Abstract
One great challenge in our understanding of TGF-β cancer biology and the successful application of TGF-β targeted therapy is that TGF-β works as both a tumor suppressor and a tumor promoter. The underlying mechanisms for its functional change remain to be elucidated. Using 4T1 mammary tumor model that shares many characteristics with human breast cancer, particularly its ability to spontaneously metastasize to the lungs, we demonstrate that Gr-1+CD11b+ cells or myeloid derived suppressor cells (MDSCs) are important mediators in TGF-β regulation of mammary tumor progression. Depletion of Gr-1+CD11b+ cells diminished the anti-tumor effect of TGF-β neutralization. Two mechanisms were involved: first, treatment with TGF-β neutralization antibody (1D11) significantly decreased the number of Gr-1+CD11b+ cells in tumor tissues and premetastatic lung. This is mediated through increased Gr-1+CD11b+ cell apoptosis. In addition, 1D11 treatment significantly decreased the expression of Th2 cytokines & Arginase 1. Interestingly, the number and property of Gr-1+CD11b+ cells in peripheral blood/draining lymph nodes correlated with tumor size and metastases in response to 1D11 treatment. Our data suggest that the efficacy of TGF-β neutralization depends on the presence of Gr-1+CD11b+ cells, and these cells could be good biomarkers for TGF-β targeted therapy.
Keywords: TGF-β, Gr-1+CD11b+ cells, breast cancer, targeted therapy, biomarker
Introduction
TGF-β is over-expressed in many advanced human cancers. It correlates with metastasis and poor prognosis.1, 2 In preclinical mouse models, systemic inhibition of TGF-β signaling significantly suppresses pulmonary metastasis.3 Targeting TGF-β signaling is also very effective in osteolytic bone metastasis.4 TGF-β promotes tumor progression through dysregulation of cyclin-dependent kinase inhibitors, alteration in cytoskeletal architecture, increases in proteases and extracellular matrix formation, decreases in immune surveillance and increases in angiogenesis. Therapeutic strategies including neutralizing antibodies and small molecule inhibitors have been developed to target TGF-β signaling.5–7 However, TGF-β is well known for its dual role in tumor progression 8–10. TGF-β functions as a tumor suppressor in early tumor development. In a number of human cancers, mutations in the genes encoding TβRI and TβRII (Tgfbr1 and Tgfbr2, respectively) or decreases in expression and phosphorylation of other components of this pathway have been reported. In different mouse tumor models, the conditional knockout of Tgfbr2 has resulted in the much more aggressive tumor progression.11–14 These data support the notion that TGF-β has a tumor suppressor function, through which it inhibits cell cycle progression, increases apoptosis, and suppresses the expression of growth factors, cytokines and chemokines.
A significant challenge to the development of successful TGF-β antagonistic treatment is to understand the cellular and molecular mechanisms by which TGF-β changes its function from a tumor suppressor to a tumor promoter.10 TGF-β regulates the infiltration of inflammatory cells and cancer associated fibroblasts into the tumor microenvironment, resulting in changes in signaling cascade in tumor cells.15–17 Additionally, TGF-β exerts systemic immune suppression and significantly inhibits host tumor immune surveillance.18, 19 Gr-1+CD11b+ cells are overproduced in tumor hosts including cancer patients. This correlates with stage of tumor progression.20, 21 Gr-1+CD11b+ cells inhibit the function of NK, B and T cells through the production of arginase and reactive oxygen species. Further, they inhibit functional maturation of dendritic cells and promote type II macrophage development. They represent one of the mechanisms by which tumors escape from immune system control and compromise the efficacy of cancer immunotherapy.22–25 There are two major subpopulations of Gr-1+CD11b+ cells: mononuclear cells (precursors for macrophages), and low-density polymorphonuclear cells (immature neutrophils). Both populations suppress antigen-specific T-cell responses, but through distinct effector molecules and signaling pathways.26 Tumor-infiltrating Gr-1+CD11b+ cells also have non-immune suppressive effects that could profoundly impact tumor progression and metastasis. For example, they produce high levels of metallic matrix proteases (MMPs) and TGF-β, which contribute to tumor angiogenesis, vasculogenesis (incorporate into tumor vasculature),21 and tumor invasion.17 Very interestingly, the production of TGF-β by myeloid cells was more important in suppressing the immune response than that by the tumor cells.27
Although Gr-1+CD11b+ cells are major resource for TGF-β production, and are very immune suppressive, it remains to be investigated as what roles of Gr-1+CD11b+ cells have in TGF-β regulation of mammary tumor progression and how do they respond to anti-TGF-β treatment. Using the 4T1 mammary tumor model, we show that treatment with an anti-TGF-β antibody (1D11) suppresses metastasis and tumor growth through Gr-1+CD11b+ cell mediated mechanisms. Depletion of Gr-1+CD11b+ cells diminished the anti-tumor effect of TGF-β neutralization. This was mediated through increased Gr-1+CD11b+ cell apoptosis in tumor microenvironment and premetastatic lung. In addition, 1D11 treatment also decreased the expression of Th2 cytokines (IL-4, IL-10), GM-CSF (which enhances Th2 cytokine production), and Arginase1. Furthermore, the number and property of Gr-1+CD11b+ cells in peripheral blood/draining lymph nodes correlated with tumor size and metastases in response to 1D11 treatment. Our data suggest that Gr-1+CD11b+ cells are important in TGF-β regulation of tumor progression and might be good biomarkers in TGF-β targeted therapy.
Materials and Methods
Cell line and mice
4T1 mammary tumor cell line was maintained per standard cell culture techniques. Balb/cANCr mice were purchased from NIH Frederick. CL4 transgenic mice specific to HA 518–526 peptide (IYSTVASSL) were from Jackson lab. All animal studies were performed under National Cancer Institute IACUC approved protocol LCBG-007.
Tumor growth and metastasis
4T1 tumor cells (5×104 cells) were injected into the #4 & 5 mammary glands of Balb/c mice. Mice were then randomized into two treatment groups. Anti-TGF-β antibody (1D11; 5 mg/kg body weight) or isotype control (13C4; 5 mg/kg body weight) was administered i.p. three times per week, starting one day after cell inoculation. For the metastasis studies, the tumors were surgically removed and weighted on day 12–14. For tumor growth studies, the tumors were removed and weighted on day 25 or 42 to evaluate the effect of 1D11 treatment. Mice were sacrificed at the end of the experiments by anesthetic overdose. Lungs were processed as described in the whole lung mounting procedure.28 Tumor nodules in lung were then counted. Representative lung samples were also fixed. Butterfly sections were then stained for HE, scanned and photographed.
Gr-1+CD11b+ cell depletion in 4T1 tumor-bearing mice
The 4T1 tumor cells (5×104 cells) were injected into the #2 mammary gland to increase the number of metastasis. To deplete Gr-1+CD11b+ cells in these tumor-bearing mice, anti-Gr-1 antibody (from RB6-8C5 hybridoma culture supernatant) or the control IgG (from Balb/c mouse serum), were purified by affinity chromatography technology in Antibody Production and Purification Unit, NCI, and the endotoxin level was < 0.1 EU per 1 μg of the antibody. The antibodies were injected into the tumor-bearing mice (i.p., 100ug per mouse) every 2 to 3 days. The percentage of Gr-1 positive cells in peripheral blood was analyzed by flow cytometry to determine the depletion efficiency. The 1D11 treatment started on day 6, and the treatment dose and schedule were the same as in the other experiments. As shown in figure 1B, the tumors were surgically removed on day 18. The tumor metastases were examined on day 42.
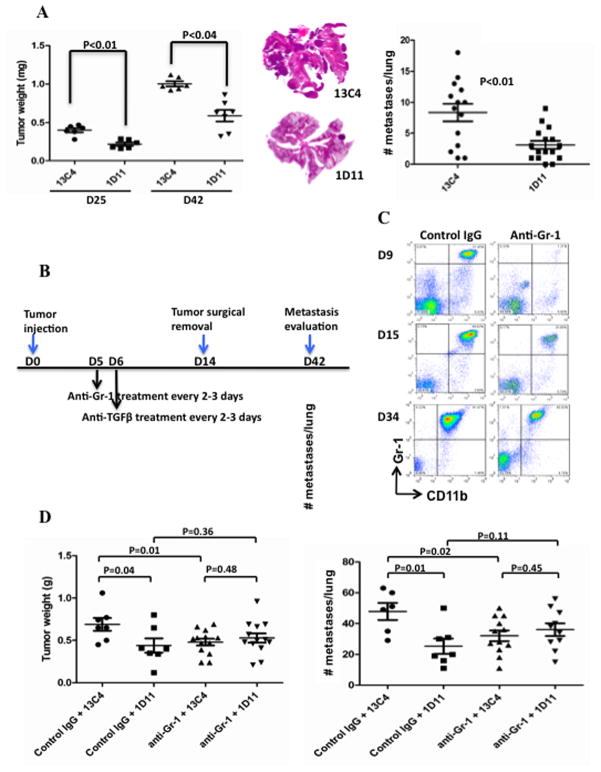
Figure 1.
Depletion of Gr-1+CD11b+ cells diminished the anti-tumor effects of TGF-β neutralization. (A) 1D11 treatment reduced tumor growth and lung metastasis of 4T1 cells. Mice bearing 4T1 tumors (#4 mammary gland) were treated (i.p.) with 1D11 or control 13C4. Tumor weight was taken at day 25 and 42 (left panel). HE staining of butter flying section of representative lungs is shown in the middle. In separate experiments for the metastasis studies, the tumors were surgically removed on day 12–14, number of lung metastasis was examined at the end of the experiment and the data are on the right panel. (B) Schematic diagram of the experimental plan for systemic depletion of Gr-1+CD11b+ cells (anti-Gr-1 antibody) in TGF-β neutralization therapy. (C) Flow cytometry analysis to examine Gr-1+CD11b+ cell depletion efficiency, shown are Gr-1+CD11b+ cells in peripheral blood on day 9, 15 and 34 after tumor injection. Nucleated blood cells are gated and analyzed by CD11b-FITC and Gr-1-PE. (D) Depletion of Gr-1+CD11b+ cells reduced tumor growth (left panel) and lung metastasis of 4T1 tumors (right panel), and diminished the anti-metastatic effects of TGF-β neutralization. Mice received 4T1 tumor cell injection in the #2 mammary gland. Bar shows mean ± SEM, each dot represents one mouse.
Flow cytometry analysis and single-cell sorting
Single cell suspensions were made from peripheral blood, lymph nodes, spleens, and bone marrow of normal and tumor-bearing mice,21 as well as tumor tissues and lungs.29 These cells were labeled with fluorescence-conjugated antibodies (BD PharMingen), CD11b-FITC, Gr-1-PE, Ly6G-APC, Ly6C-PE, CD45-Percp, CD11c-APC, Annexin V-PE, 7-AAD, and F4/80-Percp (Bio-legend), and isotype-matched IgG controls (BD PharMingen). For single-cell sorting, splenocytes or peripheral blood cells from normal or tumor-bearing mice were stained with fluorescence-labeled antibodies (CD11b-FITC, Gr-1-PE, BD PharMingen), and sorted with a FACSAria flow cytometer (BD Biosciences), or by MACS (Miltenyi Biotec) using Gr-1-PE and anti-PE conjugated microbeads according to manufacturer’s instruction. The purity of the both FACS and MACS sorted cells were more than 90%.
Immunofluorescence
Tissue sections were fixed with 4% paraformaldehyde. The slides were then incubated with anti-Gr-1 antibody (BD, 1:100 dilution) or anti-PAR antibody (BD, 1:100). Alexa flour 488 goat anti-rat (Invitrogen, 1:500) or Fluoresein horse anti-mouse (Vector Laboratories, 1:3000) antibodies were used to detect the presence of Gr-1+CD11b+ cells. The slides were mounted with Prolong Gold + DAPI (Invitrogen) and examined under fluorescence microscope. The tissue sections were stained by Histoserv, Inc (Germantown, MD) for H&E.
Bio-Plex assay
The protein samples of Gr-1+CD11b+ cells were prepared by RayBio cell lysis buffer (RayBiotech, Inc). The Bio-Rad Bio-Plex Pro Assay Mouse Th1/Th2 Group I kit, for cytokines IL-2, IL-4, IL-5, IL-10, IL-12, GM-CSF, IFN-g and TNF-α, was used to analyze the cytokines according to manufacturer’s instruction. Briefly, 50 ul of coupled premixed magnetic beads were loaded into the pre-wetted 96-well filter plate. After washing the plate two times with wash buffer by vacuum manifold, the standards, controls and samples were loaded into each well and the plate was incubated on shaker at 1000 rpm for 30 seconds, and then at 300 rpm for 30 minutes at room temperature. Then, 50 ul of detection antibody was loaded into the plate and the plate was incubated on shaker for 30 minutes at room temperature after washing the plate three times. After another 3 times wash, the 50 ul streptavidin-PE was added into each well and the plate was incubated on shaker for 10 minutes at room temperature. Then, 125 ul of assay buffer was added into each well after washing the plate three times. After another 30-second shaking, the plate was loaded into the machine, Bio-Plex 200 system (Bio-Rad), and the data was acquired and analyzed by the Bio-Plex Manger version 4.0 software (Bio-Rad).
Arginase activity assay
QuantiChrom™ Arginase Assay Kit (BioAssay Systems, Catalog #: DARG-200) was used to determine arginase activity in Gr-1+CD11b+ cells. Briefly, 106 sorted Gr-1+CD11b+ cells per sample were lysed for 10 min in 100 μL of 10 mM Tris-HCl buffer (pH 7.4) containing 1 μM pepstatin A, 1 μM leupeptin, and 0.4% (w/v) Triton X-100. The supernatant was collected after centrifugation. Then, 40 μL of sample and 10 μL of substrate buffer were combined into wells of 96-well reaction plate. Sample without substrate buffer, H2O, and Urea standard were used as blank control, ODBLANK, standard background, ODWATER, and standard, ODSTANDARD respectively. After incubation, Urea reagent and substrate buffer were added into each well. OD values were read at 520 nm. Arginase activity was calculated as = (ODSAMPLE − ODBLANK)/(ODSTANDARD − ODWATER) × [Urea Standard] × 50 × 103/(40 × t).
IFNγ ELISPOT assay
The Gr-1+CD11b+ cells from 4T1 tumor-bearing mice were sorted as described above. They were pre-treated with 1D11 or 13C4 antibodies (10μg/ml for 3 hours), and then co-cultured with splenocytes (2 × 105) from CL4 transgenic mice (3:1, splenocytes:myeloid cells). HA 518–526 peptide (1μg/ml) was added as stimulator. After 24-hour culture, IFNγ ELISPOT assay were preformed. The ELISPOT plate was scanned in ImmunoSpot (Cellular Technology Ltd. Shaker Heights, OH) and quantification was assessed using the CTL Scanning and CTL counting 4.0.
Western blot
Gr-1+CD11b+ cells sorted from spleens, lung, tumor or peripheral blood of 4T1 tumor bearing mice treated with 1D11 or 13C4 were lysed with RIPA buffer (1X PBS added 0.5% sodium deoxycholate, 0.1% SDS, 1% Nonidet P-40 and protease inhibitors). Protein concentrations were measured using Bio-Rad Dc Protein Assay reagent. Proteins were then separated on 4–12% SDS polyacrylamide gels and transferred to PVDF membranes, which were then blocked with 5% milk in Tris-buffered saline, 0.1% Tween 20 and incubated with primary antibodies (Caspase-3, 1/1000; PARP, 1/1000 and β-actin, 1/5000) overnight at 4 °C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (Sigma), and developed using an West Pico Chemiluminescent substrate kit (Pierce), and scanned in G:Box (syngene).
Quantitative RT-PCR of Arginase
Total RNA was extracted from sorted Gr-1+CD11b+ cells as described using an RNeasy Mini Kit (QIAGEN Inc). cDNA was synthesized using Invitrogen superscripttm First-strand synthesis system. Primers specific for Arg-1 and Gapdh were used and relative gene expression was determined using Bio-Rad iCycler-iQ SYBR Green PCR kit (Bio-Rad Laboratories). The comparative threshold cycle method was used to calculate gene expression normalized to Gapdh.
Statistical analysis
Two group comparison was analyzed using the Student t-test, and was expressed as mean±SE. For peripheral blood Gr-1+CD11b+ cells as biomarker experiment for anti- TGFβ cancer therapy, Mann –Whitney test was used. Differences were considered statistically significant when the p-value was < 0.05.
Results
Systemic depletion of Gr-1+CD11b+ cells diminished anti metastatic effect of TGF-β neutralization
TGF-β neutralization has been reported to inhibit tumor growth and metastasis.30–33 Because Gr-1+CD11b+ cells are the major source of TGF-β,21 we investigated whether they are responsible for TGF-β neutralization effect. We used the 4T1 mammary tumor model in which the mice were treated with TGF-β and/or Gr-1 neutralizing antibodies. The 4T1 tumor model shares many characteristics with human breast cancer, particularly its ability to spontaneously metastasize to the lungs. Mice were implanted with 5×104 4T1 cells orthotopically in the #4 mammary gland. For the metastasis studies, the tumors were surgically removed and weighted on day 12–14. For tumor growth studies, the tumors were removed and weighted on day 25 or 42 to evaluate the effect of 1D11 treatment. Treatment with the anti-TGF-β antibody (1D11), delivered intraperitoneally (i.p.), showed a significant reduction in tumor growth (Figure 1A, left panels) and lung metastasis (Figure 1A middle and right panels). There was a decreased tumor recurrence at the primary tumor injection site after 1D11 treatment (25%) compared to control antibody (13C4, 57%) (Supplementary Figure 1). Our results show the inhibitory effect of TGF-β neutralization on mammary tumor growth and metastasis, similar to previously published reports.32, 33
We next depleted Gr-1+CD11b+ cells and examined whether this would diminish the anti-tumor phenotype of TGF-β neutralization. In these experiments, the mice received 4T1 injection in the #2 mammary gland, which gave higher number of metastatic nodules. Mice received 100 ug/mouse of anti-Gr-1 antibody or control IgG (i.p.) on day 5 after tumor injection and every 2–3 days thereafter (Figure 1B). This was based on increased Gr-1+CD11b+ cell production and presence in distant lung as early as day 7 after 4T1 tumor injection.34 The TGF-β neutralization was followed from day 6 (Figure 1B). The percentage of Gr-1+CD11b+ cells in peripheral blood was examined to evaluate the depletion efficiency by flow cytometry. The depletion rate was 94%, 63%, and 56% for day 9, 15, and 34 respectively (Figure 1C). To avoid large primary tumor burden and allow metastasis to develop, the tumors were surgically removed and weighted on day 14. The anti Gr-1 treatment was withdrawn for 5 days after surgery to allow wound healing occur. The lungs were collected for whole lung mounting on day 42. Depletion of Gr-1+CD11b+ cells diminished tumor growth inhibition (Figure 1D left panel) and metastasis inhibition (Figure 1D right panel) by TGF-β neutralization. As expected, depletion of Gr-1+CD11b+ cells alone inhibited lung metastasis and tumor weights (Figure 1D), similar to that of TGF-β neutralization. No synergistic effect from combined treatment of 1D11 and anti Gr-1 was observed. These results suggest that Gr-1+CD11b+ cells likely mediate the anti metastatic effect of TGF-β neutralization.
TGF-β neutralization decreased the number of Gr-1+CD11b+ cells in tumor microenvironment, draining lymph node and premetastatic lung
We next examined mechanisms associated with Gr-1+CD11b+ cells that are responsible for decreased tumor metastasis upon TGF-β neutralization. We first looked into Gr-1+CD11b+ cell profiling in the tumor microenvironment and the immune organs (bone marrow, spleen, lymph node), as they are important in TGF-β regulation of tumor progression.17, 33, 34 We also examined the survival of Gr-1+CD11b+ cells in premetastatic lung, defined 14 days or earlier after tumor injection because these cells change the premetastatic lung into an inflammatory and proliferative environment, diminish immune protection, and promote metastasis through aberrant vasculature formation.34 We found significantly reduced number of Gr-1+CD11b+ cells in tumor microenvironment (figure 2A, with quantitative data on the right panels), draining lymph nodes (figure 2B, with immune fluorescence of Gr-1 on the right panels), and premetastatic lung (figure 2C), compared with the 13C4 treatment. No difference was found in Gr-1+CD11b+ cell numbers in bone marrow and spleen (supplementary figure 2). In two subpopulations of Gr-1+CD11b+ cells, Ly6G+CD11b, mostly immature neutrophils, and Ly6C+CD11b, mostly immature monocytes,22, 35, 36 1D11 treatment affected different subpopulations in different organs: Ly6G+ Gr-1+CD11b+ cells in tumor microenvironment and premetastatic lung (figure 2A and 2C), and Ly6C+ cells in lymph nodes (figure 2B). These results, together with data in figure 1, suggest that Gr-1+CD11b+ cells in tumor microenvironment, draining lymph nodes and premetastatic lung may be responsible for TGF-β regulation of tumor progression, and are the important target of 1D11 treatment.
Figure 2.
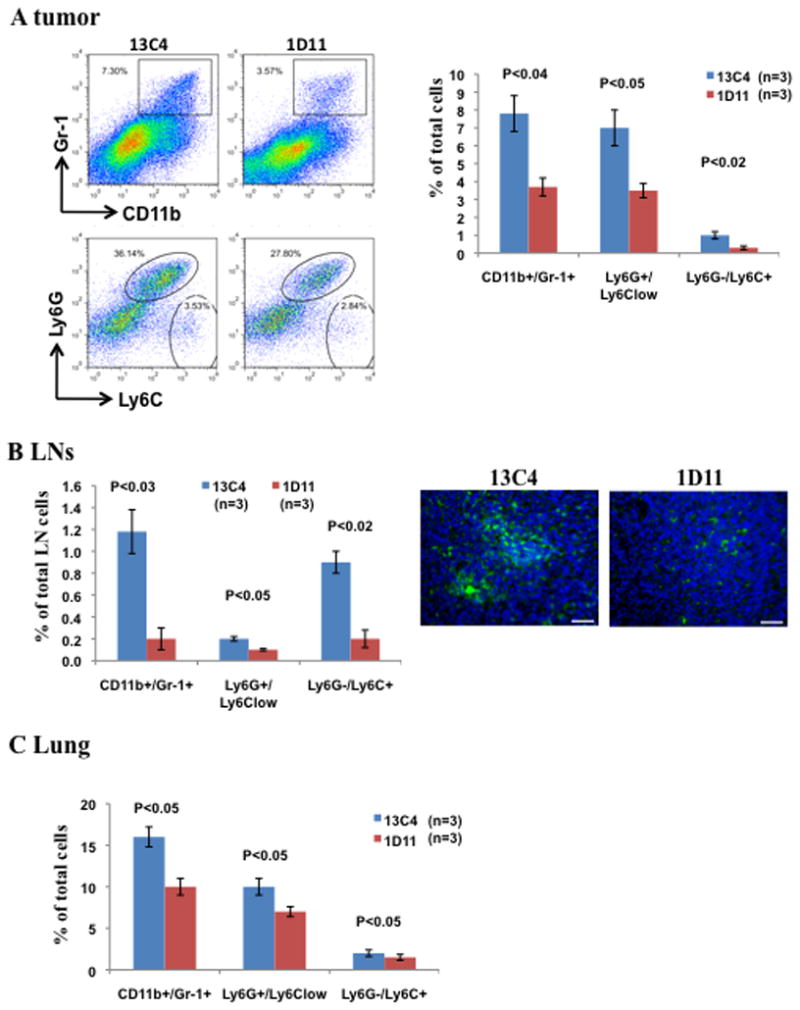
Decreased Gr-1+CD11b+ cells in tumor microenvironment, draining lymph nodes, and premetastatic lung of tumor bearing mice that received 1D11 or 13C4 treatment. The mice received orthotropic implantation of 4T1 cells (5 × 104) into #4 mammary gland. Three representative mice with average tumor size were selected from each group containing 6–8 mice for the assay, data show one of at least three repeats. Flow cytometry analysis of Gr-1+CD11b+ cells (upper panel) and subsets (gated CD11b+ cells, lower panel) in (A) 4T1 tumor tissues, one of three experiments is shown: representative dot plots (left) and statistic results (right, n=3). (B) Draining lymph nodes (n=3, left panel). Immunofluorescence staining of Gr-1+CD11b+ cells (green) in formalin-fixed draining lymph nodes are on the right. One of three independent experiments is shown. Magnification: X20. Scale bar: 200 μm (C) The lungs of 4T1 tumor bearing mice 14 days after tumor injection (n=3). One of two independent experiments is shown. Error bar = SEM.
TGF-β neutralization increased differentiation of Gr-1+CD11b+ cells into F4/80+ and decreased their Th2 cytokine expression
One potential explanation for decreased Gr-1+CD11b+ cells could be due to an increase in Gr-1+CD11b+ cell differentiation. We looked into two mature myeloid cell type: F4/80+ macrophages and CD11c+ dendritic cells upon TGF-β neutralization. We noticed that the decrease in Gr-1+CD11b+ cells is associated with a significantly increased number of macrophage (F4/80 positive) but not CD11c+ dendritic cells in the tumor, lymph nodes and premetastatic lung (figure 3A). Further examination showed significantly increased F4/80 positive cells within the Gr-1+CD11b+ cell population in tumor and premetastatic lung in mice treated with 1D11 (Figure 3B), indicating that Gr-1+CD11b+ cells, the immature myeloid cells, may differentiate into macrophages.
Figure 3.
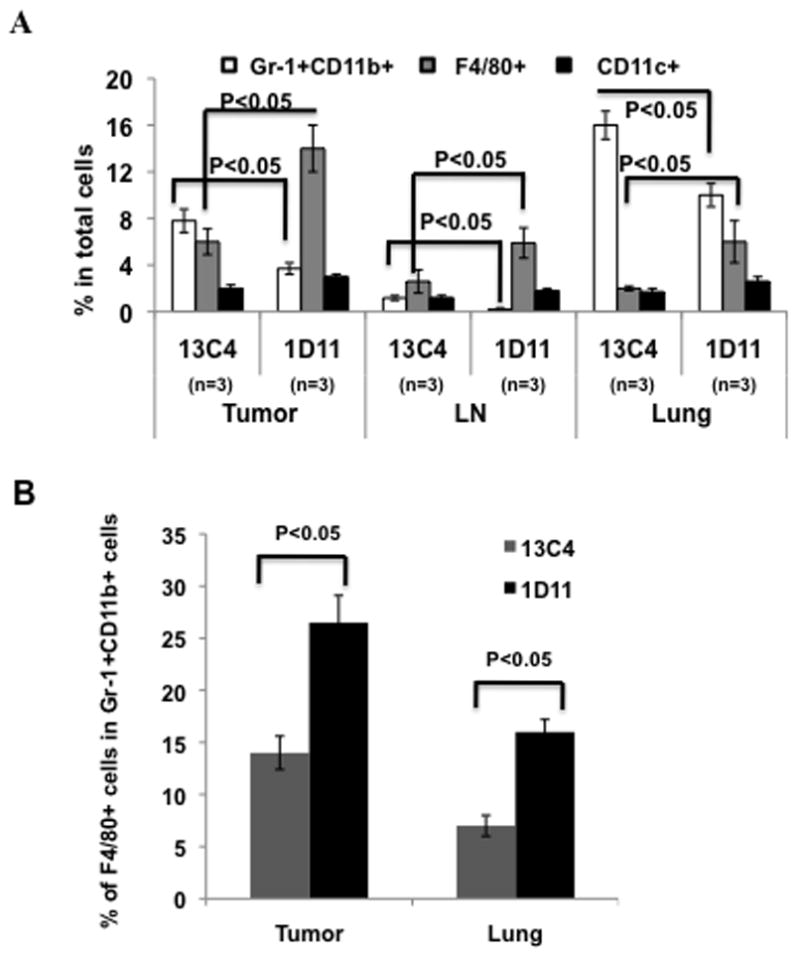
TGF-β neutralization decreased the number of Gr-1+CD11b+ cells, TH2 cytokine secretion, and immune suppressive activity. Three representative mice with average tumor size were selected from each group containing 6–8 mice for the assay, data show one of at least three repeats. (A) A decreased number of Gr-1+CD11b+ cells correlated with increased F4/80+ (Gr-1−CD11b−) cells in tumor tissue, draining lymph node (LN), and D14 lung of 4T1 tumor bearing mice. No difference in CD11c+ cells was found. One of three experiments is shown. (B) Increased F4/80 expression within Gr-1+CD11b+ cell population from tumor tissues and lungs of mice received 1D11 treatment.
To further characterize the properties and functionality of Gr-1+CD11b+ cells from tumor-bearing mice that received 1D11 treatment, we examined Th1/Th2 cytokine profile (IL-2, IL-4, IL-5, IL10, IL12, GM-CSF, IFN-γ and TNF-α) in sorted Gr-1+CD11b+ cells from peripheral blood. We utilized Bioplex technology which uses up to 100 unique fluorescently dyed beads that permit the simultaneous detection of up to 100 different types of molecules in a single well of a 96-well microplate.37 Gr-1+CD11b+ cells from 1D11 treated mice showed significantly decreased Th2 cytokine expression including IL-4, IL-10, but not IL-5. Additionally, 1D11 treatment also decreased GM-CSF, a cytokine that enhances Th2 cytokine production. There was no difference in Th1 cytokine production including IFNγ, TNF-α, IL-12, and IL-2 (figure 4A). Our results show that 1D11 inhibits the production of immune suppressive cytokines IL-4, IL-10 and GM-CSF in Gr-1+CD11b+ cells.
Figure 4.
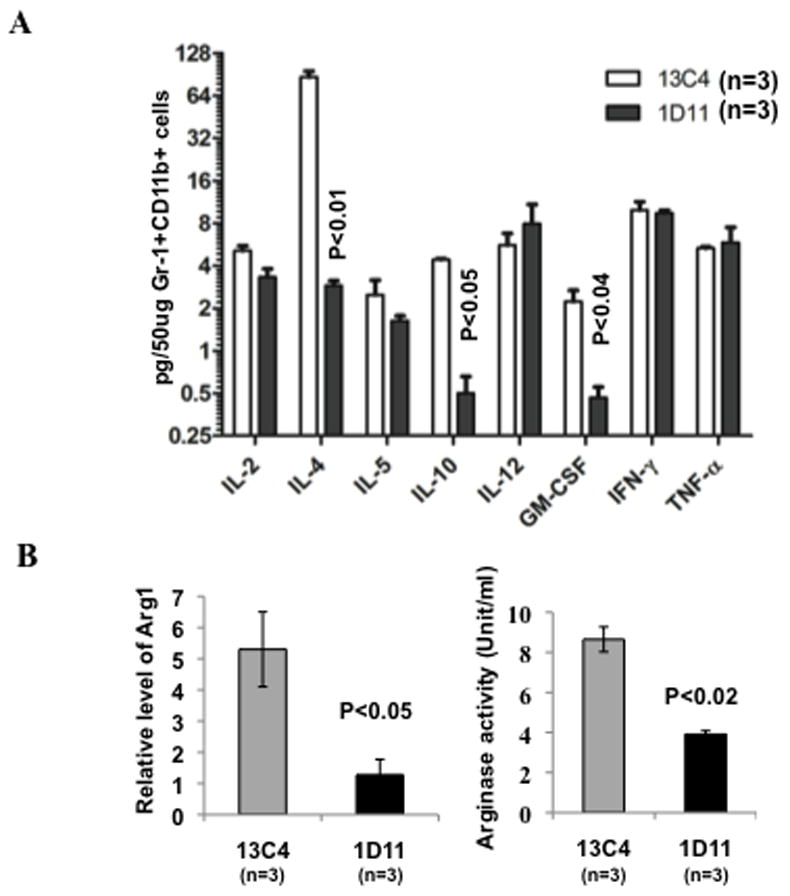
Decreased TH2 cytokine expression and immune suppressive property in Gr-1+CD11b+ cells. (A) Bioplex analysis of Th1/Th2 cytokines in FACS sorted peripheral blood Gr-1+CD11b+ cells. Three mice were used for each group. (B) Arginase expression and activity in FACS sorted blood Gr-1+CD11b+ cells. qRT-PCR of arginase expression, normalized to GAPDH, is shown on the left, arginase activity was on the right. n=3. Error bar = SEM, for all figures. Three representative mice with average tumor size were selected from each group containing 6–8 mice for the assay, data show one of at least three repeats. (C) IFNγ ELISPOT assay showing Gr-1+CD11b+ cells pre-treated with 1D11 significantly increased IFNγ production by T cells. The Gr-1+CD11b+ cells from 4T1 tumor-bearing mice were pre-treated with 1D11 or 13C4 antibodies and then co- cultured with splenocytes from CL4 transgenic mice (3:1, splenocytes:myeloid cells). HA 518–526 peptide was added to stimulate IFNγ production by T cells. Data are represented as mean ± SEM. * indicate p<0.05.
Gr-1+CD11b+ cells and T cells require L-arginine for protein synthesis, however, high level of arginase in Gr-1+CD11b+ cell could deplete their environmental L-arginine and limit L-arginine availability to T cells, which results in inhibited anti-tumor immunity.38 To examine whether neutralization of TGF-β affect L-arginine level in Gr-1+CD11b+ cells, we analyzed the Arg 1 mRNA level and the arginase activity. We found a significantly decreased Arg 1 expression (figure 4B, left panel) and arginase activity (figure 4B, right panel) after 1D11 treatment. Therefore, 1D11 treatment not only decreased Gr-1+CD11b+ cells number but also inhibited arginase mediated suppressive function of Gr-1+CD11b+ cells.
Decreased Th2 cytokine and arginase in Gr-1+CD11b+ cells after 1D11 treatment suggests that these cells may reduce immune suppressive effect on the T lymphocytes. We thus performed IFNγ ELISPOT assay in which the Gr-1+CD11b+ cells from 4T1 tumor-bearing mice were pre-treated with 1D11 or 13C4 antibodies and then co-cultured with splenocytes from CL4 transgenic mice (3:1, splenocytes:myeloid cells). We found that Gr-1+CD11b+ cells pre-treated with 1D11 significantly increased IFNγ production by T cells (figure 4C). The data suggest that 1D11 treatment also decreased immune suppressive effect of Gr-1+CD11b+ cells.
TGF-β neutralization induced apoptosis in Gr-1+CD11b+ cells in the tumor microenvironment and premetastatic lung
TGF-β is an important growth factor in hematopoiesis homeostasis. We next asked the question whether TGF-β neutralization affects Gr-1+CD11b+ cell survival and apoptosis. We observed significantly increased Annexin V expression in Gr-1+CD11b+ cells in the tumor microenvironment (figure 5A) and premetastatic lung (figure 5B) in mice that received 1D11 treatment compared to 13C4 control. The increased apoptosis was not observed in Gr-1+CD11b+ cells from blood, spleen, and lymph nodes (Supplementary Figure 3). We next looked into two prominent markers, Caspase-3 and Poly (ADP-ribose) polymerase (PARP), which are critical in apoptosis induction. Surprisingly, there was no difference in the expression of Caspase-3 between 1D11 and 13C4 treatment (figure 5C, left panel). Interestingly, there was an increased expression of PARP in Gr-1+CD11b+ cells from 1D11 treated tumors compared to 13C4 control (figure 5C, left panel). However, the PARP was not cleaved (figure 5C). PARP catalyzes the synthesis of Poly (ADP-ribose) PAR, an important signaling component of PARP dependent apoptosis. Therefore, we examined the expression of PAR in tumor sections by immunofluorescence staining. Notably, we observed an increased expression of PAR in tumors treated with 1D11 compared to 13C4 where no PAR was detected (figure 5C, right panels). Primarily, the PAR expression was localized in the invasive front where infiltration of Gr-1+CD11b+ cells was observed (data not shown). These data suggest that the therapeutic effect of TGF-β neutralization is likely dependent on the induction of Gr-1+CD11b+ cell apoptosis in the tumor microenvironment and premetastatic lung.
Figure 5.
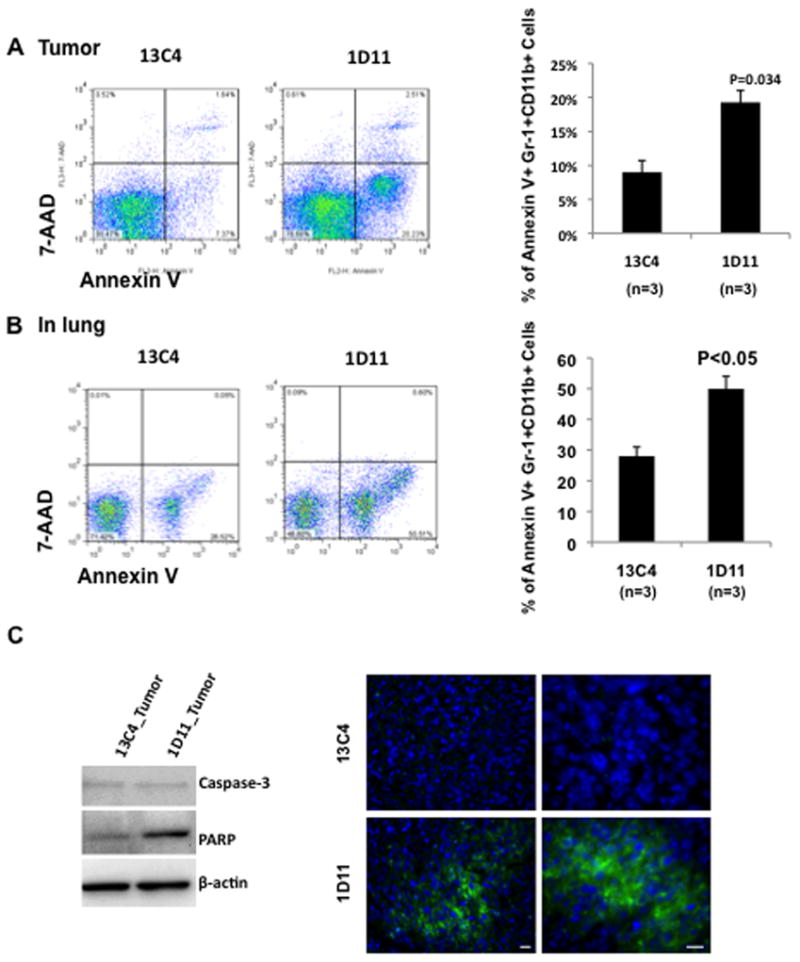
Treatment with 1D11 increased Gr-1+CD11b+ cell apoptosis in tumor microenvironment and premetastatic lung. Single cell suspension from tumor tissues (A) or D14 lung (B) were prepared and stained with Gr-1, CD11b, Annexin V and 7-AAD. The samples were then analyzed by flow cytometry. Three representative mice with average tumor size were selected from each group containing 6–8 mice for the assay, data show one of at least three repeats. Representative dot plots (gated Gr-1+CD11b+ cell, left) and statistic results (right, n=3 each for 1D11 or 13C4) are shown. Error bar = SEM. (C) Left panels: Western analysis showing an increased PARP expression in Gr-1+CD11b+ cells sorted from 1D11 treated tumors. β-actin was used as loading control. Right panels: Immunofluorescence staining of PAR in tumor tissue sections from mice that received 1D11 or 13C4 treatment. Scale bar −10 μm.
Circulating Gr-1+CD11b+ cells and effector cells as biomarkers for TGFβ-targeted therapy
Clinical studies demonstrate that the percentage of immature myeloid cells (the equivalent to the Gr-1+CD11b+ cells in mouse) in peripheral blood correlates with the stages of cancer patients.20, 21 Together with the significant responsiveness of Gr-1+CD11b+ cells to 1D11 treatment we observed in current studies lead us to investigate whether Gr-1+CD11b+ cells can be used as a biomarker in detecting the effectiveness of 1D11 treatment. We asked whether the number of Gr-1+CD11b+ cells correlates with the treatment outcome. We examined Gr-1+CD11b+ cells in peripheral blood as they are easily obtained and clinically relevant. We treated a large cohort of tumor bearing animals (20 for 13C4, and 30 for 1D11), with surgical tumor removal on day 12 after tumor injection. We then divided the animals that received 1D11 into non-responders and responders. This is based on the median of the tumor volume (figure 6A, measured at day 12 after surgical removal) or on number of lung metastasis nodules (figure 6B, on day 56 at the end of the experiments). In addition, the mouse number for each group was taken into consideration thus each group would have similar mouse number (n=14–16). For lung metastasis, non-responders were those mice with an average of 9 metastases (n=14), the responders were those with less than 4 metastases (n=16). Interestingly, there was a clear correlation of Gr-1+CD11b+ cell number with tumor size and lung metastasis. The 1D11 responders showed a significantly reduced number of Gr-1+CD11b+ cells in blood compared to the non-responders (figure 6A and 6B, right panels). The number of Gr-1+CD11b+ cells from non-responders was not significantly different from the 13C4 control treatment (figure 6B). Interestingly, mice with higher percentage (60–90%) of Gr-1+CD11b+ cells showed significantly decreased lung metastasis in response to 1D11 treatment but not those with lower number of Gr-1+CD11b+ cells (10–60%) (Supplementary Figure 4).
Figure 6.
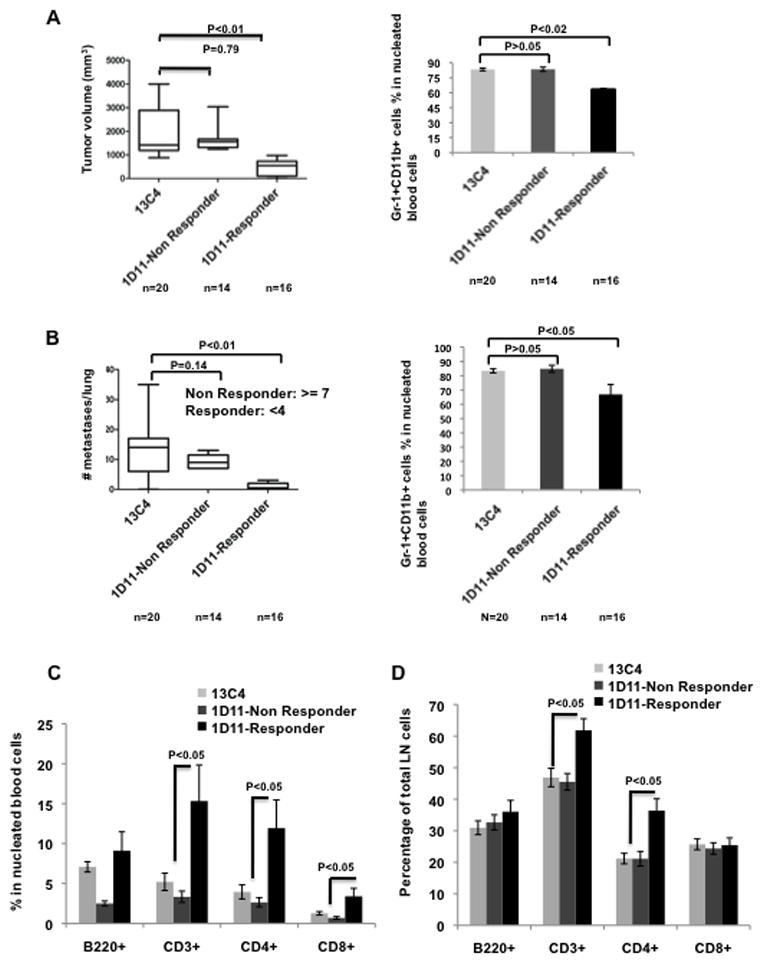
The number of Gr-1+CD11b+ cells in peripheral blood is explored as a potential biomarker for anti-TGFβ cancer therapy. A large cohort of tumor bearing mice was given 1D11 (n=30) or 13C4 (n=20) treatment. Mice in 1D11 treatment group were further divided into non-responder (n=14) and responder (n=16), according to the median of tumor volume (A, left panel) or number of lung metastasis (B, left panel), as indicated. Tumor weight, number of lung metastasis and percentage of peripheral blood Gr-1+CD11b+ cells were evaluated on Day 56 after tumor injection. The correlation of Gr-1+CD11b+ cell percentage with tumor size (A, right panel) or metastasis (B, right panel) is shown. C. The responder of 1D11 treatment showed increased CD3+, CD4+ and CD8+ T cells in peripheral blood compared to the control treatment or the non-responder group. D. The increased CD3+ and CD4+ but not CD8+ T cells were also observed in draining lymph nodes of mice treated with 1D11.
Gr-1+CD11b+ cells inhibit T lymphocyte proliferation and function under tumor condition. We next examined whether the decrease in number of Gr-1+CD11b+ cells are associated with increased T cells in the peripheral blood with 1D11 treatment. There were more CD3+ T cells, CD4 T cells, and CD8 T cells but not B cells in 1D11 treated mice than those treated with 13C4 (figure 6C). This was also true for responders compared to the non-responders (figure 6C). We further examined draining lymph nodes as they are often biopsied in clinic and can be used to monitor the treatment outcome. The increased number of CD3 and CD4 T cells was also observed (figure 6D). These data suggest that Gr-1+CD11b+ cells and their interacting effector T cells in peripheral blood as well as draining lymph nodes might be a good biomarker for TGFβ-targeted therapy.
Discussion
TGF-β exerts both anti-oncogenic and pro-oncogenic functions in cancer. Despite its complex biology, initial preclinical studies showed considerable efficacy of antibody-like TGF-β antagonists in suppressing metastasis.39, 40 Anti–TGF-β antibodies also completely inhibited tumor recurrence in a fibrosarcoma model and reduced metastasis in a colon cancer model.27 As a result of these and other studies, a number of TGF-β pathway antagonists are in late preclinical or early clinical development for treatment of patients with advanced cancer.6, 7 However, successful development of TGF-β antagonism will require a detailed understanding of which cell compartments and which biological processes are affected by TGF-β antagonism in vivo. Many questions remain to be answered. These include how patient should be selected? When such treatment should be given? What indicative biomarkers can be used? This is particularly important as TGF-β neutralization may jeopardize the homeostasis of many normal tissues and organs. Like a number of molecularly targeted therapeutics entering clinical cancer treatment, patient selection and inflammation/immune biomarkers for TGF-β antagonism therapy are to be explored.
Our studies show that Gr-1+CD11b+ cells are important mediators in tumor-promoting effect of TGF-β in mammary cancer progression. We also found that the number of Gr-1+CD11b+ cells in peripheral blood and T cells, as well as molecular properties can be used as biomarkers for TGF-β antagonism therapy. We built our studies on the 4T1 orthotopic mammary tumor model that shares many characteristics with human breast cancer, particularly its ability to spontaneously metastasize to the lungs. This model has been used as preclinical mouse model to study the effects of TGF-β neutralization on tumor metastasis.33 Here we demonstrate that deletion of Gr-1+CD11b+ cells diminished the anti-tumor effect of 1D11 treatment. The efficiency of Gr-1+CD11b+ cell depletion was very good in the earlier stage of tumor growth, but relapsed after the tumor size increased. However the effect on tumor growth and metastasis was still significant, suggesting that the Gr-1+CD11b+ cells may promote tumor in the earlier phase rather than later. We found two mechanisms for Gr-1+CD11b+ cells in response to 1D11 treatment: (1) 1D11 neutralizing TGF-β made by Gr-1+CD11b+ cells and (2) 1D11 affecting numbers and biological properties Gr-1+CD11b+ cells. Indeed, Gr-1+CD11b+ cells are a major source of TGF-β production under tumor conditions.17, 27
Gr-1+CD11b+ cells have been known to be immune suppressive. They consist of two major subpopulations: mononuclear cells (precursors for macrophages), and low-density polymorphonuclear cells (immature neutrophils).26 In addition to immune suppression, Gr-1+CD11b+ cells also infiltrate into tumors, promote tumor angiogenesis and metastasis by producing high levels of MMPs and TGFβ.21 These cells also mediate tumor refractoriness to anti-VEGF treatment.41 While these effects are distinct for each immune cell population, they are also interrelated. For example, TGF-β inhibition of CD8 cytotoxic activity is largely due to TGF-β produced by Gr-1+CD11b+ myeloid cells, which is further regulated by IL-13 produced by natural killer T cells, a novel natural killer cell type discovered in recent years.27 Very interestingly, this production of TGF-β by myeloid cells was more important in suppressing the immune response than production of TGF-β by the tumor itself. Blocking production of TGF-β in these myeloid cells (by eliminating these cells or by blocking the upstream signal from natural killer T cells or IL-13) abrogated the suppression even though TGF-β production by the tumor itself was not affected.27
TGF-β neutralization significantly decreased the number of Gr-1+CD11b+ cells in tumor tissues, draining lymph nodes, and premetastatic lung of 4T1 tumor bearing mice. The effects of 1D11 treatment on the tumor microenvironment has been previously reported.42, 43 Our data suggest two additional sites as important targets of 1D11 treatment: the draining lymph nodes and premetastatic lung. The latter is very interesting, as many therapeutic studies have not looked into the effect in the distant premetastatic organ. In fact, a recent report demonstrates an important role of TGFβ in the distant premetastatic lung. TGFβ regulates the production of the chemoattractants S100A8 and S100A9. These attract Mac1+ myeloid cells that activate mitogen-activated protein kinase p38 in tumor cells. This activation confers invasive activity via the development of pseudopodia (invadopodia).43
We found two mechanisms involving Gr-1+CD11b+ cells that are responsible for the anti TGF-β effect: 1D11 treatment skews Gr-1+CD11b+ cells to a less M2 phenotype through decreased Th2 cytokine expression, and increases Gr-1+CD11b+ cell apoptosis. The effect on the immune phenotype of the Gr-1+CD11b+ cells is not surprising as inhibition of TGF-β signaling using a small molecule inhibitor targeting kinase activity significantly modulates cytokine profiling of macrophages co-cultured with renal cell carcinoma and tumor associated neutrophils (identified as CD11b+Ly6G+ cells) in vivo.44, 45 These tumor-associated neutrophils, in fact are part of the Gr-1+CD11b+ cells. 44 Although in these studies, the Th1 cytokine was increased, whereas in our studies we found a decreased Th2 cytokine production using Bioplex technology. It has been previously reported that one of the major effects of TGF-β antagonism is to reverse the systemic immune suppression of TGF-β on the host immune system. In fact, neutralizing TGF-β in preclinical mouse models enhances CD8+ T-cell and natural killer cell-mediated anti-tumor immune response.30, 46, 47 As Gr-1+CD11b+ cells interact with multiple effector cells to exert immune suppression, our data suggest myeloid cells may play a primary and causal role in the immune effect by TGF-β targeted therapy.
Gr-1+CD11b+ myeloid cells are significantly increased in 4T1 tumor-bearing mice. In human, overproduction of immature myeloid cells are also reported, however, they are identified differently. Interestingly, the number of immature myeloid cells in peripheral blood of cancer patients correlates with the stages of tumor progression including breast, lung, and head & neck cancers.48 Our studies have further extended those findings to show that Gr-1+CD11b+ cells are important mediators in TGFβ antagonism treatment. The number and molecular properties of these Gr-1+CD11b+ cells in the peripheral blood can be used as biomarker for efficacy of the treatment outcome. This is supported by recent findings indicating that the immune/inflammatory responses are reliable markers for predicting clinical outcome in human hepatocellular carcinoma49 and colorectal tumors.50 These new understanding of TGF-β signaling in regulation of tumor microenvironment and immune response may provide useful information, particularly for patient selection and inflammation/immune biomarkers for TGF-β antagonism therapy in clinical trials.
Supplementary Material
TGF-β neutralization decreased tumor recurrences in mice received 1D11 compared to 13C4 treatment. Tumor bearing mice were treated with 1D11 (n=22) or 13C4 (n=22). The tumors were surgically removed on day 14. The mice with tumor recurrence were recorded on day 42.
No difference of Gr-1+CD11b+ cell production between 1D11 and 13C4 treatment was observed in bone marrow (A) or spleen (B) of tumor bearing mice. The Gr-1+CD11b+ cells were analyzed by flow cytometry (n=3). Bar shows median ± SEM.
No difference in Gr-1+CD11b+ cell apoptosis between 1D11 and 13C4 treatment was observed in blood, spleen and LNs of 4T1 tumor-bearing mice. Cells from peripheral blood, spleen, and LN of mice bearing 4T1 tumor were stained with Gr-1, CD11b, Annexin V and 7-AAD. The percentage of 7-AAD and Annexin positive cells within Gr-1+CD11b+ cell population was analyzed by flow cytometry. The percentage of 7-AAD and Annexin positive cells within Gr-1+CD11b+ cell population was analyzed by flow cytometry. Representative dot plots (A) and statistic results (B) are shown. Error bar = SEM. One of three independent experiments is shown.
TGF-β neutralization decreased lung metastasis in mice with peripheral Gr-1+CD11b+ cells in 60–90% but not those with peripheral Gr-1+CD11b+ cells in 10–60%. A large cohort of tumor bearing mice were given 1D11 (n=20) or 13C4 (n=20) treatment, on day 12, the tumor was surgically removed. Peripheral Gr-1+CD11b+ cell percentage and Lung metastasis were evaluated on Day 42.
Acknowledgments
We thank Barbara Taylor in FACS core of National Cancer Institute for the technical support. We thank Tina Wellington for help with animal care, and participation from Bjorn Ernst as summer intern. We appreciate the critical reading from Drs. Lalage Wakefield, Kathy Flanders, and Alexander Kovalchuk.
Grant Support
This work was supported by Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research to principal investigator Li Yang.
Footnotes
Dr. Scott Lonning is an employee in Genzyme, the manufacture for 1D11 antibody used in the study
Disclosure of Potential Conflict of Interest
No potential conflict of interest was disclosed.
References
- 1.Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10:303–60. [PubMed] [Google Scholar]
- 2.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078–93. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga CL. Inhibition of TGFbeta signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30–7. doi: 10.1016/j.gde.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Dumont N, Arteaga CL. Targeting the TGF beta signaling network in human neoplasia. Cancer Cell. 2003;3:531–6. doi: 10.1016/s1535-6108(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 6.Saunier EF, Akhurst RJ. TGF beta inhibition for cancer therapy. Curr Cancer Drug Targets. 2006;6:565–78. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 7.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3:1011–22. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 8.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 9.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–11. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, Muller WJ, Moses HL. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65:2296–302. doi: 10.1158/0008-5472.CAN-04-3272. [DOI] [PubMed] [Google Scholar]
- 12.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–27. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–60. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz NM, Upton M, Rojas A, Washington MK, Lin L, Chytil A, Sozmen EG, Madison BB, Pozzi A, Moon RT, Moses HL, Grady WM. Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res. 2006;66:9837–44. doi: 10.1158/0008-5472.CAN-06-0890. [DOI] [PubMed] [Google Scholar]
- 15.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 16.Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, Forrester E, Yang L, Wagner KU, Moses HL. Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res. 2008;68:1809–19. doi: 10.1158/0008-5472.CAN-07-5597. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGFbeta Signaling in Mammary Carcinomas Recruits Gr-1+CD11b+ Myeloid Cells that Promote Metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Debusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 24.Marx J. Cancer immunology. Cancer’s bulwark against immune attack: MDS cells. Science. 2008;319:154–6. doi: 10.1126/science.319.5860.154. [DOI] [PubMed] [Google Scholar]
- 25.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood. 2008 doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 27.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jessen KA, Liu SY, Tepper CG, Karrim J, McGoldrick ET, Rosner A, Munn RJ, Young LJ, Borowsky AD, Cardiff RD, Gregg JP. Molecular analysis of metastasis in a polyomavirus middle T mouse model: the role of osteopontin. Breast Cancer Res. 2004;6:R157–69. doi: 10.1186/bcr768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljung BM, Mayall B, Lottich C, Boyer C, Sylvester SS, Leight GS, Siegler HF, Smith HS. Cell dissociation techniques in human breast cancer--variations in tumor cell viability and DNA ploidy. Breast Cancer Res Treat. 1989;13:153–9. doi: 10.1007/BF01806527. [DOI] [PubMed] [Google Scholar]
- 30.Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Invest. 1993;92:2569–76. doi: 10.1172/JCI116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–13. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam JS, Suchar AM, Kang MJ, Stuelten CH, Tang B, Michalowska AM, Fisher LW, Fedarko NS, Jain A, Pinkas J, Lonning S, Wakefield LM. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer. Cancer Res. 2006;66:6327–35. doi: 10.1158/0008-5472.CAN-06-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–23. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–49. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–9. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–15. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b(+)Gr1(+) myeloid cells. Nat Biotechnol. 2007;25:911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 42.Hulper P, Schulz-Schaeffer W, Dullin C, Hoffmann P, Harper J, Kurtzberg L, Lonning S, Kugler W, Lakomek M, Erdlenbruch B. Tumor localization of an anti-TGF-beta antibody and its effects on gliomas. Int J Oncol. 2011;38:51–9. [PubMed] [Google Scholar]
- 43.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 44.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee GT, Hong JH, Kwak C, Woo J, Liu V, Lee C, Kim IY. Effect of dominant negative transforming growth factor-beta receptor type II on cytotoxic activity of RAW 264. 7, a murine macrophage cell line. Cancer Res. 2007;67:6717–24. doi: 10.1158/0008-5472.CAN-06-4263. [DOI] [PubMed] [Google Scholar]
- 46.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, Lawrence S, Danielpour D, et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–43. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terabe M, Ambrosino E, Takaku S, O’Konek JJ, Venzon D, Lonning S, McPherson JM, Berzofsky JA. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–9. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 49.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TGF-β neutralization decreased tumor recurrences in mice received 1D11 compared to 13C4 treatment. Tumor bearing mice were treated with 1D11 (n=22) or 13C4 (n=22). The tumors were surgically removed on day 14. The mice with tumor recurrence were recorded on day 42.
No difference of Gr-1+CD11b+ cell production between 1D11 and 13C4 treatment was observed in bone marrow (A) or spleen (B) of tumor bearing mice. The Gr-1+CD11b+ cells were analyzed by flow cytometry (n=3). Bar shows median ± SEM.
No difference in Gr-1+CD11b+ cell apoptosis between 1D11 and 13C4 treatment was observed in blood, spleen and LNs of 4T1 tumor-bearing mice. Cells from peripheral blood, spleen, and LN of mice bearing 4T1 tumor were stained with Gr-1, CD11b, Annexin V and 7-AAD. The percentage of 7-AAD and Annexin positive cells within Gr-1+CD11b+ cell population was analyzed by flow cytometry. The percentage of 7-AAD and Annexin positive cells within Gr-1+CD11b+ cell population was analyzed by flow cytometry. Representative dot plots (A) and statistic results (B) are shown. Error bar = SEM. One of three independent experiments is shown.
TGF-β neutralization decreased lung metastasis in mice with peripheral Gr-1+CD11b+ cells in 60–90% but not those with peripheral Gr-1+CD11b+ cells in 10–60%. A large cohort of tumor bearing mice were given 1D11 (n=20) or 13C4 (n=20) treatment, on day 12, the tumor was surgically removed. Peripheral Gr-1+CD11b+ cell percentage and Lung metastasis were evaluated on Day 42.