Abstract
Purpose
Many surgical patients are admitted to the intensive care unit (ICU), resulting in an increased demand, and possible waste, of resources. Patients who undergo liver resection are also transferred postoperatively to the ICU. However, this may not be necessary in all cases. This study was designed to assess the necessity of ICU admission.
Methods
The medical records of 313 patients who underwent liver resections, as performed by a single surgeon from March 2000 to December 2010 were retrospectively reviewed.
Results
Among 313 patients, 168 patients (53.7%) were treated in the ICU. 148 patients (88.1%) received only observation during the ICU care. The ICU re-admission and intensive medical treatment significantly correlated with major liver resection (odds ratio [OR], 6.481; P = 0.011), and intraoperative transfusions (OR, 7.108; P = 0.016). Patients who underwent major liver resection and intraoperative transfusion were significantly associated with need for mechanical ventilator care, longer postoperative stays in the ICU and the hospital, and hospital mortality.
Conclusion
Most patients admitted to the ICU after major liver resection just received close monitoring. Even though patients underwent major liver resection, patients without receipt of intraoperative transfusion could be sent to the general ward. Duration of ICU/hospital stay, ventilator care and mortality significantly correlated with major liver resection and intraoperative transfusion. Major liver resection and receipt of intraoperative transfusions should be considered indicators for ICU admission.
Keywords: Hepatectomy, Major resection, Intensive care units, Intraoperative transfusion
INTRODUCTION
Surgery in patients with chronic liver disease has a higher morbidity and mortality than surgery in patients without chronic liver disease [1]. The risks associated with surgery are particularly high in patients with diseases of the hepatobiliary system requiring major liver resection [2,3]. Because of improvements in the understanding of hepatic anatomy and surgical techniques, outcomes of liver resection have improved [4] with posthepatectomy mortality at less than 5% [5,6]. Postoperative management is one of the contributors to the reduction of morbidity and mortality. Key elements of perioperative management include appropriate nutritional support [7], respiratory assistance, infusion of fluid and blood components [8], and liver medications [9].
To reduce postoperative morbidity and mortality, aggressive monitoring of patients, early detection of life threatening complications, and immediate therapeutic interventions are important. Comprehensive monitoring in the intensive care unit (ICU) facilitates all of these elements of care [10]. On the other hand, many surgical patients are admitted to the ICU, resulting in an increased demand, and possible waste, of resources [11,12]. Hence, selective admission of surgical patients to the ICU has been suggested [13-15]. Bastounis et al. [16] evaluated the need for ICU admission among patients who have undergone vascular surgery. However, studies on the necessity of ICU admission among posthepatectomy patients have not been reported, to our knowledge.
The aim of this study was to assess the necessity of ICU care and suggest guidelines for determining ICU admission in these patients.
METHODS
Patients
We retrospectively analyzed the medical records of 313 patients with hepatocellular carcinoma who underwent liver resection, as performed by a single surgeon from March 2000 to December 2010. The Child-Pugh class of all patients except one was "A" class. The extent of liver resection was determined according to the indigocyanine green retention rate at 15 minutes (ICG R15) results and tumor characteristics. When there were poor prognostic characteristics following as presence of vascular invasion, satellite nodule and infiltrative type, we performed more wide resection within the limit of residual liver functions. Patients admitted to the ICU directly, without admission to a general ward after liver resection, were placed in the ICU group. Patients admitted from surgery to a general ward were placed in the non-ICU group.
Definitions
Body mass index (BMI) was divided into 4 categories according to the World Health Organization classifications for underweight (<18.5), normal (18.5 to 25), overweight (25 to 30), and obese (>30). Major liver resection was defined as resection of more than three segments of the Couinaud. Intraoperative transfusion included only red blood cell transfusion. Among complications, ascites and pleural effusions that occurred as surgical complications but resolved with medical management and without drainage were not considered complications in the analysis; only those requiring intervention were considered complications. Model for end-stage liver disease (MELD) scores were calculated according to the formula used by Freeman [17] Intensive treatments in ICU did not include only vital sign monitoring, but inotrophics use with close monitoring for maintaining blood pressure, hyperoxygenation for improving low oxygen saturation and continuous renal replacement therapy for treating acute renal failure.
Outcomes
Clinical and operative characteristics were compared between the ICU and non-ICU groups. Predictive factors for intensive treatments and the ICU re-admission were analyzed. Postoperative course was re-analyzed according to subgroups with/without having predictive factors.
Statistical analysis
Statistical analysis was performed using the SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). All continuous variables are presented as median (range), and categorical variables are presented as percentages of affected patients. A univariate analysis of the characteristics in each group was conducted using the Mann-Whitney U test for continuous variables and the chi-square test or Fisher's exact test for categorical variables. For predictive factor of special care and the ICU re-admission, multiple logistic regression analysis was used in univariate and multivariate analyses. P-values < 0.05 were considered statistically significant.
RESULTS
Baseline characteristics
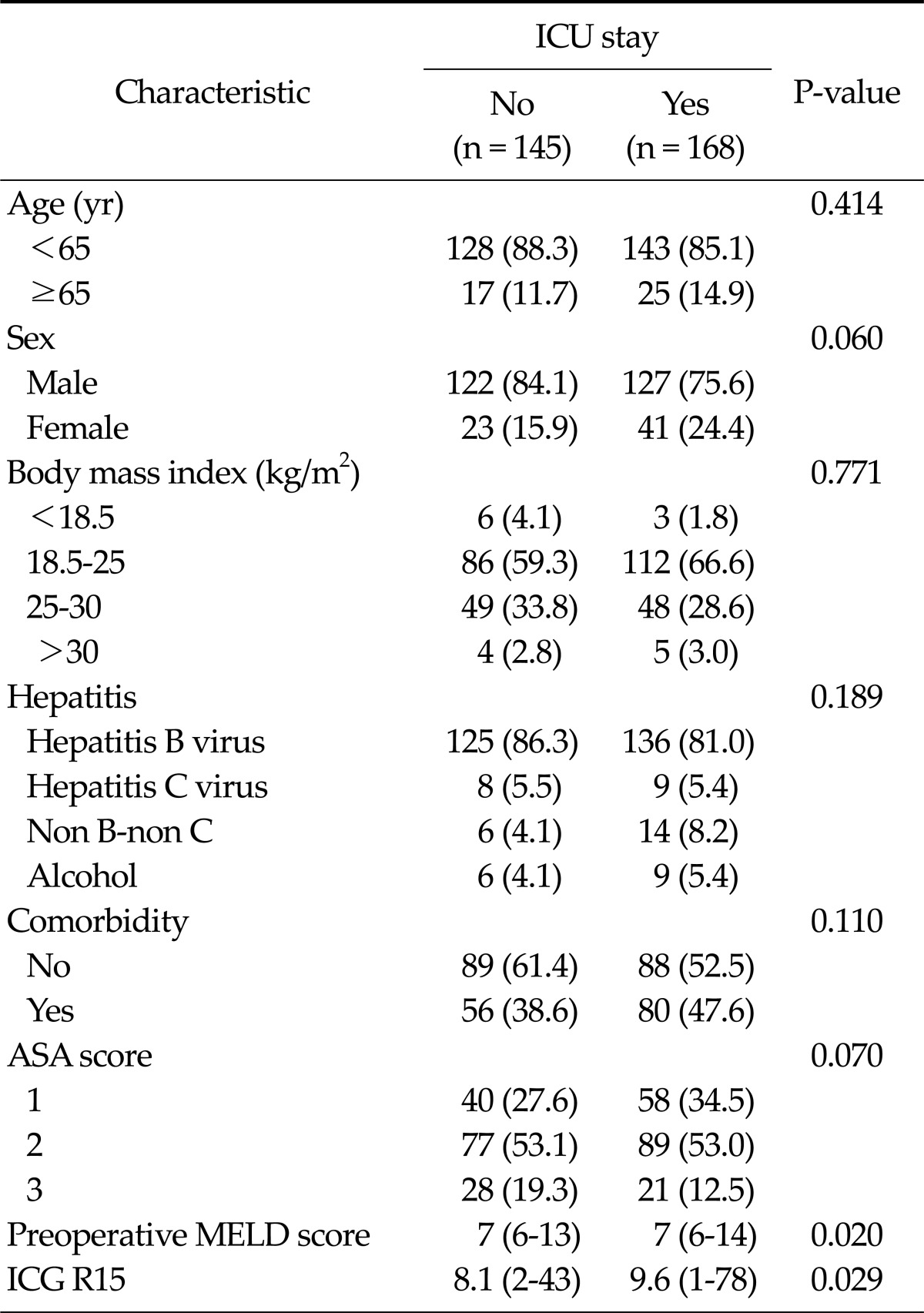
All patients had a pure hepatocellular carcinoma and underwent liver resection by a single surgeon. Baseline characteristics are presented in Table 1. Of the 313 patients, 168 patients (53.7%) were treated in the ICU. In both groups, males predominated and normal weight was most common. Hepatitis B virus was the most common cause of the underlying liver disease. The American Association of Anesthetists (ASA) score was 2 in 77 patients (53.1%) of the non-ICU group and 89 patients (53%) of the ICU group. Preoperative MELD score and ICG R15 were significantly higher in the ICU group than those in the non-ICU group.
Table 1.
Baseline characteristics according to intensive care unit (ICU) stay
Values are presented as no. of patients (%) or median (range).
ASA, American Association of Anesthetists; MELD, model for end-stage liver disease; ICG R15, indigocyanine green retension rate at 15 minutes.
Operative and postoperative characteristics
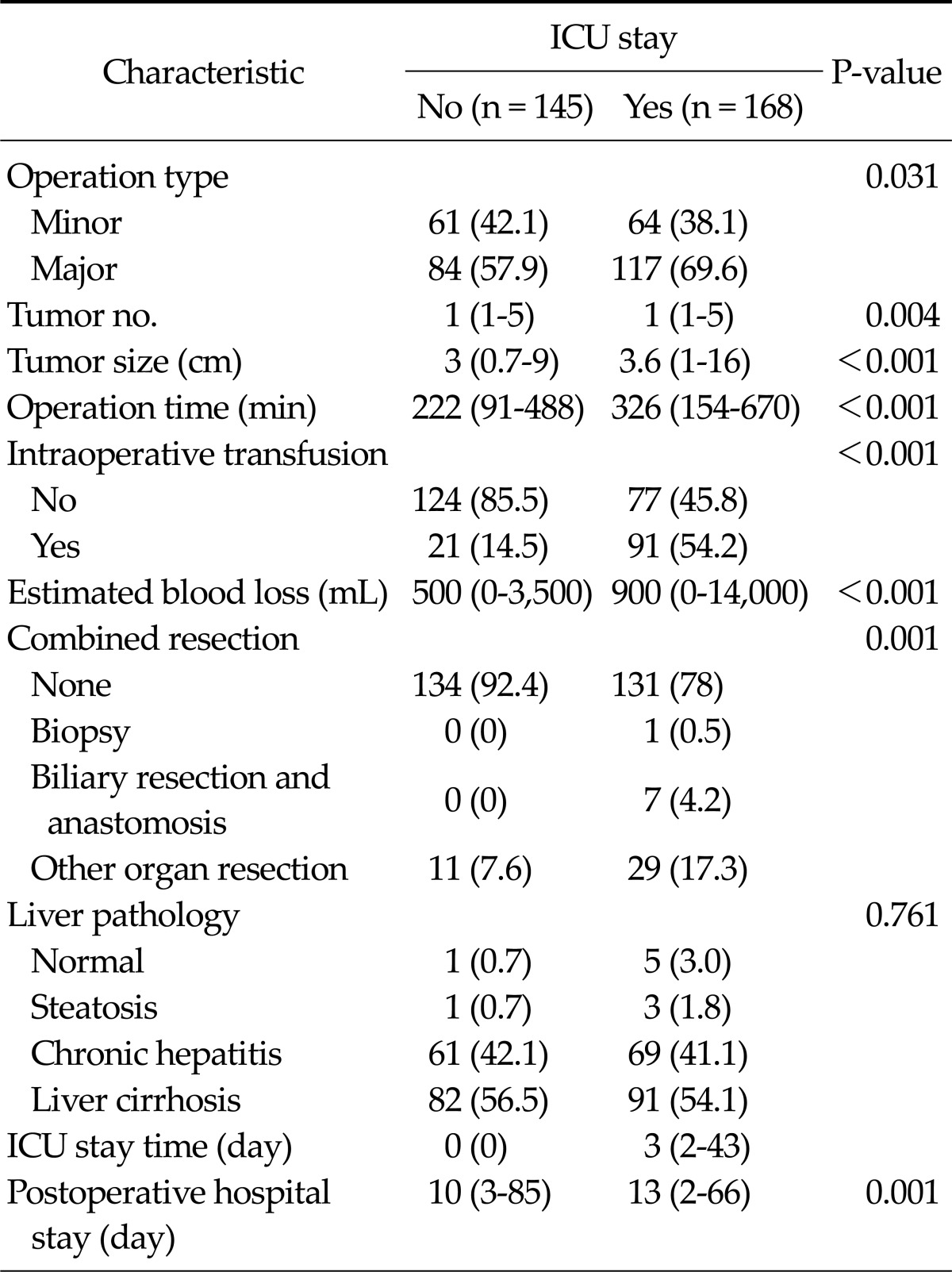
Major liver resection was performed more frequently in the ICU group (117 patients, 69.6%; P = 0.031). Median operation time and estimated blood loss in the ICU group were 326 minutes and 900 mL. The rate of intraoperative transfusion in the ICU group was higher, such as 91 patients (54.2%), than that (21 patients, 14.5%) in the non-ICU group (P < 0.001) (Table 2). Among both groups, non-tumor liver pathology did not show a significant difference. Median postoperative hospital stay (13 days) in the ICU group was significantly higher than that (10 days) in the non-ICU group (P = 0.001) (Table 2).
Table 2.
Operative and postoperative characteristics according to intensive care unit (ICU) stay
Values are presented as no. of patients (%) or median (range).
Predictive factors of intensive treatment and ICU re-admission
Among 313 patients, 22 patients (7%) received intensive treatments in the ICU group or readmitted to the ICU because of hepatic encephalopathy, pulmonary edema, variceal bleeding and re-operation. 148 patients (88.1%) in the ICU group did not received any intensive treatments and were only observed in the ICU.
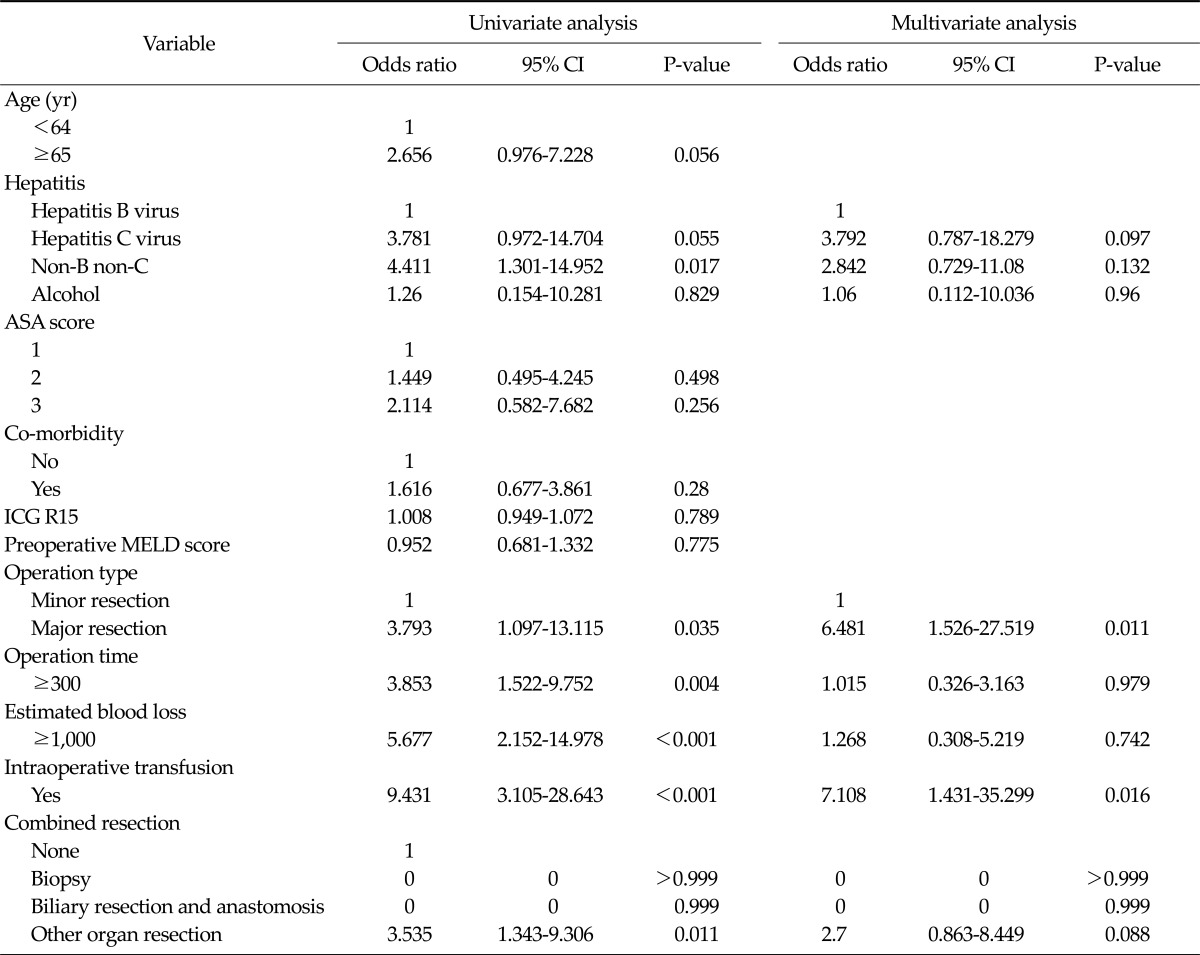
Intensive treatment and the ICU re-admission were closely associated with followings in univariate analysis: Non-B non-C liver disease (odds ratio [OR], 4.411; 95% confidence interval [CI], 1.301 to 14.952; P = 0.017), major liver resection (OR, 3.793; 95% CI, 1.097 to 13.115; P = 0.035), operation time more than 300 minutes (OR, 3.853; 95% CI, 1.522 to 9.752; P = 0.004), intraoperative transfusion (OR, 9.431; 95% CI, 3.105 to 28.643; P ≤ 0.001), and combined resection of other organ (OR, 3.535; 95% CI, 1.343 to 9.306; P = 0.011). However, multivariate analysis showed major liver resection (OR, 6.481; 95% CI, 1.526 to 27.519; P = 0.011) and intraoperative transfusion (OR, 7.108; 95% CI, 1.431 to 35.299; P = 0.016) as an independent predictive factor (Table 3).
Table 3.
Univariate and multivariate analysis of prognostic factors for intensive treatmenta) in intensive care unit (ICU) and re-admission to ICUb)
CI, confidence interval; ASA, American Association of Anaesthetists; ICG R15, indigocyanine green retension rate at 15 minutes; MELD, model for end-stage liver disease.
a)Intensive treatment included use of the inotrophics for maintain blood pressure, hyperoxygenation because of low oxygen saturation and continuous renal replacement therapy because of transient acute renal failure. b)Re-admission meant later transfer from the general ward to the ICU for problems during recovery.
The correlation with postoperative course
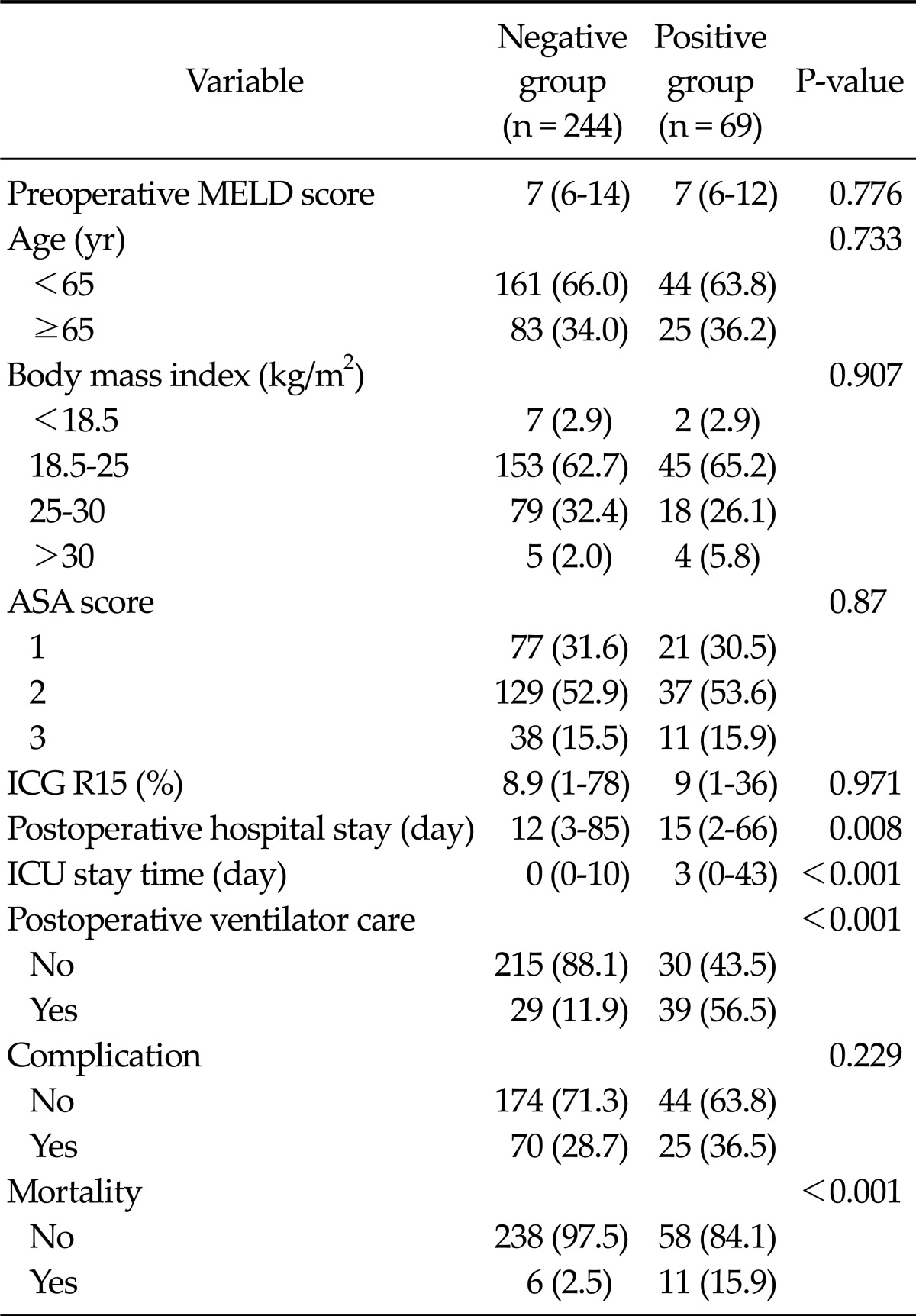
The patients were classified as positive group with predictive factors and negative group without predictive factors. Preoperative conditions (MELD score, age, BMI, ASA score, and ICG R15) did not show any difference between the both groups (Table 4).
Table 4.
The postoperative outcomes according to the intensive care unit (ICU) admission guidelinea)
Values are presented as median (range) or no. of patients (%).
ASA, American Association of Anaesthetists; MELD, model for end-stage liver disease; ICG R15, indigocyanine green retension rate at 15 minutes.
a)ICU admission guideline was major liver resection plus intraoperative transfusion.
Positive group showed longer median postoperative hospital stay (15 days) and ICU stay (3 days) than negative group (12 days and 0 days). The rate of postoperative ventilator care was higher in positive group (39/69 patients, 56.5%) than in negative group (29/244 patients, 11.9%). Complication rate did not show any significant difference between both groups. However, morality was significantly high in positive group (11 patients, 15.9%) (Table 4).
DISCUSSION
Most patients with liver cancer experience deteriorated liver function due to malignancy and/or underlying disease. In such cases, the risk of morbidity and mortality after liver resection is increased [2,3,18]. Therefore, the risk of surgery due to co-morbidity and underlying diseases should be assessed carefully prior to surgery, and the extent and technique for surgery should be optimized accordingly. In addition, postoperative care at the ICU level should be considered to provide adequate observation of vital signs and signs of potentially life-threatening complications. Preoperative liver function and general physical status were assessed using the Child-Pugh class [19,20] and ASA score [21], and residual liver function using ICG R15 [22]. The risk of surgery according to these tests has been well known, and the results of such evaluation are of help in determining the appropriateness and extent of surgery. However, guidelines for ICU vs. general-ward admission after hepatectomy have not been established.
The concept of the ICU was introduced in 1801 in the Newcastle Infirmary, England, to provide a space for seriously sick patients or patients undergoing major surgery [23]. In the 1900s, the ICU developed into a space for selective patients, and in 1923, the first ICU was built in the department of neurosurgery [24]. After the introduction of the coronary ICU in the 1960s, mortality of acute myocardial infarction was reduced by approximately 20% [25]. However, the ICU has been used excessively in caring for postoperative patients and, thus, has been considered a waste of resources and a contributing factor to unnecessarily long hospital stays in many cases [11,12]. In fact, some studies indicated that selective admission to the ICU reduced hospital stays and costs without an apparent adverse effect on postoperative morbidity and mortality. These studies then suggested guidelines for determining which postoperative patients should be admitted to the ICU [13-15]. In addition, efforts have been made to establish general guidelines for admission to and discharge from the ICU [26]. Such guidelines have been developed by the American College of Critical Care Medicine and the Society of Critical Care Medicine. Indicators for ICU admission for postoperative patients were a need for ventilator support, chronic co-morbidities that may develop into acute severe medical or surgical illnesses, and shock or hemodynamic instability [26]. However, many postoperative patients (70 to 76%) undergo monitoring only while in the ICU. This applies to posthepatectomy patients among others. In previous studies, those patients were defined as low-risk monitor (LRM) patients and "too well to benefit."
In our study, most patients (148/168 patients, 88.1%) in the ICU group underwent monitoring only. This finding suggests that all patients after liver resection may not need admission to the ICU, and the patients who are admitted to the general ward did not need ICU-level care. However, the LRM patients are not no-risk patients and guidelines for admission to the ICU in posthepatectomy patients may be needed. Even though the guidelines mentioned earlier suggest that co-morbidities indicate a need for ICU admission, co-morbidities were not associated with intensive treatment and ICU re-admission. Another study reported that co-morbidities did not affect the early outcomes of liver resection [27].
Advanced age was one of the limitations for surgery. However, according to some studies, morbidity and mortality after liver resection do not differ significantly between patients older or younger than 70 years [28]. In our study, similarly, intensive treatment and the ICU re-admission were not associated with age.
The presence of intraoperative transfusions and major liver resection were closely related with intensive treatment and ICU re-admission in univariate and multivariate analysis. Major liver resection was the most common cause of posthepatectomy liver failure. It was the most serious complication in the patients who underwent liver resection. This complication increased the cost markedly [29]. Intraoperative transfusion may affect not only oncologic outcomes but also morbidity [30]. In our study, Positive group underwent major liver resection and received intraoperative transfusion showing long ICU stay and postoperative hospital stay. They experienced more much postoperative ventilator care and mortality than negative group.
In conclusion, prior to surgery, it is very difficult to determine the need for postoperative ICU admission. If the following conditions are met, some patients need not be admitted to the ICU after hepatectomy: adequate preoperative assessment is performed, the extent and technique of the surgery are appropriately selected, and major intraoperative problems do not occur. However, patients who undergo major liver resection and intraoperative transfuision may need to have intensive treatment and ICU re-admission, in which case they may suffer extension of hospital stay, increase of cost and serious complications. Because LRM patients are not no-risk patients, further studies are needed to validate the guidelines derived from this study. However, we suggest that patients who require transfusion during major liver resection should be admitted to the ICU after liver resection for close monitoring for the development of serious complications.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084120).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Powell-Jackson P, Greenway B, Williams R. Adverse effects of exploratory laparotomy in patients with unsuspected liver disease. Br J Surg. 1982;69:449–451. doi: 10.1002/bjs.1800690805. [DOI] [PubMed] [Google Scholar]
- 2.Alfieri S, Carriero C, Caprino P, Di Giorgio A, Sgadari A, Crucitti F, et al. Avoiding early postoperative complications in liver surgery: a multivariate analysis of 254 patients consecutively observed. Dig Liver Dis. 2001;33:341–346. doi: 10.1016/s1590-8658(01)80089-x. [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Cowan JA, Jr, Knol JA, Upchurch GR., Jr Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138:185–191. doi: 10.1001/archsurg.138.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–380. doi: 10.1308/003588402760978148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547–1552. doi: 10.1056/NEJM199412083312303. [DOI] [PubMed] [Google Scholar]
- 8.Kaibori M, Saito T, Matsui K, Yamaoka M, Kamiyama Y. Impact of fresh frozen plasma on hepatectomy for hepatocellular carcinoma. Anticancer Res. 2008;28:1749–1755. [PubMed] [Google Scholar]
- 9.Kim YI, Hwang YJ, Song KE, Yun YK, Lee JW, Chun BY. Hepatocyte protection by a protease inhibitor against ischemia/reperfusion injury of human liver. J Am Coll Surg. 2002;195:41–50. doi: 10.1016/s1072-7515(01)01118-8. [DOI] [PubMed] [Google Scholar]
- 10.Goldhill DR. Preventing surgical deaths: critical care and intensive care outreach services in the postoperative period. Br J Anaesth. 2005;95:88–94. doi: 10.1093/bja/aeh281. [DOI] [PubMed] [Google Scholar]
- 11.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA, et al. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 12.Miranda DR, Rivera-Fernandez R, Nap RE. Critical care medicine in the hospital: lessons from the EURICUS-studies. Med Intensiva. 2007;31:194–203. doi: 10.1016/s0210-5691(07)74806-4. [DOI] [PubMed] [Google Scholar]
- 13.Bertges DJ, Rhee RY, Muluk SC, Trachtenberg JD, Steed DL, Webster MW, et al. Is routine use of the intensive care unit after elective infrarenal abdominal aortic aneurysm repair necessary? J Vasc Surg. 2000;32:634–642. doi: 10.1067/mva.2000.110173. [DOI] [PubMed] [Google Scholar]
- 14.Hobart DC, Nicholas GG, Reed JF, 3rd, Nastasee SA. Carotid endarterectomy outcomes research: reduced resource utilization using a clinical protocol. Cardiovasc Surg. 2000;8:446–451. doi: 10.1016/s0967-2109(00)00068-5. [DOI] [PubMed] [Google Scholar]
- 15.To EW, Tsang WM, Lai EC, Chu MC. Retrospective study on the need of intensive care unit admission after major head and neck surgery. ANZ J Surg. 2002;72:11–14. doi: 10.1046/j.1445-2197.2002.02285.x. [DOI] [PubMed] [Google Scholar]
- 16.Bastounis E, Filis K, Georgopoulos S, Bakoyannis C, Xeromeritis N, Papalambros E. Selective use of the intensive care unit after elective infrarenal abdominal aortic aneurysm repair. Int Angiol. 2003;22:308–316. [PubMed] [Google Scholar]
- 17.Freeman RB. MELD: the holy grail of organ allocation? J Hepatol. 2005;42:16–20. doi: 10.1016/j.jhep.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Das BC, Isaji S, Kawarada Y. Analysis of 100 consecutive hepatectomies: risk factors in patients with liver cirrhosis or obstructive jaundice. World J Surg. 2001;25:266–272. doi: 10.1007/s002680020059. [DOI] [PubMed] [Google Scholar]
- 19.Garrison RN, Cryer HM, Howard DA, Polk HC., Jr Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648–655. doi: 10.1097/00000658-198406000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansour A, Watson W, Shayani V, Pickleman J. Abdominal operations in patients with cirrhosis: still a major surgical challenge. Surgery. 1997;122:730–735. doi: 10.1016/s0039-6060(97)90080-5. [DOI] [PubMed] [Google Scholar]
- 21.Wolters U, Wolf T, Stutzer H, Schroder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217–222. doi: 10.1093/bja/77.2.217. [DOI] [PubMed] [Google Scholar]
- 22.Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg. 1992;163:515–518. doi: 10.1016/0002-9610(92)90400-l. [DOI] [PubMed] [Google Scholar]
- 23.Frost EA, Thomson DA. Development of the post-anaesthetic care unit. Baillieres Clin Anaesthesiol. 1994;8:749–754. [Google Scholar]
- 24.Calvin JE, Habet K, Parrillo JE. Critical care in the United States. Who are we and how did we get here? Crit Care Clin. 1997;13:363–376. doi: 10.1016/s0749-0704(05)70315-8. [DOI] [PubMed] [Google Scholar]
- 25.Lee TH, Goldman L. The coronary care unit turns 25: historical trends and future directions. Ann Intern Med. 1988;108:887–894. doi: 10.7326/0003-4819-108-6-887. [DOI] [PubMed] [Google Scholar]
- 26.Guidelines for intensive care unit admission, discharge, and triage. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Crit Care Med. 1999;27:633–638. [PubMed] [Google Scholar]
- 27.Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaibori M, Matsui K, Ishizaki M, Saito T, Kitade H, Matsui Y, et al. Hepatic resection for hepatocellular carcinoma in the elderly. J Surg Oncol. 2009;99:154–160. doi: 10.1002/jso.21221. [DOI] [PubMed] [Google Scholar]
- 29.Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg. 2011;254:907–913. doi: 10.1097/SLA.0b013e31821d4a43. [DOI] [PubMed] [Google Scholar]
- 30.Dionigi G, Boni L, Rovera F, Rausei S, Cuffari S, Cantone G, et al. Effect of perioperative blood transfusion on clinical outcomes in hepatic surgery for cancer. World J Gastroenterol. 2009;15:3976–3983. doi: 10.3748/wjg.15.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]