Abstract
Fragile X Syndrome (FXS) is the most common form of inherited mental retardation. The neuroanatomical phenotype of adult FXS patients, as well as adult Fmr1 knockout (KO) mice, includes elevated dendritic spine density and a spine morphology profile in neocortex that resembles younger individuals. Developmental studies in mouse neocortex have revealed a dynamic phenotype that varies with age, especially during the period of synaptic pruning. Here we investigated the hippocampal dentate gyrus to determine if the FXS spine phenotype is similarly tied to periods of maturation and pruning in this brain region. We used high-voltage electron microscopy to characterize Golgi-stained spines along granule cell dendrites in Fmr1 KO and wildtype (WT) mouse dentate gyrus at postnatal days 15, 21, 30, and 60. In contrast to neocortex, dendritic spine density was higher in Fmr1 KO mice across development. Interestingly, neither genotype showed specific phases of synaptogenesis or pruning, potentially explaining the phenotypic differences from neocortex. Similarly, although the KO mice showed a more immature morphological phenotype overall than WT (higher proportion of thin headed spines, lower proportion of mushroom and stubby spines), both genotypes showed gradual development, rather than impairments during specific phases of maturation. Finally, spine length showed a complex developmental pattern that differs from other brain regions examined, suggesting dynamic regulation by FMRP and other brain region-specific proteins. These findings shed new light on FMRP’s role in development and highlight the need for new techniques to further understand the mechanisms by which FMRP affects synaptic maturation.
Keywords: Plasticity, hippocampus, spine shape, activity dependent, development, High-voltage electron microscopy, stereoscopic images, Golgi impregnation
1. Introduction
Fragile X Syndrome (FXS), the most common form of inherited mental retardation, is caused by the expansion of an unstable repeat in the untranslated region of the FMR1 gene on the X chromosome (Pieretti et al., 1991). This mutation results in the absence of functional FMRP (Fragile X Mental Retardation Protein), an mRNA binding protein that plays a role in regulation of protein synthesis in neuronal cell bodies as well as near synaptic sites (Khandjian et al., 2004; Weiler et al., 2004). The majority of excitatory synapses in the brain are characterized by protrusions on the post-synapse, called dendritic spines. Disturbances in the density and shape of dendritic spines have been found in most causes of mental retardation in humans, although the specific pattern of deficits differ in each disease and the causative relationship is still unclear (Fiala et al., 2002). In the case of FXS, abnormalities have been observed in the density, length and morphology of dendritic spines in post-mortem cerebral cortical tissue from adult FXS patients (see Churchill et al., 2002; Hagerman and Hagerman, 2002). Specifically, spine density and spine length in this region are increased in FXS, and the morphology of dendritic spines in these adult patients appears immature for the brain region examined (Hinton et al., 1991; Irwin et al., 2001). Importantly, this phenotype is recapitulated in the adult cerebral cortex of the mouse model of this disease, the Fmr1 knockout (KO) mouse (Bakker et al., 1994; Irwin et al., 2002; McKinney et al., 2005). Coexistent with increased density and length of dendritic spines, adult Fmr1 KO mice exhibit an abundance of morphological spine types commonly observed in the neocortex of immature animals (more filopodia-like and thin-headed spines and fewer mushroom/stubby spines), suggestive of a deficit in spine maturation (Irwin et al., 2002; McKinney et al., 2005). Individual studies at different stages of development have shown that the FXS phenotype (increased density, length, and immature morphology) is seen in early post-natal life (1–3 weeks) in the somatosensory cortex, as well as adulthood, but is not found around 1 month of age (Galvez and Greenough, 2005; Nimchinsky et al., 2001). Between ~1 month and young adulthood (75 days), WT animals exhibited a drop in spine density and length (pruning and maturation), whereas the KO density and length remained at pre-pruning levels (Galvez and Greenough, 2005).
The dendritic spine phenotype in the neocortex of Fmr1 KO mice has been relatively well-characterized, but much less is known regarding dendritic spine development in the hippocampal formation of these animals. Expression levels of FMRP are high in the hippocampus, a brain region known to play an important role in learning and memory. Furthermore, bilateral enlargement of hippocampal volume has been reported in patients with FXS, suggesting that the absence of FMRP may lead to structural deficits in this brain region (Eichenbaum and Cohen, 2001; Hinds et al., 1993; Reiss et al., 1994). Grossman et al (2006b) examined dendritic spines in the adult hippocampal subfield CA1 and found that the spine morphology profile in adult Fmr1 KO mice appeared immature for the CA1 hippocampal region, but differed from the morphological pattern of abnormalities found in neocortex.
Here we follow dendritic spine development of granule cells in the dentate gyrus of Fmr1 KO mice, a brain region and cell type outside of neocortex where developmental plasticity is also thought to occur. Although dentate gyrus development has been examined across several species, there are discrepancies as to the timing and the nature of dendritic spine maturation (e.g., Duffy and Rakic, 1983; Meyer and Ferres-Torres, 1978; Norrholm and Ouimet, 2000, see also discussion; Steward and Falk, 1991; Yan et al., 1997). However, there is agreement that maturation in the dentate gyrus involves an increase in mushroom spines and a decrease in thin spines, more similar to the maturation seen in neocortex than the neighboring CA1. We examined spines along granule cell dendrites in dentate gyrus of Fmr1 KO and WT mice on postnatal days 14, 21, 30 and 60 to determine whether FMRP plays a role in dendritic spine pruning and maturation in this brain region. For improved visualization and more precise analysis of spine density, length and morphology, fully-impregnated Golgi-stained dendrites were examined using High Voltage Electron Microscopy (HVEM). Two angled images were taken of each dendritic segment, such that a stereoscopic image could be created, revealing the 3-dimensional structure and projection of each spine (see Figure 1). We found that the density of dendritic spines along dentate granule neurons increased with age, showing no evidence of a defined pruning phase and that, across development, spine density on these dendrites was elevated overall in Fmr1 KO mice. Morphologic development appeared delayed in the KO mice, which showed a higher overall proportion of thin headed (C/D) spines than WT mice and a lower overall proportion of stubby and mushroom-shaped (F/G) spines, a morphologic profile in KO mice that appears immature for this brain region, and more reminiscent of neocortex than hippocampal area CA1. The persistent increase in spine density in dentate gyrus in the absence of a defined pruning period argues for a role of FMRP in processes leading to individual spine maturation, rather than in controlling a global, developmental pruning phase.
Figure 1.
(A) Representative stereoscopic images of dentate granule cell dendrites from 30 day-old wildtype (WT) and Fmr1 knockout (KO) mice. Viewing with red-blue glasses yields a 3-Dimensional image. Scale bar represents 1 µm. (B) Morphology categories used to classify spines. Part B is reprinted from Grossman et al (2006b).
2. Results
2.1. Dendritic Spine Density
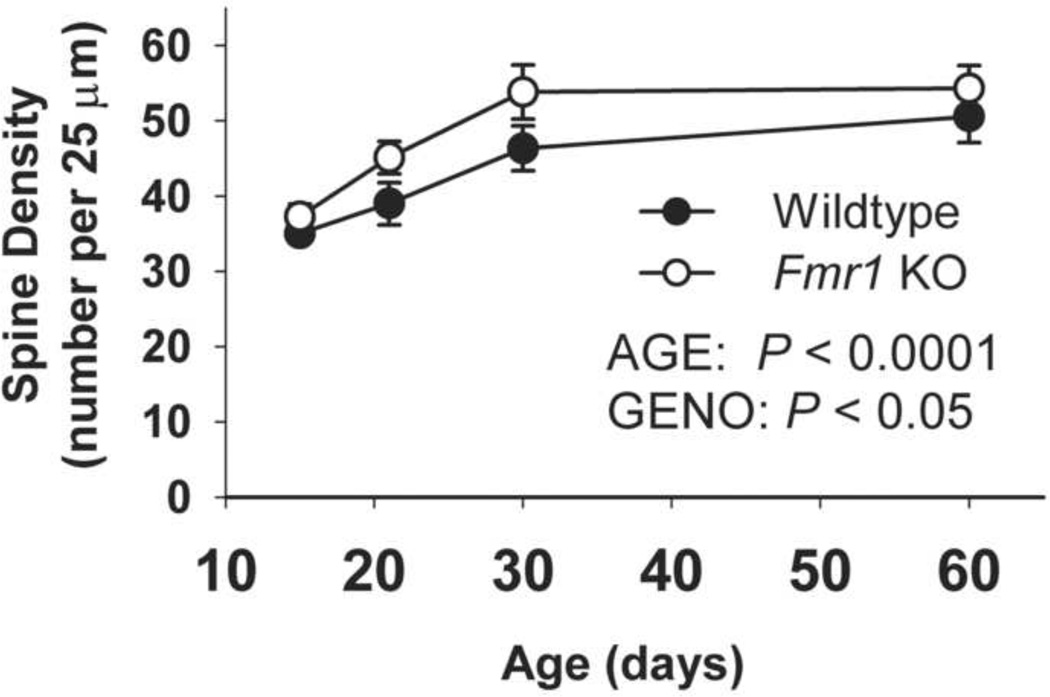
As shown in Figure 2, the density of spines along dendrites of dentate granule cell neurons increased with age overall (ANOVA main effect of AGE: F(3,26) = 14.03; p < 0.0001), and was higher in Fmr1 KO than in WT mice (GENOTYPE : F(1,26) = 7.54; p < 0.05). There was no significant AGE * GENOTYPE interaction. The elevated spine density in KO mice appeared to develop gradually, and no distinct phases of spine overproduction followed by pruning were noted in either WT or Fmr1 KO mice.
Figure 2.
Spine density along dentate granule cell dendrites of WT and Fmr1 KO mice increased with age. Spine density was higher in Fmr1 KO than in WT mice. Error bars represent S.E.M.
2.2 Spine Morphology
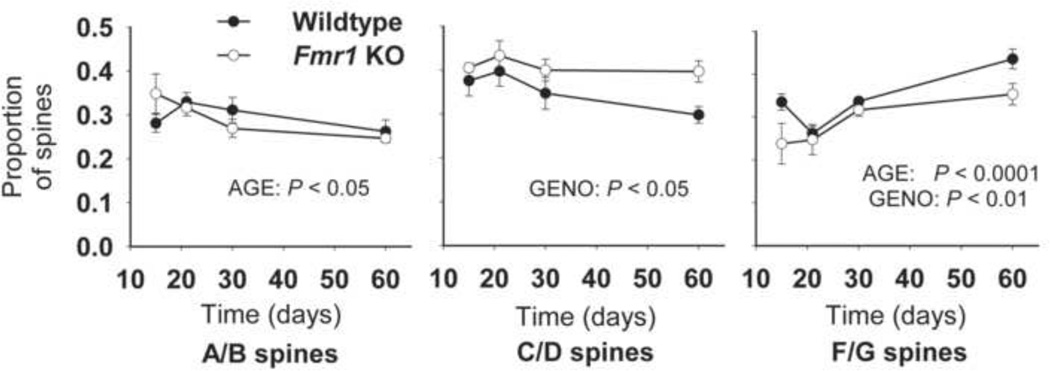
The spine morphology categories used previously by our laboratory (Figure 1B; see also Table 1 in supplemental materials) were used here to characterize the morphology of each spine, and to calculate the proportion of spines in each category that appeared along a given dendrite (see Figure 3). Across development, the proportion of thin, filopodia-like (A/B) spines decreases in mice of each genotype (AGE: F(3,26) = 3.35, p < 0.05), while the proportion of stubby and mushroom-shaped (F/G) spines increases (F(3,26) = 12.00, p < 0.0001). Overall, KO mice appear to have a higher proportion of thin, headed (C/D) spines (GENO: F(1,26) = 6.99, p < 0.05) but a lower proportion of mushroom/stubby (F/G) spines (F(1,26) = 8.77, p < 0.01) than WT mice. Spine types E (double-headed thin spines) and H (double headed branched spines) occurred rarely in this brain region and have therefore been omitted from analyses.
Figure 3.
The proportion of spines in each morphology category across development in Fmr1 KO and WT mouse dentate gyrus. In both genotypes, the proportion of thin (A/B) spines decreased with age while the proportion of stubby and mushroom-shaped (F/G) spines increased. Across ages, Fmr1 KO mice had a higher proportion of thin single-headed (C/D) spines and a lower proportion of stubby and mushroom-shaped (F/G) spines. Error bars represent S.E.M.
2.3 Spine Length
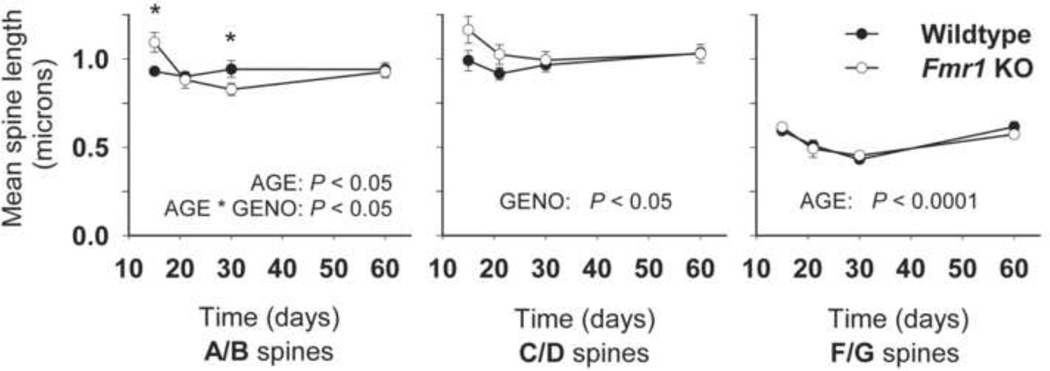
Mean spine length per animal was determined for each prominent spine type (A/B, C/D and F/G) and group differences were assessed (see Figure 4). For filopodia-like (A/B) spines, a significant AGE * GENOTYPE interaction was observed (F(3,26) = 4.19; P < 0.05), along with an effect of AGE (F(3,26) = 4.26; P < 0.05). A/B spines in Fmr1 KO mice were longer than in WT at 15 days (p < 0.05), and shorter than in WT at 30 days (p < 0.05). Early in development, the Fmr1 KO mice also had longer thin, headed (C/D) spines than did WT mice (GENO: F(1,26) = 4.65; P < 0.05). For stubby or mushroom-shaped (F/G) spines (which tended to be shorter than other spine types), there was no difference in length between WT and Fmr1 KO mice, but a main effect of AGE was noted (F(3,26) = 18.39; p < 0.0001); these spines decreased in length (appearing to be shortest at 30 days) and increased again by day 60.
Figure 4.
Mean dendritic spine length for predominant spine types along dentate granule cell dendrites in WT and Fmr1 KO mice. Error bars represent S.E.M. (* p < 0.05).
3. Discussion
The development of dendritic spines and synapses in the dentate gyrus molecular layer has been studied in several species. Some have described synaptic overproduction followed by pruning (Duffy and Rakic, 1983; Norrholm and Ouimet, 2000; Steward and Falk, 1991; Yan et al., 1997). Other researchers have not observed this pattern (Crain et al., 1973; Meyer and Ferres-Torres, 1978), and trajectories of spine development may differ even within the dentate gyrus, potentially accounting for some of the differences in the literature (Zehr et al., 2008). To our knowledge, this experiment represents one of the first investigations in the Fmr1 KO mouse of dendritic spine characteristics in non-pyramidal cells, specifically granule cells in the dentate gyrus. Our study reveals an adult spine phenotype in dentate gyrus distinct from what has been described in hippocampal subfield CA1 of these animals (Grossman et al., 2006b). The phenotype in dentate gyrus is more consistent with what has been described in the neocortex, but appears to emerge without a defined pruning phase (Galvez and Greenough, 2005; Irwin et al., 2002; McKinney et al., 2005). In contrast to the somatosensory cortex, spine density along dentate granule cells continues to increase until 60 days of age in both WT and KO mice, and is higher in KO mice across development. These findings suggest that disruption of a specific pruning phase may not be central to the emergence of the spine density phenotype. Instead, lack of FMRP may upset the regulation of constitutive processes of forming, stabilizing, or removing dendritic spines, with the resultant spine profile therefore depending on the developmental trajectory of synaptic connectivity in each brain region.
Previous findings suggest that the developmental shift in spine morphology occurs abnormally in the Fmr1 KO mice. In the visual and somatosensory cortex of adult Fmr1 KO mice, filopodia-like (A/B) and thin, headed (C/D) spines [types that are abundant in the neocortex of developing animals (e.g., Galofre and Ferrer, 1987; Murphy and Magness, 1984)] are present in greater proportions than in age-matched wildtype mice (Galvez and Greenough, 2005; McKinney et al., 2005). Data from hippocampal area CA1 of KO mice support this hypothesis: the profile of spine morphologies in area CA1 of adult knockouts is shifted toward an abundance of mushroom/stubby (F/G) spines, which are relatively more abundant in area CA1 of young rodents (Grossman et al., 2006b; Harris et al., 1992). The present findings in the dentate gyrus reveal a developmental shift from filopodia-like (A/B) to mushroom/stubby (F/G) spines in each genotype, indicating that spine development on dentate granule cells may more closely resemble neocortical pyramidal cells than it does neighboring CA1 pyramidal cells. The proportion of thin, headed (C/D) spines in Fmr1 KO mice, however, remains significantly higher than in WT mice across development, whereas the proportion of mushroom/stubby (F/G) spines is significantly lower than in WT mice, and fails to increase to WT levels with age. The spine morphology profile in the dentate gyrus of Fmr1 KO mice therefore is consistent with the hypothesis of impairments in spine development.
The excess of thin, headed (C/D) spines in Fmr1 KO mice across development suggests that FMRP is important either for limiting the rate of development of these spines or for facilitating the transformation or maturation of these spines into another spine type. In vivo studies on WT animals suggest that, while many spines remain stable across long periods of time, other spines display remarkable plasticity of shape (Grutzendler et al., 2002; Holtmaat et al., 2005). Filopodia in the neocortex have been shown to be particularly dynamic early in life, with lifetimes often shorter than a day, whereas larger, headed spines are associated with much longer lifetimes (Grutzendler et al., 2002; Zuo et al., 2005). Recently, Cruz-Martin et al. (2010) confirmed that in WT animals there is a reduction in turnover rate (the formation and elimination of new spines) during the second post-natal week. In the Fmr1 KO mouse this developmental change is delayed, lending support to the hypothesis that spine maturation is abnormal in the Fmr1 KO mouse.
Work from our laboratory and others over the past decade indicates that the Fmr1 KO mouse expresses different developmental phenotypes in multiple brain regions, suggesting that FMRP may be involved in a general regulatory process governing the balance of spine loss and formation, rather than a specific mechanism, such as spine enlargement, which would be expected to affect all neurons similarly. This is consistent with current theories regarding the molecular role of FMRP, which is thought to dynamically regulate new protein synthesis of a variety of mRNAs, including many involved in synaptic stability and spine maturation (eg: CamKII, PSD-95. see Grossman et al., 2006a). Absence of FMRP has been associated with excess basal rates of protein synthesis, as well as a deficit in specific, activity-driven protein synthesis (Bassell and Warren, 2008). It is possible that excess synthesis of particular proteins impairs plasticity of spine shape, thus permitting maintenance of inappropriate spines, including the over-abundant thin-headed spines. As different brain regions have a different cohort of mRNAs that are expressed basally, a lack of activity-dependent regulation of brain region-specific mRNAs under activity-induced conditions might arrest maturation in this “intermediate” stage, resulting in the paucity of mushroom/stubby spines seen in the dentate and neocortex of adult KO mice.
Several factors may influence interpretation of the differences in spine density, length and morphology reported here. First, the fact that new neurons are added postnatally to the dentate gyrus may introduce some variability in the relative maturity of the dendritic segments analyzed in this study (see Jones et al., 2003). Recent research has been inconsistent regarding the extent of cell proliferation, neuronal differentiation, and survival in the Frm1 KO mouse dentate gyrus (Eadie et al., 2009; Luo et al., 2010). Although future studies will be necessary to reconcile these differences, it is important to note the multiple levels at which loss of FMRP could affect neuronal development.
Second, the dendritic spine phenotype associated with FXS has traditionally been considered a deficit in postsynaptic processing, relating to FMRP’s presence and role in postsynaptic protein synthesis (Weiler et al., 2004). However, it is likely that the dendritic spine phenotype is intimately tied to the input signal, and thus the quality of that input must also be suspect. For example, when input to WT dentate gyrus granule cells was blocked, dendrites from these cells had a higher than normal proportion of filopodia-like spines (Drakew et al., 1999). Thus, to determine whether spine abnormalities reflect a primary or secondary deficit, future studies will need to assess the integrity of presynaptic inputs and neural networks in Fragile X Syndrome.
Synapses in the granule cell molecular layer receive the initial input to the hippocampal formation. Therefore, deficits in synaptic plasticity and maturation in the dentate gyrus may have a particularly negative impact on the functioning of the hippocampus, perhaps contributing to the abnormal staining patterns of mossy fiber terminal fields (Ivanco and Greenough, 2002; Mineur et al., 2002) and, farther downstream, to the dendritic spine phenotype observed in area CA1 of these animals (Grossman et al., 2006b). Such changes may cause improper development of network connectivity both within the hippocampus and throughout the brain, potentially leading to a lowered seizure threshold, deficits in learning, and other behavioral abnormalities seen in Fragile X Syndrome. Importantly, pharmacologic approaches that have been shown to rescue dendritic spine abnormalities in Fmr1 KO mice in vivo and in cell culture, have also been shown to ameliorate many aspects of the mouse behavioral phenotype, including predisposition to audiogenic seizures and abnormalities in tests of anxiety and learning (Bilousova et al., 2008; de Vrij et al., 2008; Liu et al., 2010), making research into the connection between spine abnormalities and mental retardation a continuing priority.
4. Methods and Materials
4.1 Experimental animal and tissue processing
The present study examined Fmr1 knockout (KO) mice and age-matched controls (wildtype with respect to the FMR1 allele) at 15, 21, 30, and 60 days of age. All mice were male and were backcrossed 6 times in the C57BL/6 background (B6.129P2-Fmr1tm1Cgr). Four animals per group were used at 15, 21, 30 days, whereas at 60 days, there were 5 animals per group. Animals were socially housed, with 2–4 per cage, and PCR tests confirmed the genotype of each mouse (see ref. Bakker et al., 1994). Mice were deeply anesthetized and sacrificed by transcardial perfusion with 4% paraformaldehyde. Brains were postfixed in 2% paraformaldehyde /2.5% glutaraldehyde for >24 hours, then stained with Rapid Golgi protocol described by Lee et al. (2004). Briefly, blocks containing the dentate gyrus were rinsed with 0.1 N Sodium Cacodylate buffer and stored in the dark for 4 days at 20° C in a solution of 2.25% potassium dichromate and 0.4% osmium tetroxide. Blocks were then transferred to a solution of 0.75% silver nitrate solution and kept in the dark for 3 days at 20° C. Following processing for electron microscopy (dehydration through a graded series of ethanols and then propylene oxide, followed by embedding in Araldite / LX-112), sagittal sections (75 µm thick) were made from embedded samples using a sliding microtome. These sections were re-embedded on glass slides, and allowed to cure.
Dentate granule cell dendrites that appeared fully impregnated were randomly selected from each animal under the light microscope, isolated and attached to LX-112 / Araldite blocks. Dendrites were chosen from tissue blocks extracted from both hemispheres. Tissue blocks were selected irrespective of hemisphere, with no lateralizing preference in any age or genotype. This method minimized hemispheric variation in sampling of dendrites for analysis. Sections (4 µm thick) that included the dendrite were made with a Leica Ultracut-T ultramicrotome and were mounted on copper double-mesh grids. Segments of granule cell dendrite (centered upon the middle 1/3 of the molecular layer of the dorsal blade of the dentate gyrus) were visualized using high-voltage electron microscopy (≥ 1000kV or 1MeV) as described previously (Hama et al., 1989; Hama et al., 1986; Lee et al., 2004). Data was collected from an average of 11.4 dendrites per animal with 40.4 spines per dendrite at ages 15, 21 and 30 days and an average of 5.7 dendrites per animal with 82.5 spines per dendrite at age 60 days. Micrographs were taken at 2500x for 15, 21, and 30 days animals using the 1.2MeV AEI High Voltage Electron Microscope at the Resource for the Visualization of Biological Complexity at the Wadsworth Center, Albany, NY. For 60 day animals, micrographs were taken at 2000x on the 1MeV Hitachi H-1250M located at the National Institute for Physiological Sciences, Okazaki, Japan. Pictures of dendrites were taken at tilt angles of −8°, 0°, and +8°.
Images were scanned into a computer, and Adobe Photoshop was used to crop and align images. The Stereoscopic Image Merger software program (Witvision Co., Seoul, Korea) was used to create stereoscopic (red-blue) anaglyph images, using the 0° image and either the +8° or −8° image (chosen randomly). Each micrograph was associated with a scale bar taken at the same time and magnification as the micrographs. This scale bar was used to calibrate each micrograph, thus negating the potential confound of a different magnification for the 60-day animals. For all analyses, raters employed stereoscopic (red-blue) glasses to visualize dendrites and spines.
4.2 Spine Density
Spine density was measured by a single rater who was blind to experimental group using Scion Image Beta 4.02 (Scion Corp; Fredrick, MD). Dendritic spine density was calculated as the ratio of the number of visible dendritic spines (all spines were counted, regardless of spine orientation) to the measured length of the entire visible dendrite (following as closely as possible the contour of the dendritic shaft). This value was expressed as the number of spines per 25 µm dendritic length, and was calculated for each dendrite. The spine densities of each animal’s dendrites were then averaged to get a mean spine density per animal (weighted for the number of dendrites per animal) which was used to calculate the group mean. To test for differences between group means, ANOVA was used with AGE and GENOTYPE as main effects and an AGE * GENOTYPE interaction.
4.3 Spine Morphology
Spine morphology was characterized by four raters (each blind to experimental group) using the morphological categories used previously by our laboratory (see Figure 1B, ref. Grossman et al., 2006b; Irwin et al., 2001; McKinney et al., 2005). These raters characterized the first ten spines (distinct protrusions that stayed in the horizontal plane) from the top of the dendrite for 15, 21, and 30 day animals, and the top ten and bottom ten spines for 60 day animals (as there were fewer dendrites per animal in this group, more spines per dendrite were measured). Scanning in micrographs allowed digital enhancement of magnification, thus an objective set of rules was created and followed by each of the four raters to reduce variation (see Table 1 in supplemental material). Although we have shown that spine length and spine morphology phenotypes in Fragile X are largely independent (Grossman et al., 2006b; McKinney et al., 2005), these rules clearly specify that morphology categorization was to be kept independent of spine length. The proportion of spines in each morphology category was determined for each dendrite. For the graphs in Figure 3, these values were then averaged across dendrites to obtain the proportion of spines in each category for each animal, and group means were calculated. For these data, age is an independent variable that could interact with spine type; hence analysis of variance, while more conservative than the chi-square analysis used in previous papers (Galvez and Greenough, 2005; Grossman et al., 2006b; Irwin et al., 2002; McKinney et al., 2005) was necessary to allow age to be considered in the overall analysis. The effect of genotype and development on the proportion of spines in each category was assessed using ANOVA with AGE and GENOTYPE as main effects and including the AGE*GENOTYPE interaction.
4.4 Spine Length
The length of each spine whose morphology was characterized was also measured. Using Scion Image and calibrations specific to each micrograph, spine length was measured along the center axis of the spine from the base (which was the surface of the dendritic shaft) to the tip of the spine head, following the main axis of the spine. For each animal, the mean length of spines of each predominant spine type (A/B, C/D, F/G) was determined. These values were used to calculate the group mean for each spine type. To assess the effect of genotype and development on mean length for each spine type, ANOVA was used with AGE and GENOTYPE as main effects and including the AGE * GENOTYPE interaction.
Any spine that emerged above or below the horizontal plane or protruded above or below the dendrite was deemed un-measureable, as spine length measurements would be inherently incorrect (the morphology of these spines was also not characterized).
Supplementary Material
Acknowledgements
This work was supported by FRAXA, NIH Grants MH35321, HD007333, and the Spastic Paralysis and Allied Diseases of the Central Nervous System Research Foundation. We thank David Barnard at the Resource for the Visualization of Biological Complexity at the Wadsworth Center, Albany, NY. The authors would also like to thank Kathy Bates, Julie Markham, Lisa Foster and Dack Shearer for their valuable contributions to this research.
Abbreviations
- FMRP
Fragile X Mental Retardation Protein
- LTP
Long Term Potentiation
- LTD
Long Term Depression
- FXS
Fragile X Syndrome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Bakker C, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilousova T, et al. Minocycline Promotes Dendritic Spine Maturation and Improves Behavioral Performance in the Fragile X Mouse Model. J Med Genet. 2008 doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Churchill JD, et al. A converging-methods approach to fragile X syndrome. Dev Psychobiol. 2002;40:323–338. doi: 10.1002/dev.10036. [DOI] [PubMed] [Google Scholar]
- Crain B, et al. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 1973;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, et al. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakew A, et al. Blockade of neuronal activity alters spine maturation of dentate granule cells but not their dendritic arborization. Neuroscience. 1999;94:767–774. doi: 10.1016/s0306-4522(99)00378-4. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Rakic P. Differentiation of granule cell dendrites in the dentate gyrus of the rhesus monkey: a quantitative Golgi study. J Comp Neurol. 1983;214:224–237. doi: 10.1002/cne.902140210. [DOI] [PubMed] [Google Scholar]
- Eadie BD, et al. Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol Dis. 2009;36:361–373. doi: 10.1016/j.nbd.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: memory systems of the brain, vol. New York: Oxford University Press; 2001. [Google Scholar]
- Fiala JC, et al. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Galofre E, Ferrer I. Development of dendritic spines in the Vth's layer pyramidal neurons of the rat's somatosensory cortex. A qualitative and quantitative study with the Golgi method. J Hirnforsch. 1987;28:653–659. [PubMed] [Google Scholar]
- Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- Grossman AW, et al. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006a;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, et al. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006b;1084:158–164. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, et al. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ, editors. Fragile X Syndrome: Diagnosis, Treatment, and Research. Baltimore: Johns Hopkins University Press; 2002. [Google Scholar]
- Hama K, et al. Three-dimensional morphometrical study of dendritic spines of the granule cell in the rat dentate gyrus with HVEM stereo images. J Electron Microsc Tech. 1989;12:80–87. doi: 10.1002/jemt.1060120203. [DOI] [PubMed] [Google Scholar]
- Hama K, et al. Three dimensional organization of neurons and glia cells as revealed by high voltage electron microscopic stereoscopy. Biomedical Research. 1986;7:214–251. [Google Scholar]
- Harris KM, et al. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds HL, et al. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993;3:36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, et al. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Irwin SA, et al. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet. 2002;111:140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- Irwin SA, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Greenough WT. Altered mossy fiber distributions in adult Fmr1 (FVB) knockout mice. Hippocampus. 2002;12:47–54. doi: 10.1002/hipo.10004. [DOI] [PubMed] [Google Scholar]
- Jones SP, et al. Maturation of granule cell dendrites after mossy fiber arrival in hippocampal field CA3. Hippocampus. 2003;13:413–427. doi: 10.1002/hipo.10121. [DOI] [PubMed] [Google Scholar]
- Khandjian EW, et al. Biochemical evidence for the association of fragile X mental retardation protein with brain polyribosomal ribonucleoparticles. Proc Natl Acad Sci U S A. 2004;101:13357–13362. doi: 10.1073/pnas.0405398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, et al. Morphological analysis of spine shapes of Purkinje cell dendrites in the rat cerebellum using high-voltage electron microscopy. Neurosci Lett. 2004;359:21–24. doi: 10.1016/j.neulet.2004.01.071. [DOI] [PubMed] [Google Scholar]
- Liu ZH, et al. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol. 2010:1–13. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, et al. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6:e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, et al. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am J Med Genet B Neuropsychiatr Genet. 2005;136:98–102. doi: 10.1002/ajmg.b.30183. [DOI] [PubMed] [Google Scholar]
- Meyer G, Ferres-Torres R. [Quantitative age-dependent variations in dendritic spines in the hippocampus (CA1, CA3 and fascia dentata) of the albino mouse] J Hirnforsch. 1978;19:371–378. [PubMed] [Google Scholar]
- Mineur YS, et al. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12:39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- Murphy EH, Magness R. Development of the rabbit visual cortex: a quantitative Golgi analysis. Exp Brain Res. 1984;53:304–314. doi: 10.1007/BF00238159. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, et al. Abnormal development of dendritic spines in FMR1 knockout mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res. 2000;883:205–215. doi: 10.1016/s0006-8993(00)02909-7. [DOI] [PubMed] [Google Scholar]
- Pieretti M, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Reiss AL, et al. Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology. 1994;44:1317–1324. doi: 10.1212/wnl.44.7.1317. [DOI] [PubMed] [Google Scholar]
- Steward O, Falk PM. Selective localization of polyribosomes beneath developing synapses: a quantitative analysis of the relationships between polyribosomes and developing synapses in the hippocampus and dentate gyrus. J Comp Neurol. 1991;314:545–557. doi: 10.1002/cne.903140311. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, et al. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci U S A. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, et al. Effects of neonatal serotonin depletion on the development of rat dentate granule cells. Brain Res Dev Brain Res. 1997;98:177–184. doi: 10.1016/s0165-3806(96)00176-9. [DOI] [PubMed] [Google Scholar]
- Zehr JL, et al. Adolescent development of neuron structure in dentate gyrus granule cells of male Syrian hamsters. Dev Neurobiol. 2008;68:1517–1526. doi: 10.1002/dneu.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, et al. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.