Abstract
The Chronic Kidney Disease in Children study is a cohort of about 600 children with chronic kidney disease (CKD) in the United States and Canada. The independent variable for our observations was a measurement of glomerular filtration rate (GFR) by iohexol disappearance (iGFR) at the first two visits one year apart and during alternate years thereafter. In a previous report, we had developed GFR estimating equations utilizing serum creatinine, blood urea nitrogen, height, gender and cystatin C measured by an immunoturbidimetric method; however the correlation coefficient of cystatin C and GFR (-0.69) was less robust than expected. Therefore, 495 samples were re-assayed using immunonephelometry. The reciprocal of immunonephelometric cystatin C was as well correlated with iGFR as was height/serum creatinine (both 0.88). We developed a new GFR estimating equation using a random 2/3 of 965 person-visits and applied it to the remaining 1/3 as a validation data set. In the validation data set, the correlation of the estimated GFR with iGFR was 0.92 with high precision and no bias; 91% and 45% of eGFR values were within 30% and 10% of iGFR, respectively. This equation works well in children with CKD in a range of GFR from 15 to 75 ml/min per 1.73 m2. Further studies are needed to establish the applicability to children of normal stature and muscle mass, and higher GFR.
Introduction
Cystatin C is a small molecular weight protein that is produced ubiquitously at a regular rate and its reciprocal has been shown to be highly correlated with glomerular filtration rate (GFR) [1-4]. This relationship is independent of inflammatory conditions, muscle mass, gender, body composition, and age (after 12 months) [5;6]. Cystatin C levels are slightly below 1 mg/l in the blood of healthy individuals [7]. The protein is catabolized and almost completely reabsorbed by renal proximal tubular cells, so that little is normally excreted in the urine [8]. Inter-individual variations in cystatin C account for 25% of its biological variability compared to 93% for creatinine [9]. Thus, the upper limit of the population reference interval for cystatin C is seldom more than 3-4 standard deviations (SD) from the mean value of any healthy individual (compared to 13 SD for creatinine).
Cystatin C is commonly quantified using an automated particle-enhanced turbidimetric immunoassay (PETIA) or nephelometric immunoassay (PENIA) to measure the formation of antigen-antibody complexes. Turbidimetry refers to the measurement of transmitted light at the same wavelength and direction as the incident beam, whereas nephelometry refers to detection of light scattered and leaving the solution at some angle other than that of the incident beam [10]. The nephelometric method is more sensitive [10] and performs optimally in dilute solution, making it preferable for small sample volumes encountered in the pediatric population. It should also be noted that there are discrepancies in the determination of cystatin C in the same blood samples between the Dako turbidimetric PETIA assay and the Siemens Healthcare Diagnostics (formerly Dade Behring) PENIA method [11;12] suggesting different reactivity to the antibodies against the cystatin C molecule, different standards, or different substrates [11]. Indeed, in a previous meta-analysis of the correlation between cystatin C and measured GFR [1], the mean correlation coefficient was significantly greater for the immunonephelometric assay (r=0.832) compared to other cystatin C assays (r=0.784).
Some studies in children have shown that the concentration of serum cystatin C is better correlated with GFR than is serum creatinine (Scr) [5;13]. Moreover, subtle decrements in GFR may be more readily detected by changes in serum cystatin C than by Scr [13], due in part to the shorter half-life of cystatin C. Because nephelometric cystatin C levels in serum are well correlated with GFR in adults [14;15] and in children, [2;3;16;17] we sought to develop a new GFR estimating equation using cystatin C that was measured by an immunonephelometric assay and enzymatic creatinine in children with chronic kidney disease (CKD).
The Chronic Kidney Disease in Children (CKiD) study is an NIH-funded cohort study of ~600 children with mild to moderate CKD in the United States and Canada. Its purposes are to determine risk factors for further renal progression, cardiovascular morbidity, growth failure, and neurocognitive impairment. As the primary measure of disease progression is the decline in GFR, the study determines GFR by iohexol plasma disappearance (iGFR). However to improve recruitment and retention, reduce participants’ burden and costs, it was decided to measure iGFR only during alternate annual study visits after the first two visits. Accordingly, GFR would be estimated at the other visits using equations developed from endogenous biomarkers and measurements of body habitus.
We believed it would be important to utilize cystatin C, in addition to height(ht)/Scr, in developing these GFR estimating equations. Such GFR estimating equations have been previously published using CKiD data incorporating cystatin C analyzed by DAKO immunoturbidimetry [18]. We found the ht/Scr term provided substantially more weight to the estimating equation than did the cystatin C term, indicating that the latter analyte was less well correlated with iohexol GFR (iGFR) than was ht/Scr [18]. We questioned whether the methodology of cystatin C analysis was limiting our ability to accurately predict GFR. Whereas creatinine is now being referenced to isotope dilution mass spectrometry (IDMS) [19], the process is just beginning for cystatin C [20].
Results
Paired comparisons of 495 turbidimetric and nephelometric assays of cystatin C
The median turbidimetric (DAKO) cystatin C was 1.88 (IQR = 1.54 to 2.37), compared with the nephelometric (Siemens Healthcare) median of 1.78 (IQR = 1.41 to 2.33). Figure 1 shows the box-percentile plots of the two determinations with solid lines connecting the 495 paired samples. It is clear from the Figure and the corresponding Bland-Altman analysis that the turbidimetric method had a positive bias of 6.9% (p<0.001) and a 5.2% (p=0.103) smaller standard deviation compared to the nephelometric method, and the correlation between two methods supposedly measuring the same analyte was only modest (r = 0.77). More importantly, the reciprocal of cystatin C measured by the nephelometric method showed substantially stronger correlations with iGFR (0.87 for nephelometric vs. 0.74 for turbidimetric), ht/Scr (0.82 vs. 0.70), and 1/BUN (0.66 vs. 0.54).
Figure 1.
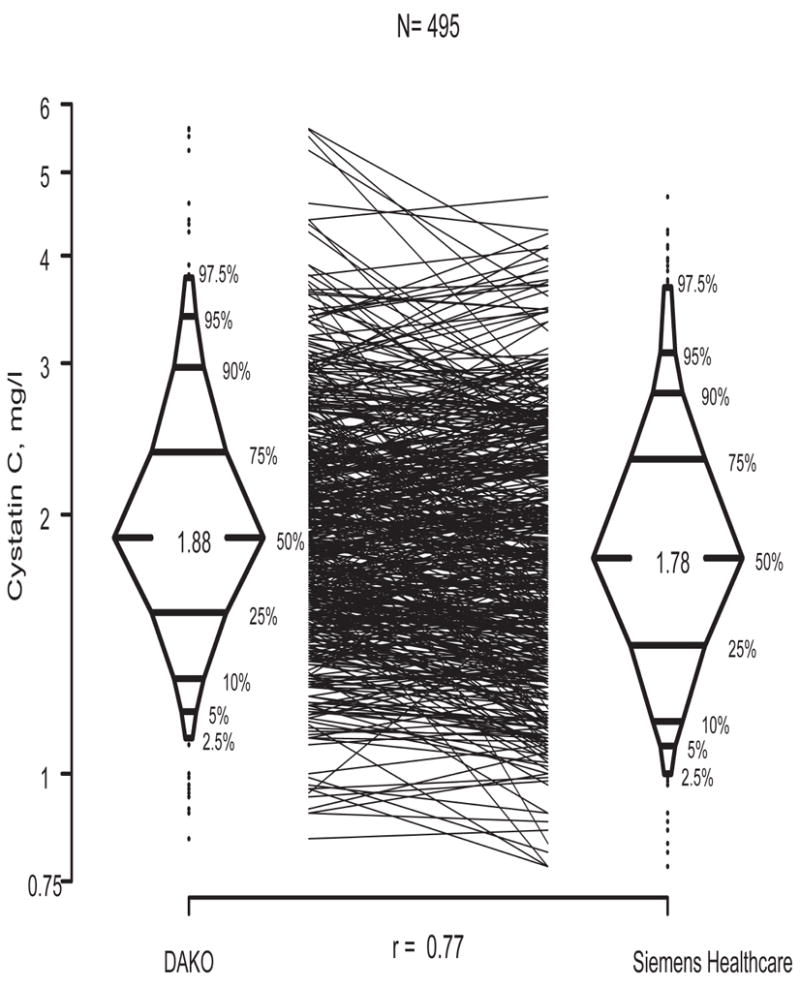
Box-percentile plots of cystatin C measured using the DAKO turbidimetric method vs. the Siemens Healthcare nephelometric method.
Comparison of univariate cystatin C and ht/Scr equations to estimate GFR
In contrast to the poorer diagnostic performance of turbidimetric cystatin C relative to ht/Scr [18], using a total of 965 person-visits with cystatin C measured by the nephelometric method, we found a univariate cystatin C based eGFR of 40.6(1.8/cysC)0.93 with an R2 of 76.1% (Figure 2), which was very close to the R2 of 77.1% provided by the univariate ht/Scr-based eGFR of 42.3(Ht/Scr)0.79 (Figure 3). Furthermore, Figure 4 shows that both methods provided median values that were within 0.7 ml/min of the median of the iGFR; and as is the case of all estimating equations, the dispersions from both equations were equally smaller relative to the dispersion in iGFR. However, the cystatin C-based equation overestimated several of the low iGFRs, and in particular, it can be seen in Figure 2 that for values of 1.8/cystatin < 1/2 (i.e., cystatin C > 3.6 mg/l), several iGFR values were substantially overestimated. On the other hand, ht/Scr performed well throughout the range of measurement (Figure 3).
Figure 2.
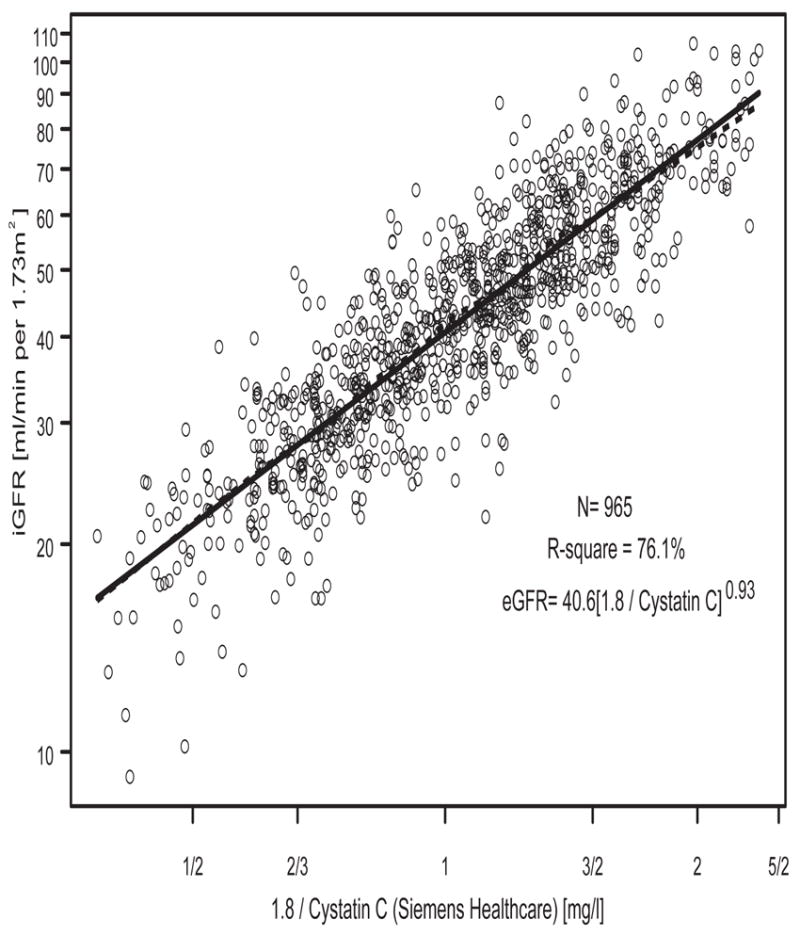
Univariate analysis of log(iGFR) vs. log(1.8/(cystatin C [Siemens Healthcare]) in N=965 person-visits.
Figure 3.
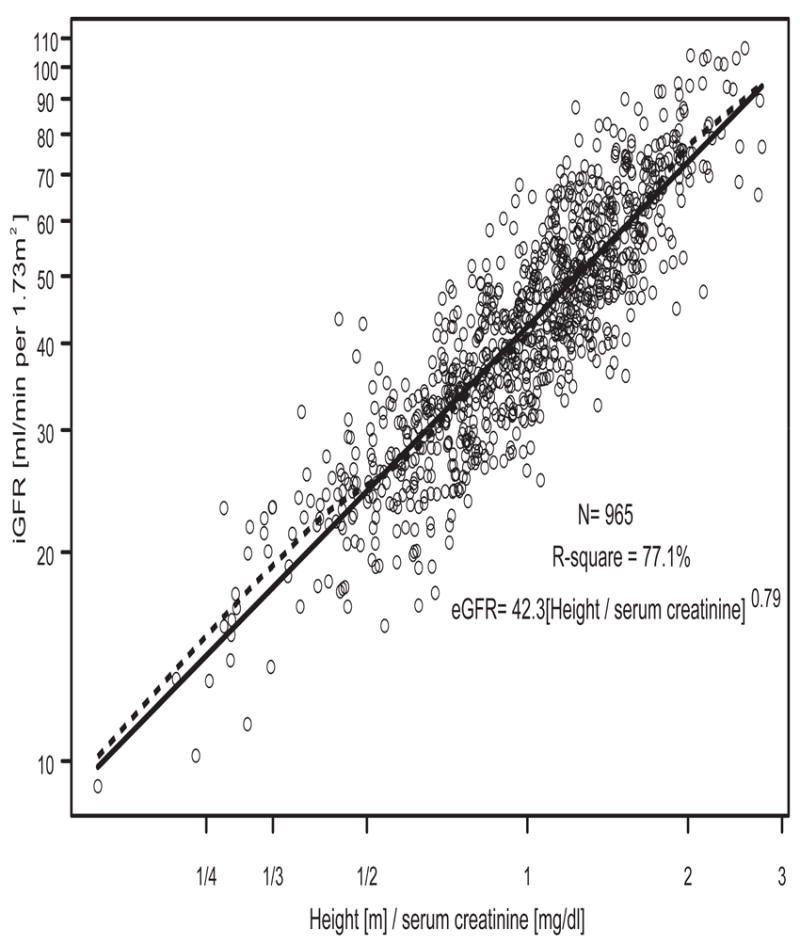
Univariate analysis of log(iGFR) vs. log(ht/Scr) in N=965 person-visits.
Figure 4.
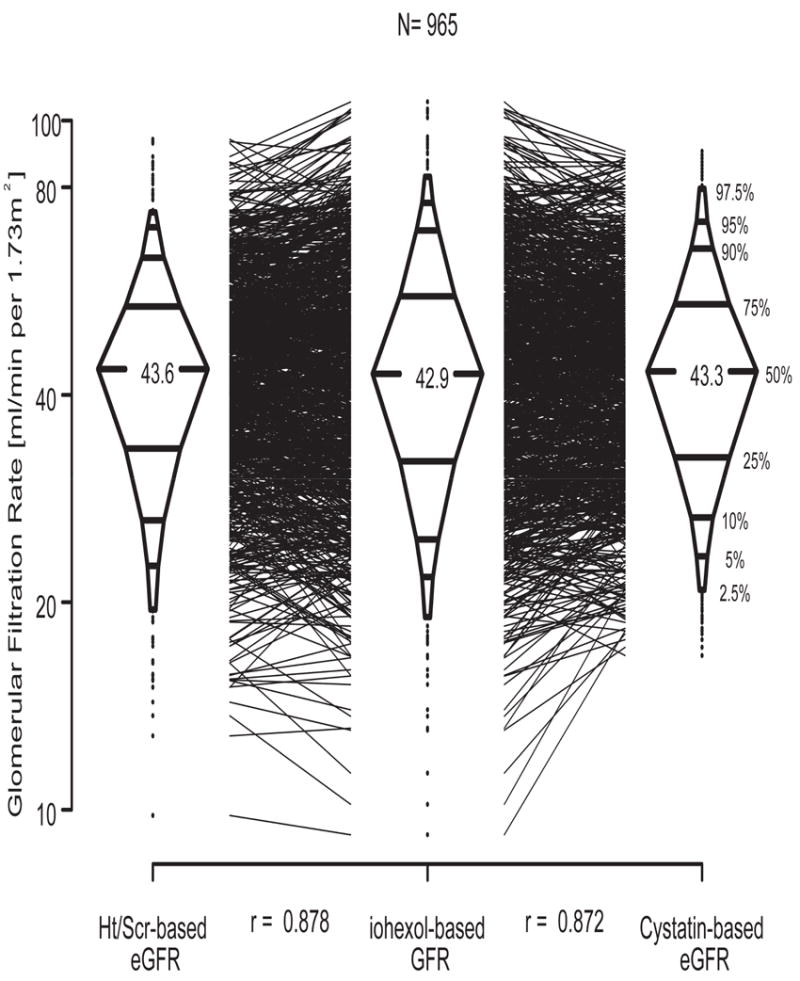
Three box-percentile plots illustrating ht/Scr-based and nephelometric cystatin C-based eGFR vs. iGFR in N=965 person-visits.
There were 176 studies in which eGFR using ht/Scr differed from eGFR using cystatin C by more than 30%, but these estimates were comparably well correlated with iGFR. There was no obvious systematic reason that explained the deviation from iGFR for either estimating equation.
Estimation of GFR from the training set
In order to develop a multivariate equation to estimate GFR including at a minimum cystatin C and ht/Scr, we selected a 2/3 random sample (n=643) from the 965 person-visits and used that as the training set with the complement 1/3 (n=322) utilized for the purposes of validating our proposed equations. The descriptive statistics of the two groups showed as expected, great comparability (Table 1). In the training set of 643 person-visits, univariate eGFR could be determined with approximately similar confidence and accuracy by using cystatin C or ht/Scr (R2 = 77.1 vs. 78.5%, Table 2); the use of BUN alone was clearly inferior. Bivariate analysis showed that the combination of cystatin C and ht/Scr (Model I & II, Table 2, Bivariate models) strongly improved the GFR estimating equation by increasing R2 to 84.3%, decreasing root mean squared error to 0.157, and further increasing accuracy such that 90.1% and 46.5% of eGFRs were within 30% and 10%, respectively, of measured iGFR; other bivariate combinations using BUN were less accurate and less precise. Whereas there was further improvement using a multivariate equation with cystatin C, ht/Scr, and BUN, the most accurate and effective GFR estimating equation incorporated a gender and a separate height term (Final model, Table 2). This equation, eGFR (ml/min per 1.73 m2) = 39.8[ht(m)/Scr(mg/dl)]0.456[1.8/cystatin C (mg/l)]0.418 [30/BUN(mg/dl)]0.079 [1.076male] [ht(m)/1.4]0.179, utilized a format that was originally published using turbidimetric cystatin C values [18], improved R2 to 86.3%, reduced the root mean squared error to 0.147, and improved accuracy so that 91.3% and 48.8% of the estimates were within 30% and 10% of measured iGFR, respectively. The approximately equal exponents for 1.8/cystatin C and ht/Scr suggest that both variables contributed equally to the GFR estimating equation.
Table 1.
Descriptive statistics* of the training and validation samples from the CKiD cohort study
| Training 2/3 random sample (n=643) | Validation 1/3 random sample (n=322) | |
|---|---|---|
| Height, m | 1.4 (1.2, 1.6) | 1.4 (1.2, 1.6) |
| Serum creatinine, mg/dl | 1.3 (1.0, 1.9) | 1.4 (1.0, 2.0) |
| Height[m] / serum creatinine[mg/dl] | 1.0 (0.8, 1.4) | 1.0 (0.7, 1.3) |
| Nephelometric cystatin C, mg/l | 1.7 (1.3, 2.3) | 1.7 (1.3, 2.3) |
| Blood Urea Nitrogen, mg/dl | 28.0 (21.0, 39.0) | 28.0 (22.0, 41.0) |
| Male | 60.5% (389) | 64.3% (207) |
| Iohexol-based GFR, ml/min per 1.73m2 | 43.3 (32.6, 55.6) | 42.4 (31.0, 55.6) |
Median (inter-quartile range) reported for continuous variables, percentages (n) reported for categorical variables.
Table 2.
Precision, goodness of fit, and agreement of estimated glomerular filtration rate (eGFR) derived from coefficients of indicated regression model; N=643 (i.e., 2/3 of 965) training set children-visits of the Chronic Kidney Disease in Children (CKiD) study.
| eGFR = a[height/Scr]b [1.8/Cystatin C]c [30/BUN]d [emale] [height/1.4]f
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | a | b | c | d | e | f |
|
R2 | % of eGFR within 30% of iGFR | % of eGFR within 10% of iGFR | |
| Univariate | |||||||||||
| I | 42.3 ± 0.3 | 0.780 ± 0.016 | 0.184 | 78.5% | 84.3% | 40.4% | |||||
| II | 40.9 ± 0.3 | 0.931 ± 0.020 | 0.190 | 77.1% | 84.9% | 42.3% | |||||
| III | 41.0 ± 0.5 | 0.613 ± 0.024 | 0.280 | 50.2% | 67.8% | 26.1% | |||||
| Bivariate | |||||||||||
| I & II | 41.6 ± 0.3 | 0.443 ± 0.026 | 0.479 ± 0.031 | 0.157 | 84.3% | 90.1% | 46.5% | ||||
| I & III | 41.9 ± 0.3 | 0.662 ± 0.021 | 0.171 ± 0.021 | 0.175 | 80.6% | 86.5% | 42.8% | ||||
| II & III | 40.8 ± 0.3 | 0.796 ± 0.027 | 0.157 ± 0.022 | 0.183 | 78.7% | 84.9% | 42.8% | ||||
| Multivariate I & II & III | 41.5 ± 0.3 | 0.417 ± 0.026 | 0.431 ± 0.032 | 0.088 ± 0.019 | 0.155 | 84.8% | 89.4% | 47.1% | |||
| Final | 39.8 ± 0.4 | 0.456 ± 0.026 | 0.418 ± 0.031 | 0.079 ± 0.018 | 1.076 ± 0.013 | 0.179 ± 0.032 | 0.147 | 86.3% | 91.3% | 48.8% | |
eGFR and iGFR (ml/min per 1.73m2); height (m); Scr, serum creatinine (mg/dl); cystatin C (mg/l); BUN, blood urea nitrogen (mg/dl); MSE, mean square error; entries for a – f are regression coefficient ± standard error
Validation of estimated GFR
We applied the estimated GFR equations described above to the remaining 1/3 validation set (322 person-visits)(Table 3). Univariate ht/Scr- and cystatin C-based equations were nearly equivalent with a correlation with iGFR of 0.84 and 0.85, respectively, but not as good as the bivariate equation using both of these parameters (r=0.90). The multivariate equation using ht/Scr, cystatin C, and BUN was slightly better but the final equation, which added a gender and additional height term, was the best. Specifically, the correlation was 0.92; 91.0% and 45.0% of eGFR values were within 30% and 10% of iGFR, respectively, and most importantly there was no bias.
Table 3.
Application of CKiD prediction equations to 1/3 validation set of 322 person-visits of the Chronic Kidney Disease in Children (CKiD) study with iGFR 44.4 ± 17.2 ml/min per 1.73m2
| Model | eGFR | Bias† | 95% LOA‡ | Correlation | % of eGFR within 30% of iGFR | % of eGFR within 10% of iGFR |
|---|---|---|---|---|---|---|
| Univariate | ||||||
| I: ht/SCr | 43.4 ± 13.5 | -1.0 | -19.2, 17.1 | 0.84 | 80.4% | 36.0% |
| II: Cystatin C | 44.7 ± 15.2 | 0.3 | -17.5, 18.1 | 0.85 | 82.6% | 37.6% |
| III: BUN | 43.0 ± 11.5 | -1.4 | -25.3, 22.5 | 0.71 | 65.5% | 27.3% |
| Bivariate | ||||||
| I & II | 44.1 ± 14.6 | -0.3 | -15.4, 14.7 | 0.90 | 88.8% | 38.2% |
| I & III | 43.4 ± 13.7 | -1.0 | -17.5, 15.5 | 0.87 | 83.9% | 35.1% |
| II & III | 44.6 ± 15.3 | 0.1 | -16.7, 17.0 | 0.87 | 82.3% | 35.4% |
| Multivariate: | ||||||
| I&II&III | 44.1 ± 14.7 | -0.4 | -14.9, 14.2 | 0.90 | 89.1% | 41.9% |
| Final | 44.2 ± 14.9 | -0.2 | -13.4, 13.0 | 0.92 | 91.0% | 45.0% |
eGFR (ml/min per 1.73m2); LOA, Limits of Agreement.
bias = average of 322 (eGFR – iGFR) values, in ml/min per 1.73 m2
95% LOA = bias ± 1.96 ·standard deviation of (eGFR – iGFR)
The univariate cystatin C GFR estimating equation was compared with others utilized in children (Table 4). The relation of eGFR versus cystatin C conforms to a hyperbolic curve for each of the formulas presented in Table 4. However, the Filler equation [4] consistently yielded higher GFR estimates for the same cystatin C value indicating a significant positive bias. The Zappitelli [21] and Hoek [22] equations slightly overestimated GFR at cystatin C values below 1.3 and slightly underestimated GFR at cystatin C values above 1.3. Overall, Zappitelli’s and Hoek’s equations showed similar lack of bias, precision, and accuracy when compared with the CKiD univariate (i.e., cystatin C) equation, but the percentage of eGFR within 30% of iGFR was higher for the CKiD equation.
Table 4.
Application of univariate cystatin C prediction equations to 1/3 validation set of 322 person-visits of the Chronic Kidney Disease in Children (CKiD) study with iGFR 44.4 ± 17.2 ml/min per 1.73m2.
| Equation | eGFR | Bias† | 95% LOA‡ | Correlation | % of eGFR within 30% of iGFR | % of eGFR within 10% of iGFR |
|---|---|---|---|---|---|---|
| CKiDa | 44.7 ± 15.2 | 0.3 | -17.5, 18.1 | 0.85 | 82.6% | 37.6% |
| Zappitelli et al.b | 43.5 ± 18.7 | -1.0 | -20.5, 18.6 | 0.85 | 77.3% | 37.6% |
| Filler et al.c | 53.5 ± 21.9 | 9.0 | -14.0, 32.0 | 0.85 | 64.3% | 21.7% |
| Hoek et al.d | 45.0 ± 18.0 | 0.6 | -18.4, 19.6 | 0.85 | 78.6% | 37.9% |
eGFR (ml/min per 1.73m2); LOA, Limits of Agreement.
bias = average of 322 (eGFR – iGFR) values, in ml/min per 1.73 m2
95% LOA = bias ± 1.96 · standard deviation of (eGFR – iGFR)
70.69(cystatin C)-0.931
75.94(cystatin C)-1.170; Am J Kidney Dis. 2006; 48:221-30.
91.62(cystatin C)-1.123; Pediatr Nephrol. 2003;18:981-5.
-4.32 + 80.35(cystatin C)-1; Nephrol. Dial. Transplant. 2003; 18:2024-2031.
We then assessed whether race, hypertension, weight, serum albumin, or steroid use were different in the person-visits when the difference between eGFR and iGFR was >10 ml/min per 1.73 m2 (“outliers”) compared to person-visits when the difference between eGFR and iGFR was ≤ 10 ml/min per 1.73 m2 using four different estimates of GFR (Scr-based, cystatin C-based, Scr- and cystatin C-based, final model). We found that the “outliers” were consistently associated with heavier weight. More specifically, there were not many measurements contributed by participants with low weight in whom eGFR underestimated iGFR. No other factor significantly predicted a poor estimate of GFR.
Discussion
Value of cystatin C in estimating GFR
The CKiD study is an observational study designed to monitor the effect of CKD progression on cardiovascular morbidity, growth failure, and neurocognitive defects. The independent variable in this study is the determination of GFR. In order to reduce costs and improve recruitment, the 5 hour iohexol plasma disappearance GFR determination is only performed in alternate years after the first two annual study visits. Accordingly, we have developed equations to estimate GFR during the years when iohexol GFR is not performed. Our bedside formula for estimating GFR, 0.413ht(cm)/Scr, has become a useful tool for recruitment and local use and for rapidly estimating GFR when cystatin C values are not available [18]. In addition, while underestimating iGFR by an average of 2.23 ml/min per 1.73 m2, our previously published multivariate GFR estimating equation [18], which utilized the DAKO turbidimetric cystatin C, was more accurate than those equations based on ht/Scr alone and performed better than other published equations within the range of GFR of the subjects in the CKiD study [18]. On the other hand, iGFR was less well correlated with turbidimetric cystatin C than with ht/Scr [18]; this finding was in contrast to the work done by Filler et al [4], which indicated the better diagnostic performance of nephelometric determination of cystatin C over ht/Scr in estimating GFR in children. Given these conflicting results, we felt that the method of measuring cystatin C should be evaluated and performance monitored.
Comparison of turbidimetric and nephelometric cystatin C measures in estimating GFR
Figure 1 showed that paired comparisons of nephelometric and turbidimetric cystatin C values were not as well correlated (r = 0.77) as might be expected for measurement of the same analyte; similar findings have been previously published [11;23] and have been suggested in a meta-analysis [1]. Furthermore, GFR and other endogenous biomarkers (Scr and BUN) were better correlated with nephelometric cystatin C than with the turbidimetric cystatin C measurement. Without comparisons to isotope dilution mass spectrometry standards, it is not possible to comment specifically on which method yields truer values of cystatin C. However, it is evident that the nephelometric method better predicts iGFR. Clearly, standardization of cystatin C calibrators, as recently done for creatinine [24], may help to reconcile some differences in the determination of cystatin C [20;25]. Such calibrators are not yet available for our immunonephelometric assay.
When one estimates GFR using cystatin C alone, the best equation was: eGFR = 40.9(1.8/cystatin C)0.931 or 70.69(cystatin C-0.931), and this provides accuracy and correlation that is comparable to estimates determined from ht/Scr (Tables 2 and 3). Compared with the univariate cystatin C GFR estimating equations of Filler [4], which was generated from children with mild CKD and a mean single slope 99mTc-DTPA single injection GFR measurement of 103 ml/min per 1.73 m2, the CKiD equation was more accurate and less biased (see Table 4). In addition, there were no major differences in bias, precision, or accuracy between the CKiD equation and the univariate cystatin C estimating equations of Zappiteli [21] and Hoek [22]. Interestingly, the Zappiteli equation was developed using children with mild to moderate CKD as indicated by a mean iothalamate infusion clearance of 74 ml/min per 1.73 m2 [21]. The Hoek equation was generated from adults with CKD and a median iothalamate renal clearance of 81 ml/min per 1.73 m2 [22]. Whereas bivariate estimating equations utilizing both cystatin C and ht/Scr proved superior to both univariate equations, as previously shown by us [18], as well as by Zappitelli et al [21] and Bouvet et al [26], improved accuracy, correlation, and precision was obtained by including ht/Scr, cystatin C, and BUN plus an extra component for height and gender (Tables 2 and 3). As seen in Tables 2 and 3, this multivariate equation performed better than any of the univariate or bivariate equations.
Consideration of univariate GFR estimating equations
Whereas the combined prediction equations provide the best accuracy and performance, there are situations in which one of the biomarkers is unusually affected, and probably diminishes its effectiveness. For example, severe reductions of muscle mass or increased muscle mass with heavy weight training will adversely affect serum creatinine without necessarily affecting GFR. Similarly, high doses of glucocorticoids, thyroid disease or significant inflammation may adversely affect cystatin C (rev. in [27]). However, in our sample, C reactive protein and white blood cell count were not significantly correlated with serum cystatin C level (data not shown). Studies in adults suggest that estimates of GFR by cystatin C are not superior to those based on creatinine [25;28;29], even if cystatin C is a better predictor of cardiovascular disease than is creatinine. Indeed, factors other than GFR affect serum cystatin C levels in adults [30;31], but this did not appear to be the case in our CKiD population.
Another issue affecting GFR estimation is the validity of the GFR measurement. The CKiD study utilizes the plasma disappearance of iohexol. Whereas renal inulin clearance is still recognized as the gold standard for measuring GFR, practical matters limit the application of such methodology in children. First, inulin is not readily available and is difficult to measure. Second, since 80% of the recruited CKiD subjects have non-glomerular disease [32], a significant fraction is likely to have vesicoureteral reflux or dysfunctional bladder emptying, making urine collections for inulin assay extremely inaccurate. Parents are also unlikely to allow biennial urine catheterization of their children to measure GFR. Thus, it would seem preferable to use plasma disappearance clearances to avoid this situation. Radioactive studies on a biennial basis also cannot be easily recommended due to the accumulated burden, causing the need to use non-radioactive agents. As iothalamate is secreted by the kidney [33], it was decided to use iohexol as the GFR agent [34]. Most studies indicate close agreement between GFR (measured by inulin clearance) and clearance of iohexol, measured as standard renal clearance or plasma disappearance [35-39], and some authors believe iohexol has become the new gold standard [36].
Optimization of the performance of the iGFR pilot study showed that blood iohexol sampling could be reduced from nine to four time points to characterize a two compartment system [34] and subsequently to three points [40] and two points [41] as the monoexponential (renal) curve could be well correlated with two compartment GFR via coefficients derived from a specific approach as developed originally by Brochner-Mortensen [42].
Use of univariate ht/Scr- and cystatin C-based GFR estimating equations
Based on the pioneering work by Grubb [43] we can recommend an alternate approach to estimating GFR in children. GFR in ml/min per 1.73 m2 is estimated from univariate formulas utilizing ht(m)/Scr and cystatin C (for ht/Scr: eGFR = 42.3(ht/Scr)0.78 or the bedside formula, eGFR = 41.3(ht/Scr), and for cys C: eGFR = 70.69(cys C)-0.931). These two eGFRs are compared and if they agree within a certain specified limit (perhaps 10 ml/min per 1.73 m2 in the CKiD population, or within 15-20% in a general pediatric population), they can be averaged to provide an accurate non-invasive eGFR for the patient or subject. If there is disagreement between the two estimates, and a reason can be found, such as decreased muscle mass, then the eGFR determined from cystatin C would be used; similarly for the use of high doses of corticosteroids, the eGFR determined from ht/Scr could be utilized. When there is no obvious reason for a greater than 10 ml/min discrepancy (or 15-20%) between eGFRs from ht/Scr and cystatin C, it may be necessary to measure GFR. Interestingly, in our CKiD population, the only variable resulting in “outliers” from the univariate (and multivariate) GFR estimating equations was heavier weight. There was no association with race, hypertension, serum albumin, or use of steroids.
Conclusions
Until a universal standardized cystatin C calibrator is available [20], the CKiD study will use the present Siemens Healthcare nephelometric method to measure cystatin C. The equation: eGFR = 39.8* [ht(m)/Scr]0.456[1.8/cysC]0.418[30/BUN]0.0791.076male[ht(m)/1.4]0.179 will be utilized to estimate GFR at study visits when iohexol is not administered. It shows high accuracy and precision and minimal bias in the CKiD population. Confirmation of the usefulness of this equation is desirable in other populations of children with CKD. Further studies are needed to examine its applicability in children with normal stature and muscle mass, and higher GFR. The use of univariate eGFR equations may serve as a reasonable alternative to the multivariate equation when there is a concern about one of the major variables, ht, Scr, or cystatin C. In children with CKD, in most cases, the bedside equation (0.413(ht/(cm)/Scr) allows rapid and reasonably accurate estimation of GFR for clinical use. When measurement and calibration is more broadly available, GFR estimates using cystatin C may also have broad clinical utility.
Methods
Study Participants & Basic Assays
The CKiD study was approved by research review boards at all of the participating sites in the United States and Canada. Eligible individuals were 1 to 16 years of age with initial estimated GFR of 30 to 90 ml/min per 1.73 m2 (estimated by the original Schwartz equation [44;45]) at each local site. Body surface area (BSA) was computed from height and weight using the formula of Haycock et al [46]. Sera was shipped to the CKiD Central Biochemistry Laboratory (CBL) at the University of Rochester Medical Center (URMC) for determination of BUN and enzymatic creatinine. Sera for cystatin C was frozen and stored at -80° C, and shipped quarterly to the Children’s Mercy Hospital (CMH) in Kansas (S. Hellerstein) through July 2008. Subsequently, frozen sera for cystatin C were directly shipped from the sites to the CBL on a quarterly basis.
Specific Assays
At the baseline and all even-numbered study visits GFR was measured by iohexol plasma disappearance [18;34;40]. Iohexol (Omnipaque™) was provided by GE Healthcare (R. Vitti, Amersham Division, Princeton, NJ). Iohexol concentrations were determined by high performance liquid chromatography (HPLC) in the URMC Toxicology laboratory [34]. The inter-assay coefficients of variation of 30 separate runs of quality control iohexol samples was 5.8% at a level of 12.1 mcg/ml and 3.0% at 117 mcg/ml. The intra-assay coefficients of variation obtained from spiked iohexol samples at two levels, six injections per level, on three different days averaged 1.95% at 14.77 mcg/ml and 1.23% at 99.25 mcg/ml. The limit of quantification was 2 mcg/ml.
The training set contained a total of 643 iGFR measurements, 498 (77.5%) measured from four iohexol concentrations taken at 10, 30, 120, and 300 minutes and 145 (22.6%) measured from three iohexol concentrations taken at 120, 240, and 300 minutes. The validation set contained a total of 322 iGFR measurements, 257 (79.8%) measured from four iohexol concentrations taken at 10, 30, 120, and 300 minutes and 65 (20.2%) measured from three iohexol concentrations taken at 120, 240, and 300 minutes. The four point iohexol GFR measurement was previously shown [34] to comprise the fewest number of blood samples needed to characterize the two compartment iohexol plasma disappearance curve. We have shown in a more recent analysis [41] that there was very good agreement between the four point iGFR measurement and the two point iGFR that characterized only the renal (slow) plasma disappearance curve along with a formula relating the slow GFR to the two-component (slow + fast) GFR.
BUN and enzymatic creatinine were analyzed on an Advia 2400 (Siemens Diagnostics, Tarrytown, NY); the creatinine assay had been validated using isotope dilution mass spectrometry reference material [19].
Cystatin C was originally measured at CMH by a turbidimetric assay (Cystatin C Kit K0071; DAKO SD, Copenhagen, Denmark). Cystatin C was subsequently measured at the CBL using a Siemens BN II nephelometer (after July 2008). Twenty μl of 1% sodium azide were added to the sera after assaying for cystatin C at CMH. Volumes in the cryovials were estimated to the nearest 100 μl and corrected by the factor: volume/(volume - 20). Other samples measured at the CBL but not previously at CMH were not diluted in this way and did not require correction for the change in volume. The Siemens nephelometric assay (order number OQNM13) was performed with a six point calibration generated from multiple dilutions of a human cystatin C calibrator obtained from human urine. The intensity of the signal is proportional to the cystatin C sample concentration. Each run included 1-3 sera of known cystatin C concentration to rule out drift of the assay. Each run of 10-60 samples was preceded and followed by measurement of quality controls of low (1.06 mg/l) and high (1.93 mg/l) cystatin C concentrations, and the runs were discarded if the quality controls changed by more than 6% over the course of the assay. The assay range is 0.195 to 7.330 mg/l; the reference range for young healthy persons ranges from 0.53 to 0.95 mg/l [47;48]. The interassay CV is 2.3-3.1%. There is no interference from bilirubin, hemolysis, or lipids. Samples can be stored at least 6 months at -80°C [47], and in preliminary studies, could be frozen and thawed a few times without change in measured concentration.
There were 646 person-visits with dual measurements of cystatin C by turbidimetry and nephelometry. There were 495 person-visits with a concomitant successful iGFR and Scr. In total there were 1580 person-visits with cystatin C measured by nephelometry, for which 965 had successful concurrent iGFR, Scr, and BUN measured.
Statistical Analyses
Bland-Altman analysis [49] in the log scale was used to compare the methods of cystatin C measurement. Univariate linear regression analyses of cystatin C concentration were performed to determine the correlation between the two cystatin C methods and iGFR, ht/Scr, and BUN.
Standard regression techniques for Gaussian data were used to determine the coefficients of GFR estimating equations after logarithmic transformation of the iGFR, cystatin C, ht/Scr, and BUN values. These continuous independent variables were centered at the median values when entered into regression models. In this way the models’ intercept represent the expected value of GFR for the group of individuals with the constellation of predictors at the centering values.
The general regression model was of the form:
where X is a constellation of continuous predictors (e.g., ht[m]/Scr[mg/dl], 1.8/Cystatin C[mg/l], 30/BUN)[mg/dl], ht[m]/1.4, Z is a constellation of categorical variables (e.g., sex), and ε follows a normal distribution with mean zero and variance σ2 [where σ2 corresponds to the expected value of the mean square error (MSE)]. Therefore, a represents the expected value of iGFR for the group whose values of the continuous predictors are at the median values of the study population (e.g., ht[m]/Scr[mg/dl]= 1, cystatin C[mg/l]= 1.8, BUN[mg/dl]= 30) and whose categorical variables are at the reference categories (e.g., female).
The estimated GFR (eGFR= a[X]b[exp(c)]Z) was obtained by using the expected values of the regression coefficients (a, b and c) along with specific values of the independent variables (X and Z) for each individual. To assess the properties of the estimating equations we calculated: (i) the RMSE (=square root of the MSE), which measures the unexplained variability of iGFR; (ii) the R2 which measures the percentage of the variability in iGFR explained by the predictors; (iii) the correlation between the observed iGFR and eGFR; (iv) the percentage of eGFR values that were within 30% and 10% of the corresponding iGFR values calculated on a 2/3 randomly chosen training sample (i.e., the one used to develop the equations, n-=643) and on a validation sample comprised of the remaining 1/3 of the person-visits (n=322).
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and The Children’s Hospital of Philadelphia (Susan Furth, MD, Ph.D.), data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.), and the Central Biochemistry Laboratory at the University of Rochester (George J. Schwartz, MD). The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK82194, U01-DK-66143, U01-DK-66174, and U01-DK-66116). The CKID website is located at http://www.statepi.jhsph.edu/ckid. The authors are grateful to Nicholas Miravalle for his care and attention given to the immunonephelometric cystatin C assay and to Dr. Tai Kwong and Mr. Brian Erway for their excellent performance and maintenance of the iohexol assay. We are also grateful to Dr. Mary Lou Gantzer of Siemens for her support in developing the immunonephelometric cystatin C assay at the Central Biochemistry Laboratory and to GE Healthcare (Dr. Rich Vitti) for providing the Omnipaque 300™ for the iohexol GFR studies.
Reference List
- 1.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 2.Ylinen EA, Ala-Houhala M, Harmoinen APT, Knip M. Cystatin C as a marker for glomerular filtration rate in pediatric patients. Pediatr Nephrol. 1999;13:506–509. doi: 10.1007/s004670050647. [DOI] [PubMed] [Google Scholar]
- 3.Stickle D, Cole B, Hock K, et al. Correlation of plasma concentrations of cystatin C and creatinine to inulin clearance in a pediatric population. Clinical Chemistry. 1998;44:1334–1338. [PubMed] [Google Scholar]
- 4.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 5.Finney H, Newman DJ, Thakkar H, et al. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Childh. 2000;82:71–75. doi: 10.1136/adc.82.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bökenkamp A, Domanetzki M, Zinck R, et al. Reference values for cystatin C serum concentrations in children. Pediatr Nephrol. 1998;12:125–129. doi: 10.1007/s004670050419. [DOI] [PubMed] [Google Scholar]
- 7.Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 1992;38:S20–S27. [PubMed] [Google Scholar]
- 8.Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409–414. doi: 10.3109/00365519609088795. [DOI] [PubMed] [Google Scholar]
- 9.Keevil BG, Kilpatrick ES, Nichols SP, Maylor PW. Biological variation of cystatin C: implications for the assessment of glomerular filtration rate. Clin Chem. 1998;44:1535–1539. [PubMed] [Google Scholar]
- 10.Hills LP, Tiffany TO. Comparison of turbidimetric and light-scattering measurements of immunoglobulins by use of a centrifugal analyzer with absorbance and fluorescence/light-scattering optics. Clin Chem. 1980;26:1459–1466. [PubMed] [Google Scholar]
- 11.Flodin M, Hansson LO, Larsson A. Variations in assay protocol for the Dako cystatin C method may change patient results by 50% without changing the results for controls. Clin Chem Lab Med. 2006;44:1481–1485. doi: 10.1515/CCLM.2006.271. [DOI] [PubMed] [Google Scholar]
- 12.Finney H, Newman DJ, Gruber W, et al. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II) Clin Chem. 1997;43:1016–1022. [PubMed] [Google Scholar]
- 13.Dworkin LD. Serum cystatin C as a marker of glomerular filtration rate. Current Opinion in Nephrology and Hypertension. 2001;10:551–553. doi: 10.1097/00041552-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kid Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 15.Christensson A, Ekberg J, Grubb A, et al. Serum cystatin C is a more sensitive and more accurate marker of glomerular filtration rate than enzymatic measurements of creatinine in renal transplantation. Nephron Physiol. 2003;94:19–27. doi: 10.1159/000071287. [DOI] [PubMed] [Google Scholar]
- 16.Filler G, Priem F, Lepage N, et al. β-Trace protein, cystatin C, β2-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clinical Chemistry. 2002;48:729–736. [PubMed] [Google Scholar]
- 17.Filler G, Priem F, Vollmer I, et al. Diagnostic sensitivity of serum cystatin for impaired glomerular filtration rate. Pediatr Nephrol. 1999;13:501–505. doi: 10.1007/s004670050646. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Kwong T, Erway B, et al. Validation of creatinine assays utilizing HPLC and IDMS traceable standards in sera of children. Pediatr Nephrol. 2009;24:113–119. doi: 10.1007/s00467-008-0957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubb A, Blirup-Jensen S, Lindstrom V, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 21.Zappitelli M, Parvex P, Joseph L, et al. Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis. 2006;48:221–230. doi: 10.1053/j.ajkd.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 22.Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 23.Flodin M, Larsson A. Performance evaluation of a particle-enhanced turbidimetric cystatin C assay on the Abbott ci8200 analyzer. Clin Biochem. 2009;42:873–876. doi: 10.1016/j.clinbiochem.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Inker LA, Okparavero A. Cystatin C as a marker of glomerular filtration rate: prospects and limitations. Curr Opin Nephrol Hypertens. 2011;20:631–639. doi: 10.1097/MNH.0b013e32834b8850. [DOI] [PubMed] [Google Scholar]
- 26.Bouvet Y, Bouissou F, Coulais Y, et al. GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol. 2006;21:1299–1306. doi: 10.1007/s00467-006-0145-z. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 28.Eriksen BO, Mathisen UD, Melsom T, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78:1305–1311. doi: 10.1038/ki.2010.321. [DOI] [PubMed] [Google Scholar]
- 29.Eriksen BO, Mathisen UD, Melsom T, et al. The Role of Cystatin C in Improving GFR Estimation in the General Population. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 32.Furth SL, Abraham AG, Jerry-Fluker J, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2132–2140. doi: 10.2215/CJN.07100810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odlind B, Hällgren R, Sohtell M, Lindström B. Is 125I iothalamate an ideal marker for glomerular filtration? Kidney Int. 1985;27:9–16. doi: 10.1038/ki.1985.3. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GJ, Furth S, Cole S, et al. Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 35.Gaspari F, Perico N, Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6:257–263. doi: 10.1681/ASN.V62257. [DOI] [PubMed] [Google Scholar]
- 36.Brown SCW, O’Reilly PH. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol. 1991;146:675–679. doi: 10.1016/s0022-5347(17)37891-6. [DOI] [PubMed] [Google Scholar]
- 37.Rahn KH, Heidenreich S, Bruckner D. How to assess glomerular function and damage in humans. J Hypertens. 1999;17:309–317. doi: 10.1097/00004872-199917030-00002. [DOI] [PubMed] [Google Scholar]
- 38.Lindblad HG, Berg UB. Comparative evaluation of iohexol and inulin clearance for glomerular filtration rate determinations. Acta Paediatr. 1994;83:418–422. doi: 10.1111/j.1651-2227.1994.tb18133.x. [DOI] [PubMed] [Google Scholar]
- 39.Berg UB, Back R, Celsi G, et al. Comparison of plasma clearance of iohexol and urinary clearance of inulin for measurement of GFR in children. Am J Kidney Dis. 2011;57:55–61. doi: 10.1053/j.ajkd.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GJ, Abraham AG, Furth SL, et al. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77:65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng DK, Schwartz GJ, Jacobson LP, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80:423–430. doi: 10.1038/ki.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30:271–274. doi: 10.3109/00365517209084290. [DOI] [PubMed] [Google Scholar]
- 43.Grubb A. Non-invasive estimation of glomerular filtration rate (GFR). The Lund model: Simultaneous use of cystatin C- and creatinine-based GFR-prediction equations, clinical data and an internal quality check. Scand J Clin Lab Invest. 2010;70:65–70. doi: 10.3109/00365511003642535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 45.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 46.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 47.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 48.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]


