Abstract
Purpose
This study examined the response of the respiratory system to three cues used to elicit increased vocal loudness to determine whether the effects of cueing, shown previously in sentence tasks, were present in connected speech tasks and to describe differences among tasks.
Methods
Fifteen young men and 15 young women produced a two-paragraph reading passage in response to four different loudness cues: comfortable loudness level, targeting 10 dB above comfortable, at what they perceived as twice their comfortable loudness, and with multi-talker noise present in the background. A short monologue was produced at comfortable loudness level and with noise in the background.
Results
Differences in respiratory strategies were demonstrated for the different cueing conditions, similar to patterns observed in sentence productions. The kinematic patterns were similar for reading and monologue; however, utterances were longer and speaking rate was slower in the monologue task.
Conclusions
The findings extend the results from sentences to connected speech and provide support for the hypothesis that “intention” or goals play a role in the control of respiratory function during speech. Respiratory kinematics were similar across tasks, when the same cue was used, except for differences related to breath group length and speech rate.
INTRODUCTION
Studying the responses of the respiratory system to changes in vocal loudness is of interest to speech scientists because changing loudness can be viewed as a natural perturbation to the speech system. It is well accepted that speakers vary vocal intensity and subglottal pressure within and across utterances (c.f. (Ladefoged, 1967; Winkworth, Davis, Ellis, & Adams, 1994). Studies that utilize changes in loudness as an experimental condition extend our current speculations about the neural control of the speech system (Tasko & McClean, 2004). Further, because many therapeutic strategies with adults with neuromotor control diseases incorporate changes to loudness, studies incorporating loudness changes provide information related to the use of cues to increase loudness with individuals with speech difficulties. The current study examined the response of the respiratory system to three cues used to elicit increased vocal loudness during reading and monologue.
Several studies have examined respiratory characteristics of speech production during connected speech tasks, including reading, conversation, monologue, and extemporaneous speech, at comfortable loudness levels (c.f., Hixon, Goldman, & Mead, 1973; Hogde & Rochet, 1989; Hoit & Hixon, 1987; Hoit, Hixon, Altman, & Morgan, 1989; Solomon & Hixon, 1993; Winkworth, Davis, Adams, & Ellis, 1995; Winkworth et al., 1994). In these studies, the connected speech tasks resulted in similar respiratory kinematics for the most part. Speech was produced in the mid-lung volume range, initiated above end expiratory level (EEL), and terminated at or above EEL. For longer utterances, speech was sometimes terminated farther below EEL than for shorter utterances. The results were similar for the rib cage and abdominal measurements, although abdominal initiations and terminations may be lower in reading than in conversation (Solomon & Hixon, 1993).
Previous studies of respiratory kinematics with increased loudness have primarily used syllable train tasks and sentence tasks (Dromey & Ramig, 1998; Huber, Chandrasekaran, & Wolstencroft, 2005; Stathopoulos & Sapienza, 1997). Stathopoulos and Sapienza (1997) demonstrated that subjects, aged four years to young adult, increased the volume at which speech was initiated when asked to speak 5 dB above comfortable loudness. Dromey and Ramig (1998) found subjects initiated speech at a higher lung volume when asked to speak at four times their comfortable loudness level. One of the only studies to examine changes to respiratory kinematics with increased loudness during connected speech was by Hixon and colleagues (1973) who reported that lung volume initiations and excursions were higher in loud reading, as compared to comfortable reading.
Increasing loudness often involves increased subglottal pressure (Holmberg, Hillman, & Perkell, 1988; Isshiki, 1964; Stathopoulos & Sapienza, 1997). At least one study has found that the alteration of respiratory pressures is the primary mechanism speakers use to increase subglottal pressure for louder speech (Finnegan, Luschei, & Hoffman, 2000). Increasing volume initiations has been suggested to be an efficient mechanism for increasing loudness. Passive recoil pressure is higher at higher lung volumes (Rahn, Otis, Chadwick, & Fenn, 1946); therefore, less muscular pressure needs to be applied to generate higher subglottal pressures for louder speech at those volumes.
However, the finding that individuals increase the volume at which they start to speak when increasing loudness has not been universally reported. Dromey and Ramig (1998) did not find significant changes in lung volume initiation when asking subjects to speak twice as loudly as comfortable, even though asking them to speak at four times comfortable loudness did result in higher speech initiations. Winkworth and Davis (1997) used the Lombard Effect as a cue to elicit louder speech in reading and monologue and found considerable variability in respiratory kinematics. The Lombard Effect is a phenomenon in which individuals automatically increase vocal loudness when background noise is present (Lane & Tranel, 1971; Pick, Siegel, Fox, Garber, & Kearney, 1989). The participants increased loudness 10-16 dB SPL due to the Lombard effect (Winkworth & Davis, 1997). On average, higher lung volume initiations and lower terminations were seen for both speech tasks when the individuals were speaking in noise. However, examination of the trial-by-trial data did not demonstrate linear increases in lung volume initiation with increased sound pressure level (SPL). Winkworth and Davis (1997) hypothesized that the amount of variability demonstrated in their study was related to the way the loud speech was elicited, which did not focus the speaker’s attention on the task of increasing loudness.
Central to Winkworth and Davis’s (1997) interpretations was the question of cueing. Cues are prompts designed to assist an individual in obtaining a desired output. They can vary in a number of ways, including the degree of cognitive, emotional, and physical demands they place on the individual. In previous studies of respiratory kinematic changes with increased loudness, several cues have been used to elicit the increased loudness. Asking an individual to target a specific SPL using an SPL meter, as was used by Stathopoulos and Sapienza (1997), provides both an external goal and external feedback and may add some cognitive load to the speech task because the individual must monitor SPL meter while speaking. When an individual is asked to speak at two or four times comfortable loudness, as in Dromey and Ramig’s (1998) study, there is no explicit external feedback about loudness; however, auditory feedback is present. When an individual speaks in background noise, as used by Winkworth and Davis (1997), the change in loudness is likely to be automatic and not under much volitional control. The noise cue is the most natural of the three cues presented here because speaking in noise is a common occurrence in everyday life. However, no explicit feedback about loudness is present, and auditory feedback is degraded to some extent due to the noise in the environment.
To extend the findings from Winkworth and Davis (1997) and further examine the role of cueing in respiratory control, Huber and colleagues (2005) examined the effects of three cues to increase loudness on respiratory kinematics in young adults producing two sentences. Both sentences were relatively short, 6 syllables and 12 syllables. That study established that speakers use different respiratory mechanisms to increase loudness depending on how they are cued. When speakers were asked to target a SPL about 10 dB above their comfortable SPL, they primarily increased lung and rib cage volume initiations and terminations, thereby utilizing higher recoil pressures to generate more subglottal pressure. When asked to speak at twice their perceived comfortable loudness, speakers did not change lung or rib cage initiations or terminations, but instead, used more expiratory muscle tension to increase subglottal pressure as demonstrated by lower abdominal volume initiations and terminations. When speaking in noise, participants used a combination of the aforementioned strategies, increasing lung and rib cage initiations and terminations and decreasing abdominal initiations and terminations, but to a lesser extent than under the other two cues. The trial-to-trial variability in respiratory kinematic data reported by Huber and colleagues (2005) in the noise condition was much lower than that reported by Winkworth and Davis (1997); however, the speech task was different. It may be that production of sentences did not elicit the variability that may be seen with a connected speech task when speaking in noise.
The data from the Huber and colleagues (2005) study, that the type of cue used to elicit an increase in loudness is associated with different respiratory kinematic patterns, also provides evidence that respiratory function for speech is finely controlled and in tune with the perceived demands of the speech task. Huber and colleagues (2005) attributed the differences in respiratory mechanisms used to support louder speech among the three cueing conditions to differences in internal targets elicited by the cues. Changes to putative internal targets have been inferred from limb studies showing changes in movement patterns utilized to achieve a task. For example, in a study by Gentilucci, Benuzzi, Bertolani, Daprati, & Gangitano (2000), reaching and grasping movements were altered as a result of the label on the target object. Objects were labeled “large” or “small,” regardless of the actual size of the object. Kinematic patterns for reaching and grasping large and small objects labeled “small” were similar. The same was true for large and small objects labeled “large.” Therefore, the label on the object had a greater effect on the movement pattern than the actual size of the object, suggesting a change in how the individuals perceived the task or the goal of the task as a result of the label. One possible interpretation of the data is that an internal representation of the goal of a movement is reflected in the kinematics associated with achieving that goal. Extending these findings to connected speech would suggest that the results of the study by Huber et al. (2005) reflect more global mechanisms in the movement control system - whether movement representations are specifically involved – and would provide support for the hypothesis that respiratory kinematics for speech are affected by the speaker’s goal.
There were two purposes of the current study. One was to examine how respiratory kinematics changed with increased loudness during a connected speech task. Specifically it was of interest whether the effect of cueing on respiratory kinematics was a function of the sentence task used by Huber and colleagues (2005) or if it was present in connected speech tasks. It was hypothesized that similar respiratory mechanisms would be demonstrated during connected speech under each of the cueing conditions as were demonstrated during the sentence tasks in Huber et al. (2005). That finding would be consistent with the view that cueing effects are relatively global in speech respiration. The finding would also prepare a platform for future studies that will attempt to identify the specific mechanisms by which cues modulate kinematic pattering in speech breathing.
Second, most of what is known about respiratory function for loud speech is based on the production of syllable trains and sentences. Therefore, the second purpose of the current study was to examine whether the type of task (reading and monologue) affected respiratory kinematics both at comfortable loudness and with background noise present. Based on previous research, it was expected that there would be few differences in task performance at comfortable intensity level. It was hypothesized that the respiratory kinematic patterns for loud speech would be similar across the reading and monologue tasks, within each cueing condition. However, it was hypothesized that if differences in utterance length existed between the two tasks, these differences would result in lower lung volume terminations and larger lung volume excursions in the task with longer breath groups.
METHODS
Participants
Thirty normal young adults, 15 women and 15 men, participated in this study. mean age of the women was 22 years, 4 months, and the mean age of the men was 22 years, 10 months. Participants reported no history of voice problems, neurological disease, head or neck surgery, formal speaking or singing training; no recent colds or infections, and they had been non-smoking for the past five years. They had a body-mass index (BMI) between 19 and 30, normal speech, language, and voice, and General North American dialect of English as judged by the PI, a certified speech-language pathologist. Participants passed a hearing screening at 30 dB HL for octave frequencies between 250 and 8000 Hz, bilaterally, completed in a quiet room. Participants had normal vital capacity (VC), forced VC, and forced expiratory volume in one second. Normal was defined as equal to or better than 80% of expected values based on age, sex, height, weight, and ethnicity as determined by algorithms running on a digital spirometer (VacuMed Discovery Handheld Spirometer) that were drawn from several sets of published normative data for spirometry.
Equipment
The acoustic signal was transduced via a condenser microphone that was connected to an SPL meter (Quest model 1700). The microphone was placed 6 inches from the participant’s mouth, at a 45 degree angle. The microphone signal was recorded to digital audiotape (DAT) and later digitized into a PC-computer using Praat (Boersma & Weenink, 2003). The signal was digitized at 44.1 kHz and resampled at 18 kHz. The resampling process applied a low-pass filter at 9000 Hz for anti-aliasing.
Respiratory kinematic data were transduced via respiratory inductive plethesmography using the Respitrace system (Ambulatory Monitoring, Inc.). The first elastic band was placed around the rib cage, just under the axilla to transduce movements of the rib cage. A second elastic band was placed around the abdomen at the level of the umbilicus, ensuring that it was below the last rib, to transduce movements of the abdomen. Respiratory kinematic data were digitized at 2000 Hz. Data from a second microphone was collected with these data so that an acoustic record would be digitized in combination with the respiratory kinematic data.
Procedures and Speech Tasks
Participants read a two-paragraph passage containing eleven sentences (Sapienza & Stathopoulos, 1995). The passage is presented in Appendix I. The reading passage was projected onto a computer screen. Each participant read the passage two times under each of the following conditions.
COMF: Participants were instructed to read the passage at their comfortable pitch and loudness.
COMF+10: Participants were instructed to read the passage while maintaining a specific SPL, using an SPL meter for feedback. The readout from the SPL meter was projected onto a television screen that was placed next to the computer screen displaying the reading passage. The targeted level was set at 10 dB above the participant’s comfortable SPL (+/- 2 dB).
2XCOMF: Participants were instructed to read the paragraph at what they felt was “twice their comfortable loudness.”
NOISE: Participants were instructed to read the paragraph while noise was played in the background. The noise consisted of multi-talker babbling noise (AUDiTEC of St. Louis), played at 70 dBA relative to the participants’ ears. The speakers were placed in front of the participants, approximately 39 inches away.
These three cues to increase loudness have been used in previous studies of respiratory kinematic patterns with increased SPL. The conditions were devised to elicit about the same amount of SPL increase. Winkworth and Davis (1997) found that their subjects increased SPL by at least 10 dB when multi-talker noise at 70 dBA was played while they were speaking. Psychophysical literature has demonstrated that individuals perceive a sound that is 10 dB louder than a referent as “twice as loud” (Stevens, 1955). Therefore, it was hypothesized that the cue to speak at “twice comfortable loudness” would elicit an SPL increase of 10 dB. The target for the SPL meter condition was set at 10 dB above comfortable based on the expectation that the other two cues would elicit approximately a 10 dB increase in SPL.
Participants also produced a monologue for approximately 1 minute, 30 seconds in the COMF and NOISE conditions. To ensure that the participants did not become fatigued during the experimental session, the monologue was not completed in the COMF+10 and 2XCOMF conditions. The examiners responded to the participant as necessary to maintain the flow of the monologue. For example, the examiners nodded their head and made eye contact with the participant. If the participant paused for more than a few seconds, the examiners asked a question such as “Can you tell me more about what happened?”, but that was rarely necessary. The role of the examiner was not large enough to truly call the interaction a conversational exchange.
The COMF condition was always completed before the loud conditions. The order of the three loud conditions for the reading task (COMF+10, 2XCOMF, and NOISE) was counterbalanced across participants. The monologue task was completed after the reading task.
Calibration of the Respiratory Kinematic Signals
Because lung volume change reflects the combined effect of changes in RC and AB volumes (Konno & Mead, 1967), the sum of the RC and AB signals, corrected for the respective RC and AB contributions to lung volume (LV) change, was computed. Two non-speech tasks, rest breathing and “speech-like” breathing, were used to determine the RC and AB contributions to LV change. Data was collected for two 45-second periods of rest breathing and three 45-second periods of “speech-like” breathing. For the “speech-like” breathing, participants were instructed to read the sentence “You buy Bobby a puppy now if he wants one” silently, one time per breath. Lung volume data was collected during rest breathing and “speech-like” breathing using a spirometer (VacuMed Universal Ventilation Meter). The spirometer used has a very small dead space, and therefore, does not cause a change to respiratory patterns due to a build-up of carbon dioxide. The spirometric (SP) data were digitized along with the RC and AB signals at 2000 Hz.
Signals from the SP, RC, and AB collected during rest breathing and “speech-like” breathing were used to determine the correction factors for the RC and AB. The solution for the correction factors (k1 and k2) in the following formula was determined using each set of RC, AB, and SP data points in the two breathing tasks: SP = k1(RC) + k2(AB).
The Moore-Penrose pseudoinverse function was used in Matlab to calculate the solution for the correction factors with the least error.
The correction factors were verified by visually checking the calculated sum signal (k1(RC) + k2(AB)) against the original SP signal for several consecutive “speech-like” breathing cycles. An estimated lung volume signal was then computed for each point during the reading and monologue tasks using the formula: Estimated Lung Volume (LV) = k1(RC) + k2(AB).
Participants also produced maximum capacity tasks (Hoit & Hixon, 1987). A minimum of three vital capacity tasks (in addition to the ones used for subject inclusion) were completed with the bands in place. The largest of these was used to determine lung capacity (VC). A minimum of three maximum abdomen (AB) in and maximum AB-out maneuvers were completed at EEL (Hoit & Hixon, 1987). The largest AB-in coupled with the largest AB-out maneuver were used to determine AB capacity. Measurements during speech production were expressed as a percent of capacity so that comparisons could be made across individuals of differing sizes.
Measurements
Sound Pressure Level
Average SPL was measured using Praat (Boersma & Weenink, 2003) across each sentence of the reading passage and each breath group in the monologue.
Timing Measurements
Number of syllables (#SYL) was computed for each breath group. This measurement provided an indication of utterance length.
Syllables per second (SYL/SEC) was computed by dividing #SYL by the duration for each breath group. Duration was measured as the time of speech onset to the time of speech offset that were determined while measuring the initiations and terminations in Matlab. This measure reflected speech rate.
Respiratory Kinematic Measurements
Respiratory kinematic measurements were made using algorithms written in Matlab (Mathworks). Before any measurements were made, the respiratory kinematic signals were low-pass filtered at 40 Hz to remove noise. The microphone signal was displayed with the kinematic signals for measurement.
Lung volume initiations (LVI) and abdominal volume initiations (ABVI) were defined by the point where voicing began for each utterance, as indicated by the microphone signal.
Lung volume terminations (LVT) and abdominal volume terminations (ABVT) were defined by the point where voicing ended for each utterance, as indicated by the microphone signal.
Lung volume excursions (LVE) and abdominal volume excursions (ABVE) were calculated as the volume at initiation minus the volume at termination.
Once initiation and termination points were chosen, the acoustic signal was played back to ensure no portion of an utterance was cut-off in choosing the initiations and terminations. Initiations and terminations from the LV and AB signals were measured relative to EEL. EEL was measured from troughs of three steady rest breaths prior to the start of each trial. Initiations, terminations, and excursions were expressed as a percent of VC and ABC, respectively.
Rib cage volume measurements were not included because the results were similar to those in the lung volume measurements. This finding was expected due to the close correlation between lung volume and rib cage volume. Abdominal measurements were included to provide information regarding abdominal volume and to infer abdominal muscle activity during speech.
Statistics
For each task (reading and monologue), means were computed for each participant for each condition (COMF, COMF+10, 2XCOMF, and NOISE). To address purpose 1, changes across the conditions, the differences in the means were assessed in two-factor repeated measures analyses of variance (ANOVA), separately for the tasks, reading and monologue. The ANOVAs examining condition effects for the two tasks were done separately because the COMF+10 and 2XCOMF conditions were not completed in the monologue task. The factors were condition (cue) and sex. Sex was included as a factor in the statistical analyses because differences in respiratory kinematics have been reported in studies using syllable trains and sentences (Huber et al., 2005; Huber & Stathopoulos, 2003; Stathopoulos & Sapienza, 1997) and studies of normal respiratory physiology have demonstrated that women have lower static recoil pressures than men (Bode, Dosman, Martin, Ghezzo, & Macklem, 1976). Tukey HSD tests were completed for all factors and interactions that were significant in the ANOVA. The alpha level for the ANOVAs and the Tukey HSD tests was set at p < 0.003, based on an alpha adjustment for the 18 ANOVAs completed (0.05/18 = 0.003).
To statistically examine differences between the two tasks (reading and monologue), the means from COMF and NOISE for both tasks were assessed in a one-factor repeated measures analysis of variance (ANOVA). The factor was task. Tukey HSD tests were completed for all factors and interactions that were significant in the ANOVA. The alpha level for the ANOVAs and the Tukey HSD tests was set at p < 0.006, based on an alpha adjustment for the 9 ANOVAs completed (0.05/9 = 0.006).
Inter-measurer reliability was completed on 2 male and 2 female participants, who were randomly chosen for remeasurement. Reading (504 utterances, 14.8% of utterances) and monologue (220 utterances, 15.8%) were remeasured. Independent t-tests (degrees of freedom = 1434) were completed on the two sets of measurements. Mean differences (MD) and alpha levels from the t-tests between the two sets of measurements were as follows, SPL: MD = 0.10 dB, p = 0.76, # SYL: MD = 0.17 syllables, p = 0.64; SYL/SEC: MD = 0.03 syllables/second, p = 0.56; LVI: MD = 0.04 %VC, p = 0.931; LVT: MD = −0.03 %VC, p = 0.94; LVE: MD = 0.07 %VC, p = 0.83; ABVI: MD = −0.04 %ABC, p = 0.91; ABVT: MD = 0.02 %ABC, p = 0.95; ABVE: MD = −0.06 %ABC, p = 0.80. The small mean differences and the lack of significant differences between the two sets of measurements indicate good inter-rater reliability.
RESULTS
The statistical summary for the reading task is in Table 1, and the summary for the monologue task is in Table 2. The statistical summary for the task effect is presented in Table 3.
Table 1.
Statistical Summary for Effects of Condition in the Reading Task.
| Measures | Condition (3, 84) |
Sex (1, 28) |
Condition X Sex (3, 84) |
|||
|---|---|---|---|---|---|---|
| F | P | F | p | F | p | |
| Sound Pressure Level | 134.1 | 0.000* | 2.7 | 0.112 | 0.1 | 0.937 |
| Number of Syllables | 1.3 | 0.266 | 0.7 | 0.405 | 1.9 | 0.131 |
| Syllables Per Second | 9.0 | 0.000* | 1.1 | 0.310 | 1.0 | 0.400 |
| Lung Volume Initiation | 6.6 | 0.000* | 9.2 | 0.005 | 0.7 | 0.547 |
| Lung Volume Termination | 2.1 | 0.107 | 13.8 | 0.001* | 0.5 | 0.646 |
| Lung Volume Excursion | 16.2 | 0.000* | 0.3 | 0.609 | 0.8 | 0.501 |
| Abdominal Volume Initiation | 5.7 | 0.001* | 0.3 | 0.606 | 3.0 | 0.036 |
| Abdominal Volume Termination | 3.8 | 0.013 | 0.1 | 0.749 | 1.9 | 0.127 |
| Abdominal Volume Excursion | 20.5 | 0.000* | 0.2 | 0.674 | 0.7 | 0.558 |
Degrees of freedom in parentheses. F = F-ratio, p = level of significance.
Asterisk indicates significance at the p ≤ 0.003 level.
Table 2.
Statistical Summary for Effects of Condition in the Monologue Task.
| Measures | Condition (1, 27) |
Sex (1, 27) |
Condition X Sex (1, 27) |
|||
|---|---|---|---|---|---|---|
| F | P | F | p | F | p | |
| Sound Pressure Level | 134.77 | 0.000* | 0.22 | 0.647 | 2.56 | 0.122 |
| Number of Syllables | 1.0 | 0.328 | 2.1 | 0.160 | 0.0 | 0.896 |
| Syllables Per Second | 0.8 | 0.381 | 2.2 | 0.149 | 0.5 | 0.490 |
| Lung Volume Initiation | 9.0 | 0.006 | 8.0 | 0.009 | 2.6 | 0.118 |
| Lung Volume Termination | 1.1 | 0.301 | 8.8 | 0.006 | 1.9 | 0.174 |
| Lung Volume Excursion | 24.7 | 0.000* | 0.0 | 0.934 | 0.0 | 0.827 |
| Abdominal Volume Initiation | 3.1 | 0.91 | 0.0 | 0.865 | 0.1 | 0.744 |
| Abdominal Volume Termination | 0.7 | 0.407 | 0.0 | 0.827 | 0.0 | 0.850 |
| Abdominal Volume Excursion | 5.3 | 0.029 | 0.6 | 0.459 | 0.1 | 0.748 |
Degrees of freedom in parentheses. F = F-ratio, p = level of significance.
Asterisk indicates significance at the p ≤ 0.003 level.
Table 3.
Statistical Summary for Effects of Task.
| Measures | Task | |
|---|---|---|
| F | p | |
| Sound Pressure Level | 4.23 | 0.044 |
| Number of Syllables | 282.6 | 0.000* |
| Syllables per Second | 63.7 | 0.000* |
| Lung Volume Initiation | 3.7 | 0.065 |
| Lung Volume Termination | 0.9 | 0.353 |
| Lung Volume Excursion | 50.7 | 0.000* |
| Abdominal Volume Initiation | 1.8 | 0.191 |
| Abdominal Volume Termination | 0.02 | 0.901 |
| Abdominal Volume Excursion | 8.8 | 0.006* |
Degrees of freedom were (1, 29). F = F-ratio, p = level of significance. Asterisk indicates significance at the p ≤ 0.006 level.
Sound Pressure Level (Figure 1)
Figure 1.

Sound pressure level in decibels (dB) by task and condition. Symbols with error bars represent means and standard deviations for conditions. Filled symbols represent reading; open symbols represent monologue. Asterisk indicates significantly different from the COMF condition within task. Carrot indicates significantly different from the NOISE condition during reading.
In reading, all three loud conditions were produced at a significantly higher SPL than the COMF condition. Additionally, SPL in the NOISE condition was significantly higher than in the 2XCOMF condition. In the monologue task, the NOISE condition was produced at a significantly higher SPL than the COMF condition. There were no significant differences in SPL between reading and monologue.
Number of Syllables
There were no significant effects of condition for either reading or monologue tasks. Significantly fewer syllables (mean (M) = 14.1 syllables (standard deviation (SD) = 3.9)) were produced per breath group during reading than during monologue (M = 18.6 syllables (SD = 5.4)).
Syllables per Second (Figure 2)
Figure 2.

Syllables per second by task and condition. Symbols with error bars represent means and standard deviations for conditions. Filled symbols represent reading; open symbols represent monologue. Asterisk indicates significantly different from the COMF condition within task.
In the reading task, significantly fewer syllables per second were produced in the COMF+10 and NOISE conditions than in COMF. For the monologue task, there were no significant condition effects. Significantly fewer syllables per second were produced in the monologue than in reading.
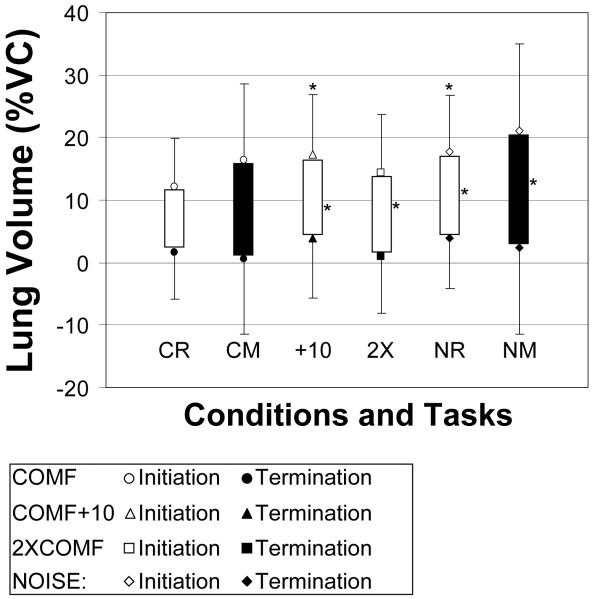
Lung Volume Initiation (Figure 3)
Figure 3.
Lung Volume Measurements by task and condition. Initiations represented by unfilled symbols; terminations represented by filled symbols; excursions represented by bars (unfilled bars = reading (R), filled bars = monologue (M)). Symbols with error bars represent means and standard deviations. Asterisk indicates significantly different from the COMF condition within task. Values in percent vital capacity (%VC) and relative to end expiratory level.
For the reading task, LVI was significantly higher in the COMF+10 and NOISE conditions as compared to the COMF condition. For the monologue task, there were no significant effects. There were no significant differences in LVI between the reading and monologue tasks.
Lung Volume Termination
For reading, there was a significant sex effect, but no significant differences among the conditions. For women, LVT (M = 7 %VC, SD = 7.1 %VC) was significantly higher than for men (M = −1 %VC, SD = 8.0 %VC). For the monologue task, there were no significant effects. There were no significant differences in LVT between the reading and monologue tasks. Lung Volume Excursion (Figure 3): In reading, LVE was significantly larger in all three loud conditions as compared to the COMF condition. During monologue, LVE was significantly larger in the NOISE condition as compared to the COMF condition. Mean LVE was significantly larger in monologue than in reading.
Abdominal Volume Initiation (Figure 4)
Figure 4.
Abdominal Volume Measurements by task and condition. Initiations represented by unfilled symbols; terminations represented by filled symbols; excursions represented by bars (unfilled bars = reading (R), filled bars = monologue (M)). Symbols with error bars represent means and standard deviations. Asterisk indicates significantly different from the COMF condition within task. Values in percent abdominal capacity (%ABC) and relative to end expiratory level.
During reading, ABVI was significantly lower in 2XCOMF condition than in the COMF condition. During monologue, there were no significant effects. There were no significant differences in ABVI between the reading and monologue tasks.
Abdominal Volume Termination
During reading and monologue, there were no significant effects. There were no significant differences in ABVT between the reading and monologue tasks.
Abdominal Volume Excursion (Figure 4)
During reading, ABVE was significantly higher in all three loud conditions than in the COMF condition. During monologue, there were no significant effects. Mean ABVE was significantly larger in the monologue task than in the reading task.
Variability
The pattern of initiating speech at a higher lung volume, as compared to COMF, was used fairly consistently in the COMF+10 and NOISE conditions. In the COMF+10 condition, LVI was higher than in the COMF condition for 68% of the utterances. In the NOISE condition during reading, LVI was higher than in the COMF condition for 70% of the utterances. In the NOISE condition during monologue, LVI was higher than in the COMF condition for 67% of the utterances. In the 2XCOMF condition, LVI was higher than in the COMF condition for only 57% of the utterances.
In contrast, the most consistent abdominal initiation patterns were seen in the 2XCOMF condition during reading. In the 2XCOMF condition, ABVI was lower than in the COMF condition for 67% of the utterances. The COMF+10 and NOISE conditions during reading demonstrated less consistency. In the COMF+10 condition, ABVI was lower than in the COMF condition for 60% of the utterances. Similarly, in the NOISE condition during reading, ABVI was lower than in the COMF condition for 58% of the utterances. However, the NOISE condition during monologue demonstrated a consistent pattern in the opposite direction, ABVI was higher than in the COMF condition for 70% of the utterances.
DISCUSSION
There were two purposes of the current study. One was to examine the effect of cueing on respiratory kinematics in a connected speech task. Cues did alter respiratory kinematics, regardless of the task (reading and monologue). During the COMF+10 and NOISE conditions, participants tended to use higher LVI than during the COMF condition. During the 2XCOMF condition, participants tended to use lower ABVI than during the COMF condition. Differences across conditions were more pronounced in the reading task than in the monologue task. The respiratory kinematic patterns used in the different cueing conditions were similar to those demonstrated for sentence tasks, extending the findings from Huber et al. (2005) to connected speech.
The second purpose of the current study was to examine whether the type of speech task affected respiratory kinematics both at comfortable intensity and with background noise present. Respiratory kinematics were similar across reading and monologue; however, there were a few differences between reading and monologue regardless of condition (COMF and NOISE). The participants produced more syllables per breath group and spoke more slowly in the monologue task than the reading task. These differences in breath group length resulted in larger excursions in the monologue task, but not lower terminations.
Differences among the Three Cues to Increase Loudness
As in the Huber et al. (2005) study, SPL increased in all of the loud conditions by about 10 dB above COMF for both reading and monologue. During reading, the NOISE condition elicited a somewhat larger increase over COMF (about 3 dB) than the 2XCOMF condition.
Because the amount of SPL change across the loud conditions was similar, it can be assumed that about the same increase in subglottal pressure was required across the loud conditions. This assumption is based on the fact that previous studies have shown there to be a linear relationship between subglottal pressure and SPL (Finnegan et al., 2000; Tanaka & Gould, 1983; Tang & Stathopoulos, 1995). However, as with the sentence tasks (Huber et al., 2005), the strategy the individuals used to generate these pressures and increased SPL were different depending on how the participants were cued to increase SPL. Although the laryngeal system was likely to be involved in the changes in loudness across the three conditions as well, the differences in respiratory kinematics clearly indicate that the cues did affect how the respiratory system was used to support the need for higher subglottal pressure in the louder speech conditions. These data extend the findings from Huber and colleagues (2005) to connected speech.
Participants significantly increased LVI in the COMF+10 and NOISE conditions during reading. This strategy allowed participants to take advantage of greater recoil pressures available at higher lung volumes in order to generate higher pressures for louder speech. Huber and colleagues (2005) also found significantly higher LVI during the COMF+10 condition, but not during the NOISE condition.
Although the change in LVI was not significant for the NOISE condition in the monologue task, mean LVI did increase (see Figure 3). The variability around initiations and terminations is larger in the monologue task than in the reading task, possibly due to a greater variability in the lengths of the utterances in the monologue task (which is a less constrained task than reading). The lengths of the utterances in the monologue task were more varied, as demonstrated by the greater standard deviation for the number of syllables per breath group. This variability around mean LVI may have affected the statistical result. The trend for increased LVI is present in the data from the NOISE condition in the monologue task, and the statistic approaches significance [p=0.006].
The respiratory mechanism used to increase subglottal pressure in the 2XCOMF condition was distinctly different from the one used in the COMF+10 and NOISE conditions. The lower ABVI in the 2XCOMF condition as compared to COMF suggests that participants primarily used a strategy of generating pressure for loud speech by increasing expiratory pressure through abdominal muscle contraction.
Several differences exist across the three cue sets that might conceivably have contributed to the results. Among the most obvious differences is the type of feedback that participants received (COMF+10: quantitative, visual, external; 2XCOMF: qualitative, auditory, internal; and NOISE: limited, degraded auditory feedback). Also the cues might have required allocation of different amounts of attention to the task. Arguably, either of these differences are candidates to explain the modulations in the results as a function of cue type. However, apart from the fact that it is not clear how such differences would translate to the particular patterns seen, this possibility seems unlikely in any straightforward way, based on the data. The logic is as follows. Potentially, the COMF+10 condition required the most attention, may have induced a greater cognitive load, and provided the most explicit feedback. The NOISE condition presumably involved more automatic processing, requiring little direct attention to increasing loudness volitionally, and it provided the least amount of feedback due to signal degradation. The 2XCOMF condition could be seen as intermediate between the other two conditions on the noted parameters. Less attention would have been required to speak at twice comfortable loudness than to target a specific SPL using an SPL meter, but the change in loudness was not automatic as in the NOISE condition. Also, although feedback was not as specific as in the COMF+10 condition, the auditory feedback was less degraded in the 2XCOMF condition than in the NOISE condition. Yet, data from the COMF+10 and NOISE conditions are very similar and data from the 2XCOMF condition are different. Thus, there is clearly no linear relationship between these hypothetical variables of interest (feedback, attention) and the results.
A potentially more satisfying explanation for the results has to do with possible differences in participants’ perceptions of task “difficulty” across the cueing conditions. As in the other two conditions, the participants spoke approximately 10 dB louder in the 2XCOMF condition than in the COMF condition. A 10 dB increase in loudness was expected in the 2XCOMF condition because individuals label sounds that increase by 10 dB as sounding twice as loud as a reference intensity level (Stevens, 1955). A 10 dB increase as a result of the 2XCOMF cue suggests that participants had a clear target for how loud they needed to be. However, the speakers did not prepare in advance for the required increase in subglottal pressure and begin to speak at a higher lung volume, as they did in the COMF+10 and NOISE conditions. Speaking “twice as loud as comfortable” may not have seemed as difficult to participants as targeting 90 dB on the SPL meter (as in the COMF+10 condition) or speaking in loud noise. Therefore, they may not have prepared as much for this condition in advance of beginning each utterance. Starting at a lower lung volume in the 2XCOMF condition, but maintaining a similar SPL level, necessitated the use of more expiratory muscle pressure to generate adequate pressure for speech. Clearly, these explanations are speculative at this point. However, the value of the present results is that they demonstrate the presence of a cueing effect, which is important from both clinical and research perspectives. The effect of cueing suggests that cognitive factors such as “intention” or goals do play a role in regulating movement even in gestures as biologically fundamental as breathing (e.g., Lee, 1998). Further, they may pave the way for future research specifically designed to assess the intervening mechanisms.
Consistency of Respiratory Strategy Used
Use of the higher LVI strategy was fairly consistent across subjects and utterances in both the COMF+10 and NOISE conditions. Even though the difference was not significant during the NOISE condition in the monologue, participants did increase LVI on a consistent basis. Data from Huber and colleagues (2005) also demonstrated consistent increases in LVI in the COMF+10 and NOISE conditions. Participants were more consistent in the Huber and colleagues study than in the current study. However, a lower consistency in connected speech tasks was expected. The volume at which speech is initiated depends to some extent on the volume of air in the lungs when speech is terminated for the previous utterance (Winkworth et al., 1994). The variability in LVI should be higher in reading and monologue because the length of the preceding utterance varied, whereas the length was fixed for the sentence tasks.
The findings regarding the consistency with which the strategy of increasing LVI was used in the current study and the Huber et al. (2005) study are in contrast with the data from Winkworth and Davis (1997). Winkworth and Davis (1997) found that women were not consistent in their use of this strategy when speaking in noise. The difference in consistency between the data from the current study in the NOISE condition and Winkworth and Davis (1997) may be related to how the noise was presented. In the Winkworth and Davis (1997) study, the noise was presented via headphones, rather than in the free-field. The headphones may have interfered more with the speaker’s auditory feedback than the noise in the free-field did, increasing the variability of the speaker’s movements. Based on the data from this study and from Huber and colleagues (2005), a more natural cue to increase loudness, such as speaking in noise, does not elicit more variable respiratory mechanisms. Respiratory patterns are consistent, suggesting global control of the mechanisms used to support increased loudness, even though it is not completely clear what those mechanisms are.
Effects of Condition on Speaking Rate
In the NOISE condition, it seemed there were two important considerations for speech. Not only did participants increase SPL, but they also slowed their speech rate in the NOISE condition as compared to the COMF condition. Huber et al. (2005) found that speech rate was reduced in the NOISE condition and posited that this slower speech rate was related to the speakers’ attempts to improve transmission of the message in spite of the background noise. Slower speech rates in noise may improve the intelligibility of speech produced in noise (Van Summers, Pisoni, Bernacki, Pedlow, & Stokes, 1988). Van Summers and colleagues (1988) presented speech produced in noise and speech produced in quiet to listeners at similar signal-to-noise ratios and found that the speech produced in noise was more intelligible than the speech produced in quiet, possibly due to the slower speech rate.
However, if speech rate was reduced to improve intelligibility, the speech rate effect should be present in the current data for both reading and monologue. Although individuals did speak more slowly in the NOISE condition when reading, speech rate did not change from the COMF condition to the NOISE condition in the monologue task. The lack of change in speech rate in the monologue task was surprising because the Lombard Effect has been shown to be greater as communicative demand increases (Lane & Tranel, 1971). The monologue task should have had a greater communicative demand than the reading task because the participants were constructing a novel message in the monologue task. The lack of change in speech rate during the monologue task may be due to the slow rate used during the COMF condition for the monologue task. The slow rate used during the monologue task was most likely related to the language formulation demands present for that task (Solomon & Hixon, 1993). Participants may not have needed to further reduce their speech rate in the NOISE condition during the monologue task in order to be intelligible.
In the current study, participants also spoke more slowly in the COMF+10 condition as compared to the COMF condition. This effect was not present in the sentence data of Huber et al. (2005). The slower rate in the COMF+10 condition was most likely due to how the SPL meter and reading passage were presented to participants. Participants had to simultaneously watch the SPL meter on a television screen and read the passage from a computer screen. Even though the television screen showing the SPL meter was positioned next to the computer screen, speakers may have needed to read more slowly due to the need to shift their visual focus from the reading passage to the SPL meter.
Speech Task-Related Differences
There were several differences between the reading and monologue tasks in the current study. The monologue task resulted in longer breath groups than the reading task did. According to mean data presented in earlier studies (Hogde & Rochet, 1989; Hoit & Hixon, 1987; Hoit et al., 1989; Solomon & Hixon, 1993), this finding was not expected. In those studies, the mean length of the breath groups in reading was longer than the mean in conversation/extemporaneous speech. Because the extemporaneous speech task used by Hoit and Hixon (1987) and Hoit and colleagues (1989) was very similar to the monologue task used in the current study, the difference between the current study and previous studies may have more to do with the reading task utilized and the length of the sentences in the reading task. Speakers take breaths most often at sentence boundaries when reading (Winkworth et al., 1994). If the sentences were longer in the reading passage used in the current study than in other studies, participants would have been more likely to use longer breath groups.
Participants spoke at a slower rate in the monologue task than in the reading task, as was demonstrated by Hodge and Rochet (1989) and Solomon and Hixon (1993) as well. Solomon and Hixon (1993) hypothesize that the slower speech rate in monologue is due to the fact that more linguistic formulation required in monologue than in reading.
Both LVE and ABVE were larger in the monologue task than in the reading task in the current study. Hixon and colleagues (1973) also found larger LVE during loud reading. The larger LVE and ABVE in reading in the current study are most likely due to the longer breath groups and slower rate in the monologue task. Solomon and Hixon (1993) found that the reading task, which resulted in longer breath groups in their study, was associated with larger ABVE than the monologue task. Additionally, Dromey and Ramig (1998) reported that when they asked their participants to speak more slowly, LVE increased.
Because most of what is known about respiratory function for loud speech is based on the production of syllable trains and sentences, it was of interest to compare the results in the current study to those from previous studies using syllable trains and sentences (see Table 4). The lung volume data from the reading and monologue tasks are similar to findings for sentences and syllable trains. Similarly, the abdominal data in the current study are comparable across tasks.
Table 4.
Comparison across Studies of Respiratory Kinematics with Increased Loudness.
| Measure | COMF+10 | 2XCOMF | NOISE |
|---|---|---|---|
| Lung Volume Initiations |
a, c, d: increased | a, d, e: no change | a, b: increased d: no change |
| Lung Volume Terminations |
a, c: no change | d. increased | a, d, e: no change a, b, d: no change |
| Lung Volume Excursions |
a, c: increased d: no change |
a: increased d, e: no change |
a, b, d: increased |
| Abdominal Volume Initiations |
a, c, d: no change | a, d: decreased | a, b, d: no change |
| Abdominal Volume Terminations |
a, c, d: no change | a: no change d: decreased |
a, b, d: no change |
| Abdominal Volume Excursions |
a: increased c, d: no change |
a: increased d: no change |
a: increased b, d: no change |
current study during reading
current study during monologue
Stathopoulos and Sapienza (1997) – target +5 dB above comfortable intensity
Huber et al. (2005) – used same cues are current study
Dromey and Ramig (1998) – twice as loud as comfortable (no abdominal measurements made)
One consistent difference between the connected speech tasks and the sentence and syllable train tasks is that LVE and ABVE both increased with increased loudness, regardless of cue, in the reading task. LVE also increased significantly in NOISE in the monologue task, and the trend for a larger ABVE in NOISE is present in the monologue data as well. The changes in LVE and ABVE are less consistent in the syllable train and sentence tasks (see Table 4). The consistent increase in excursions with increased loudness in the connected speech tasks may be related to utterance length. As utterance length increases, increasing loudness may tax the respiratory system more, requiring larger excursions. Because the sentence and syllable train tasks are shorter (12 syllables or less) than the average breath group in the connected speech tasks, they may not be long enough to really tax the respiratory system at higher intensities.
The findings, both similarities and differences in respiratory kinematics, across speech tasks are an important finding for several reasons. First, these findings suggest that the cognitive mechanisms (such as the perceived difficulty of reaching the target loudness) and utterance length may be the primary determinants in neural control of respiratory movements. The overarching speech task (whether the person is planning to say one utterance or several in a row) may not be as important for neural control and planning. This hypothesis would make sense from the standpoint of natural speech production. Often when conversing with another individual, we do not know how many utterances we will be producing, although we know the message we are trying to convey. This hypothesis would be further bolstered if future studies demonstrate that respiratory kinematics for longer sentence tasks are more similar to kinematics for utterances from the connected speech tasks. Second, from a technical standpoint, sentence tasks are much easier to collect and analyze, especially when examining multiple speech subsystems and when collecting data from clinical populations.
In summary, respiratory kinematic patterns differed depending on the cue used to elicit louder speech. This finding extends the results of Huber et al. (2005) to connected speech and provides support for the hypothesis that “intention” or goals play a role in the control of respiratory kinematic movements. These data indicate that cue must be considered when collecting data for research studies or in clinical situations. Further, respiratory strategies for supporting loud speech were similar between the reading and monologue tasks and between the connected speech tasks and previous data on syllable trains and sentence productions, when the same cue was used. However, there were differences present among the tasks that were associated with differences in utterance length and speech rate. These results suggest that respiration for speech may be more impacted by the length of the utterance, the target loudness of the utterance, and the rate of speech than by length of the speaking task (i.e., 1-2 utterances versus many utterances produced consecutively).
Grants and Acknowledgements
This research was funded by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders, grant # 1R03DC05731-01. I would like to thank the reviewers, the associate editor, and the editor for their careful and thoughtful review of this manuscript that provided for great improvement.
Appendix I: Reading Passage
“The Papa Passage” (Sapienza & Stathopoulos, 1995)
Papa was a great man. Working all his life as a carpenter, he built homes for other people. Papa was an excellent craftsman. Anyone who worked with Papa knew that he was an honest man. Papa gave himself to his work, toiling daily for small amounts of money. No one disliked Papa. In fact, neighbors used to bring Papa apples, pears, and other fruits, especially around the holidays.
I remember Papa for his kind ways. What I remember was the manner in which Papa dressed, the way he carried himself. Papa was such a strong man. Devoted to his family, especially his children, Papa worked night and day to provide for us. Although we never showed Papa our appreciation on a daily basis, I know that he felt our love, or so I hope.
REFERENCES
- Bode FR, Dosman J, Martin RR, Ghezzo H, Macklem PT. Age and sex differences in lung elasticity, and in closing capacity in nonsmokers. Journal of Applied Physiology. 1976;41:129–135. doi: 10.1152/jappl.1976.41.2.129. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat (Version 4.1) Institute of Phonetic Sciences; Amsterdam: 2003. [Google Scholar]
- Dromey C, Ramig LO. Intentional changes in sound pressure level and rate: Their impact on measures of respiration, phonation, and articulation. Journal of Speech, Language, and Hearing Research. 1998;41:1003–1018. doi: 10.1044/jslhr.4105.1003. [DOI] [PubMed] [Google Scholar]
- Finnegan EM, Luschei ES, Hoffman HT. Modulations in respiratory and laryngeal activity associated with changes in vocal intensity during speech. Journal of Speech, Language, and Hearing Research. 2000;43:934–950. doi: 10.1044/jslhr.4304.934. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Benuzzi F, Bertolani L, Daprati E, Gangitano M. Language and motor control. Experimental Brain Research. 2000;133:468–490. doi: 10.1007/s002210000431. [DOI] [PubMed] [Google Scholar]
- Hixon TJ, Goldman MD, Mead J. Kinematics of the chest wall during speech production: Volume displacements of the rib cage, abdomen, and lung. Journal of Speech and Hearing Research. 1973;16:78–115. doi: 10.1044/jshr.1601.78. [DOI] [PubMed] [Google Scholar]
- Hogde MM, Rochet AP. Characteristics of speech breathing in young women. Journal of Speech and Hearing Research. 1989;32:466–480. doi: 10.1044/jshr.3203.466. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ. Age and speech breathing. Journal of Speech and Hearing Research. 1987;30:351–366. doi: 10.1044/jshr.3003.351. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ, Altman ME, Morgan WJ. Speech breathing in women. Journal of Speech and Hearing Research. 1989;32:353–365. doi: 10.1044/jshr.3202.353. [DOI] [PubMed] [Google Scholar]
- Holmberg EB, Hillman RE, Perkell JS. Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. The Journal of the Acoustical Society of America. 1988;84:511–529. doi: 10.1121/1.396829. [DOI] [PubMed] [Google Scholar]
- Huber JE, Chandrasekaran B, Wolstencroft JJ. Changes to respiratory mechanisms during speech as a result of different cues to increase loudness. Journal of Applied Physiology. 2005;98:2177–2184. doi: 10.1152/japplphysiol.01239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Stathopoulos ET. Respiratory and laryngeal responses to an oral air pressure bleed during speech. Journal of Speech, Language, and Hearing Research. 2003;46:1207–1220. doi: 10.1044/1092-4388(2003/094). [DOI] [PubMed] [Google Scholar]
- Isshiki N. Regulatory mechanism of voice intensity variation. Journal of Speech and Hearing Research. 1964;7:17–29. doi: 10.1044/jshr.0701.17. [DOI] [PubMed] [Google Scholar]
- Konno K, Mead J. Measurement of the separate volume changes of the rib cage and the abdomen during breathing. Journal of Applied Physiology. 1967;22:407–422. doi: 10.1152/jappl.1967.22.3.407. [DOI] [PubMed] [Google Scholar]
- Ladefoged P. Three Areas of Experimental Phonetics. Oxford University Press; London: 1967. [Google Scholar]
- Lane H, Tranel B. The Lombard Sign and the role of hearing in speech. Journal of Speech and Hearing Research. 1971;14:677–709. [Google Scholar]
- Lee TD. On the dynamics of motor learning research. Research Quarterly for Exercise and Sport. 1998;69:334–337. doi: 10.1080/02701367.1998.10607707. [DOI] [PubMed] [Google Scholar]
- Pick HL, Jr., Siegel GM, Fox PW, Garber SR, Kearney JK. Inhibiting the Lombard effect. The Journal of the Acoustical Society of America. 1989;85:894–900. doi: 10.1121/1.397561. [DOI] [PubMed] [Google Scholar]
- Rahn H, Otis AB, Chadwick LE, Fenn WO. The pressure-volume diagram of the thorax and lung. American Journal of Physiology. 1946;146:161–178. doi: 10.1152/ajplegacy.1946.146.2.161. [DOI] [PubMed] [Google Scholar]
- Sapienza CM, Stathopoulos ET. Speech task effects on acoustic and aerodynamic measures of women with vocal nodules. Journal of Voice. 1995;9:413–418. doi: 10.1016/s0892-1997(05)80203-6. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Hixon TJ. Speech breathing in Parkinson’s disease. Journal of Speech and Hearing Research. 1993;36:294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- Stathopoulos ET, Sapienza CM. Developmental changes in laryngeal and respiratory function with variations in sound pressure level. Journal of Speech, Language, and Hearing Research. 1997;40:595–614. doi: 10.1044/jslhr.4003.595. [DOI] [PubMed] [Google Scholar]
- Stevens SS. The measurement of loudness. The Journal of the Acoustical Society of America. 1955;27:815–829. [Google Scholar]
- Tanaka S, Gould WJ. Relationship between vocal intensity and noninvasively obtained aerodynamic parameters in normal adults. The Journal of the Acoustical Society of America. 1983;73:1316–1321. doi: 10.1121/1.389235. [DOI] [PubMed] [Google Scholar]
- Tang J, Stathopoulos ET. Vocal efficiency as a function of vocal intensity: A study of children, women, and men. The Journal of the Acoustical Society of America. 1995;97:1885–1892. doi: 10.1121/1.412062. [DOI] [PubMed] [Google Scholar]
- Tasko SM, McClean MD. Variations in articulatory movement with changes in speech task. Journal of Speech, Language, and Hearing Research. 2004;47:85–100. doi: 10.1044/1092-4388(2004/008). [DOI] [PubMed] [Google Scholar]
- Van Summers W, Pisoni DB, Bernacki RH, Pedlow RI, Stokes MA. Effects of noise on speech production: Acoustic and perceptual analyses. The Journal of the Acoustical Society of America. 1988;84:917–928. doi: 10.1121/1.396660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkworth AL, Davis PJ. Speech breathing and the Lombard effect. Journal of Speech, Language, and Hearing Research. 1997;40:159–169. doi: 10.1044/jslhr.4001.159. [DOI] [PubMed] [Google Scholar]
- Winkworth AL, Davis PJ, Adams RD, Ellis E. Breathing patterns during spontaneous speech. Journal of Speech and Hearing Research. 1995;38:124–144. doi: 10.1044/jshr.3801.124. [DOI] [PubMed] [Google Scholar]
- Winkworth AL, Davis PJ, Ellis E, Adams RD. Variability and consistency in speech breathing during reading: Lung volumes, speech intensity, and linguistic factors. Journal of Speech and Hearing Research. 1994;37:535–556. doi: 10.1044/jshr.3703.535. [DOI] [PubMed] [Google Scholar]