2 (4.7) | 89.4 (5.8) | 90.8 (5.4) | ||
| YA Long | 78.7 (3.9) | 88.5 (4.6) | 87.5 (5.4) | 89.6 (5.6) | |||
|
| |||||||
| Duration (sec) | Condition | 8.10 (<0.001*) | None | 1.87 (0.72) | 1.96 (0.81)c | 1.92 (0.74) | 2.00 (0.79)c |
| Condition X | 0.73 (0.534) | OA | 2.02 (0.78) | 2.15 (0.92) | 2.08 (0.80) | 2.20 (0.89) | |
| Age | YA | 1.75 (0.65) | 1.82 (0.68) | 1.79 (0.67) | 1.85 (0.67) | ||
|
| |||||||
| Duration (sec) | Condition X | 0.78 (0.508) | Short | 1.24 (0.19) | 1.31 (0.25) | 1.30 (0.24) | 1.36 (0.28) |
| Sentence | Long | 2.50 (0.44) | 2.62 (0.62) | 2.55 (0.50) | 2.65 (0.59) | ||
| 0.49 (0.692) | OA Short | 1.32 (0.18) | 1.41 (0.25) | 1.39 (0.25) | 1.47 (0.30) | ||
| Condition X | OA Long | 2.71 (0.45) | 2.88 (0.72) | 2.77 (0.50) | 2.93 (0.65) | ||
| Age X Sentence | YA Short | 1.17 (0.17) | 1.23 (0.22) | 1.22 (0.21) | 1.27 (0.23) | ||
| YA Long | 2.34 (0.36) | 2.40 (0.44) | 2.37 (0.42) | 2.42 (0.42) | |||
|
| |||||||
| Lung Volume Initiation (%VC) |
Condition | 8.44 (<0.001*) | None | 27.4 (14.4) | 33.5 (19.6)c | 32.6 (20.1)c | 32.6 (17.5)c |
| Condition X | 1.37 (0.253) | OA | 31.8 (15.6) | 37.4 (21.4) | 39.4 (23.2) | 38.3 (18.5) | |
| Age | YA | 23.7 (12.2) | 30.1 (17.4) | 26.9 (15.1) | 27.9 (15.2) | ||
| Condition X | 2.95 (0.035) | Short | 25.8 (12.2) | 33.0 (19.6) | 30.0 (19.7) | 32.2 (17.4) | |
| Sentence | Long | 29.1 (16.2) | 33.9 (19.7) | 35.2 (20.4) | 33.1 (17.7) | ||
|
| |||||||
| Lung Volume Initiation (%VC) |
3.70 (0.004*) | OA Short | 28.9 (11.1) | 38.7 (20.7)c | 36.3 (21.9)c | 37.8 (18.0)c | |
| Condition X | OA Long | 34.8 (18.9) | 36.2 (22.5) | 42.6 (24.5)c | 38.8 (19.5) | ||
| Age X Sentence | YA Short | 23.2 (12.6) | 28.2 (17.5) | 24.7 (16.3) | 27.5 (15.6) | ||
| YA Long | 24.3 (12.0) | 32.0 (17.3)c | 29.0 (13.7) | 28.3 (14.9) | |||
|
| |||||||
| Lung Volume Termination (%VC) |
Condition | 2.97 (0.034) | None | 18.5 (14.0) | 22.5 (20.5) | 21.7 (20.2) | 20.7 (17.5) |
| Condition X | 1.27 (0.288) | OA | 21.2 (15.6) | 23.6 (23.5) | 26.3 (23.4) | 23.5 (19.6) | |
| Age | YA | 16.1 (12.3) | 21.7 (17.1) | 17.8 (16.2) | 18.3 (15.3) | ||
| Condition X | 3.24 (0.024) | Short | 19.8 (11.7) | 25.6 (19.4) | 22.7 (20.0) | 23.9 (16.7) | |
| Sentence | Long | 17.2 (16.0) | 19.4 (20.6) | 20.7 (20.5) | 17.5 (17.9) | ||
| 1.91 (0.130) | OA Short | 21.6 (10.7) | 29.5 (21.2) | 27.3 (22.3) | 27.5 (17.8) | ||
| Condition X | OA Long | 20.9 (19.6) | 17.7 (24.6) | 25.3 (24.8) | 19.4 (20.9) | ||
| Age X Sentence | YA Short | 18.2 (12.5) | 22.5 (17.5) | 18.8 (17.2) | 20.8 (15) | ||
| YA Long | 14.0 (11.8) | 20.9 (17.0) | 16.8 (15.4) | 15.8 (15) | |||
|
| |||||||
| Lung Volume Excursion (%VC) |
Condition | 16.28 (<0.001*) | None | 9.0 (5.2) | 10.9 (6.6)c | 10.9 (6.6)c | 12.0 (7.1)c |
| Condition X | 2.94 (0.035) | OA | 10.6 (5.7) | 13.9 (7.8) | 13.1 (7.0) | 14.8 (8.0) | |
| Age | YA | 7.6 (4.4) | 8.5 (4.2) | 9.0 (5.8) | 9.6 (5.2) | ||
| Condition X | 2.78 (0.043) | Short | 6.0 (3.0) | 7.3 (3.8) | 7.3 (3.9) | 8.3 (5.1) | |
| Sentence | Long | 11.9 (5.3) | 14.5 (16.9) | 14.5 (6.8) | 15.6 (7.6) | ||
| 1.91 (0.130) | OA Short | 7.3 (3.4) | 9.2 (4.4) | 8.9 (4.4) | 10.2 (4.6) | ||
| Condition X | OA Long | 13.9 (5.6) | 18.5 (7.6) | 17.3 (6.6) | 19.4 (8.2) | ||
| Age X Sentence | YA Short | 4.9 (2.1) | 5.8 (2.2) | 5.9 (2.9) | 6.7 (2.8) | ||
| YA Long | 10.2 (4.5) | 11.2 (3.9) | 12.1 (6.2) | 12.4 (5.5) | |||
|
| |||||||
| %VC Expended per Syllable |
Condition | 16.93 (<0.001*) | None | 1.00 (0.47) | 1.21 (0.60)c | 1.21 (0.61)c | 1.34 (0.66)c |
| Condition X | 2.62 (0.053) | OA | 1.19 (0.52) | 1.54 (0.68) | 1.47 (0.65) | 1.66 (0.72) | |
| Age | YA | 0.84 (0.36) | 0.94 (0.34) | 1.00 (0.50) | 1.08 (0.46) | ||
|
| |||||||
| %VC Expended per Syllable |
Condition X | 0.93 (.430) | Short | 1.00 (0.50) | 1.22 (0.63) | 1.21 (0.66) | 1.39 (0.68) |
| Sentence | Long | 0.99 (0.44) | 1.21 (0.58) | 1.21 (0.57) | 1.30 (0.64) | ||
| 0.42 (0.737) | OA Short | 1.22 (0.57) | 1.53 (0.74) | 1.49 (0.74) | 1.71 (0.76) | ||
| Condition X | OA Long | 1.16 (0.47) | 1.54 (0.63) | 1.44 (0.55) | 1.62 (0.68) | ||
| Age X Sentence | YA Short | 0.82 (0.34) | 0.96 (0.37) | 0.98 (0.48) | 1.12 (0.47) | ||
| YA Long | 0.85 (0.37) | 0.93 (0.32) | 1.01 (0.52) | 1.03 (0.46) | |||
|
| |||||||
| Rib Cage Volume Initiation (%RCC) |
Condition | 13.82 (<0.001*) | None | 27.1 (16.0) | 34.6 (20.6)c | 35.2 (20.6)c | 34.6 (18.4)c |
| Condition X | 1.78 (0.154) | OA | 31.6 (18.3) | 39.1 (23.3) | 42.8 (22.6) | 39.2 (20.0) | |
| Age | YA | 23.4 (12.8) | 30.8 (17.3) | 29.0 (16.5) | 30.7 (16.1) | ||
| Condition X | 4.22 (0.007*) | Short | 24.6 (13.8) | 33.7 (21.4) | 33.1 (20.1) | 34.6 (19.3) | |
| Sentence | Long | 29.6 (17.8) | 35.4 (19.9) | 37.4 (21.0) | 34.6 (17.6) | ||
|
| |||||||
| Rib Cage Volume Initiation (%RCC) |
6.51 (<0.001*) | OA Short | 26.8 (15.1) | 39.8 (24.4)c | 40.0 (21.4)c | 39.8 (21.0)c | |
| Condition X | OA Long | 36.3 (20.2)2x | 38.4 (22.7)2x | 45.6 (23.7) | 38.5 (19.3)2x | ||
| Age X Sentence | YA Short | 22.8 (14.5) | 28.6 (17.3) | 27.4 (17.3) | 30.2 (15.6)c | ||
| YA Long | 23.9 (13.3) | 33.0 (17.3)c | 30.5 (15.8)c | 31.3 (15.6)c | |||
|
| |||||||
| Rib Cage Volume Termination (%RCC) |
Condition | 8.73 (<0.001*) | None | 18.9 (14.4) | 24.6 (19.2)c | 25.7 (19.1)c | 24.1 (16.6)c |
| Condition X | 2.31 (0.078) | OA | 22.1 (16.4) | 27.6 (21.7) | 32.4 (20.9) | 27.9 (18.3) | |
| Age | YA | 16.3 (11.9) | 22.1 (16.6) | 20.1 (15.6) | 21.0 (14.5) | ||
| Condition X | 3.75 (0.012) | Short | 19.1 (12.8) | 26.9 (19.8)c | 26.5 (19.5)c | 26.8 (17.2)c | |
| Sentence | Long | 18.8 (15.9) | 22.3 (18.5) | 24.9 (18.9)c | 21.4 (15.7) | ||
|
| |||||||
| Rib Cage Volume Termination (%RCC) |
5.77 (0.001*) | OA Short | 20.2 (13.9) | 31.8 (22.0)c | 32.8 (21.0)c | 31.4 (18.3)c | |
| Condition X | OA Long | 24.0 (18.7)2x | 23.3 (21.0)2x | 32.0 (21.2) | 24.3 (17.9)2x | ||
| Age X Sentence | YA Short | 18.2 (12.0) | 22.7 (17.1) | 21.2 (16.8) | 23.1 (15.4) | ||
| YA Long | 14.4 (11.7) | 21.6 (16.4)c | 18.9 (14.5) | 18.9 (13.5) | |||
|
| |||||||
| Rib Cage Volume Excursion (%RCC) |
Condition | 8.24 (<0.001*) | None | 8.2 (5.1) | 10.0 (6.6)c | 9.6 (6.6)c | 10.5 (7.0)c |
| Condition X | 1.22 (0.304) | OA | 9.5 (6.1) | 11.6 (8.5) | 10.4 (8.4) | 11.3 (8.9) | |
| Age | YA | 7.0 (3.7) | 8.7 (4.2) | 8.9 (4.6) | 9.8 (4.9) | ||
| Condition X | 1.17 (0.324) | Short | 5.5 (3.2) | 6.9 (4.6) | 6.7 (4.6) | 7.7 (5.0) | |
| Sentence | Long | 10.8 (5.2) | 13.1 (7.0) | 12.5 (7.1) | 13.2 (7.6) | ||
| 0.47 (0.707) | OA Short | 6.6 (4.2) | 8.0 (6.1) | 7.2 (6.2) | 8.4 (6.3) | ||
| Condition X | OA Long | 12.3 (6.5) | 15.1 (9.2) | 13.5 (9.2) | 14.2 (10.2) | ||
| Age X Sentence | YA Short | 4.6 (1.8) | 5.9 (2.6) | 6.2 (2.7) | 7.2 (3.7) | ||
| YA Long | 9.5 (3.4) | 11.4 (3.7) | 11.6 (4.6) | 12.4 (4.6) | |||
|
| |||||||
| Abdominal Volume Initiation (%ABC) |
Condition | 3.01 (0.032) | None | 11.2 (8.4) | 9.3 (12.9) | 7.9 (11.8) | 9.7 (10.2) |
| Condition X | 3.19 (0.025) | OA | 11.5 (7.4) | 8.7 (12.8) | 9.8 (11.8) | 12.5 (9.5) | |
| Age | YA | 10.9 (9.2) | 9.9 (13.0) | 6.3 (11.8) | 7.4 (10.3) | ||
| Condition X | 0.09 (0.964) | Short | 11.0 (8.3) | 8.6 (14.0) | 7.2 (11.2) | 9.2 (10.3) | |
| Sentence | Long | 11.3 (8.6) | 10.1 (11.7) | 8.6 (12.5) | 10.2 (10.2) | ||
| 1.52 (0.213) | OA Short | 11.2 (8.0) | 9.3 (3.1) | 8.6 (11.3) | 11.6 (8.6) | ||
| Condition X | OA Long | 11.9 (7.0) | 8.1 (12.7) | 11.1 (12.4) | 13.4 (10.5) | ||
| Age X Sentence | YA Short | 10.9 (8.7) | 7.9 (14.9) | 6.1 (11.3) | 7.3 (11.4) | ||
| YA Long | 10.9 (9.9) | 11.8 (10.7) | 6.5 (12.4) | 7.5 (9.3) | |||
|
| |||||||
| Abdominal Volume Termination (%ABC) |
Condition | 4.92 (0.003*) | None | 6.4 (8.8) | 3.5 (15.0) | 2.3 (12.1)c | 3.7 (10.5) |
| Condition X | 1.55 (0.205) | OA | 5.8 (7.6) | 0.5 (16.9) | 1.7 (12.3) | 3.3 (10.5) | |
| Age | YA | 6.9 (9.7) | 6.0 (12.9) | 2.7 (12.0) | 4.0 (10.6) | ||
|
| |||||||
| Abdominal Volume Termination (%ABC) |
Condition X | 0.01 (0.998) | Short | 7.9 (8.5) | 5.0 (15.1) | 3.8 (11.7) | 5.3 (9.6) |
| Sentence | Long | 4.8 (8.9) | 2.0 (14.9) | 0.7 (12.4) | 2.0 (11.2) | ||
| 2.27 (0.083) | OA Short | 7.4 (8.0) | 4.4 (14.5) | 3.5 (12.2) | 5.4 (9.0) | ||
| Condition X | OA Long | 4.2 (6.9) | -3.4 (18.4) | -0.1 (12.3) | 1.3 (11.7) | ||
| Age X Sentence | YA Short | 8.4 (9.0) | 5.5 (15.8) | 4.1 (11.4) | 5.3 (10.3) | ||
| YA Long | 5.4 (10.3) | 6.5 (9.2) | 1.3 (12.6) | 2.7 (10.9) | |||
|
| |||||||
| Abdominal Volume Excursion (%ABC) |
Condition | 2.72 (0.046) | None | 4.8 (4.5) | 5.8 (7.2) | 5.7 (7.1) | 6.0 (7.4) |
| Condition X | 4.95 (0.003*) | OA | 5.8 (4.7) | 8.2 (8.1) | 8.1 (7.1) | 9.2 (7.9)c | |
| Age | YA | 4.0 (4.2) | 3.9 (5.7) | 3.6 (6.5) | 3.4 (6.0) | ||
| Condition X | 1.73 (0.163) | Short | 3.1 (2.8) | 3.6 (8.9) | 3.4 (4.2) | 3.9 (5.5) | |
| Sentence | Long | 6.5 (5.3) | 8.1 (8.9) | 7.9 (8.6) | 8.2 (5.6) | ||
|
| |||||||
| Abdominal | 1.65 (0.180) | OA Short | 3.8 (3.0) | 4.9 (3.7) | 5.1 (3.5) | 6.2 (5.6) | |
| Volume | Condition X | OA Long | 7.7 (5.3) | 11.5 (9.9) | 11.2 (8.4) | 12.2 (8.8) | |
| Excursion | Age X Sentence | YA Short | 2.5 (2.5) | 2.5 (3.6) | 2.0 (4.3) | 2.0 (4.6) | |
| (%ABC) | YA Long | 5.4 (5.1) | 5.3 (7.0) | 5.2 (7.9) | 4.9 (6.9) | ||
Degrees of freedom = [3, 153]. SD = standard deviation, F = F-ratio, p = level of significance. Asterisk indicates significance at thep < 0.01 level; for significant main effect and interaction effects of condition
indicates significantly different from the COMF condition within the row of data and
indicates significantly different from the 2XCOMF condition within the row of data. OA = older adults, YA = younger adults, dB = decibels, sec = seconds, %VC, %RCC, %ABC = percent of vital, rib cage, and abdominal capacities.
Table 4.
Statistical Summary for Non-Significant Interaction Effects. Data presented in the following format: F-ratio (level of significance).
| Measures | Sent by Sex |
Sent by Age by Sex |
Cond by Sex |
Cond by Age by Sex |
Cond by Sent by Sex |
Cond by Sent by Age by Sex |
|---|---|---|---|---|---|---|
| df | [1,51] | [1,51] | [3,153] | [3,153] | [3,153] | [3,153] |
| SPL | 1.6 (0.21) | 0.04 (0.84) | 0.4 (0.76) | 0.2 (0.88) | 2.2 (0.21) | 1.0 (0.38) |
| Duration | 5.7 (0.02) | 0.4 (0.51) | 2.1 (0.11) | 0.6 (0.61) | 2.9 (0.04) | 1.5 (0.21) |
| LVI | 0.6 (0.03) | 0.2 (0.66) | 0.4 (0.75) | 2.0 (0.11) | 1.6 (0.19) | 0.9 (0.46) |
| LVT | 0.2 (0.70) | 0.01 (0.99) | 0.03 (0.99) | 2.0 (0.11) | 0.9 (0.41) | 0.8 (0.49) |
| LVE | 0.9 (0.36) | 1.2 (0.27) | 2.6 (0.05) | 0.7 (0.53) | 0.5 (0.71) | 0.5 (0.67) |
| %VC/SYL | 0.3 (0.61) | 0.6 (0.46) | 3.1 (0.03) | 1.0 (0.38) | 1.4 (0.26) | 1.4 (0.24) |
| RCVI | 0.6 (0.45) | 0.01 (0.99) | 1.6 (0.18) | 0.6 (0.64) | 1.2 (0.32) | 0.1 (0.98) |
| RCVT | 0.01 (0.98) | 0.01 (0.92) | 1.8 (0.15) | 1.2 (0.33) | 1.2 (0.32) | 0.36 (0.79) |
| RCVE | 2.9 (0.09) | 0.07 (0.80) | 2.7 (0.05) | 1.6 (0.19) | 1.1 (0.34) | 2.0 (0.12) |
| ABVI | 2.6 (0.12) | 0.91 (0.35) | 1.9 (0.13) | 1.2 (0.30) | 1.2 (0.32) | 1.6 (0.18) |
| ABVT | 1.7 (0.20) | 0.12 (0.73) | 2.3 (0.08) | 1.2 (0.30) | 0.8 (0.52) | 1.6 (0.20) |
| ABVE | 0.2 (0.64) | 4.2 (0.05) | 0.9 (0.44) | 0.4 (0.78) | 0.9 (0.45) | 0.7 (0.57) |
Sent = sentence, Cond = condition, df = degrees of freedom. SPL = sound pressure level, LV = lung volume, RCV = rib cage volume, ABV = abdominal volume, I = initiation, T = termination, E = excursion, %VC/SYL = percent vital capacity expended per syllable.
Table 5.
Summary of Effect Sizes for Significant Pairwise Comparisons.
| Measure | Effect | Comparison | Effect Size |
|---|---|---|---|
| Sound Pressure Level |
Condition | COMF – COMF+10 | 1.45 |
| COMF – 2XCOMF | 1.35 | ||
| COMF – NOISE | 1.53 | ||
| Sentence | Short – Long Sentence | −0.14 | |
|
| |||
| Duration | Age by Sentence | Long Sentence: OA – YA | 0.87 |
| Condition | COMF – COMF+10 | 0.12 | |
| COMF – NOISE | 0.18 | ||
|
| |||
| Lung Volume Initiation |
Age by Sex | Men: OA – YA | 1.09 |
| Condition by Sentence by Age |
OA Short Sentence: COMF – COMF+10 | 0.58 | |
| OA Short Sentence: COMF – 2XCOMF | 0.42 | ||
| OA Short Sentence: COMF – NOISE | 0.58 | ||
| OA Long Sentence: COMF – 2XCOMF | 0.35 | ||
| YA Long Sentence COMF – COMF+10 | 0.51 | ||
|
| |||
| Lung Volume Termination |
Age by Sex | Men: OA – YA | 0.84 |
| Condition by Sentence by Age |
OA Short Sentence: COMF – COMF+10 | 0.46 | |
|
| |||
| Lung Volume Excursion |
Age by Sentence | OA: Short – Long | 1.37 |
| YA: Short – Long | 1.38 | ||
| Long: OA – YA | 0.92 | ||
| Short: OA – YA | 0.88 | ||
| Condition | COMF – COMF+10 | 0.32 | |
| COMF – 2XCOMF | 0.32 | ||
| COMF – NOISE | 0.48 | ||
|
| |||
| Percent of Vital Capacity Expended per Syllable |
Age | OA – YA | 0.90 |
| Condition | COMF – COMF+10 | 0.39 | |
| COMF – 2XCOMF | 0.38 | ||
| COMF – NOISE | 0.59 | ||
|
| |||
| Rib Cage Volume Initiation |
Age by Sex | Men: OA – YA | 1.43 |
| Condition by Sentence by Age |
OA Short: COMF – COMF+10 | 0.63 | |
| OA Short: COMF – 2XCOMF | 0.70 | ||
| OA Short: COMF – NOISE | 0.70 | ||
| YA Short: COMF – NOISE | 0.49 | ||
| OA Long: 2XCOMF – COMF | −0.31 | ||
| OA Long: 2XCOMF – COMF+10 | −0.41 | ||
| Condition by Sentence by Age |
OA Long: 2XCOMF – NOISE | −0.32 | |
| YA Long: COMF – COMF+10 | 0.62 | ||
| YA Long: COMF – 2XCOMF | 0.69 | ||
| YA Long: COMF – NOISE | 0.68 | ||
|
| |||
| Rib Cage Volume Termination |
Age by Sex | Men: OA – YA | 1.33 |
| Condition by Sentence by Age |
OA Short: COMF – COMF+10 | 0.38 | |
| OA Short: COMF – 2XCOMF | 0.30 | ||
| OA Short: COMF – NOISE | 0.49 | ||
| OA Long: 2XCOMF – COMF | −0.41 | ||
| OA Long: 2XCOMF – COMF+10 | −0.40 | ||
| OA Long: 2XCOMF – NOISE | −0.39 | ||
| YA Long: COMF – COMF+10 | 0.50 | ||
|
| |||
| Rib Cage Volume Excursion |
Condition | COMF – COMF+10 | 0.31 |
| COMF – 2XCOMF | 0.24 | ||
| COMF – NOISE | 0.37 | ||
| Sentence | Long – Short | 0.98 | |
|
| |||
| Abdominal Volume Termination |
Condition | COMF – 2XCOMF | −0.39 |
| Sentence | Long – Short | −0.27 | |
|
| |||
| Abdominal Volume Excursion |
Age Condition by Age |
OA -YA | 0.63 |
| OA: COMF – NOISE | 0.52 | ||
| Sentence | Long – Short | 0.66 | |
OA = older adults, YA = younger adults
Hypothesis 1: Age- and Sex-Related Differences
Sound Pressure Level (SPL)
For SPL, the main effects for age and sex and the age by sex interaction effect did not reach statistical significance.
Duration
For duration, there was a significant main effect of age and a significant sentence by age interaction effect. For the interaction effect, the duration of the long sentence for the older adults was significantly longer than the duration of the long sentence for the younger adults, but the difference between the two groups for the short sentence was not statistically significant.
Lung Volume Initiation (LVI), Termination (LVT), and Excursion (LVE)
For LVI, there were nearly significant effects of age and age by sex (see Table 1). For the interaction effect, LVI was significantly higher for older men than younger men [p=0.005], but the difference between older and younger women was not statistically significant [p=1.00]. For LVT, there was a significant age by sex interaction effect. For that interaction, the post-hoc Tukey comparison between the older men and the younger men neared significance [p=0.028], with older men having higher LVT than younger men. The difference between older and younger women was not statistically significant.
For LVE, there were significant main effects of age and sentence and a significant sentence by age interaction effect. Relative to the interaction effect, both the older adults and the younger adults used significantly larger LVE for the long sentence as compared to the short sentence. Older adults used significantly larger LVE for the long sentence than the younger adults. Even though the alpha level for the comparison between the groups for the short sentence did not reach statistical significance [p=0.20], the effect size suggested these two means differed (see Table 5).
Percent Vital Capacity Expended per Syllable (%VC/SYL)
For %VC/SYL, there was a significant age effect. Older adults used significantly larger %VC/SYL than the younger adults.
Rib Cage Volume Initiation (RCVI), Termination (RCVT), and Excursion (RCVE)
For RCVI, there were nearly significant age and age by sex effects (see Table 1). For the interaction effect, RCVI was significantly higher for older men than younger men (p=0.006), but the difference between older and younger women was not statistically significant (p=1.00) (see Figure 1). For RCVT, there was a significant age by sex interaction effect. Mean RCVT was significantly higher for older men than younger men, but the difference between older and younger women was not statistically significant (see Figure 1). The main effects for age and sex and the age by sex interaction effect did not reach statistical significance for RVCE.
Figure 1.
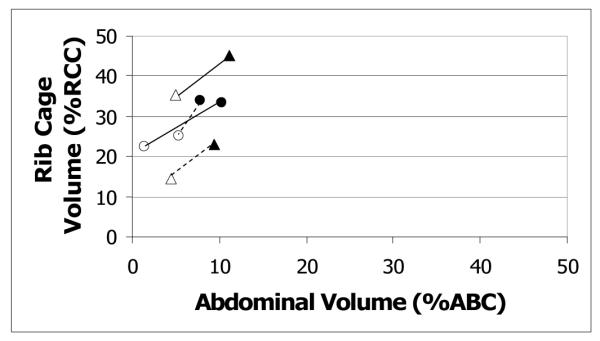
RC by AB Kinematics plot: age by sex interaction. Filled symbols represent initiations, and unfilled symbols represent terminations. Circles represent women; triangles represent men. Initiation and termination points are connected by solid lines for older adults and dotted lines for younger adults. Values are expressed in percent rib cage and abdominal capacity (%RCC, %ABC), relative to end expiratory level.
Abdominal Volume Initiation (ABVI), Termination (ABVT), and Excursion (ABVE)
The main effects for age and sex and the age by sex interaction effect did not reach statistical significance for ABVI or ABVT. For ABVE, there was a significant main effect of age. Older adults used significantly larger ABVE than the younger adults (see Figure 1). There was also a significant age by condition interaction, which is described below under hypothesis 2.
Hypothesis 2: Condition-Related Differences
Sound Pressure Level (SPL)
For SPL, there was a significant main effect of condition. Compared to COMF, the three loud conditions were produced at a significantly higher SPL. The differences among the high SPL conditions were not statistically significant.
Duration
For duration, there was a significant main effect of condition. Compared to COMF, the sentences produced in the COMF+10 and NOISE conditions had significantly longer duration. However, only the effect size for the NOISE comparison approaches a value suggesting a true significant difference in duration (see Table 5). The difference between COMF and 2XCOMF did not reach statistical significance.
Lung and Rib Cage Volume Initiations (LVI and RCVI) and Terminations (LVT and RCVT)
There was a main effect of condition for LVI, RCVI, and RCVT. There was a significant interaction between condition and sentence for RCVI. The interaction between condition and sentence was nearly significant for RCVT. There was a significant interaction among condition, sentence, and age for LVI, LVT, RCVI, and RCVT. A description of the three-way interaction for the four measurements is presented below.
Condition by Sentence by Age Effects
LVI and RCVI for short sentence
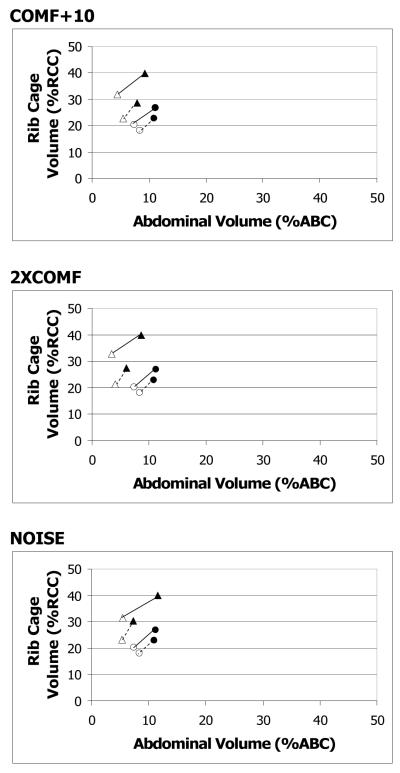
For all three loud conditions, the older adults significantly increased LVI and RCVI as compared to COMF. The differences among the conditions for the younger adults for LVI were not statistically significant; however, RCVI was significantly higher in NOISE as compared to COMF (see Figure 2).
Figure 2.
RC by AB Kinematics plot for short sentence: sentence by age by condition interaction. Filled symbols represent initiations, and unfilled symbols represent terminations. Circles represent the COMF condition. Triangles represent the loud conditions. Initiation and termination points are connected by solid lines for older adults and dotted lines for younger adults. Values are expressed in percent rib cage and abdominal capacity (%RCC, %ABC), relative to end expiratory level.
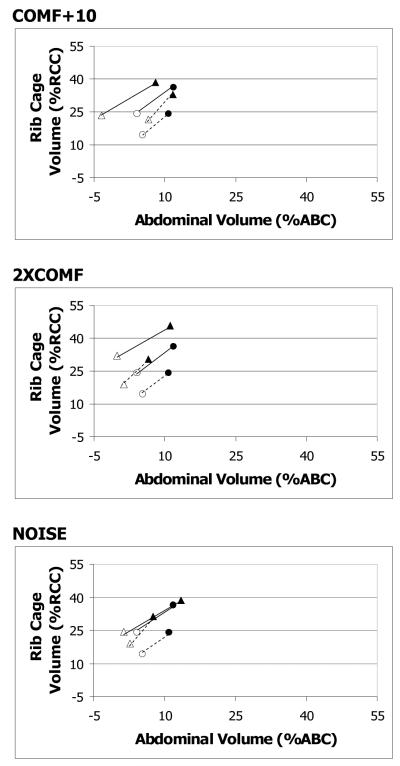
LVI and RCVI for long sentence
Older adults significantly increased LVI for 2XCOMF as compared to COMF. Younger adults significantly increased LVI for COMF+10 as compared to COMF. Older adults significantly increased RCVI for 2XCOMF as compared to all three other conditions (see Figure 3). Younger adults significantly increased RCVI for all three loud conditions as compared to COMF (see Figure 3).
Figure 3.
RC by AB Kinematics plot for long sentence: sentence by age by condition interaction. Filled symbols represent initiations, and unfilled symbols represent terminations. Circles represent the COMF condition. Triangles represent the loud conditions. Initiation and termination points are connected by solid lines for older adults and dotted lines for younger adults. Values are expressed in percent rib cage and abdominal capacity (%RCC, %ABC), relative to end expiratory level.
LVT and RCVT for short sentence
For the older adults, LVT was significantly higher in COMF+10 as compared to COMF. Older adults increased RCVT significantly for all three loud conditions as compared to COMF (see Figure 2). The differences among the conditions for LVT and RCVT for the younger adults were not statistically significant (see Figure 2).
LVT and RCVT for long sentence
The differences among the conditions in LVT for the older adults and the younger adults did not reach statistical significance. Older adults significantly increased RCVT for 2XCOMF as compared to all three other conditions (see Figure 3). Younger adults increased RCVT during COMF+10 as compared to COMF (see Figure 3).
Lung Volume Excursion (LVE)
For LVE, there was a significant main effect of condition. As compared to COMF, LVE was significantly higher in all three loud conditions for both groups.
Rib Cage Volume Excursion (RCVE)
For RCVE, there was a significant main effect of condition. As compared to COMF, RCVE was significantly higher in all three loud conditions for both groups (see Figures 2 and 3).
Percent Vital Capacity Expended per Syllable (%VC/SYL)
For %VC/SYL, there was a significant main effect of condition. As compared to COMF, significantly larger %VC/SYL was used in all three loud conditions for both groups.
Abdominal Initiation (ABVI), Termination (ABVT), and Excursion (ABVE)
The main effect of condition and the condition-related interaction effects did not reach statistical significance for ABVI. For ABVT, there was a significant main effect of condition. Mean ABVT was significantly lower in 2XCOMF than in COMF for both groups (see Figures 2 and 3). For ABVE, there was a significant condition by age interaction effect. For the older adults, ABVE was significantly higher in NOISE than in COMF, but the differences between COMF and the other two loud conditions were not statistically significant (see Figures 2 and 3). For the younger adults, the differences in ABVE across the conditions did not reach statistical significance (see Figures 2 and 3).
Sentence Length Effects
There were significant main effects of sentence length for SPL, RCVE, ABVT, and ABVE that did not interact with age, sex, or condition and, thus, did not relate to either of the two main hypotheses of the study. Mean SPL was significantly higher for the long sentence than for the short sentence, but the difference between the sentences was very small, about 1 dB, and the effect size was small, suggesting the effect was not truly significant (see Table 5). Significantly larger RCVE and ABVE were used for the long sentence than the short one. The long sentence ended at significantly lower ABVT than the short one.
DISCUSSION
The first hypothesis of the study was that, due to reduced lung recoil pressures, older adults will begin to speak at higher lung volumes than younger adults to take advantage of the greater recoil pressure available at higher lung volumes. This difference was hypothesized to be more prominent in older men than older women, due to the potential for larger age-related changes to lung recoil in men than in women. The results of the study supported this hypothesis for the most part.
Age-related changes were more pronounced in men. Older men initiated and terminated speech at higher lung and rib cage volumes than younger men, but this finding was not present in the women’s data. It is possible that the sex difference in these effects is related to differences in the way men and women age. Anatomic studies have demonstrated greater changes in the respiratory and laryngeal systems in men than in women as they get older (Bode et al., 1976; Hirano et al., 1983; Kahane, 1983, 1987; Ximenes Filho et al., 2003), and these differences have been shown to result in larger functional effects in men (Hoit & Hixon, 1987, 1992; Hoit et al., 1989; Melcon et al., 1989; Ramig & Ringel, 1983). The result of these differences in aging may be that age-related changes to the respiratory and laryngeal systems in women are not significant enough to result in a difference in respiratory support for speech, but are for men.
There were some age-related respiratory changes which were present for both older men and women. Older adults used greater respiratory volumes per breath group than the younger adults, as has been found previously (Hoit & Hixon, 1987; Hoit et al., 1989; Sperry & Klich, 1992); however, this effect was only significant for the long sentence. Increased volume expenditure may be due to reduced glottal closure in the older adults (Biever & Bless, 1989; Hoit & Hixon, 1987; Honjo & Isshiki, 1980). Reduced glottal closure leads to reduced laryngeal resistance and typically allows more air to flow through the glottis during the closed phase of vocal fold vibration, increasing the amount of lung volume expended for speech (Hoit & Hixon, 1987; Hoit et al., 1989). The finding that the older adults expended a greater percent of vital capacity than the younger adults supports this interpretation. However, since laryngeal function was not examined in the current study, this interpretation can only be inferred.
Alternatively, the duration of the utterances may explain the increased volume expenditures for the older adults. The duration of the long sentence was longer for the older adults than the younger adults. Speaking over a longer duration could also result in larger volume expenditure if flow is constant. This explanation is supported by the fact that the effect of duration was only significant in the long sentence, similar to the effects on volume excursions. Sperry and Klich (1992) reported a similar finding in their study in which older participants paused more frequently and for longer duration, particularly as sentence length increased. Pausing was not measured in this study, but may explain the longer duration of the long sentence for older adults as compared to the younger adults.
Even though both men and women expended a greater volume, only men significantly increased volume initiations and terminations. In compensating for the greater volume expenditures, older men may have needed to increase lung and rib cage volumes more than older women did. Older men may experience greater increases in lung compliance, and therefore lower recoil pressures, than older women. Respiratory recoil pressures are higher at higher lung volumes (Rahn, Otis, Chadwick, & Fenn, 1946), so speaking at a higher lung volume would allow older men to take advantage of higher recoil pressure to support speech and produce adequate subglottal pressures, even with reduced laryngeal airway resistance.
The second hypothesis was that older adults would use different kinematic mechanisms depending on how they were cued to increase loudness and that they would use respiratory kinematic mechanisms similar to those used by young adults to increase loudness under the three different cueing conditions, although the actual volumes used might differ due to age-related changes to recoil and muscle strength. The data from the current study partially supported this hypothesis. The older adults did demonstrate different kinematic patterns depending on how they were cued to increase loudness, as was found previously for the younger adults (Huber et al., 2005). Some of the measurements indicated that the older adults used the respiratory system to increase loudness in a similar manner to the younger adults under the different cueing conditions, although the actual volumes used by the two groups differed, as discussed above.
Both the older adults and the younger adults increased LVE and RCVE and expended more %VC/SYL in the three loud conditions. This finding was not unexpected. Since louder speech requires greater subglottal pressures (Baker et al., 2001; Holmberg et al., 1988; Stathopoulos & Sapienza, 1997), a larger lung volume would be likely to be expended to generate greater pressures.
Both groups slowed speech rate in the COMF+10 and NOISE conditions, as demonstrated in the duration data. In the NOISE condition, this reduced speech rate may be employed to improve the intelligibility of the message in noise (Huber et al., 2005; Summers, Pisoni, Bernacki, Pedlow, & Stokes, 1988). It is unclear why there was a significant effect for the COMF+10 condition. This effect was not present for the younger adults alone (Huber et al., 2005), and the effect size for this comparison in the current study was very small (See Table 5).
Both groups also used a lower ABVT during the 2XCOMF condition. A lower ABVT in the 2XCOMF condition indicates that the abdominal cavity was more compressed at the termination of speech in this cueing condition. These data suggest that one of the mechanisms used by both groups in 2XCOMF to maintain pressure for speech was to generate expiratory muscle pressure.
However, surprisingly, some of the patterns used in response to the three cues were not the same for the younger and older adults. Since there were no differences in SPL across the three loud conditions or between the two age groups, differences in kinematic patterns across the loud conditions or groups can be attributed to cueing, rather than to a difference in overall SPL.
For the short sentence, older adults increased LVI, RCVI, and RCVT in all three loud conditions (see Figure 2). In contrast, the younger adults only increased RCVI and only in NOISE (see Figure 2). Older adults may have needed to increase initiations to generate enough pressure for loud speech due to age-related reductions in the amount of recoil pressure available, whereas the younger adults may have been able to generate enough pressure for the short sentence without increasing the volume at speech initiation.
Differences between the two groups in responses to the cues are best demonstrated in the data from the long sentence, possibly because the long sentence may have been more taxing to the speakers than the short sentence. Older adults increased LVI, RCVI, and RCVT in 2XCOMF when producing the long sentence (see Figure 3). The higher initiations in the 2XCOMF condition suggest that older adults prepared in advance for the increased pressure required in the 2XCOMF condition and inspired to a higher lung volume before beginning each utterance. Respiratory kinematics differed most from COMF in 2XCOMF as compared to the other two loud conditions for older adults. Younger adults significantly increased LVI and RCVT in COMF+10 (see Figure 3), but not in the other two loud conditions. For younger adults, the largest effect on respiratory kinematics was in COMF+10. These results suggest that while the younger adults prepared in advance to generate higher pressures in COMF+10, the older adults prepared in advance most for 2XCOMF.
Older adults used a significantly larger ABVE in NOISE than in COMF. Abdominal volume excursion for the older adults increased in COMF+10 and 2XCOMF as well (see Figures 2 and 3), but not significantly. There was little change in ABVE across the conditions for the younger adults (see Figures 2 and 3). Older adults, overall, moved the abdomen more than the younger adults did. Higher ABVI and lower ABVT for all three loud conditions resulted in the increase in excursions (see Figures 2 and 3).
The differences in use of the abdomen, especially in loud speech, may relate to age-related changes in elastic recoil. Since elastic recoil is reduced with aging, the older adults would need to use a muscular mechanism to help displace air volumes from the lungs during speech and produce adequate subglottal pressure for loud speech. From the abdominal data, it appears that the older adults used greater expiratory muscle pressure to increase pressure for loud speech, especially in NOISE, than the younger adults. Younger adults may not need to utilize muscular mechanisms as much as the older adults when increasing loudness since they have more passive recoil force available.
The requirement for more abdominal muscle effort could lead to greater fatigue or a reduced ability to maintain louder speech for longer periods of time in the older adults. This is functionally important. In daily life, individuals almost never speak in quiet environments. Noise from a multitude of sources is often in the background during conversation. The noise used in the current study, multi-talker babbling, was much like the background noise present during everyday conversations. The finding that NOISE caused the greatest amount of change in abdominal kinematics suggests that speech production may be more work for the older adults than for the younger adults.
The data from the current study are comparable to data from previous studies when comparing within task (single sentence). Dromey and Ramig (1998) used this type of a task; however, their data are not reported relative to EEL so can not be directly compared to the data from the present study. If we assume that EEL is at about 35% VC, then we can subtract 35% from the data reported by Dromey and Ramig to estimate their values relative to EEL. Their means would then be LVI = 27.1 %VC and LVT = 18.8 %VC at comfortable loudness. Means for males and females are not presented separately and only young adults were studied in the Dromey and Ramig study. Our young adult data are comparable to these means (LVI = 23 %VC and LVT = 16 %VC). The type of task may affect the kinematic patterns used, particularly when a task requires longer utterances than those used in the current study. In the present study, mean LVI for young men in the COMF condition was 17-19 %VC. The initiations are close to the means reported by Hoit and Hixon (1987) for young men (7.5-11 %VC). The terminations for this paper are not comparable, most likely due to the fact that the speech tasks used by Hoit and Hixon were connected speech tasks and the utterance lengths were longer than 12 syllables, according to their tables approximately 16-21 syllables. Data from young adults collected in our laboratory using the same collection and analysis procedures but during connected speech tasks (Huber, 2007) are comparable to previous studies (Hodge & Rochet, 1989; Hoit & Hixon, 1987; Hoit et al., 1989; Winkworth, Davis, Ellis, & Adams, 1994).
In summary, normal age-related changes to the respiratory system affect respiratory function for speech, more so for men than women. Differences in respiratory kinematics between age groups were more prominent in the longer utterance. Older adults used different respiratory mechanisms in response to the three cues to increase loudness, as has been shown in younger adults (Huber et al., 2005). Some of the mechanisms used by the older adults to support increased loudness in response to the cues differed from those used by the younger adults. Respiratory kinematics in the 2XCOMF condition were most different from COMF for the older adults, whereas the kinematics in the COMF+10 condition were most different from COMF for the younger adults. Older adults tended to use more abdominal movement (and potentially more abdominal effort) to provide higher subglottal pressure for loud speech than in the younger adults, especially when talking in noise. Possible explanations for changes to respiratory support for speech with aging include reduced chest wall compliance, elastic recoil of the lungs, and laryngeal closure.
These results have clinical application. Clinicians need to be aware that normal age-related changes to the respiratory system do result in functional changes for speech production. Respiratory function in older patients should be compared with what is expected for normal older adults, thereby distinguishing normal age-related changes to respiratory function from those due to disease processes. Because repeated sentence production is not a natural speech task, replication of these results with more natural speech tasks, controlling for utterance length, would enhance their external validity and practical applicability. The data from the different cues to increase loudness suggest that several cues should be considered when providing therapy to individuals with low vocal loudness, and the cue(s) that elicit(s) the most normal respiratory patterns for each client should be utilized.
Grants and Acknowledgements
This research was funded by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders, grant # 1R03DC05731, and a Research Support Incentive Grant from the Center on Aging and the Life Course at Purdue University.
REFERENCES
- Baker KK, Ramig LO, Sapir S, Luschei ES, Smith ME. Control of vocal loudness in young and old adults. Journal of Speech, Language, and Hearing Research. 2001;44:297–305. doi: 10.1044/1092-4388(2001/024). [DOI] [PubMed] [Google Scholar]
- Berry JK, Vitalo CA, Larson JL, Patel M, Kim MJ. Respiratory muscle strength in older adults. Nursing Research. 1996;45(3):154–159. doi: 10.1097/00006199-199605000-00006. [DOI] [PubMed] [Google Scholar]
- Biever DM, Bless DM. Vibratory characteristics of the vocal folds in young adult and geriatric women. Journal of Voice. 1989;3(2):120–131. [Google Scholar]
- Bode FR, Dosman J, Martin RR, Ghezzo H, Macklem PT. Age and sex differences in lung elasticity, and in closing capacity in nonsmokers. Journal of Applied Physiology. 1976;41(2):129–135. doi: 10.1152/jappl.1976.41.2.129. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat (Version 4.1) Institute of Phonetic Sciences; Amsterdam: 2003. [Google Scholar]
- Chadha TS, Watson H, Birch S, Jenouri GA, Schneider AW, Cohn MA, et al. Validation of respiratory inductive plethsmography using different calibration procedures. Am. Rev. Respir. Dis. 1982;125:644–649. doi: 10.1164/arrd.1982.125.6.644. [DOI] [PubMed] [Google Scholar]
- Draper MH, Ladefoged P, Whitteridge D. Respiratory muscle in speech. Journal of Speech and Hearing Research. 1959;2(1):16–27. doi: 10.1044/jshr.0201.16. [DOI] [PubMed] [Google Scholar]
- Dromey C, Ramig LO. Intentional changes in sound pressure level and rate: Their impact on measures of respiration, phonation, and articulation. Journal of Speech, Language, and Hearing Research. 1998;41:1003–1018. doi: 10.1044/jslhr.4105.1003. [DOI] [PubMed] [Google Scholar]
- Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE. Respiratory muscle strength in the elderly. American Journal of Respiratory and Critical Care Medicine. 1994;149:430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- Finnegan EM, Luschei ES, Hoffman HT. Modulations in respiratory and laryngeal activity associated with changes in vocal intensity during speech. Journal of Speech, Language, and Hearing Research. 2000;43:934–950. doi: 10.1044/jslhr.4304.934. [DOI] [PubMed] [Google Scholar]
- Fox CM, Morrison CE, Ramig LO, Sapir S. Current perspectives on the Lee Silverman Voice Treatment Program (LSVT®) for individuals with idiopathic Parkinson’s disease. American Journal of Speech-Language Pathology. 2002;11:111–123. [Google Scholar]
- Frank NR, Mead J, Ferris BG., Jr. The mechanical behavior of the lungs in healthy elderly persons. The Journal of Clinical Investigation. 1957;36:1680–1687. doi: 10.1172/JCI103569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Kurita S, Nakashima T. Growth, development, and aging of human vocal folds. In: Bless DM, Abbs JH, editors. Vocal Fold Physiology: Contemporary Research and Clinical Issues. College-Holl Press; San Diego, CA: 1983. pp. 22–43. [Google Scholar]
- Hixon TJ, Goldman MD, Mead J. Kinematics of the chest wall during speech production: Volume displacements of the rib cage, abdomen, and lung. Journal of Speech and Hearing Research. 1973;16:78–115. doi: 10.1044/jshr.1601.78. [DOI] [PubMed] [Google Scholar]
- Hodge MM, Rochet AP. Characteristics of speech breathing in young women. Journal of Speech and Hearing Research. 1989;32:466–480. doi: 10.1044/jshr.3203.466. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ. Age and speech breathing. Journal of Speech and Hearing Research. 1987;30:351–366. doi: 10.1044/jshr.3003.351. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ. Age and laryngeal airway resistance during vowel production in women. Journal of Speech and Hearing Research. 1992;35:309–313. doi: 10.1044/jshr.3502.309. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ, Altman ME, Morgan WJ. Speech breathing in women. Journal of Speech and Hearing Research. 1989;32:353–365. doi: 10.1044/jshr.3202.353. [DOI] [PubMed] [Google Scholar]
- Holmberg EB, Hillman RE, Perkell JS. Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. The Journal of the Acoustical Society of America. 1988;84(2):511–529. doi: 10.1121/1.396829. [DOI] [PubMed] [Google Scholar]
- Holmes LC, Leeper HA, Nicholson IR. Laryngeal airway resistance of older men and women as a function of vocal sound pressure level. Journal of Speech and Hearing Research. 1994;37:789–799. doi: 10.1044/jshr.3704.789. [DOI] [PubMed] [Google Scholar]
- Honjo I, Isshiki N. Laryngoscopic and voice characteristics of aged persons. Archives of Otolaryngology. 1980;106:149–150. doi: 10.1001/archotol.1980.00790270013003. [DOI] [PubMed] [Google Scholar]
- Huber JE. Effects of cues to increase sound pressure level on respiratory kinematic patterns during connected speech. Journal of Speech, Language, and Hearing Research. 2007;50:621–634. doi: 10.1044/1092-4388(2007/044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Chandrasekaran B, Wolstencroft JJ. Changes to respiratory mechanisms during speech as a result of different cues to increase loudness. Journal of Applied Physiology. 2005;98:2177–2184. doi: 10.1152/japplphysiol.01239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Stathopoulos ET. Respiratory and laryngeal responses to an oral air pressure bleed during speech. Journal of Speech, Language, and Hearing Research. 2003;46:1207–1220. doi: 10.1044/1092-4388(2003/094). [DOI] [PubMed] [Google Scholar]
- Kahane JC. Postnatal development and aging of the human larynx. Seminars in Speech and Language. 1983;4:189–201. [Google Scholar]
- Kahane JC. Connective tissue changes in the larynx and their effects on voice. Journal of Voice. 1987;1(1):27–30. [Google Scholar]
- Kleinow J, Smith A, Ramig LO. Speech motor stability in IPD: Effects of rate and loudness manipulations. Journal of Speech, Language, and Hearing Research. 2001;44:1041–1051. doi: 10.1044/1092-4388(2001/082). [DOI] [PubMed] [Google Scholar]
- Konno K, Mead J. Measurement of the separate volume changes of the rib cage and the abdomen during breathing. Journal of Applied Physiology. 1967;22(3):407–422. doi: 10.1152/jappl.1967.22.3.407. [DOI] [PubMed] [Google Scholar]
- Lieberman P. Intonation, Perception, and Language. The M.I.T. Press; Cambridge, MA: 1967. [Google Scholar]
- Melcon MC, Hoit JD, Hixon TJ. Age and laryngeal airway resistance during vowel production. Journal of Speech and Hearing Disorders. 1989;54:282–286. doi: 10.1044/jshd.5402.282. [DOI] [PubMed] [Google Scholar]
- Mittman C, Edelman NH, Norris AH, Shock NW. Relationship between chest wall and pulmonary compliance with age. Journal of Applied Physiology. 1965;20:1211–1216. [Google Scholar]
- Pick HL, Jr., Siegel GM, Fox PW, Garber SR, Kearney JK. Inhibiting the Lombard effect. The Journal of the Acoustical Society of America. 1989;85(2):894–900. doi: 10.1121/1.397561. [DOI] [PubMed] [Google Scholar]
- Rahn H, Otis AB, Chadwick LE, Fenn WO. The pressure-volume diagram of the thorax and lung. American Journal of Physiology. 1946;146(6):161–178. doi: 10.1152/ajplegacy.1946.146.2.161. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Countryman S, Thompson LL, Horii Y. Comparison of two forms of intensive speech treatment for Parkinson Disease. Journal of Speech and Hearing Research. 1995;38:1232–1251. doi: 10.1044/jshr.3806.1232. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Ringel RL. Effects of physiological aging on selected acoustic characteristics of voice. Journal of Speech and Hearing Research. 1983;26:22–30. doi: 10.1044/jshr.2601.22. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, et al. Intensive voice treatment (LSVT®) for patients with Parkinson’s disease: a 2 year follow up. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell NK, Stathopoulos ET. Lung volume changes in children and adults during speech production. Journal of Speech and Hearing Research. 1988;31:146–155. doi: 10.1044/jshr.3102.146. [DOI] [PubMed] [Google Scholar]
- Sapienza CM, Dutka J. Glottal airflow characteristics of women’s voice production along an aging continuum. Journal of Speech and Hearing Research. 1996;39:322–328. doi: 10.1044/jshr.3902.322. [DOI] [PubMed] [Google Scholar]
- Segre R. Senescence of the voice. EENT Monthly. 1971;50:223–227. [PubMed] [Google Scholar]
- Sperry EE, Klich RJ. Speech breathing in senescent and younger women during oral reading. Journal of Speech and Hearing Research. 1992;35:1246–1255. doi: 10.1044/jshr.3506.1246. [DOI] [PubMed] [Google Scholar]
- Stathopoulos ET, Sapienza CM. Developmental changes in laryngeal and respiratory function with variations in sound pressure level. Journal of Speech, Language, and Hearing Research. 1997;40:595–614. doi: 10.1044/jslhr.4003.595. [DOI] [PubMed] [Google Scholar]
- Stevens SS. The measurement of loudness. The Journal of the Acoustical Society of America. 1955;27:815–829. [Google Scholar]
- Summers WV, Pisoni DB, Bernacki RH, Pedlow RI, Stokes MA. Effects of noise on speech production: Acoustic and perceptual analyses. The Journal of the Acoustical Society of America. 1988;84(3):917–928. doi: 10.1121/1.396660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolep K, Kelsen SG. Effect of aging on respiratory skeletal muscles. Clinics in Chest Medicine. 1993;14(3):363–378. [PubMed] [Google Scholar]
- Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25:37–42. [PubMed] [Google Scholar]
- Vinters HV. Aging and the human nervous system. In: Birren JE, Warner Schaie K, editors. Handbook of the Psychology of Aging. 5th ed. Academic Press; San Diego, CA: 2001. pp. 135–160. [Google Scholar]
- Winkworth AL, Davis PJ, Ellis E, Adams RD. Variability and consistency in speech breathing during reading: Lung volumes, speech intensity, and linguistic factors. Journal of Speech and Hearing Research. 1994;37:535–556. doi: 10.1044/jshr.3703.535. [DOI] [PubMed] [Google Scholar]
- Ximenes Filho JA, Tsuji DH, do Nascimento PH, Sennes LU. Histologic changes in human vocal folds correlated with aging: A histomorphometric study. Annals of Otology, Rhinology, and Laryngology. 2003;112:894–898. doi: 10.1177/000348940311201012. [DOI] [PubMed] [Google Scholar]