Abstract
Neurological manifestations have been reported in Kikuchi–Fujimoto disease (KFD). Characteristics of brain lesions are not defined. In addition, no biological indexes are known to help clinicians along the diagnosis process. The authors describe encephalitis associated with KFD. Brain MRI, positron emission tomography (PET) scan and a large biological assessment including interferon α (INF-α) level measurement in cerebrospinal fluid (CSF) were performed. A 39-year-old man with chronic headaches developed diplopia, slow ideation and behavioural disturbances. MRI showed brain lesions particularly in the pontine region and internal temporal lobes with enhancement of the perivacular space and the walls of the lateral ventricle. The IFN-α level was increased in the CSF without viral infection. Cervical and mediastinal adenitis were evident as a hypermetabolic focus on a PET scan, and biopsy confirmed the diagnosis of KFD. The encephalitis spontaneously remitted. The authors characterised brain lesions especially related to KFD in association with increased of IFN-α level in the CSF.
Background
Neurological manifestations associated with Kikuchi–Fujimoto disease (KFD) are poorly documented. We contribute to better characterise KFD encephalitis and provide new aspects to facilitate the diagnosis process.
Case presentation
A 39-year-old man presented with a 3-month history of chronic holocephalic headaches. Relatives reported behavioural disturbances and personality changes including irritability. The patient described episodes of diplopia, memory disturbances, difficulty concentrating and a short period of confusion (during which he was unable to recognise his family members) that had occurred over the last month. The worsening of neurological status was associated with fatigue, lack of appetite and night sweats.
The patient was admitted to the hospital for an inclusive neurological investigation. He was a native of Réunion Island but had resided in continental France since the age of 6. He had history of cervical lymphadenopathy with thrombopaenia and leucopenia at age 17.
The patient was apyretic and alert but presented with slow ideation. His cognitive assessment revealed difficulties with short-term memory and attentional tasks. Deep tendon reflexes were present. Strength, sensation, coordination and oculomotricity were normal. There were no photophobia or neck stiffness despite having sustained headaches.
Investigations
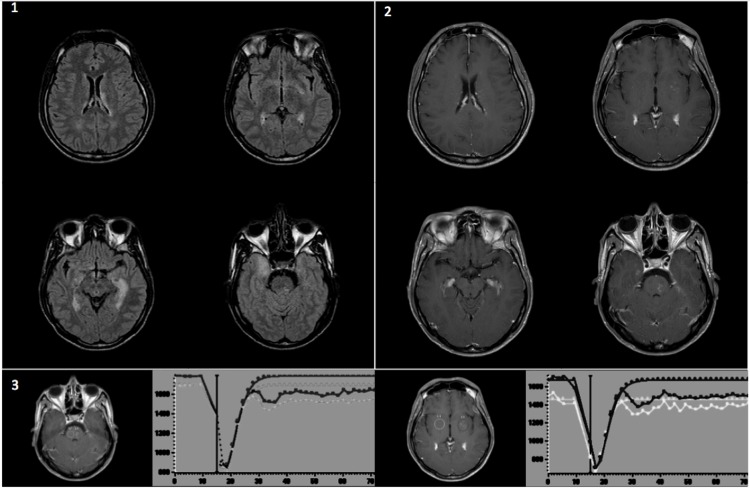
T2 and fluid-attenuated inverse-recovery sequences of brain MRI revealed hyperintense lesions in the internal temporal lobes, lenticular nuclei, left internal capsule and pontine region. These lesions (especially the peri-vascular spaces) and the lateral ventricle walls were enhanced with gadolinium on T1 weighted images. Dynamic susceptibility contrast imaging (perfusion sequences) was normal (figure 1). The EEG was normal.
Figure 1.
MRI of the brain. 1) Axial fluid-attenuated inverse-recovery (FLAIR) sequence; 2) axial gadolinium-enhanced T1-weighted images; 3) Dynamic susceptibility contrast imaging (perfusion sequences) of areas with perivascular gadolinium enhancement (black circles) compared with control areas (gray and white circles).
A laboratory blood panel revealed normocytic anaemia (10.6 g/dl), leucopenia (2.900/mm3), neutropaenia (1.300/mm3), lymphopaenia (1.200/mm3), polyclonal hyperglobulinaemia (16 g/l), hyperferritinaemia (1200 ng/ml) and an erythrocyte sedimentation rate (ESR) of 45 mm/h.
Lumbar puncture (LP) yielded clear cerebrospinal fluid (CSF) with 33 white blood cells/mm3 (100% lymphocytes), a protein concentration of 167 mg/dl and a glucose concentration of 54 mg/dl. Intrathecal immunoglobulin synthesis was absent in CSF, and IL6, IL10 levels were normal. The lymphocyte phenotype was polyclonal and displayed CD3+ reactive T lymphocytes. Interferon α (IFN-α) levels increased, with a value of 37 UI/ml (N<2 UI/ml). CSF cultures for bacteria and fungi were negative. PCR performed on CSF to detect bacteria or viruses were also negative (Mycobacteria, Chlamydia pneumoniae, Mycoplasma pneumoniae, Legionella pneumoniae, Bordetella pertussis, Enterovirus, Epstein–Barr virus (EBV), Herpes simplex virus 1 & 2, Varicella zoster virus, Cytomegalovirus (CMV), John Cunningham virus, Human herpes virus 6 & 8, Influenza virus type A (including H5N1) & B, Respiratory syncytial virus type A & B, Parainfluenza virus, Coronavirus, Rhinovirus, Adenovirus, Metapneumovirus). Quantitative PCR was negative for EBV. Cellular MRC5 culture with CSF showed no evidence of viral infection. Serological tests for HIV, EBV, CMV, Parvovirus B19, Hepatitis A, B and C virus, Toxoplasma, Treponema pallidum, Borrelia burgdorferi were negative. The result of a 10U Mantoux test for tuberculosis was anergic, and the ECA was 55 UI/l (N<52) in blood and undetectable in CSF.
Treatment with acyclovir was initiated after the first LP and was stopped when a second LP was performed 8 days later: 15 white blood cells/mm3 (70% lymphocytes), protein concentration of 97 mg/dl, glucose concentration of 57 mg/dl and IFN-α level increased to 50 UI/ml.
Tests for antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody and anti-DNA were negative. The assessment for paraneoplasic syndrome using an antineuronal antibody was also negative.
The body CAT scan displayed mediastinal adenopathy (size <1 cm). The PET scan showed hypermetabolic mediastinal and cervical lymphadenopathies but no cerebral fixation.
A biopsy of one cervical lymphadenopathy was performed. Histological analysis showed eosinophilic necrotic areas with apoptosis that were associated with immunoblastic cells and histiocytes. Lymphoma and infection were excluded. These results led to the diagnosis of KFD.
Outcome and follow-up
A month later, the disease spontaneously remitted. Neurological examination was normal. Brain MRI showed a decrease on T2 and gadolinium enhancement. Anaemia (10.6 g/dl) was observed with a neutrophil and platelet rates at 1.562/mm3 and 166 000/mm3. The autoimmune assessment remained normal. Two months later, brain MRI, CSF INF-α and blood tests were normal.
Discussion
We report a case of encephalitis associated with KFD and describe a high level of IFN-α in CSF.
KFD is defined by histological criteria corresponding to histocytic necrotising lymphadenitis.1 2
The adenitis is usually localised to the cervical region. Fever is frequently observed in association with other general symptoms such as asthenia, anorexia, weight loss and night sweats. Inflammatory syndrome with increased ESR is present in 16% of cases, and leucopenia (including neutropaenia and lymphopaenia) occurs in 18% of cases. An increase of CD4/CD8 is often observed. Hypergamma and high level of ferritin are sometimes described. Evolution is spontaneously favourable, and recurrences are very rare.3–5
Neurological manifestations in KFD are not common. Aseptic lymphocytic meningitis was described in 9.8% of cases in Japan and can represent the first sign of KFD (45% of those patients did not present Kerning’s sign and neck stiffness).
Five cases of encephalitis, sometimes limited to the cerebellum and brain stem, have been previously associated with meningitis.6 7 Cerebellar ataxia, diplopia and slowing of ideation are commonly observed.8 In one case, epileptic manifestations have been associated with confusion.9
KFD can be confused with lymphoma, tuberculosis or lupus. These were screened for and excluded in our case. The internal temporal brain lesions led to rule out infection and limbic encephalitis. An exhaustive search for infectious agents was negative. The EEG did not show epileptic features, and the serum was negative for antineuronal antibodies.
Little information is available regarding MRI of KFD in the literature. Brain stem lesions and internal temporal lesions have been previously described.9 10 This topography may therefore be of consideration as a feature of KFD brain lesions. In addition, we found an enhancement of the ventricular wall and perivascular space inside the parenchymal lesion, without abnormality of relative cerebral blood volume in the perfusion sequence.
An increase of IFN-α level in the CSF was observed without viral infection. Neurological symptoms have been described in association with IFN-α treatment used in hepatitis C and carcinoma, but the MRI lesions observed in these cases were different from those observed in KFD.11–13
An upregulation of the IFN-α type I response has been hypothesised in KFD and could lead to an increase of IFN-α level in the CSF.
In conclusion, we characterised brain lesions especially related to KFD. An increased level of IFN-α in CSF without viral infection also seems to be an important feature of KFD. A diagnosis of KFD is important to consider because it often goes into spontaneous remission, although corticotherapy is sometimes necessary in more severe cases.
Learning points.
Lesions of limbic and pontine regions were observed in KFD encephalitis.
MRI perivascular enhancement was another aspect.
Increased level of IFN-α in CSF without viral infection was a particular feature.
TEP could help to identify the adenitis to perform the biopsy.
KFD spontaneously recovers in most cases.
Acknowledgments
Robert Ghnassia M.D., Véronique Meignin M.D., Julien Savatovsky M.D.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytosis: a clinicopathological study. Acta Hematol Jpn 1972;35:379–80. [Google Scholar]
- 2.García CE, Girdhar-Gopal HV, Dorfman DM. Kikuchi-Fujimoto disease of the neck. Update. Ann Otol Rhinol Laryngol 1993;102:11–5. [DOI] [PubMed] [Google Scholar]
- 3.Kucukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol 2007;26:50–4. [DOI] [PubMed] [Google Scholar]
- 4.Kuo TT. Kikuchi’s disease (histiocytic necrotizing lymphadenitis). A clinicopathologic study of 79 cases with an analysis of histologic subtypes, immunohistology, and DNA ploidy. Am J Surg Pathol 1995;19:798–809. [DOI] [PubMed] [Google Scholar]
- 5.Lin HC, Su CY, Huang CC, et al. Kikuchi’s disease: a review and analysis of 61 cases. Otolaryngol Head Neck Surg 2003;128:650–3. [DOI] [PubMed] [Google Scholar]
- 6.Sato Y, Kuno H, Oizumi K. Histiocytic necrotizing lymphadenitis (Kikuchi’s disease) with aseptic meningitis. J Neurol Sci 1999;163:187–91. [DOI] [PubMed] [Google Scholar]
- 7.Shafqat S, Memon SB, Hyder S, et al. Brainstem encephalitis with Kikuchi-Fujimoto disease. J Coll Physicians Surg Pak 2003;13:663–4. [PubMed] [Google Scholar]
- 8.Moon JS, Il Kim G, Koo YH, et al. Kinetic tremor and cerebellar ataxia as initial manifestations of Kikuchi-Fujimoto’s disease. J Neurol Sci 2009;277:181–3. [DOI] [PubMed] [Google Scholar]
- 9.Avkan-Oguz V, Yapar N, Ozakbas S, et al. A case of fever of unknown origin: co-existence of Kikuchi-Fujimoto disease and acute disseminated encephalomyelitis (ADEM). Intern Med 2010;49:1823–6. [DOI] [PubMed] [Google Scholar]
- 10.Lays-Son L, Espinoza-Martinez Luis. Encephalitis associated to Kikuchi-Fujimoto’s disease in a young woman: a case report. Neurology Asia 2011;16:81–3. [Google Scholar]
- 11.Smedley H, Katrak M, Sikora K, et al. Neurological effects of recombinant human interferon. Br Med J (Clin Res Ed) 1983;286:262–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulbricht D, Metz RJ, Ries F, et al. [Alpha]-interferon encephalopathy. Neurology 2003;61:1301. [DOI] [PubMed] [Google Scholar]
- 13.Höftberger R, Garzuly F, Dienes HP, et al. Fulminant central nervous system demyelination associated with interferon-alpha therapy and hepatitis C virus infection. Mult Scler 2007;13:1100–6. [DOI] [PubMed] [Google Scholar]