Abstract
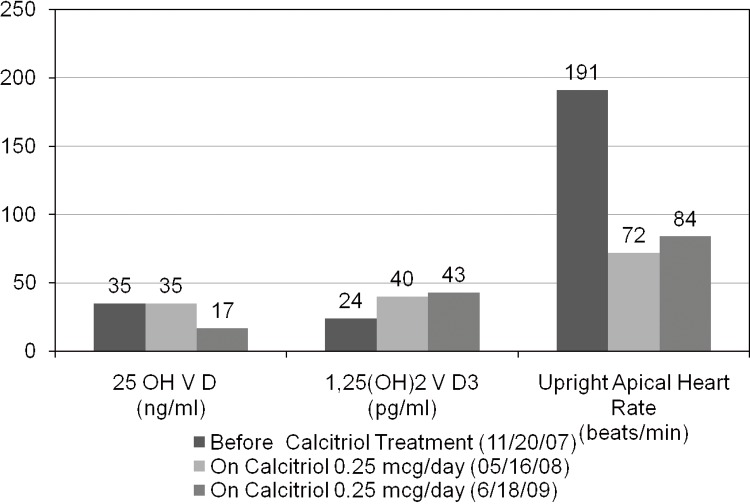
A 37-year-old woman presented with a history of reactive hypoglycaemia, non-classic adrenal hyperplasia (NCAH), osteopenia and fibromyalgia. After several months of palpitations, postural orthostatic tachycardia syndrome (POTS) was diagnosed by tilt table studies. Her heart rate (HR) reached 191 bpm at 60 degrees from horizontal. Investigation suggested increase in epinephrine and norepinephrine levels in response to tilt table. Her 25(OH) vitamin D level measured by immunoextraction radioimmunoassay was 35 pg/ ml (normal 9–54 pg/ml) while her 1,25(OH)2 vitamin D3 level was 24 pg/ml (normal 30–67 pg/ml). Accordingly, she was started on calcitriol 0.25 mcg orally daily. At her next visit after 5 months, she reported remarkable improvement in her palpitations and had been working full time for the past 4 months. HR both seated and upright was 72 bpm. After 3 months, her 1,25(OH)2 vitamin D3 level on calcitriol was 40 pg/ml. The authors suggest that 1-α hydroxylation defects should be sought and treated, if present, with calcitriol in patients with POTS.
Background
Postural orthostatic tachycardia syndrome (POTS) is an increase in heart rate (HR) from supine to upright position of >30 beats/min (bpm) or HR >120 bpm with head up tilt. POTS is an autonomic disturbance characterised by symptoms of orthostatic intolerance, mainly lightheadedness, fatigue, sweating, tremor, anxiety, palpitations, exercise intolerance and near syncope. POTS may cause other symptoms related to hypoperfusion of the upper portion of the body, for example, chest pain, dyspnoea, headache, muscle weakness and visual disturbances. Autonomic dysfunction may cause nausea, vomiting, constipation, diarrhoea and abdominal discomfort.1 2 Inappropriate levels of epinephrine (E) and norepinephrine (N) lead to anxiety-like symptoms for example, flushing, overheating, nervousness and chills. The symptoms lead to limitation of activities, impacting the patient’s life daily. POTS is most common in the age group 12–50 years with a female to male ratio of 5:1. It occurs most commonly after stress, such as sepsis, pregnancy, fever, surgery or trauma. Several aetiologies for POTS have been suggested including: decreased α-1 receptor sensitivity,3 β-1 receptor supersensitivity1 and impaired vascular innervation. The Vanderbilt Medical Center group has reported that a missense mutation of Ala457Pro (C1369G) in exon 9 of the norepinephrine transporter (NET) gene results in loss of function which has been associated with POTS. In humans, the NET gene is located on chromosome 16 locus 16q12.2.4
POTS has been reported in patients with chronic fatigue syndrome, restless leg syndrome, and Ehlers–Danlos syndrome (the latter has also been associated with mutations in the tenascin portion of the 21-hydroxylase pseudogene; it should be noted in this regard that our patient has non-classical 21-hydroxylase deficiency, which, may be associated with DNA exchange between the active gene and the pseudogene). A 1-α hydroxylation defect is a feature of chronic renal failure and vitamin D resistant rickets, while vitamin D deficiency is reported in fibromyalgia, a frequent co-morbidity of POTS.5–7
POTS is diagnosed with tilt table test. A blood test may be performed during the course of tilt table testing to check for abnormally high levels of catecholamines present in some patients with POTS.
Case presentation
The patient is a 37-year-old woman with a medical history of reactive hypoglycaemia, non-classic adrenal hyperplasia (NCAH), aldosterone synthase deficiency, osteopenia and fibromyalgia. She presented with severe postural palpitations and profound exertional fatigue in 2001. She reported that she is too fatigued to do daily activities and she has been essentially bedridden. She continued to have episodes of nausea, blurry vision, unrefreshing sleep and symptoms of heaviness in her legs and ankles. The patient was diagnosed with POTS via tilt table study with simultaneous plasma catecholamine levels (table 1; catecholamine data not shown because they were collected at another center and were unavailable to us). She was treated with several recommended options as follows:
Table 1.
Tilt table test results
| Time (min) | Tilt angle (°) | BP (mm Hg) | HR (bpm) | Comment |
|---|---|---|---|---|
| 1 | 60 | 112/80 | 191 | Severe palpitations, lightheaded |
| 5 | 60 | 134/90 | 141 | Symptomatic improvement |
| 10 | 60 | 168/104 | 114 | |
| 15 | 60 | 135/80 | 132 | Mild palpitations |
| 20 | 0 | 130/78 | 104 |
BP, blood pressure; HR, heart rate.
propanolol 20 mg q6h, cortisone acetate 12.5 mg qAM and 25 mg qPM, clonazepam 0.5 mg four times a day, fludocortisone acetate 0.05 mg four times a day, sertraline hydrochloride 25 mg four times a day, epoetin α (to aim for haematocrit of 50%) 10 000 U alternating with 12 000 U weekly, midodrine 10 mg three times a day, hypoglycaemic, low-fat, low cholesterol, portfolio diet. All these modalities slightly improved her symptoms, but she continued to be disabled. On tilt table testing at the time of diagnosis her HR reached 191 bpm at 60 degrees from the horizontal. Typically, at office visits the differential between the seated and upright positions in her apical HR was 10–20 bpm and her upright apical HR usually exceeded 90 bpm accompanied by palpitations and lightheadedness.
After completing the initial informed consent process, serum was collected for vitamin D metabolites. Her 25(OH) vitamin D by immunoextraction RIA was adequate at 35 ng/ml (9–54), while her 1,25(OH)2 vitamin D3 by immunoextraction RIA was low at 24 pg/ml (normal range 30–67 pg/ml). Her estimated glomerular filtration rate was normal. Accordingly, she was started on calcitriol 0.25 mcg orally daily. At her next visit, 5 months later, she reported that her postural palpitations had virtually ceased and that she had been working full time for the past four months. Her apical HR both seated and upright was 72 bpm (figure 1). At her subsequent visit, 3 months later, her apical HR both seated and upright was 78 bpm. Her propanolol dose was tapered from 15 to 20 mg every 6 h. Her 1,25(OH)2 vitamin D3 level on calcitriol was 40 pg/ml. Her relevant tilt table results are given in table 1 below.
Figure 1.
Change in apical heart rate after calcitriol treatment.
Investigations
The patient came to the office in 06/09 with a slight increase in palpitations. We again checked her vitamin D metabolite levels. Her 1,25(OH)2 vitamin D3 level was 43 pg/ml, while her 25(OH) vitamin D had fallen to 17 ng/ml. Three weeks after adding cholecalciferol 400 IU orally daily the patient reported that palpitations had again resolved.
The changes in the patient’s blood pressure before and during calcitriol treatment are shown in table 2 below
Table 2.
Blood pressure readings before and during calcitriol treatment
| Calcitriol treatment | Seated blood pressure (mm Hg) | Upright blood pressure (mm Hg) |
|---|---|---|
| Before calcitriol treatment (2007) | 90/70 | 95/70 |
| On calcitriol 0.25 mcg/day (2008) | 80/60 | 80/60 |
| On calcitriol 0.25 mcg/day (2009) | 98/58 | 80/58 |
Treatment
Treatment is included in the case presentation and investigations above to provide a smoother flow.
Outcome and follow-up
We followed the patient from 2007 to 2009 and results are shown as above in figure 1. The results showed that repletion of both calcitriol and 25(OH) vitamin D helps to improve symptoms in patient with POTS.
Discussion
In the above patient, treatment with calcitriol improved the symptoms and physical findings of POTS. We found that the data from reported experiments on vitamin D deficient rats were consistent with findings in our patient. Antiel et al and Baksi et al’s studies reported that a vitamin D deficient diet increases norepinephrine levels in the body.7–10 Brion et al’s study reported that vitamin D deficiency decreases the activity of the enzyme phenylethanolamine n-methyltransferase (PNMT), which converts N to E. Hence, N levels are relatively higher than E levels.11 The question then raised is why β-1 expression is more prominent than α-1 expression in POTS. Dr Novelli et al’s study12 and Baksi et al’s study13 reported that a vitamin D-deficient diet induces a decrease in pressor response to N, which explains why there is an apparent β-1-receptor super-expression despite the relative increase in N, which is mostly a α-1 agonist. This, coupled with a decrease in E and decreased α-1 receptor expression, results in a tendency to postural tachycardia.
Learning points.
Published experimental data provide evidence that vitamin D deficiency could cause the development of POTS symptoms.14
Vitamin D deficiency causes an alteration of catecholamine levels via a change in PNMT activity in the sympathetic nervous system causing higher levels of norepinephrine than epinephrine.
Vitamin D deficiency results in α-1 adrenergic resistance manifesting as a tachycardic rather than a hypertensive response to N.
This patient with POTS has a defect in 1-α hydroxylase expression rendering her deficient in 1,25(OH)2 vitamin D3. Calcitriol repletion in this patient resulted in resolution of postural tachycardia and accompanying symptoms.
This patient’s last visit showed low 25(OH) vitamin D level and recurrence of palpitations. Although most 25(OH) D is converted to 1,25(OH)2 D3 in the kidney, it is recognised that many other tissues in the body (brain, colon, prostate, breast, immune cells, heart, vasculature) have the capacity to convert 25(OH) D to 1,25(OH)2 D3. These other tissue types partially rely on this local production of 1,25(OH)2 D3 to help control cell growth, differentiation and activation. Therefore, for the body to maintain homeostasis, 25(OH) D level must be adequate, regardless of 1,25(OH)2 D3 concentration. We recommend normalising 25(OH) D levels as well if low levels are found.13 14
It is possible that mutations in the gene or promoter for PNMT are linked with a loss of function mutation in the gene for 1-α hydroxylase or with a defect in its transcription, translation or post-translation expression. This possibility is unlikely, however, in our patient because her postural tachycardia resolved when she was replete in both vitamin D metabolites.
Fibroblast growth factor-23 (FGF-23) is known to downregulate the expression of 1-α hydroxylase. Increased FGF-23 might be suspected if hypophosphatemia/hyperphosphaturia is present. However, there is no data available linking or refuting FGF-23 involvement with POTS. Serum inorganic phosphate was normal in our patient and 24 h urinary phosphate has been ordered for her next visit.15
We recommend measurement of both vitamin D metabolites in patients with POTS and repletion of both calcitriol and 25(OH) vitamin D.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Stewart JM, Gewitz MH, Weldon A, et al. Orthostatic intolerance in adolescent chronic fatigue syndrome. Pediatrics 1999;103:116–21. [DOI] [PubMed] [Google Scholar]
- 2.Grubb BP, Kosinski DJ, Boehm K, et al. The postural orthostatic tachycardia syndrome: a neurocardiogenic variant identified during head-up tilt table testing. Pacing Clin Electrophysiol 1997;20:2205–12. [DOI] [PubMed] [Google Scholar]
- 3.Gordon VM, Opfer-Gehrking TL, Novak V, et al. Hemodynamic and symptomatic effects of acute interventions on tilt in patients with postural tachycardia syndrome. Clin Auton Res 2000;10:29–33. [DOI] [PubMed] [Google Scholar]
- 4.Grubb BP. Orthostatic Intolerance. National Dysautonomia Research Foundation Patient Conference. July 2000, Minneapolis, Minnesota. [Google Scholar]
- 5.Robertson D, Shannon JR, Biaggioni I, et al. ; Neurolab Autonomic Team. Orthostatic intolerance and the postural tachycardia syndrome: genetic and environment pathophysiologies. Neurolab Autonomic Team. Pflugers Arch 2000;441(2–3 Suppl):R48–51. [DOI] [PubMed] [Google Scholar]
- 6.Karas B, Grubb BP, Boehm K, et al. The postural orthostatic tachycardia syndrome: a potentially treatable cause of chronic fatigue, exercise intolerance, and cognitive impairment in adolescents. Pacing Clin Electrophysiol 2000;23:344–51. [DOI] [PubMed] [Google Scholar]
- 7.Antiel RM, Caudill JS, Burkhardt BE, et al. Iron insufficiency and hypovitaminosis D in adolescents with chronic fatigue and orthostatic intolerance. South Med J 2011;104:609–11. [DOI] [PubMed] [Google Scholar]
- 8.Baksi SN, Hughes MJ. Chronic vitamin D deficiency in the weanling rat alters catecholamine metabolism in the cortex. Brain Res 1982;242:387–90. [DOI] [PubMed] [Google Scholar]
- 9.Baksi SN, Hughes MJ. Alteration of adrenal catecholamine levels in the rat after dietary calcium and vitamin D deficiencies. J Auton Nerv Syst 1984;11:393–6. [DOI] [PubMed] [Google Scholar]
- 10.Baksi SN, Hughes MJ. Deficiency in dietary vitamin D, not calcium, alters noradrenergic responsiveness in rat atria in vitro. J Mol Cell Cardiol 1986;18:653–6. [DOI] [PubMed] [Google Scholar]
- 11.Brion H, Parvez S, Parvez C, et al. , Effects of glucocorticoids upon adrenal and urinary epinephrine and NE and activity of enzyme PNMT in rats made partially deficient in Vitamin D. Role of vitamin D supplementation. Horm Mat 1978;10:556–60. [DOI] [PubMed] [Google Scholar]
- 12.De Novellis V, Loffreda A, Vitagliano S, et al. Effects of dietary vitamin D deficiency on the cardiovascular system. Res Commun Chem Pathol Pharmacol 1994;83:125–44. [PubMed] [Google Scholar]
- 13.Baksi SN. Altered pressor response to norepinephrine in calcium- and vitamin D-deficient rats. Clin Exp Hypertens A 1988;10:811–32. [DOI] [PubMed] [Google Scholar]
- 14.Nemerovski CW, Dorsch MP, Simpson RU, et al. Vitamin D and cardiovascular disease. Pharmacotherapy 2009;29:691–708. [DOI] [PubMed] [Google Scholar]
- 15.Perwad F, Zhang MY. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 2007;293:F1577–83. [DOI] [PubMed] [Google Scholar]